Members of the B group of ABC transporters are required for polar auxin transport through various plant tissues and organs. This report clarifies how the TWD1 immunophilin affects auxin transport and root growth by affecting B-group ABC transporters.
Abstract
Multidrug resistance ABC transporters in plants are required for polar transport of the hormone auxin (indole-3-acetic acid). They are studied in animals primarily because their overexpression confers resistance to anticancer agents. Immunophilins are studied in both plants and animals for their roles in folding and trafficking of proteins, particularly those with signal transducing functions and susceptibility to immunosuppressant drugs. Previous genetic and molecular studies in Arabidopsis thaliana established a physical and functional interaction between some ABCB transporters and the TWISTED DWARF1 (TWD1) immunophilin. In this work, confocal microscopy of fluorescently tagged TWD1 shows it to reside at the endoplasmic reticulum (ER). Mutations in TWD1 caused mislocalization of ABCB1, ABCB4, and ABCB19 to the ER instead of the plasma membrane as shown by confocal microscopy of fluorescently tagged fusion proteins and transmission electron microscopy of immunogold-labeled samples in the case of ABCB19. Localization of the unrelated PIN-FORMED2 auxin transporter or plasma membrane marker proteins was not affected by loss of TWD1. Abnormal spread of auxin signaling into the elongation zone of twd1 roots, attributable to mislocalized ABCB transporters and suppressed by an auxin transport inhibitor, appeared to cause the twisted cell files characteristic of twd1 roots.
INTRODUCTION
Auxin (indole-3-acetic acid) is a central regulator of plant growth and development. Its biosynthesis is largely restricted to shoot and root apices, whereas its sites of action are widespread (Woodward and Bartel, 2005). A specialized, directionally biased transport mechanism creates the auxin concentration gradients that contribute to plant developmental patterns. The framework for understanding this unique polar transport phenomenon was first based on thermodynamic and kinetic considerations (Goldsmith, 1977) and then augmented with important molecular and genetic findings (Vieten et al., 2007). According to the model, protonated auxin moves readily inward across the plasma membrane from the acidic apoplast, assisted by the AUX1 permease (Kramer, 2004). In the cytoplasm, due to the near-neutral pH, the anionic form of indole-3-acetic acid predominates. The auxin anion is thermodynamically poised to move outward passively. Plasma membrane PIN proteins and multidrug resistance-like ABC transporters are physically interacting components of the mechanism that allows efflux of the auxin anion (Blakeslee et al., 2007). A strong bias in the direction of auxin efflux results from a distinctly asymmetric (i.e., polar) localization of PIN proteins in each cell (Petrášek and Friml, 2009). The cumulative effect at the tissue level of cellular PIN localization is a macroscopic auxin transport stream that sets in motion or regulates numerous aspects of plant growth and development. Unlike PINs, the ABC transporters are uniformly distributed in cells, yet they are required for polar auxin transport (Noh et al., 2001; Wu et al., 2007). Their role may be to enhance PIN-mediated efflux at subcellular sites of colocalization.
Because the phenomenon of polar transport through tissues emerges from cellular-level behavior of the auxin transport proteins, their processing, maturation, and targeting is an important topic. The polarized distribution of PINs is established and maintained by a dynamic protein trafficking mechanism that involves recycling through endosomes (Kleine-Vehn and Friml, 2008). Much less is known about the maturation and targeting to the plasma membrane of the required ABC protein components of the mechanism. An opportunity to learn about the maturation and trafficking of ABCB19 (hereafter B19), an ABC transporter with a large effect on polar auxin transport streams in stems, petioles, and roots (Lewis et al., 2007, 2009; Noh et al., 2001), was provided by the finding that its cytoplasmic C terminus physically interacts with an immunophilin-like protein known as TWISTED DWARF1 (TWD1) (Geisler et al., 2003). The immunophilins to which TWD1 is related were first identified in mammals as targets of FK506 immunosuppressants and are now thought to facilitate protein folding and maturation by virtue of cis-trans peptidyl-prolyl isomerase (PPI) activity and interaction with the HSP90 chaperone (Barik, 2006). TWD1, also called FKBP42 (for FK506 Binding Protein 42), is an example of a multidomain FKBP-type immunophilin (Romano et al., 2005; Geisler and Bailly, 2007). In addition to a PPI-like domain, it possesses a tetratricopeptide repeat domain, a calmodulin binding domain, and a hydrophic domain that is believed to serve as a membrane anchor (He et al., 2004). The site of interaction with B19 was mapped to the PPI domain at the N terminus of TWD1 (Geisler et al., 2003). Although this domain in TWD1 lacks PPIase activity due to missing or misplaced critical amino acids (Kamphausen et al., 2002; Weiergräber et al., 2006), the functional relevance of its interaction with B19 and the closely related ABCB1 protein (hereafter B1) is supported by genetic evidence (Geisler et al., 2003). The twisted dwarf phenotype of twd1 knockout mutants overlaps with that of b1 b19 double mutants to an extent that suggests a functional relationship between the proteins (Noh et al., 2001; Geisler et al., 2003; Pérez-Pérez et al., 2004). However, the existing biochemical evidence is less clear. Coexpression in HeLa cells showed TWD1 enhanced B19-mediated auxin export, but the same experiment in yeast cells gave the opposite result (Bouchard et al., 2006). This work clarifies how the TWD1 immunophilin affects auxin transport and root growth by affecting B-group ABC transporters. Because ABCB1, the most similar ABC transporter in humans, is responsible for drug resistance in tumors (Szakács et al., 2006), this study may have also a broader significance in cancer research.
