Abstract
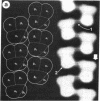
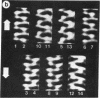
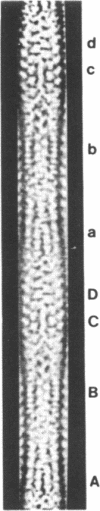
Sickle cell anemia results from the formation of hemoglobin S fibers in erythrocytes, and a greater understanding of the structure of these fibers should provide insights into the basis of the disease and aid in the development of effective antisickling agents. Improved reconstructions from electron micrographs of negatively stained single hemoglobin S fibers or embedded fiber bundles reveal that the 14 strands of the fiber are organized into pairs. The strands in each of the seven pairs are half-staggered, and from longitudinal views the polarity of each pair can be determined. The positions of the pairs and their polarities (three in one orientation; four in the opposite orientation) suggest a close relationship with the crystals of deoxyhemoglobin S composed of antiparallel pairs of half-staggered strands.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Crepeau R. H., Dykes G., Garrell R., Edelstein S. J. Diameter of haemoglobin S fibres in sickled cells. Nature. 1978 Aug 10;274(5671):616–617. doi: 10.1038/274616a0. [DOI] [PubMed] [Google Scholar]
- Crepeau R. H., Edelstein S. J. Polarity of the 14-strand fibers of sickle cell hemoglobin determined by cross-correlation methods. Ultramicroscopy. 1984;13(1-2):11–18. doi: 10.1016/0304-3991(84)90052-4. [DOI] [PubMed] [Google Scholar]
- Dykes G. W., Crepeau R. H., Edelstein S. J. Three-dimensional reconstruction of the 14-filament fibers of hemoglobin S. J Mol Biol. 1979 Jun 5;130(4):451–472. doi: 10.1016/0022-2836(79)90434-0. [DOI] [PubMed] [Google Scholar]
- Dykes G., Crepeau R. H., Edelstein S. J. Three-dimensional reconstruction of the fibres of sickle cell haemoglobin. Nature. 1978 Apr 6;272(5653):506–510. doi: 10.1038/272506a0. [DOI] [PubMed] [Google Scholar]
- Edelstein S. J. Molecular topology in crystals and fibers of hemoglobin S. J Mol Biol. 1981 Aug 25;150(4):557–575. doi: 10.1016/0022-2836(81)90381-8. [DOI] [PubMed] [Google Scholar]
- Garrell R. L., Crepeau R. H., Edelstein S. J. Cross-sectional views of hemoglobin S fibers by electron microscopy and computer modeling. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1140–1144. doi: 10.1073/pnas.76.3.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofrichter J., Hendricker D. G., Eaton W. A. Structure of hemoglobin S fibers: optical determination of the molecular orientation in sickled erythrocytes. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3604–3608. doi: 10.1073/pnas.70.12.3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huisman T. H., Dozy A. M. Studies on the heterogeneity of hemoglobin. IX. The use of Tris(hydroxymethyl)aminomethanehcl buffers in the anion-exchange chromatography of hemoglobins. J Chromatogr. 1965 Jul;19(1):160–169. doi: 10.1016/s0021-9673(01)99434-8. [DOI] [PubMed] [Google Scholar]
- Magdoff-Fairchild B., Chiu C. C. X-ray diffraction studies of fibers and crystals of deoxygenated sickle cell hemoglobin. Proc Natl Acad Sci U S A. 1979 Jan;76(1):223–226. doi: 10.1073/pnas.76.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magdoff-Fairchild B., Swerdlow P. H., Bertles J. F. Intermolecular organization of deoxygenated sickle haemoglobin determined by x-ray diffraction. Nature. 1972 Sep 22;239(5369):217–219. doi: 10.1038/239217a0. [DOI] [PubMed] [Google Scholar]
- Makinen M. W., Sigountos C. W. Structural basis and dynamics of the fiber-to-crystal transition of sickle cell hemoglobin. J Mol Biol. 1984 Sep 15;178(2):439–476. doi: 10.1016/0022-2836(84)90152-9. [DOI] [PubMed] [Google Scholar]
- Nagel R. L., Johnson J., Bookchin R. M., Garel M. C., Rosa J., Schiliro G., Wajcman H., Labie D., Moo-Penn W., Castro O. Beta-chain contact sites in the haemoglobin S polymer. Nature. 1980 Feb 28;283(5750):832–834. doi: 10.1038/283832a0. [DOI] [PubMed] [Google Scholar]
- Potel M. J., Wellems T. E., Vassar R. J., Deer B., Josephs R. Macrofiber structure and the dynamics of sickle cell hemoglobin crystallization. J Mol Biol. 1984 Aug 25;177(4):819–839. doi: 10.1016/0022-2836(84)90050-0. [DOI] [PubMed] [Google Scholar]
- Pumphrey J. G., Steinhardt J. Formation of needle-like aggregates in stirred solutions of hemoglobin S1. Biochem Biophys Res Commun. 1976 Mar 8;69(1):99–105. doi: 10.1016/s0006-291x(76)80278-1. [DOI] [PubMed] [Google Scholar]
- Rosen L. S., Magdoff-Fairchild B. X-ray diffraction studies of 14-filament models of deoxygenated sickle cell hemoglobin fibers. Models based on electron micrograph reconstructions. J Mol Biol. 1985 Jun 25;183(4):565–574. doi: 10.1016/0022-2836(85)90172-x. [DOI] [PubMed] [Google Scholar]
- Wellems T. E., Josephs R. Crystallization of deoxyhemoglobin S by fiber alignment and fusion. J Mol Biol. 1979 Dec 15;135(3):651–674. doi: 10.1016/0022-2836(79)90170-0. [DOI] [PubMed] [Google Scholar]
- Wishner B. C., Ward K. B., Lattman E. E., Love W. E. Crystal structure of sickle-cell deoxyhemoglobin at 5 A resolution. J Mol Biol. 1975 Oct 15;98(1):179–194. doi: 10.1016/s0022-2836(75)80108-2. [DOI] [PubMed] [Google Scholar]