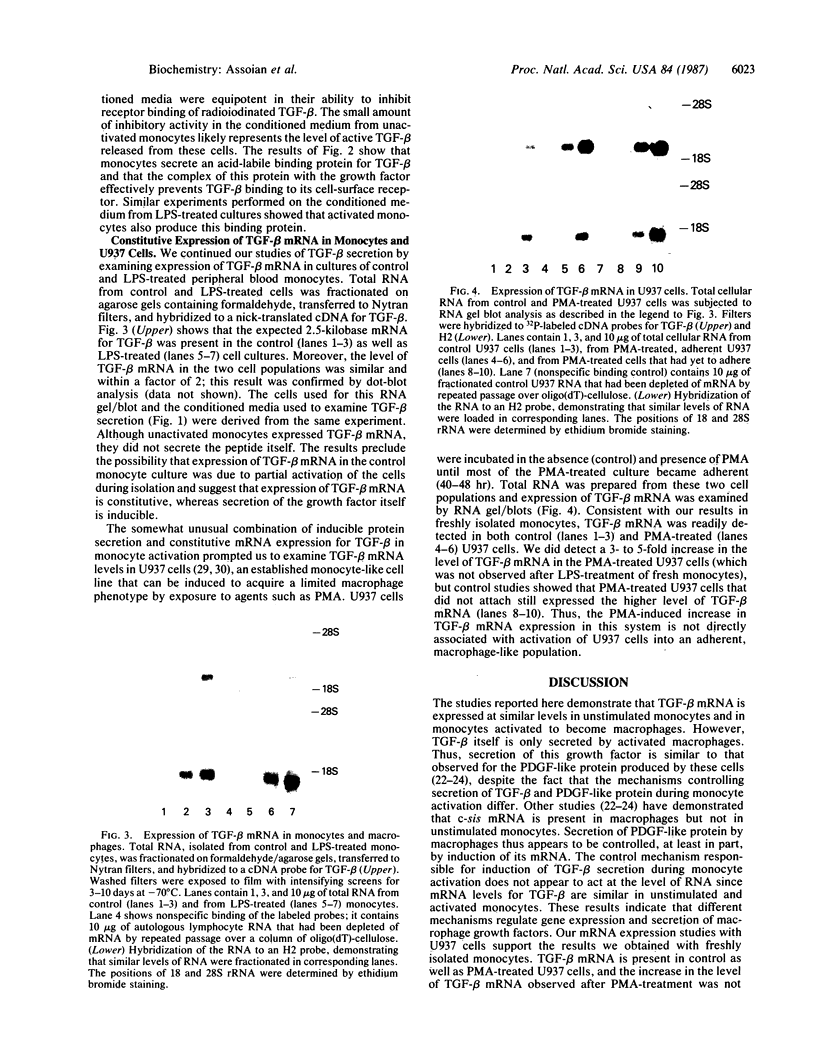
Abstract
Alveolar macrophages activated with concanavalin A and peripheral blood monocytes activated with lipopolysaccharide secrete type beta transforming growth factor (TGF-beta). There is minimal TGF-beta secretion in unactivated monocytes, even though TGF-beta mRNA is expressed in these cells at a level similar to that in activated, lipopolysaccharide-treated cultures. U937 lymphoma cells, which have monocytic characteristics, also express mRNA for TGF-beta. Freshly isolated monocytes, both control and lipopolysaccharide-treated, secrete an acid-labile binding protein that inhibits TGF-beta action. We conclude the following: (i) that expression of TGF-beta mRNA is unrelated to monocyte activation, (ii) that secretion of TGF-beta is induced by monocyte activation, and (iii) that cosecretion of TGF-beta and its monocyte/macrophage-derived binding protein may modulate growth factor action. In contrast, monocytic expression of other growth factor genes, such as the B chain of platelet-derived growth factor, is not constitutive and requires activation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anzano M. A., Roberts A. B., Meyers C. A., Komoriya A., Lamb L. C., Smith J. M., Sporn M. B. Synergistic interaction of two classes of transforming growth factors from murine sarcoma cells. Cancer Res. 1982 Nov;42(11):4776–4778. [PubMed] [Google Scholar]
- Assoian R. K. Biphasic effects of type beta transforming growth factor on epidermal growth factor receptors in NRK fibroblasts. Functional consequences for epidermal growth factor-stimulated mitosis. J Biol Chem. 1985 Aug 15;260(17):9613–9617. [PubMed] [Google Scholar]
- Assoian R. K., Grotendorst G. R., Miller D. M., Sporn M. B. Cellular transformation by coordinated action of three peptide growth factors from human platelets. 1984 Jun 28-Jul 4Nature. 309(5971):804–806. doi: 10.1038/309804a0. [DOI] [PubMed] [Google Scholar]
- Assoian R. K., Komoriya A., Meyers C. A., Miller D. M., Sporn M. B. Transforming growth factor-beta in human platelets. Identification of a major storage site, purification, and characterization. J Biol Chem. 1983 Jun 10;258(11):7155–7160. [PubMed] [Google Scholar]
- Assoian R. K., Sporn M. B. Type beta transforming growth factor in human platelets: release during platelet degranulation and action on vascular smooth muscle cells. J Cell Biol. 1986 Apr;102(4):1217–1223. doi: 10.1083/jcb.102.4.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs C. B., Proper J. A., Tucker R. F., Moses H. L. Serum contains a platelet-derived transforming growth factor. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5312–5316. doi: 10.1073/pnas.79.17.5312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosman D., Khoury G., Jay G. Three classes of mouse H-2 messenger RNA distinguished by analysis of cDNA clones. Nature. 1982 Jan 7;295(5844):73–76. doi: 10.1038/295073a0. [DOI] [PubMed] [Google Scholar]
- Deeley R. G., Gordon J. I., Burns A. T., Mullinix K. P., Binastein M., Goldberg R. F. Primary activation of the vitellogenin gene in the rooster. J Biol Chem. 1977 Nov 25;252(22):8310–8319. [PubMed] [Google Scholar]
- Derynck R., Jarrett J. A., Chen E. Y., Eaton D. H., Bell J. R., Assoian R. K., Roberts A. B., Sporn M. B., Goeddel D. V. Human transforming growth factor-beta complementary DNA sequence and expression in normal and transformed cells. Nature. 1985 Aug 22;316(6030):701–705. doi: 10.1038/316701a0. [DOI] [PubMed] [Google Scholar]
- Frolik C. A., Wakefield L. M., Smith D. M., Sporn M. B. Characterization of a membrane receptor for transforming growth factor-beta in normal rat kidney fibroblasts. J Biol Chem. 1984 Sep 10;259(17):10995–11000. [PubMed] [Google Scholar]