RESULTS
ABCB Transporters Are Mislocalized in twd1 Mutants
The central hypothesis tested here is that proper localization of auxin-related ABCB transporters requires TWD1. A corollary to this would be that twd1 phenotypes are due to aberrant auxin patterns resulting from mislocalization of ABCB transporters. To test this hypothesis, two different green fluorescent protein (GFP) fusions of B19 in two different Arabidopsis thaliana ecotypes were studied in root apices by laser scanning confocal microscopy. The GFP-B19 plant line was generated by Wu et al. (2007) in the Wassilewskija (Ws) background, and the B19-GFP line was generated by Mravec et al. (2008) in the Columbia (Col) background. Both protein fusions rescue b19 mutations (Wu et al., 2007; Mravec et al., 2008; Lewis et al., 2009) and show very similar expression patterns (Figures 1A and 1C). Both GFP fusion proteins were mostly restricted to the plasma membranes (Figures 1E and 1G) as previously reported. Crossing these transgenic lines to different twd1 mutant alleles in the appropriate genetic background demonstrated a large effect on B19 localization. Throughout the root tip, GFP-tagged B19 created a more diffuse signal in twd1-1 and twd1-3, very distinct from the wild-type distribution (Figures 1B and 1D). Also, overall levels of B19 appeared lower in twd1 compared with the wild type (Figures 1A to 1D). Imaging at higher resolution and higher detector gain settings revealed a strong GFP signal from what appears to be the endomembrane system and, to a lesser extent, the plasma membrane in twd1 mutant cells (Figures 1F and 1H). This was not due to a general mislocalization effect since the localization of the plasma membrane markers 29-1 and 37-26 fused to GFP was not affected by the twd1 mutations (Figures 1I to 1P).
Figure 1.
Mislocalization of ABCB19, but Not Plasma Membrane Markers, in twd1 Mutants.
(A) to (D) B19 molecules tagged with GFP at the N terminus (GFP-B19) in the Ws ecotype ([A] and [B]) or C terminus (B19-GFP) in the Col-0 ecotype ([C] and [D]) show an expression pattern similar to the wild type (WT) in two separate twd1 mutants, though the B19 levels are slightly lower in the mutant backgrounds.
(E) to (F) Higher magnification images showed mislocalized B19 in both twd1 alleles ([F] and [H]) compared with the wild type ([E] and [G]).
(I) to (L) Localization of plasma membrane marker proteins 29-1 ([I] and [J]) and 37-26 ([K] and [L]) fused to GFP were not affected by twd1 mutation.
(M) to (P) Higher-magnification images showed normal localization pattern of the two plasma membrane markers in twd1 background.
Cortical cells were used for subcellular localization in (E) to (H) and (M) to (P). Genotype is Ws in (A) and (E), twd1-1 in (B) and (F), Col in (C), (G), (I), (K), (M), and (O), and twd1-3 in (D), (H), (J), (L), (N), and (P). Bars = 50 μm in (A) to (D) and (I) to (L) and 10 μm in (E) to (H) and (M) to (P).
To investigate B19 subcellular localization with a second technology, transmission electron microscopy was performed on immunogold-labeled thin sections of root tips expressing GFP-B19. In the wild-type background, the anti-GFP antibody recognized epitopes at the plasma membrane (Figure 2A) of all 25 root cells analyzed, but it did not label the endoplasmic reticulum (ER; Figure 2B). In the 30 root cells analyzed from twd1-1 plants, 27 cells showed some gold particles at or near the plasma membrane (Figure 2C), but in 25 of these cells, the large majority of the gold labeling was present at the ER (Figures 2D to 2F). The GFP antibody occasionally cross-reacted with the cell wall of nontransgenic plants (Figure 2G).
Figure 2.
Localization of Immunogold-Labeled GFP-B19 Determined by Transmission Electron Microscopy.
(A) Wild-type root cells showed anti-GFP labeling mostly on the plasma membrane (arrows).
(B) Label was not detected on cytoplasmic structures such as ER, Golgi, or tonoplast surrounding the vacuole (V) in the wild type.
(C) twd1-1 mutant root cells showed some labeling at or near the plasma membrane (arrows).
(D) to (F) Unlike the wild type, twd1-1 samples showed significant labeling on the ER membranes (arrowheads).
(G) An image from a wild-type seedling serves as negative control. The anti-GFP antibody can bind nonspecifically to the cell wall (CW).
Bars = 200 nm.
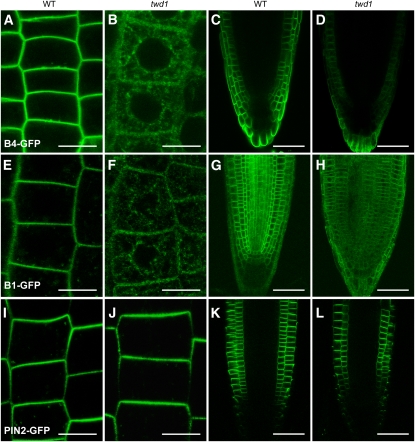
The B subfamily of ABC transporters in Arabidopsis contains 20 members. In addition to B19, ABCB1 and ABCB4 (hereafter, B1 and B4, respectively) have also been shown to affect polar auxin transport (Noh et al., 2001; Santelia et al., 2005; Cho et al., 2007; Lewis et al., 2007). The C terminus of B1 was shown to interact with TWD1, so it too may be affected by loss of TWD1. The C terminus of B4 is similar in sequence to that of B19, so TWD1 may be expected to influence its intracellular localization as well. Figures 3A to 3D show that GFP-tagged B4, confined to the plasma membrane in the wild type, adopted the same ER localization pattern as B19 in twd1 plants. Figures 3E to 3H show that the same is true of B1. B4 is required for auxin transport from the root tip toward the root base (Lewis et al., 2007), a process that also requires the PIN2 auxin efflux carrier in the same cells (Chen et al., 1998). However, PIN2 localization was not affected by the loss of TWD1 (Figures 3I to 3L), consistent with a previous study using antibodies and fixed tissues that found PIN1 and PIN2 to be normally localized in twd1 roots (Bouchard et al., 2006). Thus, whereas TWD1 is required for trafficking of at least two ABCB proteins involved in auxin distribution, it is not required for trafficking of the PIN components of the auxin transport machinery in the very same cells.
