Abstract
Plasmodium transmission from the human host to the mosquito depends on the ability of gametocytes to differentiate into ookinetes, the invasive form of the parasite that invades and establishes infection in the mosquito midgut. The biology of P. falciparum ookinetes is poorly understood, because sufficient quantities of this stage of this parasite species have not been obtained for detailed study. This report details methods to optimize production of P. falciparum sexual stage parasites, including ookinetes. Flow cytometric sorting was used to separate diploid/tetraploid zygotes and ookinetes from haploid gametetocytes and unfertilized gametes based on DNA content. Consistent production of 106–107 P. falciparum ookinetes per 10 mL culture was observed, with ookinete transformation present in 10–40% of all parasite forms. Transmission electron micrographs of cultured parasites confirmed ookinete development.
Introduction
Malaria affects more than 500 million people and kills an estimated 900,000 people each year.1 Interest in the potential of transmission-blocking vaccines to control or even eradicate malaria has recently increased; such vaccines work by inducing antibodies against components of the parasite's sexual stage forms, hence reducing infectivity of the reservoir, humans, for the obligate vector, Anopheles mosquitoes.2–11 More detailed understanding of Plasmodium falciparum transmission stage biology, particularly ookinetes, would directly contribute to human transmission-blocking vaccine development.
Plasmodium sexual development occurs in the mosquito midgut. Mature gametocytes taken up with the mosquito blood meal emerge from erythrocytes as gametes. Male microgametes fertilize female macrogametes to generate zygotes. The parasite transforms into a motile, constitutively secreting and invasive ookinete at the same time that it is undergoing genetic recombination.12–16 Sexual stage-specific antigens are potential targets for transmission-blocking antibodies, which has been most robustly shown in animal models of malaria.16,17 Because of experimental challenges, the biology of few sexual stage antigens of P. falciparum is understood in any detail.18 Recent successes have been achieved in generating P. falciparum sexual stage parasites in vitro, including a recent study by Ghosh and others.19,20 However, a detailed study of the biology of transmission-blocking antigens is still limited by the inability to generate large quantities of P. falciparum sexual stage parasites, particularly ookinetes, in vitro.
Here, we report optimization of methods that allow for the consistent generation of 105–106 P. falciparum ookinetes per 10 mL in vitro culture.19,21–25 Flow cytometry sorting based on detection of DNA content allowed for the enrichment of different sexual state parasite forms that allow for the direct study of the different stages of P. falciparum sexual stage parasites.
High-Yield Ookinete Production
The P. falciparum strain NF54 was maintained in continuous asexual culture according to standard protocol.26 Human blood used for in vitro culture was freshly drawn from volunteers after informed consent according to a protocol approved by the University of California, San Diego Human Subjects Protection Program. Gametocytes were cultured as previously described27 (Appendix), and the overall procedure is schematized in Figure 1. Morphologically mature microgametocytes were seen as early as 12 days for microgametocytes and 14 days macrogametocytes and as late as 22 days for both. To compensate for this lack of synchronization, gametocyte cultures were started 2–3 days apart to ensure that cultures containing mature microgametocytes could be mixed with cultures containing mature macrogametocytes.26–29
Figure 1.
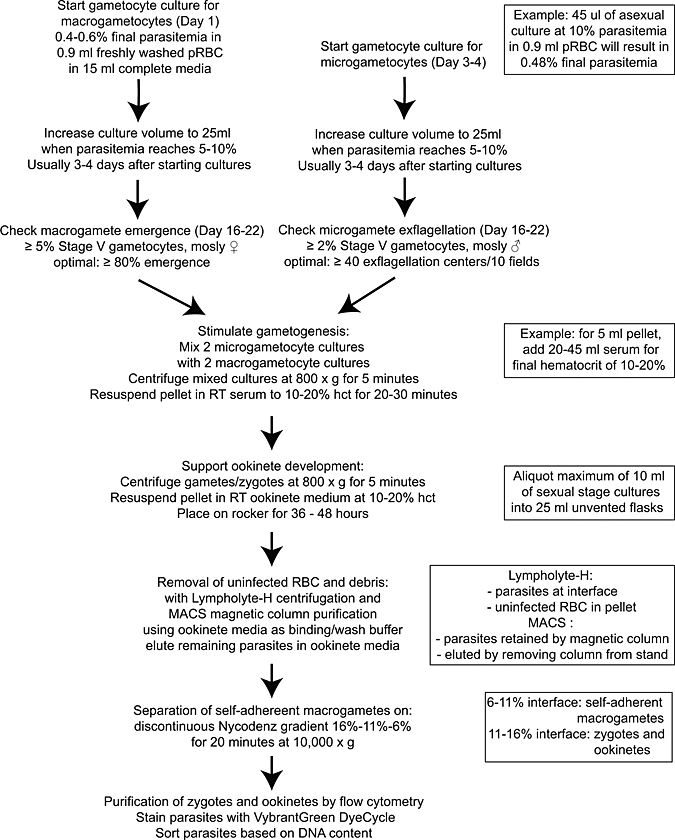
Flow chart of P. falciparum sexual stage parasite culture protocol. pRBC = packed red blood cells; ♂ = male; ♀ = female; Δ = heat inactivated; RT = room temperature (19–21°C); hct = hematocrit.
On days 14–17 of culture, mature microgametocytes were tested for the ability to exflagellate and emerge under standard conditions (Appendix).30 Two gametocyte cultures with microgametocytes confirmed to be functional (≥ 4 exflagellation centers per 40× field) were combined with two gametocyte cultures with emergence-competent macrogametocytes (≥ 80% macrogamete emergence). The combined parasites were centrifuged, and the pellet was resuspended in heat-inactivated AB+ human serum to 10–20% hematocrit at 19–23°C (ambient laboratory temperature) for 30 minutes for gamete maturation and zygote fertilization. Sexual stage parasites were centrifuged, and the pellet was resuspended in freshly prepared filter-sterilized ookinete medium (RPMI–1640, 25 mM Hepes, 2 mM l-glutamine, 2 g/L NaHCO3, 50 mg/L hypoxanthine, 15% heat-inactivated AB+ human serum or heat-inactivated fetal bovine serum [FBS], pH 8.2–8.4, with NaOH) to 10–20% hematocrit. Centrifugation steps were done at 19–23°C and 800 × g without brake. Parasites in ookinete medium were transferred in 10-mL aliquots to 25-cm2 flasks and gently rocked at 19–23°C for 36–48 hours, although ookinetes could be seen in culture for up to 72 hours in ookinete medium.
