Abstract
In this Commentary, the roles of uridine adenosine tetraphosphate as an endothelium-derived contracting or relaxing factor described in the paper by Tölle et al. are considered and put into the wider context of the mechanisms of control of vascular tone by purinergic signalling via receptors located on both smooth muscle and endothelial cells.
Keywords: purinergic, sympathetic nerves, endothelial cells, cotransmission, ATP, UTP, adenosine, Up4A
For many years control of vascular tone was understood to be via antagonistic sympathetic adrenergic vasoconstrictor and parasympathetic cholinergic vasodilator nerves. Then the seminal discovery by Furchgott of control of vascular tone via endothelium-derived relaxing factors opened up a whole new field. Endothelium-derived contracting factors were also identified.
Dual control of vascular tone by ATP was recognized (Burnstock and Kennedy, 1986). ATP released as a cotransmitter with noradrenaline from sympathetic nerves to produce contraction of vascular smooth muscle via P2X (ion channel) receptors; while ATP released from endothelial cells in response to changes in blood flow (shear stress) and hypoxia to act on P2Y (G protein-coupled) receptors on endothelial cells to release nitric oxide (NO) resulting in vasodilation (Burnstock, 1990). Subsequently, more purinoceptor subtypes were recognized on both vascular smooth muscle (P2X1 (in some vessels P2X2 and P2X4), P2Y1 and P2Y2) and endothelial cells (in various vessels P2X1, P2X2, P2X3, P2X4 and P2Y1, P2Y2, P2Y4 and P2Y11) in different vessels, mediated by pyrimidines as well as purines and diadenosine polyphosphates (see Burnstock, 2008). In 2005, uridine adenosine tetraphosphate (Up4A) and adenosine tetraphosphate were shown to be endothelial contracting factors probably acting via P2X1 receptors on smooth muscle (Jankowski et al., 2005; Tölle et al., 2008).
A paper by the same group in this issue of the BJP, extends this finding to show that in the rat isolated perfused kidney model, in addition to smooth muscle P2X1 receptor-mediated constrictor activation, Up4A showed dose-dependent P2Y2 receptor-mediated long-lasting vasoconstriction. Further, they demonstrated that vasoconstriction by Up4A was followed by vasodilation mediated by P2Y1 and P2Y2 receptor activation of endothelial cells leading to release of NO. Whether the actions of Up4A described in this paper are confined to vessels in the kidney or are more widely utilized in the vascular system needs to be resolved. Nevertheless, this work is important in emphasizing the complex and variable purinergic pathways involved in control of vascular tone in different vessels and its relevance to both the physiology and pathophysiology of the vascular system. Figure 1 illustrates the current knowledge of the involvement of purinergic receptor subtypes in these regulatory mechanisms and highlights the new information produced by the paper by Tölle et al. (2010).
Figure 1.
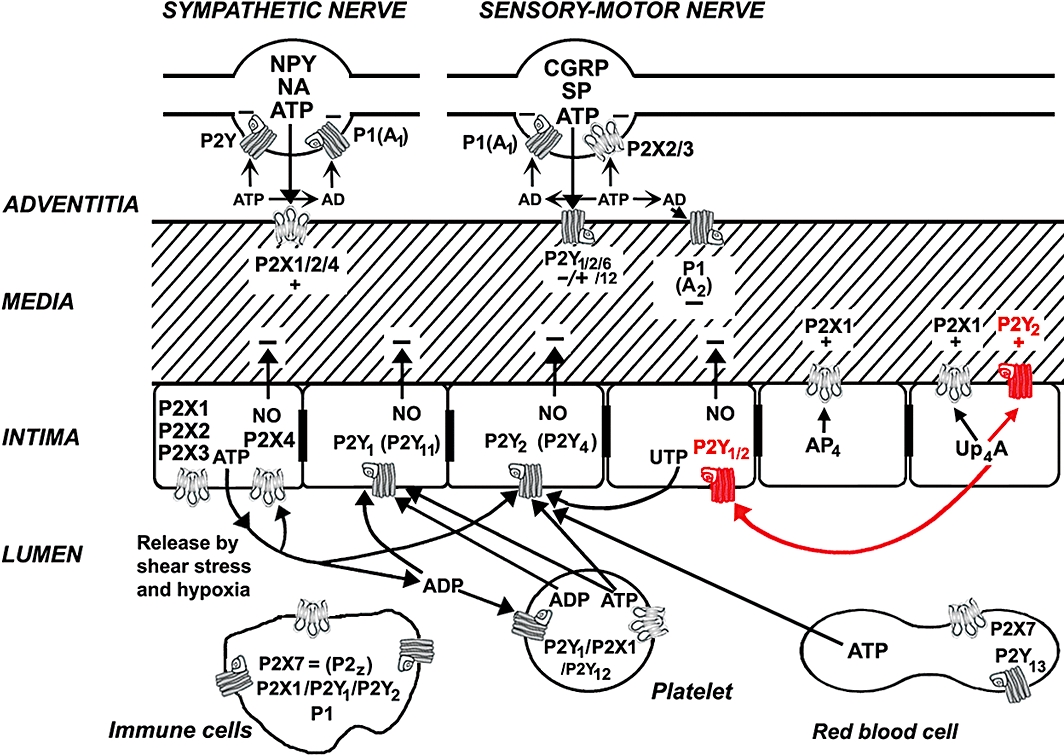