Figure 3.
Mislocalization of ABCB1 and ABCB4 but Not PIN2 in twd1 Mutants.
(A) and (E) ABCB4-GFP (B4-GFP) in Col and ABCB1-GFP (B1-GFP) in Ws were uniformly distributed around the periphery of wild-type (WT) cells, consistent with plasma membrane localization.
(B) and (F) B4-GFP in twd1-3 and B1-GFP in twd1-1 cells was prevalent in cytoplasmic structures resembling the ER.
(C) and (D) Lower B4-GFP signal in twd1-3 mutant compared with the wild type (Col).
(G) and (H) Lower B1-GFP signal in twd1-1 mutant compared with the wild type (Ws).
(I) PIN2-GFP signal was restricted to the periphery of wild-type (Col) cells, consistent with plasma membrane localization.
(J) The twd1-3 mutation did not affect PIN2-GFP localization.
(K) and (L) PIN2-GFP signal is not affected by TWD1 mutation (twd1-3) compared with the wild type (Col).
Confocal images were acquired at the same magnification and equal detector gain settings for each chromophore in the wild type and the twd1 mutant. Cortical cells were used for subcellular localization. Bars = 10 μm in (A), (B), (E), (F), (I), and (J) and 50 μm in (C), (D), (G), (H), (K), and (L).
The drug brefeldin A (BFA) affects membrane trafficking, providing an independent test of whether B4 and B19 were shifted from the plasma membrane to the ER in twd1. Plasma membrane proteins that cycle to and from the endosomal system at some frequency will localize to large cytoplasmic aggregations in response to BFA. This was observed for GFP-B19, B4-GFP, and PIN2-GFP in a wild-type background (Figures 4A, 4C, and 4E). No such accumulations of GFP-B19 or B4-GFP occurred in twd1 seedlings (Figures 4B and 4D), presumably because those proteins were not at the plasma membrane when the drug was applied. Instead, their distribution continued to resemble that of the ERYFP marker, which also did not shift in response to BFA (Figures 4G and 4H). PIN2-GFP, by contrast, localized to BFA bodies in the wild-type and twd1 backgrounds (Figures 4E and 4F). These results provide independent support for the conclusion that B4 and B19 are absent from the plasma membrane or are present at greatly reduced levels in twd1. The results also show the TWD1 independence of PIN2 trafficking and localization.
Figure 4.
TWD1 and Effects of BFA.
(A) GFP-B19 accumulated in large cytoplasmic bodies after 1.5 h of treatment with 20 μM BFA in the wild type (WT; Ws).
(B) GFP-B19 did not localize to BFA bodies in twd1-1.
(C) B4-GFP localized to BFA bodies in the wild type (Col).
(D) B4-GFP did not localize to BFA bodies in twd1-3.
(E) PIN2-GFP localized BFA bodies in the wild type (Col).
(F) PIN2-GFP localized BFA bodies in twd1-3.
(G) The ER marker ERYFP after mock treatment (DMSO solvent only).
(H) ERYFP did not localize to BFA bodies.
Cortical cells are shown in each panel. Bars = 10 μm.
TWD1 Is Localized to the ER
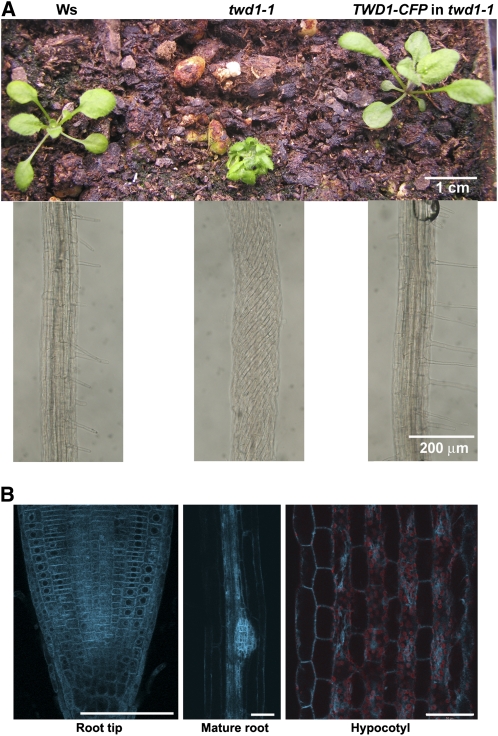
The mislocalization of the ABCB transporters in the twd1 mutants led us to hypothesize that TWD1 assists the passage of B1, B4, and B19 from the site of their translation at the ER to the secretory pathway that delivers integral membrane proteins to the plasma membrane. A TWD1-CFP (for cyan fluorescent protein) fusion construct under the control of the TWD1 promoter was introduced into the genome of twd1-1. The fusion construct completely rescued the many visible twd1 phenotypes (Figure 5A), indicating that it was functional. This reporter transgene was crossed into a plant expressing an ER marker (ERYFP), a Golgi marker (GYFP), or a vacuolar membrane (tonoplast) marker (δ-TIP-GFP) (Cutler et al., 2000; Nelson et al., 2007). Figure 6A shows that the TWD1-CFP distribution in root cells was a near perfect match with the ERYFP signal. No overlap between TWD1-CFP and the GYFP Golgi marker was detected (Figure 6B). Confocal images of cells or portions of cells in the root elongation zone showed that the δ-TIP-GFP marker was present at the ER as well as the vacuolar membrane. TWD1-CFP coresided with δ-TIP-GFP at the ER but not at the tonoplast, which can be seen distinctly in Figures 6C and 6D at the regions marked by arrowheads. Plants expressing the plasma membrane marker 29-1-GFP were crossed to the TWD1-CFP line. Confocal analysis of the coexpressing plants showed that TWD1-CFP did not overlap the plasma membrane (Figure 6E). If TWD1 were in the plasma membrane, it would be expected to enter BFA bodies, but Figure 6F shows that only the 29-1 marker was present in BFA bodies. Previous reports of TWD1 protein residing at the plasma membrane or tonoplast (Kamphausen et al., 2002; Bouchard et al., 2006) may be partially correct, but the multifaceted evidence presented here indicates that TWD1 resides and functions in processing of ABCB membrane proteins predominantly at the ER (Figures 1 to 4).