The generation of large quantities of P. falciparum ookinetes was dependent on the presence of mature micro- and macrogametes, which, in turn, was dependent on the production of mature micro- and macrogametocytes (Figure 2). Gametocyte cultures contained up to 8.5% gametocytemia, and 50–90% of these gametocytes were stage V gametocytes (Table 1). Optimized cultures had an average ookinete density of 25%, with a yield of 5–50 × 106 ookinetes per 10 mL of ookinete culture (Table 2). This yield is at least 4- to 8-fold better than previously described methods.23,31
Figure 2.
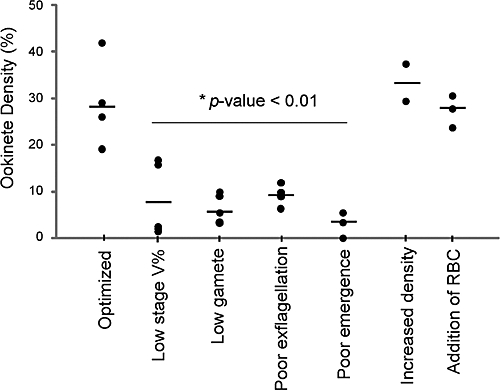
Gametocyte and gamete maturity significantly affect ookinete production. Ookinete density in sexual stage cultures depends on gametocytemia and gamete maturation. Sexual stage parasite cultures containing 19–42% ookinetes were consistently produced by mixing ≥ 5% macrogametocytemic cultures with ≥ 2% microgametocytemic cultures at a final hematocrit of 10–20%. Optimized = use of cultures with less than optimized parameters resulted in sexual stage cultures with ookinete densities of 3–17%. Low stage V%* = gametocyte cultures with less than 2% mature gametocytemia ookinetes. Low gamete* = cultures with low rates of both gamete emergence and exflagellation, defined as < 80% emergence and less than an average of four exflagellating centers per 40× field. Low exflagellation* = cultures with less than an average of four exflagellating centers per 40× field mixed with cultures exhibiting ≥ 80% macrogamete emergence. Low emergence* = cultures exhibiting ≥ 80% macrogamete emergence mixed with microgamete cultures with an average of four exflagellating centers per 40× field. Other factors that reportedly improved parasite transmission to mosquitoes, namely increasing parasite density and adding fresh drawn erythrocytes to the cultures, did not significantly improve ookinete development in vitro compared with other optimized conditions described here.29 Increased density = increased density of sexual stage cultures by increasing final hematocrit from 10% to 20% (P value < 0.842). Addition of RBC = addition of erythrocytes drawn from volunteers and washed within 24 hours of sexual stage parasite cultures (P value = 0.489). Statistical significance was determined by analysis of variance (ANOVA). Asterisk indicates P value < 0.01. Of note, lack of a statistically significant difference may be caused by low sample numbers, particularly in the increased density and addition of RBC groups.
Table 1.
In vitro P. falciparum gametocyte production
| Culture number | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Asexual parasitemia | 0.3% | 0% | 0% | 0.4% | 0.4% |
| Immature gametocytemia | 3% | 0.5% | 2.2% | 1% | 2% |
| Stage V gametocytemia | 5% | 6% | 6.3% | 5% | 5% |
| Total gametocyte yield | 20 × 107 | 3.3 × 107 | 7.5 × 107 | 5 × 107 | 10 × 107 |
Gametocytemia of unpurified P. falciparum gametocyte cultures was determined when the majority of gametocytes were mature, as early as 16 days and as late as 22 days of culture; 1,100–2,400 cells were counted in 10 fields. Average yields were 2–20 × 107 gametocytes per 25 mL culture. Asexual stage-to-gametocyte transformation efficiencies for five representative gametocyte cultures used for ookinete preparations are shown.
Table 2.
P. falciparum ookinete densities resulting from in vitro culture
| Culture number | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Gametocytes (%) | 39% | 45% | 37% | 32% | 13% |
| Macrogametes (%)* | 24% | 16% | 12% | 31% | 67% |
| Round forms (%)† | 11% | 9% | 9% | 11% | 8% |
| Ookinetes (%) | 26% | 31% | 42% | 26% | 12% |
| Ookinete yield | 10 × 106 | 57 × 106 | 31 × 106 | 10 × 106 | 24 × 106 |
Purified sexual stage parasites were stained with Leukostat and examined by light microscopy to determine sexual stage parasite densities; 300–1,000 parasites in 10 fields were counted. Yields were 5–60 × 106 ookinetes per 10 mL culture. Transformation efficiencies for five representative cultures are shown.
Self-adherent macrogametes.
Other round forms including non–self-adherent macrogametes, zygotes, and retort ookinetes that could not be definitively distinguished from each other by light microscopy.
Purification of P. Falciparum Sexual Stage Parasites
P. falciparum sexual stage cultures were produced as mixtures of uninfected red blood cells, asexual stage parasites, gametocytes, macrogametes, zygotes, and ookinetes. Stage-specific enrichment was approached using multiple purification methods, including single-step density centrifugation, magnetic separation, discontinuous density gradient centrifugation, and flow cytometry sorting. The majority of uninfected erythrocytes were removed from sexual stage parasite cultures by single-step density gradient centrifugation (Lympholyte-H; Cedarlane Laboratories, Burlington, NC) according to manufacturer's instructions. Parasites were collected from the gradient interface, washed two times in ookinete medium, and further purified by magnetic separation. Purification of parasites using density gradient removed approximately 90% of red blood cells. Further purification of parasites using magnetic separation resulted in removal of approximately 95–99% of red blood cells and facilitated sorting of parasites by flow cytometry. A MidiMACS magnetic separator with an LD-50 column was used to positively select for condensed hemozoin-containing gametocytes, macrogametes, zygotes, and ookinetes as well as any remaining asexual stage schizonts, according to manufacturer's instructions (Miltenyi Biotec, Bergisch Gladbach, Germany).32 Magnetic purification of sexual stage parasite cultures was done at 19–23°C using ookinete medium instead of the manufacturer's recommended MACS® buffer. Magnet-retained sexual stage parasites were washed, eluted from the magnetic column, and then, centrifuged at 800 × g. The cell pellet was resuspended in 100–500 μL of ookinete culture medium and then placed on a 6–11% or 11–16% Nycodenz gradient (Sigma-Aldrich, St. Louis, MO).30,33 Briefly, 400 μL of 6% Nycodenz, 400 μL of 11% Nycodenz, and 400 μL of 16% Nycodenz were layered in a 1.5-mL microcentrifuge tube; 200 μL of resuspended parasites were layered on top of the three-step gradient and centrifuged at 10,000 × g for 10 minutes at 4°C with no brake. The 6–11% contained self-adherent macrogametes, consistent with previous reports,34 whereas the 11–16% interface contained ookinetes, zygotes, non–self-adherent macrogametes, and untransformed gametocytes.