- Ignotz R. A., Massagué J. Transforming growth factor-beta stimulates the expression of fibronectin and collagen and their incorporation into the extracellular matrix. J Biol Chem. 1986 Mar 25;261(9):4337–4345. [PubMed] [Google Scholar]
- Kehrl J. H., Wakefield L. M., Roberts A. B., Jakowlew S., Alvarez-Mon M., Derynck R., Sporn M. B., Fauci A. S. Production of transforming growth factor beta by human T lymphocytes and its potential role in the regulation of T cell growth. J Exp Med. 1986 May 1;163(5):1037–1050. doi: 10.1084/jem.163.5.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence D. A., Pircher R., Krycève-Martinerie C., Jullien P. Normal embryo fibroblasts release transforming growth factors in a latent form. J Cell Physiol. 1984 Oct;121(1):184–188. doi: 10.1002/jcp.1041210123. [DOI] [PubMed] [Google Scholar]
- Lawrence W. T., Norton J. A., Sporn M. B., Gorschboth C., Grotendorst G. R. The reversal of an Adriamycin induced healing impairment with chemoattractants and growth factors. Ann Surg. 1986 Feb;203(2):142–147. doi: 10.1097/00000658-198602000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibovich S. J., Ross R. The role of the macrophage in wound repair. A study with hydrocortisone and antimacrophage serum. Am J Pathol. 1975 Jan;78(1):71–100. [PMC free article] [PubMed] [Google Scholar]
- Leof E. B., Proper J. A., Goustin A. S., Shipley G. D., DiCorleto P. E., Moses H. L. Induction of c-sis mRNA and activity similar to platelet-derived growth factor by transforming growth factor beta: a proposed model for indirect mitogenesis involving autocrine activity. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2453–2457. doi: 10.1073/pnas.83.8.2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley T. J., Anagnou N. P., Pepe G., Nienhuis A. W. RNA processing errors in patients with beta-thalassemia. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4775–4779. doi: 10.1073/pnas.79.15.4775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin T. R., Altman L. C., Albert R. K., Henderson W. R. Leukotriene B4 production by the human alveolar macrophage: a potential mechanism for amplifying inflammation in the lung. Am Rev Respir Dis. 1984 Jan;129(1):106–111. doi: 10.1164/arrd.1984.129.1.106. [DOI] [PubMed] [Google Scholar]
- Martinet Y., Bitterman P. B., Mornex J. F., Grotendorst G. R., Martin G. R., Crystal R. G. Activated human monocytes express the c-sis proto-oncogene and release a mediator showing PDGF-like activity. Nature. 1986 Jan 9;319(6049):158–160. doi: 10.1038/319158a0. [DOI] [PubMed] [Google Scholar]
- Massagué J., Kelly B., Mottola C. Stimulation by insulin-like growth factors is required for cellular transformation by type beta transforming growth factor. J Biol Chem. 1985 Apr 25;260(8):4551–4554. [PubMed] [Google Scholar]
- Masui T., Wakefield L. M., Lechner J. F., LaVeck M. A., Sporn M. B., Harris C. C. Type beta transforming growth factor is the primary differentiation-inducing serum factor for normal human bronchial epithelial cells. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2438–2442. doi: 10.1073/pnas.83.8.2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mornex J. F., Martinet Y., Yamauchi K., Bitterman P. B., Grotendorst G. R., Chytil-Weir A., Martin G. R., Crystal R. G. Spontaneous expression of the c-sis gene and release of a platelet-derived growth factorlike molecule by human alveolar macrophages. J Clin Invest. 1986 Jul;78(1):61–66. doi: 10.1172/JCI112574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F., Murray H. W., Cohn Z. A. The macrophage as an effector cell. N Engl J Med. 1980 Sep 11;303(11):622–626. doi: 10.1056/NEJM198009113031106. [DOI] [PubMed] [Google Scholar]
- Pircher R., Lawrence D. A., Jullien P. Latent beta-transforming growth factor in nontransformed and Kirsten sarcoma virus-transformed normal rat kidney cells, clone 49F. Cancer Res. 1984 Dec;44(12 Pt 1):5538–5543. [PubMed] [Google Scholar]