Figure 5.
TWD1 Localization, Shown in Vivo by a TWD1-CFP That Rescued twd1 Phenotypes.
(A) Top panel shows seedlings grown in the same pot for 15 d in continuous light at room temperature. Bottom panel shows the root twisting phenotype in 6-d-old seedlings grown on agar plates.
(B) TWD1-CFP in the meristem, mature root, and hypocotyl of a light-grown transgenic twd1-1 seedling harboring TWD1-CFP. Red fluorescence from chloroplasts was unmixed from the CFP signal and overlaid to show hypocotyl structure. Bars = 50 μm.
Figure 6.
Localization of TWD1-CFP to the ER.
(A) TWD1-CFP overlapped with the ER marker (ERYFP).
(B) TWD1-CFP did not overlap with the Golgi marker (GYFP).
(C) TWD1-CFP did not overlap with the tonoplast marker δ-TIP-GFP at the vacuole (arrows).
(D) Projecting a stack of six confocal optical sections onto a plane shows that TWD1-CFP and δ-TIP-GFP overlapped at the ER membranes but not at the vacuole (arrows).
(E) The plasma membrane marker 29-1 occupies the narrow gap in TWD1-CFP signal between cells (arrows) and nowhere overlaps with TWD1-CFP.
(F) Same as (E), following 1.5 h treatment with BFA. The arrows indicate the BFA bodies.
Confocal microscopy was performed using cortical cells ([A] and [B]) and epidermal cells ([E] and [F]) in the root transition zone and epidermal cells in the mature root close to the elongation zone in (C) and (D). Bars = 10 μm.
TWD1 and the B1, B4, and B19 Transporters Have Overlapping Expression Patterns
A widespread role for TWD1 in ABCB trafficking and, therefore, in auxin distribution is supported by its broad expression in the root apex (Figure 5B), which completely overlaps with the combined expression patterns of B1, B4, and B19 (Figures 1 and 3) (Cho et al., 2007; Lewis et al., 2007). TWD1 and B19 expression patterns also overlap in lateral root primordia, the central cylinder of the mature root, and in the seedling hypocotyl (Figure 5B) (Wu et al., 2007, 2010).
Mislocalization of ABCB Transporters in twd1 Impairs Auxin Transport
A critical and widespread role for TWD1 in localization of the auxin transport machinery indicated by the results in Figures 1 to 4 is consistent with the severe developmental abnormalities of twd1 mutants described previously (Geisler et al., 2003; Pérez-Pérez et al., 2004). The phenotypes, particularly the twisting of the root cell files, are explored here in detail to learn about their relationship to ABCB-dependent auxin transport. Figure 7A shows that twd1 and b1 b19 mutants are both dwarf as adult plants, as reported previously (Geisler et al., 2003). The primary roots of twd1 and b1 b19 seedlings are similarly stunted and agravitropic compared with the wild type (Figure 7B). However, these mutations do not visibly alter the anatomy of the root apex (Figure 7C), indicating that the meristem produces new cells that undergo cell fate specification. Twisting of epidermal cell files (Figures 7D and 7E) becomes evident in twd1 seedlings in the elongation zone behind the root apex and in the hypocotyl. The twisting of cell files in most parts of b1 b19 roots is indistinguishable from that in the twd1 mutant. The twisting phenotype in the double mutant is less obvious in the shoot compared with the twd1 mutant, except in the hypocotyls of etiolated seedlings. The several additional twd1 phenotypes in the shoot not shared by b1 b19 double mutants may be due to mislocalizaton of other ABCB transporters, such as B4, in twd1.
Figure 7.
Similar Phenotypes Displayed by b1 b19 and twd1 Mutants.
(A) Dwarf phenotype of b1 b19 (middle) and twd1 (right) plants (30 d old) compared with the wild type (left).
(B) Reduced gravity-directed growth in b1 b19 and twd1 seedlings (5 d old) compared with the wild type.
(C) Anatomy of primary root tips in b1 b19 and twd1 mutants is like the wild type (confocal microscopy).
(D) Twisted cell files in mature region of b1 b19 and twd1 roots (confocal microscopy).
(E) Twisted hypocotyl cell files in etiolated b1 b19 and twd1 seedlings but not the wild type (environmental scanning electron microscopy).
Bars = 5 cm in (A), 2 cm in (B), 50 μm in (C) and (D), and 100 μm in (E).