Plasmodium zygotes and ookinetes are the only Plasmodium developmental stage cells known to be diploid/tetraploid.35,36 This difference in ploidy was exploited to separate P. falciparum zygotes and ookinetes from other sexual stage forms using flow cytometric sorting. Parasites from the 11–16% Nycodenz gradient interface were washed and stained with Vybrant DyeCycle Green stain according to manufacturer's instructions (Molecular Probes; Invitrogen, Carlsbad, CA). Approximately 107–108 parasites were then passed through a 30-μm filter to remove clumps of self-adherent cells and were sorted by fluorescence intensity using a MoFlo high-speed sorter (Dako, Glostrup, Denmark). Stained parasites were analyzed by flow cytometry and divided into four subgroups based on fluorescence (Figure 3). Region R3 showed enrichment for gametocytes, and R4 showed enrichment for non-adherent macrogametes. The finding that macrogametes could be separated from gametocytes by DNA content was surprising but consistent with previous fluorometric studies.37–41 Region R6 was enriched for a mixture of zygotes and ookinetes (Figure 3). The finding that the majority of zygotes and ookinetes had more than or equal to four times as much DNA as region R3 is consistent with our current understanding that meiotic division occurs shortly after gamete fusion.36,37,42,43 It is possible that further refinements of flow cytometric sorting (for example, incorporation of forward- and side-scatter parameters to separate round forms from elongated forms based on size or clumping) might be able to separate zygotes from ookinetes.
Figure 3.
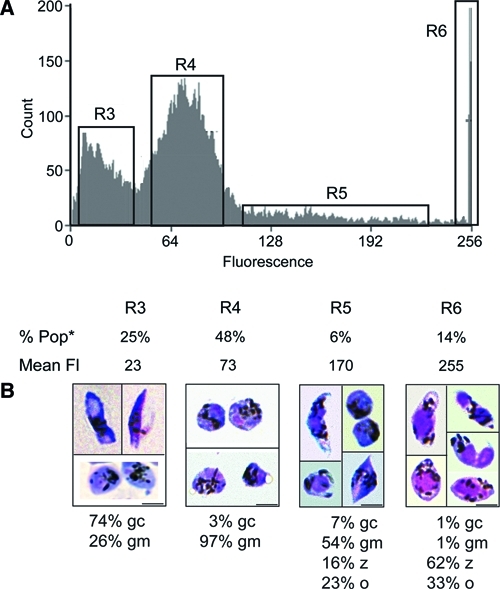
Flow cytometry sorting of P. falciparum sexual stage parasites, zygotes, and ookinetes based on DNA content. Flow cytometry-sorted samples were stained and analyzed by light microscopy to determine the dominant sexual stage form in each of the fluorescent regions populations identified by fluorescence intensity. (A) Vybrant Dyecycle Green-stained sexual stage parasites were analyzed by flow cytometry. Parasites showed differential fluorescence patterns divided into regions R3, R4, R5, and R6 based on fluorescence intensity. R3 and R4 constituted distinct fluorescent peaks. Percent of cells (% Pop) and mean fluorescence (Mean Fl) for each region are shown (*7% of the total cells counted are not contained within the four labeled gates shown and thus, could not be included in % Pop). (B) Sorted parasites from each population were stained with Leukostat and examined under light microscopy for quantification of parasite forms; 300–1,000 parasites were counted per sorted population. Gametocytes (gc) were the dominant form found in region R3; non–self-adherent round forms (gm) were found in region R4. Region R6 contained ookinetes (o) and round forms and likely contained retort ookinetes and zygotes (z), which expressed four times the fluorescence as regions R3 and R4 and represent gametocytes and likely, macrogametes. Less than 1% of parasite forms sorted into R3, and R4 was asexual stage parasite forms; 3–5% of all parasites sorted in R6 were asexual forms, mostly schizonts. (Scale bars: 5 μm.)
Pfs25 and Chitinase (PfCHT1) Detection in Gametocytes and Ookinetes
To see if Pfs25 or PfCHT1 could be used as markers to classify elongated parasites as gametocytes or ookinetes (Figure 4A and B), gametocytes and in vitro-generated ookinete cultures were examined by an immunofluorescence assay (IFA) (Figure 4C–H) using previously characterized antibodies to Pfs2544 and chitinase,45 two proteins presumed to be zygote/ookinete and ookinete-specific, respectively (Appendix).46,47 By IFA, immunoglobulin G (IgG) negative control antibody did not generate fluorescence signal in either gametocytes or ookinetes, whereas antibodies to P. falciparum chitinase (PfCHT1) and Pfs25 produced fluorescent signals in gametocytes (as previously described for Pfs25)48 and ookinetes (Figure 4C–H). The fluorescence signal was observed to be stronger in ookinetes than in gametocytes, with a distinct pattern consistent with surface localization of Pfs25 notable in ookinetes but not gametocytes.
Figure 4.