- ROSS R., BENDITT E. P. Wound healing and collagen formation. I. Sequential changes in components of guinea pig skin wounds observed in the electron microscope. J Biophys Biochem Cytol. 1961 Dec;11:677–700. doi: 10.1083/jcb.11.3.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph P., Williams N., Moore M. A., Litcofsky P. B. Induction of antibody-dependent and nonspecific tumor killing in human monocytic leukemia cells by nonlymphocyte factors and phorbol ester. Cell Immunol. 1982 Aug;71(2):215–223. doi: 10.1016/0008-8749(82)90257-x. [DOI] [PubMed] [Google Scholar]
- Roberts A. B., Anzano M. A., Lamb L. C., Smith J. M., Sporn M. B. New class of transforming growth factors potentiated by epidermal growth factor: isolation from non-neoplastic tissues. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5339–5343. doi: 10.1073/pnas.78.9.5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A. B., Anzano M. A., Wakefield L. M., Roche N. S., Stern D. F., Sporn M. B. Type beta transforming growth factor: a bifunctional regulator of cellular growth. Proc Natl Acad Sci U S A. 1985 Jan;82(1):119–123. doi: 10.1073/pnas.82.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A. B., Sporn M. B., Assoian R. K., Smith J. M., Roche N. S., Wakefield L. M., Heine U. I., Liotta L. A., Falanga V., Kehrl J. H. Transforming growth factor type beta: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4167–4171. doi: 10.1073/pnas.83.12.4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R. The fibroblast and wound repair. Biol Rev Camb Philos Soc. 1968 Feb;43(1):51–96. doi: 10.1111/j.1469-185x.1968.tb01109.x. [DOI] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis--an update. N Engl J Med. 1986 Feb 20;314(8):488–500. doi: 10.1056/NEJM198602203140806. [DOI] [PubMed] [Google Scholar]
- Seyedin S. M., Thompson A. Y., Bentz H., Rosen D. M., McPherson J. M., Conti A., Siegel N. R., Galluppi G. R., Piez K. A. Cartilage-inducing factor-A. Apparent identity to transforming growth factor-beta. J Biol Chem. 1986 May 5;261(13):5693–5695. [PubMed] [Google Scholar]
- Shimokado K., Raines E. W., Madtes D. K., Barrett T. B., Benditt E. P., Ross R. A significant part of macrophage-derived growth factor consists of at least two forms of PDGF. Cell. 1985 Nov;43(1):277–286. doi: 10.1016/0092-8674(85)90033-9. [DOI] [PubMed] [Google Scholar]
- Sporn M. B., Harris E. D., Jr Proliferative diseases. Am J Med. 1981 Jun;70(6):1231–1235. doi: 10.1016/0002-9343(81)90832-9. [DOI] [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B., Shull J. H., Smith J. M., Ward J. M., Sodek J. Polypeptide transforming growth factors isolated from bovine sources and used for wound healing in vivo. Science. 1983 Mar 18;219(4590):1329–1331. doi: 10.1126/science.6572416. [DOI] [PubMed] [Google Scholar]
- Stevenson H. C., Dekaban G. A., Miller P. J., Benyajati C., Pearson M. L. Analysis of human blood monocyte activation at the level of gene expression. Expression of alpha interferon genes during activation of human monocytes by poly IC/LC and muramyl dipeptide. J Exp Med. 1985 Mar 1;161(3):503–513. doi: 10.1084/jem.161.3.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson H. C. Isolation of human mononuclear leukocyte subsets by countercurrent centrifugal elutriation. Methods Enzymol. 1984;108:242–249. doi: 10.1016/s0076-6879(84)08087-3. [DOI] [PubMed] [Google Scholar]
- Sundström C., Nilsson K. Establishment and characterization of a human histiocytic lymphoma cell line (U-937). Int J Cancer. 1976 May 15;17(5):565–577. doi: 10.1002/ijc.2910170504. [DOI] [PubMed] [Google Scholar]
- Tucker R. F., Shipley G. D., Moses H. L., Holley R. W. Growth inhibitor from BSC-1 cells closely related to platelet type beta transforming growth factor. Science. 1984 Nov 9;226(4675):705–707. doi: 10.1126/science.6093254. [DOI] [PubMed] [Google Scholar]