Next, the role of auxin distribution in the root elongation zone phenotype was addressed. ProDR5:GFP reporter gene activity visualized in confocal optical slices acquired near the elongation zone where the cell files begin to twist showed substantially more auxin signaling in twd1 and b1 b19 double mutants than in the wild type (Figure 8A). Treatment for 2 d with 1 μM naphthylphthalamic acid (NPA), an inhibitor of polar auxin transport, restored wild-type auxin signaling distribution in both b1 b19 and twd1 mutants (Figure 8B). The primary roots elongated in the NPA-containing medium, although at a slower rate. The cell files became much less twisted in the new portion of the primary roots formed during the treatment (Figure 8C).
Figure 8.
Twisted Phenotype in twd1 Roots Correlates Spatially and Pharmacologically with Spread of Auxin Signaling into the Elongation Zone.
(A) Abnormal spread of auxin-responsive ProDR5:GFP expression in the periphery of primary root tips of the wild type, b1 b19, and twd1 mutants. The arrow points to where the cell files begin to twist. Bar = 200 μm.
(B) Auxin-responsive ProDR5:GFP expression with NPA or DMSO treatment in primary root tips of the wild type, b19, b1 b19, and twd1 mutants. Bars = 50 μm.
(C) Root morphology of the wild type, b19, b1 b19, and twd1 mutants with or without NPA treatment. Bars = 50 μm.
DISCUSSION
One of the conclusions reached here is that TWD1/FKBP42 functions in the passage of at least three ABCB components of the auxin distribution apparatus from the ER into a secretion pathway that delivers them to the plasma membrane (Vitale and Denecke, 1999). Because TWD1 interacts with the cytoplasmically localized C terminus of B19, it is expected to perform this function on the cytoplasmic face of the ER membrane. A role for TWD1 in creating functional B19 is consistent with its previously reported interaction with the chaperone Hsp90 (Kamphausen et al., 2002) but is not consistent with other reports emphasizing plasma membrane or tonoplast localization (Geisler et al., 2003, 2004). The ER localization reported here may be given extra credence because it was obtained by in vivo confocal microscopy of a functional TWD1-CFP driven by the native promoter (Figures 5 and 6) and independently supported by the subcellular phenotypes of the twd1 mutants (Figures 1 to 4).
PIN2 localization was not affected by the twd1 mutation (Figures 3I to 3L, 4E, and 4F). If the PIN/ABCB complex documented in previous studies (Blakeslee et al., 2007) was initiated at the ER, loss of TWD1 would be expected to affect both interacting partners similarly. This was not observed. Instead, it seems different trafficking mechanisms for PINs and ABCBs (Titapiwatanakun et al., 2009) maintain their separateness until they reach the plasma membrane, where an interaction that affects function takes place.
Our results also raise an interesting point about the relative importance of PIN and ABCB components of the auxin transport mechanism, a topic that has been explored by technically difficult combinations of heterologous coexpression and mutant plant analyses. Perhaps because the issue is difficult to dissect, the reported results have ranged from somewhat synergistic PIN and ABCB activities (Blakeslee et al., 2007) to PINs being primary over ABCB or rate-limiting (Petrášek et al., 2006). Geisler et al. (2003) showed the twd1 mutant to have only 14% of wild-type polar auxin transport activity. Here, Figures 1 to 4 show highly mislocalized ABCB proteins but normally localized PIN proteins (Figures 3I and 3L, 4E, and 4F; Bouchard et al., 2006). The simplest explanation of these results is that correctly localized ABCBs are required for PINs to produce normal polar auxin transport. If PIN proteins in general are not regulated properly when ABCB proteins are mislocalized, as in twd1, or missing, as in b1 b19, deviations from the normal auxin signaling pattern as shown in Figure 8A may be observed. This is consistent with PIN regulation being the main in planta function of ABCBs with respect to polar auxin transport and suggests how a uniformly localized membrane protein can be required for a polar transport process.
The abnormal auxin distribution in the lateral root cap in the mutants, which may be related to the previously proposed effect of B19 on return of auxin to the stele (Lewis et al., 2007), reached the distal end of the elongation zone where the cell files begin to twist. It is possible that the lateral root cap containing high auxin in twd1 serves as an auxin reservoir to provide growth-stimulating levels to epidermal cells in the elongation zone. The spread of auxin signaling in the root apices of b1 b19 and twd1 is typically not uniform but more pronounced on one side of the root. This asymmetry, especially if it dynamically moves around the circumference of the root, may create the twisting cell files. NPA suppressed this auxin signaling spread (Figure 8B), perhaps by blocking PIN2 function (Blakeslee et al., 2007), and suppressed the cell file twisting (Figure 8C), indicating that the latter is caused by the former. This may also be the basis of the hypocotyl twisting phenotype caused by induced PIN1 overexpression, which was also suppressed by NPA treatment (Mravec et al., 2008). How NPA suppresses the spread of auxin signaling is not addressed by these experiments, but inhibition of the misregulated PIN proteins is a possibility to be tested by further experimentation.
While the PIN proteins may continue to function in twd1, the ABCB transporters appear not to function at the ER where they mislocalize in twd1 because their genetic knockout (b1 b19) phenocopies twd1 (Figure 7; Geisler et al., 2003). Unlike PIN5, which appears to mediate auxin transport across the ER membrane to affect cytoplasmic homeostasis (Mravec et al., 2009), the ER-localized ABCB transporters do not contribute to this process to an extent that affects visible phenotypes.