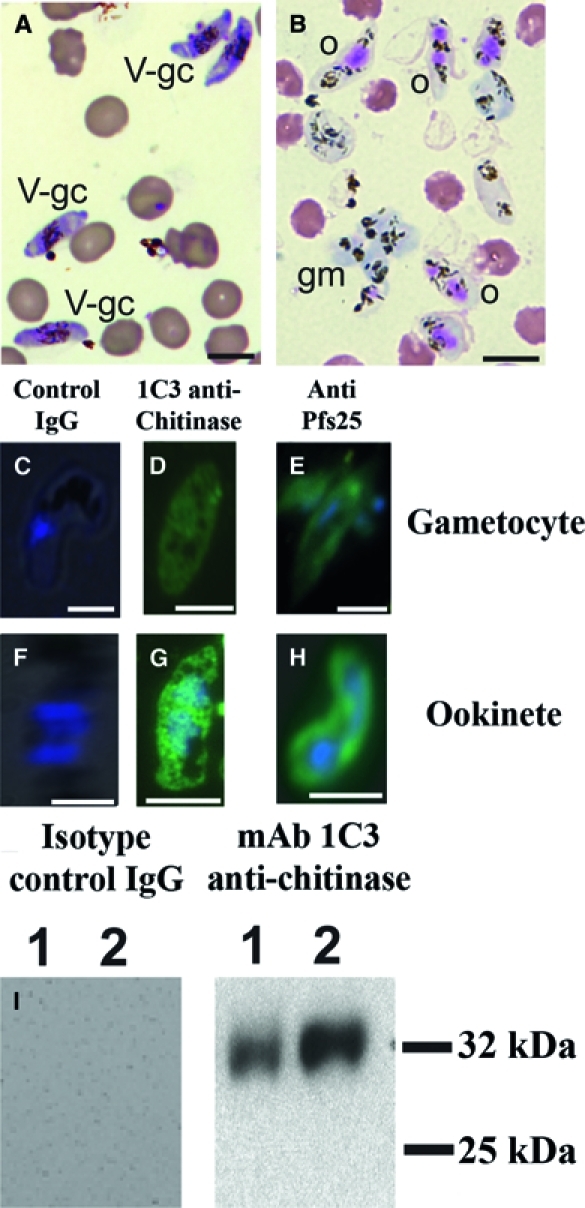
Light and fluorescence microscopy of in vitro cultured P. falciparum sexual stage parasites. (A) Leukostat-stained thin smears of unpurified gametocyte culture contained stage V gametocytes (V-gc). (B) Leukostat-stained thin smears of in vitro cultured P. falciparum sexual stage parasites. Ookinetes (o) were identified by the presence of one to two large eosinophilic nuclei and lack of a surrounding erythrocyte membrane. Round forms include macrogametes and zygotes. Self-adherent macrogametes (gm) were slightly basophilic and often found in clusters. Non-adherent macrogametes were not definitively distinguished from zygotes and retort ookinetes. Hemozoins appeared as dark brown pigment crystals. (C–H) Gametocytes from gametocyte cultures and ookinetes from sexual stage cultures were probed with antibodies to chitinase and Pfs25. DAPI (blue) was used to stain nuclear material; gametocytes contained one nucleus, whereas ookinetes contained one to two nuclei. (C) Gametocytes and (F) ookinetes probed with IgG isotype control antibody showed no reaction. (D) Gametocytes and (G) ookinetes probed with 1C3 monoclonal antibody against chitinase45 (green) showed a diffuse, intracellular staining pattern in both gametocytes and ookinetes. (E) Gametocytes and (H) ookinetes probed with antibody against Pfs2558 (green) showed an intracellular pattern in gametocytes and both intracellular and surface staining patterns with ookinetes. (I) Chitinase detected in gametocytes and a 72-hour cultured mixed zygotes and ookinetes sample by Western immunoblot. Equal numbers of cells gametocytes (lane 1) and untransformed gametes plus zygotes plus ookinetes (lane 2) were lysed in SDS, separated by SDS-PAGE, and probed with antibody against the P. falciparum chitinase PfCHT1 using monoclonal antibody 1C3.45 IgG isotype control antibody did not produce a band in either sample. Antibody against PfCHT1 recognized an approximately 32-kDa band in both gametocyte and zygote plus ookinete samples and quantitatively more after 72 hours of cultures than in gametocytes.
Western immunoblots using the monoclonal antibody 1C3 to the P. falciparum chitinase PfCHT1 detected the protein in mixed gametocytes, untransformed gametes, and early zygotes directly and in 72-hour ookinete cultures (Figure 4I), with an increased intensity of PfCHT1 seen in mature ookinetes.
The quantitative and non-qualitative aspects of these finding indicate that neither the presence of chitinase nor Pfs25 protein, as detected by IFA or Western immunoblot, unequivocally distinguishes P. falciparum ookinetes from gametocytes.
Ultrastructural Analysis of P. Falciparum Sexual Stage Parasites
Transmission electron microscopy (TEM) of sexual stage cultures was done to determine whether elongated, banana-shaped parasites were either gametocytes or ookinetes (Figure 5 and Appendix). Parasites submitted for TEM were enriched by magnetic and density gradient separation techniques. Approximately 30% of parasites were ookinetes as determined by light microscopic examination of Leukostat-stained slides. Qualitative analysis of cultivated parasites by TEM showed the presence of round parasite sections, which could have been cross-sections of macrogametes, zygotes, gametocytes, or ookinetes, as well as elongated parasite sections, which could have been gametocytes and ookinetes. Cross-sections of gametocytes and ookinetes could be identified by the presence of subpellicular microtubules, which supports the characteristic banana-shaped forms. Gametocytes had a surrounding erythrocyte membrane, whereas cross-sections of ookinetes did not.41,49–55
Figure 5.
