Abstract
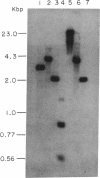
A cDNA encoding the vitamin D-dependent rat intestinal calcium-binding protein has been isolated by screening a rat intestinal cDNA library. The cDNA is 406 nucleotides long and appears to contain all the sequences of the mRNA. The cDNA includes the entire protein coding region. It consists of 237 nucleotides coding for 79 amino acids, including the starting methionine, flanked by 62 and 107 noncoding nucleotides at the 5' and 3' ends, respectively. Using the cloned cDNA, we have isolated a genomic clone from a rat liver genomic library. Restriction mapping and Southern analysis using synthetic oligonucleotides localized the gene to a 4.0-kilobase-pair HindIII fragment.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brawerman G. The Role of the poly(A) sequence in mammalian messenger RNA. CRC Crit Rev Biochem. 1981;10(1):1–38. doi: 10.3109/10409238109114634. [DOI] [PubMed] [Google Scholar]
- Corradino R. A. 1,25-Dihydroxycholecalciferol: inhibition of action in organ-cultured intestine by actinomycin D and alpha-amanitin. Nature. 1973 May 4;243(5401):41–43. doi: 10.1038/243041a0. [DOI] [PubMed] [Google Scholar]
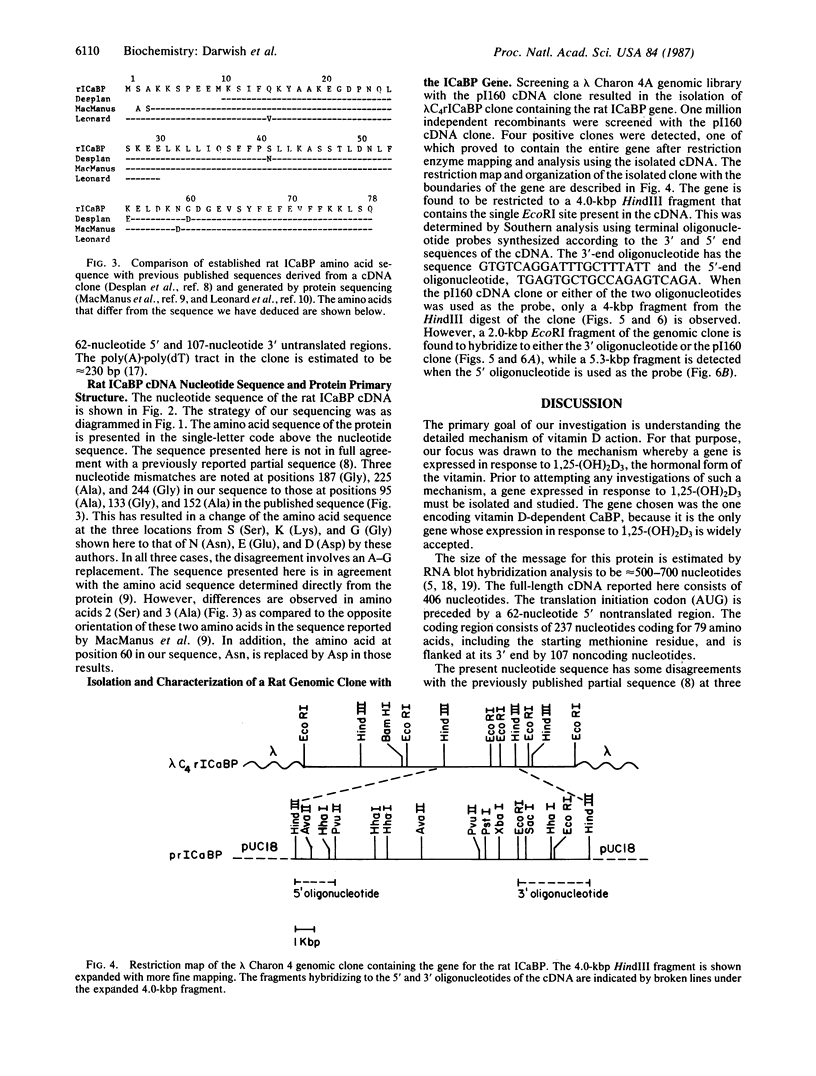
- Desplan C., Heidmann O., Lillie J. W., Auffray C., Thomasset M. Sequence of rat intestinal vitamin D-dependent calcium-binding protein derived from a cDNA clone. Evolutionary implications. J Biol Chem. 1983 Nov 25;258(22):13502–13505. [PubMed] [Google Scholar]
- Drescher D., DeLuca H. F. Vitamin D stimulated calcium binding protein from rat intestinal mucosa. Purification and some properties. Biochemistry. 1971 Jun 8;10(12):2302–2307. doi: 10.1021/bi00788a019. [DOI] [PubMed] [Google Scholar]
- Fullmer C. S., Wasserman R. H. The amino acid sequence of bovine intestinal calcium-binding protein. J Biol Chem. 1981 Jun 10;256(11):5669–5674. [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Hattori M., Sakaki Y. Dideoxy sequencing method using denatured plasmid templates. Anal Biochem. 1986 Feb 1;152(2):232–238. doi: 10.1016/0003-2697(86)90403-3. [DOI] [PubMed] [Google Scholar]
- Hofmann T., Kawakami M., Hitchman A. J., Harrison J. E., Dorrington K. J. The amino acid sequence of porcine intestinal calcium-binding protein. Can J Biochem. 1979 Jun;57(6):737–748. doi: 10.1139/o79-092. [DOI] [PubMed] [Google Scholar]