Protein structure studies give reason to believe that the relationship between TWD1 and ABCB maturation indicated by this work may be general rather than restricted to B1, B4, and B19. Fitting the x-ray structure of TWD1 to a homology model of the C terminus of the Arabidopsis ABCB1 transporter highlighted the nucleotide binding domain (NBD) as the possible site of interaction with TWD1 (Granzin et al., 2006). Because the NBD is the region most conserved between all the ABCB group members, TWD1 may interact with and affect the processing of many or all 20 members of the ABCB group. However, the modeling did not include the variable sequences extending from the NBD because there is no structural template for these member-specific regions. X-ray structures of full C termini of ABCB transporters are needed to understand how and where TWD1 interacts at the atomic level. This site of interaction could be important in biomedical fields because if an analogous interaction with an immunophilin is important to the process of human MDR1 (HsABCB1) maturation, drugs designed to interfere with it could prevent plasma membrane accumulation of the protein in tumor cells and thereby suppress the phenomenon of multidrug resistance that greatly impedes chemotherapies (Szakács et al., 2006).
METHODS
Plant Materials, Culture, and Treatments
The following Arabidopsis thaliana genotypes were used: wild-type plants of ecotypes Ws and Col-0; mutants b19-1, b1-1 b19-1 (Noh et al., 2001); b19-3 (Lewis et al., 2007); b1-100 (Lin and Wang, 2005); twd1-1 (Geisler et al., 2003); twd1-3 or ucu2-4 (Pérez-Pérez et al., 2004); transgenic lines ProB19:GFP-B19 (Wu et al., 2007); ProB19:B19-GFP and ProB1:B1-GFP (Mravec et al., 2008); ProB4:B4-GFP (Cho et al., 2007); ProPIN2:PIN2-GFP (Abas et al., 2006); 29-1-GFP and 37-26-GFP (Cutler et al., 2000); ERYFP, GYFP, and δ-TIP-GFP (Nelson et al., 2007). The transgenic lines were crossed with either with twd1-1 or twd1-3 dependent on their ecotypes. All marker plant lines used were homozygous for the transgene in the F3 generation.
For microscopy experiments, seedlings were grown on 1% bacto-agar medium, pH 5.8, containing half-strength Murashige and Skoog medium (2.15 g L−1 Murashige and Skoog salts; Sigma-Aldrich) at 50 μmol m–2 s–1 constant white light at 22°C for 6 d. In the case of plants used for NPA treatment, 4-d-old seedlings were transferred to agar plates containing the indicated concentration of NPA (Chem Service) and grown at the same condition for 2 d. The plates were kept vertical all the time.
Transgene Construction
PCR product containing eCFP was amplified from plasmid pCam-35S-eCFP-C1 (kindly provided by E. Nielsen at the University of Michigan) using two oligonucleotide primers, 5′-GTCCGATCGACCGGTGAATTCCCCGGGGTGAGCAAGGGCGAGGAGCTG-3′ and 5′-GTCAAGCTTCTTGTACAGCTCGTCCATGCC-3′, and was digested by PvuI and HindIII (restriction sites are underlined in the primer sequences). To generate binary vector pEGAD-eCFP, eGFP and the 35S promoter in the pEGAD vector were removed using HindIII and PacI, and the resulting vector was religated with the PCR product.
To generate a TWD1 promoter-driven eCFP construct (ProTWD1:TWD1-GFP), the genomic TWD1 DNA including 2.3-kb fragment of 5′ untranslated region was amplified by PCR from an Arabidopsis BAC (MIL23) using two oligonucleotide primers, 5′-CGAACCGGTGACTCCAATGGTGAAGGAGGTG-3′ and 5′-CGAACCGGTATCTGCTTTAACTCTGTGGCGTCG-3′. After digestion with AgeI (restriction sites are underlined in the primer sequences), the fragment was fused in frame to the N-terminal end of eCFP in pEGAD-eCFP vector using its AgeI site. The vector was introduced into twd1-3 plants, and lines homozygous for the transgene were obtained in the T3 generation as described previously (Wu et al., 2007).
Microscopy
Confocal microscopy was performed on a Zeiss LSM 510 laser scanning confocal microscope equipped with a Meta detector. To show the root outlines, roots were immersed in 25 μg mL−1 propidium iodide solution for 6 s. For BFA treatment, seedlings were treated in 1 mM KCl and 1 mM CaCl2 solution containing 50 μM BFA (Sigma-Aldrich). If not otherwise specified, subcellular localization was performed in cortical cells ~250 to 300 μm from the tip shown by the arrow in Figure 8A. Optics employed were a C-Apochromat ×40 water immersion lens or a plan-Apochromat ×63 oil immersion lens. The sample was excited with the 488-nm laser line for GFP, YFP, and propidium iodide signal and 458-nm laser line for CFP signal from a 30-mW argon gas laser. The fluorescence was captured in 10-nm bandwidths and then linear unmixing was performed to isolate the GFP, YFP, or CFP signal from the stained background. Channel mode detection was used to record the emission of GFP and YFP (excitation 488 nm; emission 505 to 550 nm) or CFP (excitation 458 nm; emission 473 to 495 nm) for subcellular localization. The detector pinhole was set at 1 Airy unit, and excitation power at 33.7%. To produce confocal images that are directly comparable between twd1 and the wild type, the same detector gain must be used. The gain setting that produced no signal in the nucleus of twd1, where the fluorescent protein is absent, was used for each mutant/wild-type comparison. For transmission electron microscopy, root tips were high-pressure frozen/freeze-substituted as by (Otegui and Austin, 2007). For immunolabeling, high-pressure frozen roots were substituted in 0.2% uranyl acetate (Electron Microscopy Sciences) plus 0.2% glutaraldehyde (Electron Microscopy Sciences) in acetone at −80°C for 72 h and warmed to −50°C for 24 h. After several acetone rinses, these samples were infiltrated with Lowicryl HM20 (Electron Microscopy Sciences) for 72 h and polymerized at −50°C under UV light for 48 h. Sections were mounted on Formvar-coated nickel grids and blocked for 20 min with a 5% (w/v) solution of nonfat milk in PBS containing 0.1% Tween 20. The sections were incubated in anti-GFP antibodies (Follet-Gueye et al., 2003) (1:10 in PBS-Tween 20) for 1 h, rinsed in PBS containing 0.5% Tween 20, and then transferred to the secondary antibody (anti-rabbit IgG 1:10) conjugated to 15-nm gold particles (Ted Pella) for 1 h. Ten sections of two root tips of each genotype were analyzed (~60 cells in for each genotype). Control labeling was performed by omitting the primary antibodies. Scanning electron microscopy was performed with an FEI Quanta 200 environmental scanning electron microscope on fresh, unfixed, 3-d-old etiolated seedlings.