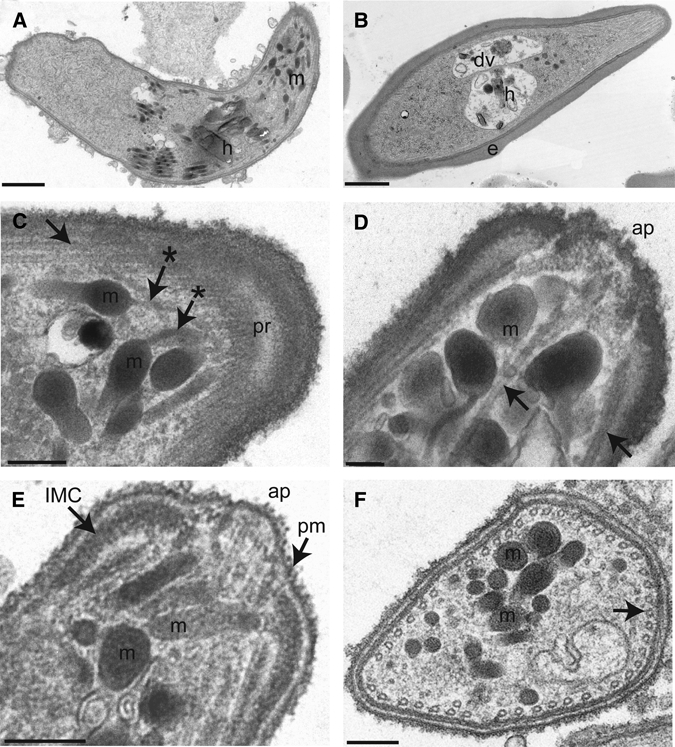
Ultrastructural features of the P. falciparum ookinete. (A) Sagittal section of an ookinete shows micronemes (m), seen as electron-dense round or cigar-shaped organelles, and a hemazoin crystal (h). (Scale bar: 1 μm.) (B) For comparison, a gametocyte within an infected erythrocyte (e) is seen with a discrete digestive vacuole (dv) with hemozoin (h). (Scale bar: 1 μm.) (C) Tangential section of the apical end of an ookinete showed microtubules (→), which converge at the polar ring (pr). This section showed narrow ducts of micronemes (m) that appear to track to the apical end (*→). (Scale bar: 200 nm.) (D) Transverse, midline section of an ookinete apical end showed the apical pore (ap), micronemes (m), and microtubules (→). (Scale bar: 200 nm.) (E) Transverse section of an ookinete showed micronemes (m) as well as the apical pore (ap) located between two electron-dense regions representing the inner membrane complex (IMC) underlying the plasma membrane (pm). (Scale bar: 200 nm.) (F) Transverse cross-section near the apical end of an ookinete shows micronemes (m) as well as microtubules (→) that circumferentially line the subpellicular space of the parasite. (Scale bar: 200 nm.)
Examination of erythrocyte-free elongated parasites showed definitive ookinete ultrastructure, including the apical complex and a pellicle, which was not observed in gametocytes (Figure 5). The apical complex consists of an apical polar ring and micronemes. The polar ring serves to anchor the subpellicular microtubules.55 Micronemes are round, protein-dense, membrane-bound organelles that have been shown to contain proteins secreted through their ducts near the apical end.56,57 The ookinete pellicle includes the parasite plasma membrane, the inner membrane complex, and subpellicular microtubules.35
The method presented here consistently produced large quantities of sexual stage forms of the human malaria parasite P. falciparum. Currently, standard methods for consistently generating P. falciparum zygotes and ookinetes require feeding gametocytes to Anopheles mosquitoes followed by dissection of these sexual stage forms from mosquito midguts. Despite electron microscopic proof of P. falciparum ookinete production, a limitation of the present report is that the ability of in vitro-generated P. falciparum ookinetes to produce oocysts in mosquitoes was not verified. Nonetheless, this improved production of P. falciparum sexual stage parasites is a significant step to understanding biological details of ookinetes specific to this human malaria parasite. This work comes at a critical juncture as interest increases in malaria control efforts based on the potential of Plasmodium transmission-blocking vaccines.
Acknowledgments
This work was supported by US Public Health Service Grants T32GM007198 (to V.B.), K24AI068903 (to J.M.V.), and R01AI45999 (to J.M.V.). The authors thank M. G. Farquhar, K. Kudlicka, and T. Meerloo, Core Electron Microscopy Facility (US Public Health Service Grants R01CA100768, R01DK017724, and R01DK017780). The authors thank C. A. Spina, J. Nordberg, and M. O'Keefe of the flow cytometry core of the Center for Acquired Immunodefieciency Syndrome (AIDS) Research at the University of California San Diego (supported by Grant S10RR027933 and P30AI036214). The authors thank S. L. Hoffman for critical comments on the manuscript and Sanaria (Rockville, MD) for this NF54 strain of P. falciparum. The authors thank R. E. Sinden for assistance with advice on the interpretation of electron micrographs. The authors thank S. R. Abeles, J. W. M. Theisen, N. V. Dharia, and T. A. Aguilera for scientific discussion. We particularly thank Paula Maguina for the logistical and scientific support for the work carried out here.
Appendix: Methods
P. falciparum gametocyte cultures.
The NF54 isolate of P. falciparum used in these experiments was a gift from Stephen Hoffmann (Sanaria, Rockville, MD). NF54 is one of the 13 initial Nijmegen Falciparum (NF) isolates derived from a Dutch patient.52–54 This strain was maintained in continuous asexual culture according to standard protocol26 with the exception that no antibiotics were used in the complete medium: RPMI 1,640, 25 mM Hepes, 2 mM l-glutamine, 2.4 g/L NaHCO3, 50 mg/L hypoxanthine, and 10% heat-inactivated AB+ human serum (Interstate Blood Bank, Memphis, TN). Asynchronous asexual stage cultures at 8–15% parasitemia were used to start gametocyte cultures. Gametocyte cultures were started by diluting asexual stage parasite cultures into 0.9 mL of freshly washed packed human red cells to generate a final concentration of 0.4–0.6% parasitemia in 15 mL total volume. Dilution of asexual cultures at 8–10% parasitemia generated gametocyte cultures with the highest yields. Spent gametocyte culture medium was removed and replaced daily with 15 mL of 37oC complete medium until cultures reached 5–10% parasitemia, usually by day 3–4.29,55,56 At this point, spent gametocyte culture medium was replaced with 25 mL of 37oC complete medium for the remainder of the culture period. Additionally, approximately 10 mL of spent medium were left in the culture flask during each medium change.57 Gametocyte cultures were maintained in a low oxygen environment by gassing the cultures with filtered 5% O2, 5% CO2, and 90% N2.
Exflagellation and emergence assays.
Mature macrogametocytes were tested for the ability to emerge using a modified exflagellation protocol: instead of examining slides for emergence in real time, blood smears were made 1 hour after gametogenesis, fixed with methanol, and stained with a modified Wright stain. The ratio of gametes to gametocytes was determined by counting thin smears 1 hour post-emergence.
Leukostat-stained light microscopy.
A modified Wright stain using Leukostat dyes was used to stain parasite thin smears. Smears were stained for 15 seconds in Leukostat 1 (0.1% eosin Y, 0.4% Na2HPO4, and 0.1% formaldehyde), rinsed in distilled water, and stained for 30 seconds in Leukostat 2 (0.04% methylene blue, 0.04% Azure A, KH2PO4, and Na2HPO4). Slides were then rinsed in distilled water and left to air dry.
Immunofluroescence of cultured parasites.