- Hunziker W. The 28-kDa vitamin D-dependent calcium-binding protein has a six-domain structure. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7578–7582. doi: 10.1073/pnas.83.20.7578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler M. A., Lamm L., Jarnagin K., DeLuca H. F. 1,25-Dihydroxyvitamin D3-stimulated mRNAs in rat small intestine. Arch Biochem Biophys. 1986 Dec;251(2):403–412. doi: 10.1016/0003-9861(86)90346-2. [DOI] [PubMed] [Google Scholar]
- Leonard W. J., Strauss A. W., Go M. F., Alpers D. H., Gordon J. I. Biosynthesis and compartmentalization of rat-intestinal vitamin-D-dependent calcium-binding protein. Eur J Biochem. 1984 Mar 15;139(3):561–571. doi: 10.1111/j.1432-1033.1984.tb08042.x. [DOI] [PubMed] [Google Scholar]
- MacManus J. P., Watson D. C., Yaguchi M. The purification and complete amino acid sequence of the 9000-Mr Ca2+-binding protein from rat placenta. Identity with the vitamin D-dependent intestinal Ca2+-binding protein. Biochem J. 1986 Apr 15;235(2):585–595. doi: 10.1042/bj2350585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perret C., Desplan C., Thomasset M. Cholecalcin (a 9-kDa cholecalciferol-induced calcium-binding protein) messenger RNA. Distribution and induction by calcitriol in the rat digestive tract. Eur J Biochem. 1985 Jul 1;150(1):211–217. doi: 10.1111/j.1432-1033.1985.tb09009.x. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent T. D., Wu J. R., Sala-Trepat J. M., Wallace R. B., Reyes A. A., Bonner J. The rat serum albumin gene: analysis of cloned sequences. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3256–3260. doi: 10.1073/pnas.76.7.3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumpf W. E., Sar M., Reid F. A., Tanaka Y., DeLuca H. F. Target cells for 1,25-dihydroxyvitamin D3 in intestinal tract, stomach, kidney, skin, pituitary, and parathyroid. Science. 1979 Dec 7;206(4423):1188–1190. doi: 10.1126/science.505004. [DOI] [PubMed] [Google Scholar]
- Thomasset M., Desplan C., Parkes O. Rat vitamin-D-dependent calcium-binding proteins. Specificity of mRNAs coding for the 7500-Mr protein from duodenum and the 28000-Mr protein from kidney and cerebellum. Eur J Biochem. 1983 Jan 1;129(3):519–524. [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Wasserman R. H., Taylor A. N. Vitamin d3-induced calcium-binding protein in chick intestinal mucosa. Science. 1966 May 6;152(3723):791–793. doi: 10.1126/science.152.3723.791. [DOI] [PubMed] [Google Scholar]