Accession Numbers
The Arabidopsis Genome Initiative locus identifier for the ABCB19 gene (formerly MDR1 or PGP19) is At3g28860, ABCB4 (formerly MDR4 or PGP4) is At2g47000, ABCB1 gene (formerly PGP1) is At2g36910, and TWD1 is At3g21640.
Acknowledgments
We thank Daniel G. Lewis for assisting with the scanning electron microscopy imaging, Guang Wu and Misuk Cho for advice and help, and Jiri Friml, Hyung-Taeg Cho, and the ABRC for providing plant materials. This work was supported by National Science Foundation Grant IOS-0921071 to E.P.S. and G.W. and National Science Foundation Grant MCB-0843151 to M.S.O.
References
- Abas L., Benjamins R., Malenica N., Paciorek T., Wiśniewska J., Wirniewska J., Moulinier-Anzola J.C., Sieberer T., Friml J., Luschnig C. (2006). Intracellular trafficking and proteolysis of the Arabidopsis auxin-efflux facilitator PIN2 are involved in root gravitropism. Nat. Cell Biol. 8: 249–256 [DOI] [PubMed] [Google Scholar]
- Barik S. (2006). Immunophilins: for the love of proteins. Cell. Mol. Life Sci. 63: 2889–2900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakeslee J.J., et al. (2007). Interactions among PIN-FORMED and P-glycoprotein auxin transporters in Arabidopsis. Plant Cell 19: 131–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard R., Bailly A., Blakeslee J.J., Oehring S.C., Vincenzetti V., Lee O.R., Paponov I., Palme K., Mancuso S., Murphy A.S., Schulz B., Geisler M. (2006). Immunophilin-like TWISTED DWARF1 modulates auxin efflux activities of Arabidopsis P-glycoproteins. J. Biol. Chem. 281: 30603–30612 [DOI] [PubMed] [Google Scholar]
- Chen R., Hilson P., Sedbrook J., Rosen E., Caspar T., Masson P.H. (1998). The Arabidopsis thaliana AGRAVITROPIC 1 gene encodes a component of the polar-auxin-transport efflux carrier. Proc. Natl. Acad. Sci. USA 95: 15112–15117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho M., Lee S.H., Cho H.T. (2007). P-glycoprotein4 displays auxin efflux transporter-like action in Arabidopsis root hair cells and tobacco cells. Plant Cell 19: 3930–3943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler S.R., Ehrhardt D.W., Griffitts J.S., Somerville C.R. (2000). Random GFP:cDNA fusions enable visualization of subcellular structures in cells of Arabidopsis at a high frequency. Proc. Natl. Acad. Sci. USA 97: 3718–3723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Follet-Gueye M.L., Pagny S., Faye L., Gomord V., Driouich A. (2003). An improved chemical fixation method suitable for immunogold localization of green fluorescent protein in the Golgi apparatus of tobacco Bright Yellow (BY-2) cells. J. Histochem. Cytochem. 51: 931–940 [DOI] [PubMed] [Google Scholar]
- Geisler M., Bailly A. (2007). Tête-à-tête: The function of FKBPs in plant development. Trends Plant Sci. 12: 465–473 [DOI] [PubMed] [Google Scholar]
- Geisler M., Girin M., Brandt S., Vincenzetti V., Plaza S., Paris N., Kobae Y., Maeshima M., Billion K., Kolukisaoglu Ü.H., Schulz B., Martinoia E. (2004). Arabidopsis immunophilin-like TWD1 functionally interacts with vacuolar ABC transporters. Mol. Biol. Cell 15: 3393–3405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler M., et al. (2003). TWISTED DWARF1, a unique plasma membrane-anchored immunophilin-like protein, interacts with Arabidopsis multidrug resistance-like transporters AtPGP1 and AtPGP19. Mol. Biol. Cell 14: 4238–4249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith M.H.M. (1977). The polar transport of auxin. Annu. Rev. Plant Physiol. 28: 439–478 [Google Scholar]
- Granzin J., Eckhoff A., Weiergräber O.H. (2006). Crystal structure of a multi-domain immunophilin from Arabidopsis thaliana: A paradigm for regulation of plant ABC transporters. J. Mol. Biol. 364: 799–809 [DOI] [PubMed] [Google Scholar]
- He Z., Li L., Luan S. (2004). Immunophilins and parvulins. Superfamily of peptidyl prolyl isomerases in Arabidopsis. Plant Physiol. 134: 1248–1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamphausen T., Fanghänel J., Neumann D., Schulz B., Rahfeld J.U. (2002). Characterization of Arabidopsis thaliana AtFKBP42 that is membrane-bound and interacts with Hsp90. Plant J. 32: 263–276 [DOI] [PubMed] [Google Scholar]
- Kleine-Vehn J., Friml J. (2008). Polar targeting and endocytic recycling in auxin-dependent plant development. Annu. Rev. Cell Dev. Biol. 24: 447–473 [DOI] [PubMed] [Google Scholar]
- Kramer E.M. (2004). PIN and AUX/LAX proteins: Their role in auxin accumulation. Trends Plant Sci. 9: 578–582 [DOI] [PubMed] [Google Scholar]