Cultured parasites were fixed and permeabilized on glass slides and probed with antibodies against chitinase (1C3), Pfs25 4B7 (MRA-28; deposited by David C. Kaslow, Malaria Research and Reference Reagent Resource Center, Manassas, VA), or mouse IgG negative control as previously described42 with fluorescein isothiocyanate (FITC)-conjugated anti-mouse secondary and 3 μM DAPI (Molecular Probes; Invitrogen, Carlsbad, CA).
TEM of cultured parasites.
Sexual stage parasite samples were depleted of uninfected red blood cells and asexual parasite forms by centrifugation on Lympholyte-H single-step density gradient and washed three times with ookinete medium. Parasites were then centrifuged and resuspended in 500 L of ookinete medium. These cells were further prepared for TEM according to standard protocol.58 Resulting parasite blocks were cut with a Reichert ultramicrotome, stained with 1% uranyl acetate and lead nitrate, examined using a JEOL 1200EX II transmission electron microscope (JEOL, Peabody, MA), and photographed using a Gatan digital camera (Gatan, Pleasanton, CA).
Disclaimer: The authors declare no conflict of interest.
Footnotes
Authors' addresses: Viengngeun Bounkeua, Fengwu Li, and Joseph M. Vinetz, Division of Infectious Diseases, Department of Medicine, University of California San Diego, La Jolla, CA.
References
- 1.World Health Organization . World Malaria Report. Geneva, Switzerland: World Health Organization; 2008. [Google Scholar]
- 2.Saul A. Mosquito stage, transmission blocking vaccines for malaria. Curr Opin Infect Dis. 2007;20:476–481. doi: 10.1097/QCO.0b013e3282a95e12. [DOI] [PubMed] [Google Scholar]
- 3.Gamage-Mendis AC, Rajakaruna J, Carter R, Mendis KN. Transmission blocking immunity to human Plasmodium vivax malaria in an endemic population in Kataragama, Sri Lanka. Parasite Immunol. 1992;14:385–396. doi: 10.1111/j.1365-3024.1992.tb00013.x. [DOI] [PubMed] [Google Scholar]
- 4.Kaslow DC. Immunogenicity of Plasmodium falciparum sexual stage antigens: implications for the design of a transmission blocking vaccine. Immunol Lett. 1990;25:83–86. doi: 10.1016/0165-2478(90)90096-9. [DOI] [PubMed] [Google Scholar]
- 5.Vermeulen AN, Ponnudurai T, Beckers PJ, Verhave JP, Smits MA, Meuwissen JH. Sequential expression of antigens on sexual stages of Plasmodium falciparum accessible to transmission-blocking antibodies in the mosquito. J Exp Med. 1985;162:1460–1476. doi: 10.1084/jem.162.5.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vermeulen AN, Roeffen WF, Henderik JB, Ponnudurai T, Beckers PJ, Meuwissen JH. Plasmodium falciparum transmission blocking monoclonal antibodies recognize monovalently expressed epitopes. Dev Biol Stand. 1985;62:91–97. [PubMed] [Google Scholar]
- 7.Hisaeda H, Stowers AW, Tsuboi T, Collins WE, Sattabongkot JS, Suwanabun N, Torii M, Kaslow DC. Antibodies to malaria vaccine candidates Pvs25 and Pvs28 completely block the ability of Plasmodium vivax to infect mosquitoes. Infect Immun. 2000;68:6618–6623. doi: 10.1128/iai.68.12.6618-6623.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duffy PE, Kaslow DC. A novel malaria protein, Pfs28, and Pfs25 are genetically linked and synergistic as falciparum malaria transmission-blocking vaccines. Infect Immun. 1997;65:1109–1113. doi: 10.1128/iai.65.3.1109-1113.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duffy PE, Pimenta P, Kaslow DC. Pgs28 belongs to a family of epidermal growth factor-like antigens that are targets of malaria transmission-blocking antibodies. J Exp Med. 1993;177:505–510. doi: 10.1084/jem.177.2.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barr PJ, Green KM, Gibson HL, Bathurst IC, Quakyi IA, Kaslow DC. Recombinant Pfs25 protein of Plasmodium falciparum elicits malaria transmission-blocking immunity in experimental animals. J Exp Med. 1991;174:1203–1208. doi: 10.1084/jem.174.5.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaslow DC, Isaacs SN, Quakyi IA, Gwadz RW, Moss B, Keister DB. Induction of Plasmodium falciparum transmission-blocking antibodies by recombinant vaccinia virus. Science. 1991;252:1310–1313. doi: 10.1126/science.1925544. [DOI] [PubMed] [Google Scholar]
- 12.Baton LA, Ranford-Cartwright LC. Spreading the seeds of million-murdering death: metamorphoses of malaria in the mosquito. Trends Parasitol. 2005;21:573–580. doi: 10.1016/j.pt.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 13.Poudel SS, Newman RA, Vaughan JA. Rodent Plasmodium: population dynamics of early sporogony within Anopheles stephensi mosquitoes. J Parasitol. 2008;94:999–1008. doi: 10.1645/GE-1407.1. [DOI] [PubMed] [Google Scholar]
- 14.Zollner GE, Ponsa N, Garman GW, Poudel S, Bell JA, Sattabongkot J, Coleman RE, Vaughan JA. Population dynamics of sporogony for Plasmodium vivax parasites from western Thailand developing within three species of colonized Anopheles mosquitoes. Malar J. 2006;5:68. doi: 10.1186/1475-2875-5-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sinden RE, Billingsley PF. Plasmodium invasion of mosquito cells: hawk or dove? Trends Parasitol. 2001;17:209–212. doi: 10.1016/s1471-4922(01)01928-6. [DOI] [PubMed] [Google Scholar]
- 16.Carter R, Chen DH. Malaria transmission blocked by immunisation with gametes of the malaria parasite. Nature. 1976;263:57–60. doi: 10.1038/263057a0. [DOI] [PubMed] [Google Scholar]
- 17.Gwadz RW. Successful immunization against the sexual stages of Plasmodium gallinaceum. Science. 1976;193:1150–1151. doi: 10.1126/science.959832. [DOI] [PubMed] [Google Scholar]