Schematic diagram illustrating the main receptor subtypes for purines and pyrimidines present in blood vessels involved in control of vascular tone. ATP is released as a cotransmitter with noradrenaline (NA) and neuropeptide Y (NPY) from sympathetic nerves in the adventitia to act at smooth muscle P2X1 receptors and, in some vessels, P2X2, P2X4 and P2Y1, P2Y2 and P2Y6 receptors, resulting in vasoconstriction (and rarely vasodilation); ATP is released with calcitonin gene-related peptide (CGRP) and substance P (SP) from sensory-motor nerves during ‘axon reflex’ activity to act on smooth muscle P2Y receptors, resulting in either vasodilatation or vasoconstriction. P1 (A1) receptors on nerve terminals of sympathetic and sensory nerves mediate adenosine (arising from ecto-enzymatic breakdown of ATP) modulation of transmitter release. P2X2/3 receptors are present on a subpopulation of sensory nerve terminals. P1 (A2) receptors on vascular smooth muscle mediate vasodilatation. Endothelial cells release ATP and UTP during shear stress and hypoxia to act on P2Y1, P2Y2 and sometimes P2Y4, P2Y11, P2X1, P2X2, P2X3 and P2X4 receptors, leading to the production of nitric oxide (NO) and subsequent vasodilatation. Adenosine tetraphosphate (AP4) activates P2X1 receptors to excite smooth muscle. ATP, after its release from aggregating platelets, also acts, together with its breakdown product ADP, on these endothelial receptors. Blood-borne platelets possess P2Y1 and P2Y12 ADP-selective receptors as well as P2X1 receptors. Immune cells of various kinds possess P2X7 as well as P1, P2X1, P2Y1 and P2Y2 receptors. ATP released from red blood cells, which express P2X7 and P2Y13 receptors, is also involved in some circumstances. The additional involvements of uridine adenosine tetraphosphate (Up4A) described in the paper by Tölle et al. 2010 are indicated in red. (This figure is modified from Burnstock G (1996). J Auton Pharmacol16: 295–302 with permission from Blackwell Science Ltd, UK.)
Glossary
Abbreviations
- NO
nitric oxide
- Up4A
uridine adenosine tetraphosphate
Supporting Information
Supporting Information: Teaching Materials; Fig 1 as PowerPoint slide.
References
- Burnstock G. Dual control of local blood flow by purines. Ann N Y Acad Sci. 1990;603:31–44. doi: 10.1111/j.1749-6632.1990.tb37659.x. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Dual control of vascular tone and remodelling by ATP released from nerves and endothelial cells. Pharmacol Rep. 2008;60:12–20. [PubMed] [Google Scholar]
- Burnstock G, Kennedy C. A dual function for adenosine 5′-triphosphate in the regulation of vascular tone. Excitatory cotransmitter with noradrenaline from perivascular nerves and locally released inhibitory intravascular agent. Circ Res. 1986;58:319–330. doi: 10.1161/01.res.58.3.319. [DOI] [PubMed] [Google Scholar]
- Jankowski V, Tölle M, Vanholder R, Schonfelder G, van der Giet M, Henning L, et al. Uridine adenosine tetraphosphate: a novel endothelium-derived vasoconstrictive factor. Nat Med. 2005;11:223–227. doi: 10.1038/nm1188. [DOI] [PubMed] [Google Scholar]
- Tölle M, Jankowski V, Schuchardt M, Wiedon A, Huang T, Hub F, et al. Adenosine 5′-tetraphosphate is a highly potent purinergic endothelium-derived vasoconstrictor. Circ Res. 2008;103:1100–1108. doi: 10.1161/CIRCRESAHA.108.177865. [DOI] [PubMed] [Google Scholar]
- Tölle M, Schuchardt M, Wiedon A, Huang T, Klöckel L, Jankowski J, et al. Differential effects of uridine adenosine tetraphosphate on purinergic receptors in the rat isolated perfused kidney. Br J Pharmacol. 2010;161:530–540. doi: 10.1111/j.1476-5381.2010.00914.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