- Lewis D.R., Miller N.D., Splitt B.L., Wu G., Spalding E.P. (2007). Separating the roles of acropetal and basipetal auxin transport on gravitropism with mutations in two Arabidopsis multidrug resistance-like ABC transporter genes. Plant Cell 19: 1838–1850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis D.R., Wu G., Ljung K., Spalding E.P. (2009). Auxin transport into cotyledons and cotyledon growth depend similarly on the ABCB19 Multidrug Resistance-like transporter. Plant J. 60: 91–101 [DOI] [PubMed] [Google Scholar]
- Lin R., Wang H. (2005). Two homologous ATP-binding cassette transporter proteins, AtMDR1 and AtPGP1, regulate Arabidopsis photomorphogenesis and root development by mediating polar auxin transport. Plant Physiol. 138: 949–964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mravec J., Kubes M., Bielach A., Gaykova V., Petrášek J., Skůpa P., Chand S., Benková E., Zazímalová E., Friml J. (2008). Interaction of PIN and PGP transport mechanisms in auxin distribution-dependent development. Development 135: 3345–3354 [DOI] [PubMed] [Google Scholar]
- Mravec J., et al. (2009). Subcellular homeostasis of phytohormone auxin is mediated by the ER-localized PIN5 transporter. Nature 459: 1136–1140 [DOI] [PubMed] [Google Scholar]
- Nelson B.K., Cai X., Nebenführ A. (2007). A multicolored set of in vivo organelle markers for co-localization studies in Arabidopsis and other plants. Plant J. 51: 1126–1136 [DOI] [PubMed] [Google Scholar]
- Noh B., Murphy A.S., Spalding E.P. (2001). Multidrug resistance-like genes of Arabidopsis required for auxin transport and auxin-mediated development. Plant Cell 13: 2441–2454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otegui M.S., Austin J.R. (2007). Visualization of membrane-cytoskeletal interactions during plant cytokinesis. Methods Cell Biol. 79: 221–240 [DOI] [PubMed] [Google Scholar]
- Pérez-Pérez J.M., Ponce M.R., Micol J.L. (2004). The ULTRACURVATA2 gene of Arabidopsis encodes an FK506-binding protein involved in auxin and brassinosteroid signaling. Plant Physiol. 134: 101–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrášek J., Friml J. (2009). Auxin transport routes in plant development. Development 136: 2675–2688 [DOI] [PubMed] [Google Scholar]
- Petrášek J., et al. (2006). PIN proteins perform a rate-limiting function in cellular auxin efflux. Science 312: 914–918 [DOI] [PubMed] [Google Scholar]
- Romano P., Gray J., Horton P., Luan S. (2005). Plant immunophilins: Functional versatility beyond protein maturation. New Phytol. 166: 753–769 [DOI] [PubMed] [Google Scholar]
- Santelia D., Vincenzetti V., Azzarello E., Bovet L., Fukao Y., Düchtig P., Mancuso S., Martinoia E., Geisler M. (2005). MDR-like ABC transporter AtPGP4 is involved in auxin-mediated lateral root and root hair development. FEBS Lett. 579: 5399–5406 [DOI] [PubMed] [Google Scholar]
- Szakács G., Paterson J.K., Ludwig J.A., Booth-Genthe C., Gottesman M.M. (2006). Targeting multidrug resistance in cancer. Nat. Rev. Drug Discov. 5: 219–234 [DOI] [PubMed] [Google Scholar]
- Titapiwatanakun B., et al. (2009). ABCB19/PGP19 stabilises PIN1 in membrane microdomains in Arabidopsis. Plant J. 57: 27–44 [DOI] [PubMed] [Google Scholar]
- Vieten A., Sauer M., Brewer P.B., Friml J. (2007). Molecular and cellular aspects of auxin-transport-mediated development. Trends Plant Sci. 12: 160–168 [DOI] [PubMed] [Google Scholar]
- Vitale A., Denecke J. (1999). The endoplasmic reticulum-gateway of the secretory pathway. Plant Cell 11: 615–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiergräber O.H., Eckhoff A., Granzin J. (2006). Crystal structure of a plant immunophilin domain involved in regulation of MDR-type ABC transporters. FEBS Lett. 580: 251–255 [DOI] [PubMed] [Google Scholar]
- Woodward A.W., Bartel B. (2005). Auxin: Regulation, action, and interaction. Ann. Bot. (Lond.) 95: 707–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G., Cameron J.N., Ljung K., Spalding E.P. (2010). A role for ABCB19-mediated polar auxin transport in seedling photomorphogenesis mediated by cryptochrome 1 and phytochrome B. Plant J. 62: 179–191 [DOI] [PubMed] [Google Scholar]
- Wu G., Lewis D.R., Spalding E.P. (2007). Mutations in Arabidopsis multidrug resistance-like ABC transporters separate the roles of acropetal and basipetal auxin transport in lateral root development. Plant Cell 19: 1826–1837 [DOI] [PMC free article] [PubMed] [Google Scholar]