- 18.Lavazec C, Bourgouin C. Mosquito-based transmission blocking vaccines for interrupting Plasmodium development. Microbes Infect. 2008;10:845–849. doi: 10.1016/j.micinf.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 19.Dinglasan RR, Alaganan A, Ghosh AK, Saito A, van Kuppevelt TH, Jacobs-Lorena M. Plasmodium falciparum ookinetes require mosquito midgut chondroitin sulfate proteoglycans for cell invasion. Proc Natl Acad Sci USA. 2007;104:15882–15887. doi: 10.1073/pnas.0706340104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghosh AK, Dinglasan RR, Ikadai H, Jacobs-Lorena M. An improved method for the in vitro differentiation of Plasmodium falciparum gametocytes into ookinetes. Malar J. 2010;9:194. doi: 10.1186/1475-2875-9-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Warburg A, Miller LH. Sporogonic development of a malaria parasite in vitro. Science. 1992;255:448–450. doi: 10.1126/science.1734521. [DOI] [PubMed] [Google Scholar]
- 22.Hurd H, Al-Olayan E, Butcher GA. In vitro methods for culturing vertebrate and mosquito stages of Plasmodium. Microbes Infect. 2003;5:321–327. doi: 10.1016/s1286-4579(03)00034-0. [DOI] [PubMed] [Google Scholar]
- 23.Carter E, Suhrbier A, Beckers P, Sinden R. The in vitro cultivation of Plasmodium falciparum ookinetes, and their enrichment of Nycodenz density gradients. Parasitology. 1987;95:25–30. doi: 10.1017/s0031182000057516. [DOI] [PubMed] [Google Scholar]
- 24.Ono T, Nakabayashi T. Gametocytogenesis induction in cultured Plasmodium falciparum and further development of the gametocytes to ookinetes in prolonged culture. Parasitol Res. 1989;75:348–352. doi: 10.1007/BF00931129. [DOI] [PubMed] [Google Scholar]
- 25.Zhou Y, Ramachandran V, Kumar KA, Westenberger S, Refour P, Zhou B, Li F, Young JA, Chen K, Plouffe D, Henson K, Nussenzweig V, Carlton J, Vinetz JM, Duraisingh MT, Winzeler EA. Evidence-based annotation of the malaria parasite's genome using comparative expression profiling. PLoS One. 2008;3:e1570. doi: 10.1371/journal.pone.0001570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Read M, Hyde JE. Simple in vitro cultivation of the malaria parasite Plasmodium falciparum (erythrocytic stages) suitable for large-scale preparations. Methods Mol Biol. 1993;21:43–55. doi: 10.1385/0-89603-239-6:43. [DOI] [PubMed] [Google Scholar]
- 27.Ifediba T, Vanderberg JP. Complete in vitro maturation of Plasmodium falciparum gametocytes. Nature. 1981;294:364–366. doi: 10.1038/294364a0. [DOI] [PubMed] [Google Scholar]
- 28.Looker BW, Taylor-Robinson AW. In: Methods in Malaria Research. 4th ed. Ljungstrom HP, Schlichtherle M, Scherf A, Wahlgren A, editors. Manassas, VA: MR4/ATCC; 2004. pp. 96–99.http://www.mr4.org/Portals/3/Pdfs/ProtocolBook/Methods_in_malaria_research.pdf (Cultivation of Plasmodium falciparum gametocytes for mosquito infectivity studies). Available at. Accessed October 15, 2010. [Google Scholar]
- 29.Graves PM, Carter R, McNeill KM. Gametocyte production in cloned lines of Plasmodium falciparum. Am J Trop Med Hyg. 1984;33:1045–1050. doi: 10.4269/ajtmh.1984.33.1045. [DOI] [PubMed] [Google Scholar]
- 30.Carter R, Ranford-Cartwright L, Alano P. The culture and preparation of gametocytes of Plasmodium falciparum for immunochemical, molecular, and mosquito infectivity studies. Methods Mol Biol. 1993;21:67–88. doi: 10.1385/0-89603-239-6:67. [DOI] [PubMed] [Google Scholar]
- 31.Ghosh AK, Dinglasan RR, Ikadai H, Jacobs-Lorena MMJ. An improved method for the in vitro differentiation of Plasmodium falciparum gametocytes into ookinetes. Malar J. 2010;9:194. doi: 10.1186/1475-2875-9-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fivelman QL, McRobert L, Sharp S, Taylor CJ, Saeed M, Swales CA, Sutherland CJ, Baker DA. Improved synchronous production of Plasmodium falciparum gametocytes in vitro. Mol Biochem Parasitol. 2007;154:119–123. doi: 10.1016/j.molbiopara.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 33.Vermeulen AN, Ponnudurai T, Beckers PJ, Verhave JP, Smits MA, Meuwissen JH. Sequential expression of antigens on sexual stages of Plasmodium falciparum accessible to transmission-blocking antibodies in the mosquito. J Exp Med. 1985;162:1460–1476. doi: 10.1084/jem.162.5.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vermeulen AN, Ponnudurai T, Lensen AH, Roeffen WF, Meuwissen JE. The purification of Plasmodium falciparum macrogametes and/or zygotes prepared from in vitro cultures. Trans R Soc Trop Med Hyg. 1983;77:753–755. doi: 10.1016/0035-9203(83)90280-8. [DOI] [PubMed] [Google Scholar]
- 35.Canning EU, Sinden RE. The organization of the ookinete and observations on nuclear division in oocysts of Plasmodium berghei. Parasitology. 1973;67:29–40. doi: 10.1017/s0031182000046266. [DOI] [PubMed] [Google Scholar]
- 36.Janse CJ, van der Klooster PF, van der Kaay HJ, van der Ploeg M, Overdulve JP. DNA synthesis in Plasmodium berghei during asexual and sexual development. Mol Biochem Parasitol. 1986;20:173–182. doi: 10.1016/0166-6851(86)90029-0. [DOI] [PubMed] [Google Scholar]
- 37.Janse CJ, Ponnudurai T, Lensen AH, Meuwissen JH, Ramesar J, Van der Ploeg M, Overdulve JP. DNA synthesis in gametocytes of Plasmodium falciparum. Parasitology. 1988;96:1–7. doi: 10.1017/s0031182000081609. [DOI] [PubMed] [Google Scholar]
- 38.Zhao H, Traganos F, Dobrucki J, Wlodkowic D, Darzynkiewicz Z. Induction of DNA damage response by the supravital probes of nucleic acids. Cytometry A. 2009;75:510–519. doi: 10.1002/cyto.a.20727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sinden RE. The cell biology of sexual development in Plasmodium. Parasitology. 1983;86:7–28. doi: 10.1017/s0031182000050824. [DOI] [PubMed] [Google Scholar]
- 40.Sinden RE. Sexual development of malarial parasites. Adv Parasitol. 1983;22:154–208. doi: 10.1016/s0065-308x(08)60462-5. [DOI] [PubMed] [Google Scholar]
- 41.Sinden RE, Canning EU, Bray RS, Smalley ME. Gametocyte and gamete development in Plasmodium falciparum. Proc R Soc Lond B Biol Sci. 1978;201:375–399. doi: 10.1098/rspb.1978.0051. [DOI] [PubMed] [Google Scholar]
- 42.Reininger L, Billker O, Tewari R, Mukhopadhyay A, Fennell C, Dorin-Semblat D, Doerig C, Goldring D, Harmse L, Ranford-Cartwright L, Packer J, Doerig C. A NIMA-related protein kinase is essential for completion of the sexual cycle of malaria parasites. J Biol Chem. 2005;280:31957–31964. doi: 10.1074/jbc.M504523200. [DOI] [PubMed] [Google Scholar]
- 43.Sinden RE. Gametocytogenesis in Plasmodium spp., and observations on the meiotic division. Ann Soc Belg Med Trop. 1985;65((Suppl 2)):21–33. [PubMed] [Google Scholar]
- 44.Kaslow D, Shiloach J. Production, purification and immunogenicity of a malaria transmission-blocking vaccine candidate: TBV25H expressed in yeast and purified using nickel-NTA agarose. Biotechnology. 1994;12:494–499. doi: 10.1038/nbt0594-494. [DOI] [PubMed] [Google Scholar]
- 45.Vinetz JM, Dave SK, Specht CA, Brameld KA, Hayward RE, Fidock DA. The chitinase PfCHT1 from the human malaria parasite Plasmodium falciparum lacks proenzyme and chitin-binding domains and displays unique substrate preferences. Proc Natl Acad Sci USA. 1999;96:14061–14066. doi: 10.1073/pnas.96.24.14061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kaslow DC, Quakyi IA, Syin C, Raum MG, Keister DB, Coligan JE, McCutchan TF, Miller LH. A vaccine candidate from the sexual stage of human malaria that contains EGF-like domains. Nature. 1988;333:74–76. doi: 10.1038/333074a0. [DOI] [PubMed] [Google Scholar]
- 47.Vinetz JM, Valenzuela JG, Specht CA, Aravind L, Langer RC, Ribeiro JM, Kaslow DC. Chitinases of the avian malaria parasite Plasmodium gallinaceum, a class of enzymes necessary for parasite invasion of the mosquito midgut. J Biol Chem. 2000;275:10331–10341. doi: 10.1074/jbc.275.14.10331. [DOI] [PubMed] [Google Scholar]
- 48.Scholz SM, Simon N, Lavazec C, Dude MA, Templeton TJ, Pradel G. PfCCp proteins of Plasmodium falciparum: gametocyte-specific expression and role in complement-mediated inhibition of exflagellation. Int J Parasitol. 2008;38:327–340. doi: 10.1016/j.ijpara.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 49.Sinden RE, Winger L, Carter EH, Hartley RH, Tirawanchai N, Davies CS, Moore J, Sluiters JF. Ookinete antigens of Plasmodium berghei: a light and electron-microscope immunogold study of expression of the 21 kDa determinant recognized by a transmission-blocking antibody. Proc R Soc Lond B Biol Sci. 1987;230:443–458. doi: 10.1098/rspb.1987.0028. [DOI] [PubMed] [Google Scholar]
- 50.Sinden RE, Hartley RH, Winger L. The development of Plasmodium ookinetes in vitro: an ultrastructural study including a description of meiotic division. Parasitology. 1985;91:227–244. doi: 10.1017/s0031182000057334. [DOI] [PubMed] [Google Scholar]
- 51.Sinden RE. A cell biologist's view of host cell recognition and invasion by malarial parasites. Trans R Soc Trop Med Hyg. 1985;79:598–605. doi: 10.1016/0035-9203(85)90165-8. [DOI] [PubMed] [Google Scholar]
- 52.Sinden RE, Strong K. An ultrastructural study of the sporogonic development of Plasmodium falciparum in Anopheles gambiae. Trans R Soc Trop Med Hyg. 1978;72:477–491. doi: 10.1016/0035-9203(78)90167-0. [DOI] [PubMed] [Google Scholar]
- 53.Sinden RE. Gametocytogenesis in Plasmodium spp., and observations on the meiotic division. Ann Soc Belg Med Trop. 1985;65:21–33. [PubMed] [Google Scholar]
- 54.Sinden RE, Hartley RH, Gametogenesis NJK. Gametogenesis in Plasmodium; the inhibitory effects of anticytoskeletal agents. Int J Parasitol. 1985;15:211–217. doi: 10.1016/0020-7519(85)90089-x. [DOI] [PubMed] [Google Scholar]
- 55.Raibaud ALP, Paul REL, Mercati D, Brey PT, Sinden RE, Heuser JE, Dallai R. Cryofracture electron microscopy of the ookinete pellicle of Plasmodium gallinaceum reveals the existence of novel pores in teh alveolar membranes. J Struct Biol. 2001;135:47–57. doi: 10.1006/jsbi.2001.4396. [DOI] [PubMed] [Google Scholar]
- 56.Bannister LH, Hopkins JM, Dluzewski AR, Margos G, Williams IT, Blackman MJ, Kocken CH, Thomas AW, Mitchell GH. Plasmodium falciparum apical membrane antigen 1 (PfAMA-1) is translocated within micronemes along subpellicular microtubules during merozoite development. J Cell Sci. 2003;116:3825–3834. doi: 10.1242/jcs.00665. [DOI] [PubMed] [Google Scholar]
- 57.Schrevel J, Asfaux-Foucher G, Hopkins JM, Robert V, Bourgouin C, Prensier G, Bannister LH. Vesicle trafficking during sporozoite development in Plasmodium berghei: ultrastructural evidence for a novel trafficking mechanism. Parasitology. 2008;135:1–12. doi: 10.1017/S0031182007003629. [DOI] [PubMed] [Google Scholar]
- 58.Kaslow DC, Shiloach J. Production, purification and immunogenicity of a malaria transmission-blocking vaccine candidate: TBV25H expressed in yeast and purified using nickel-NTA agarose. Biotechnology. 1994;12:494–499. doi: 10.1038/nbt0594-494. [DOI] [PubMed] [Google Scholar]


