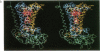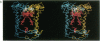Abstract
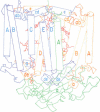
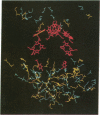
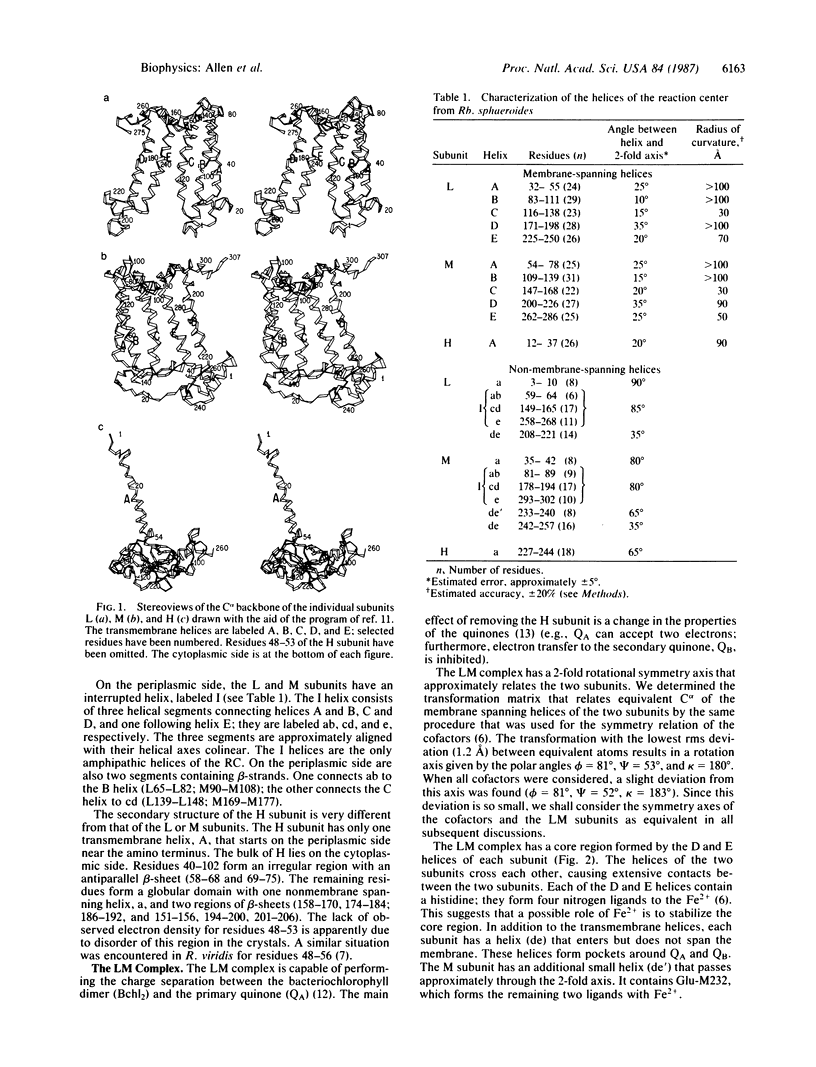
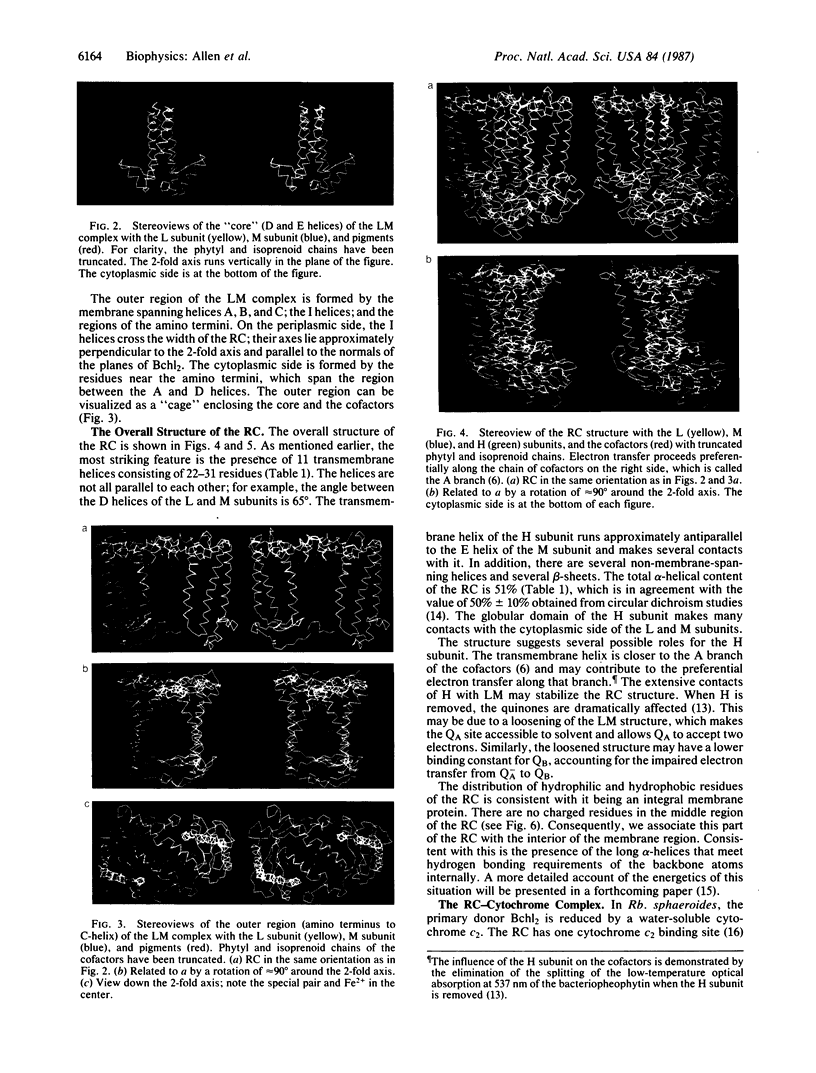
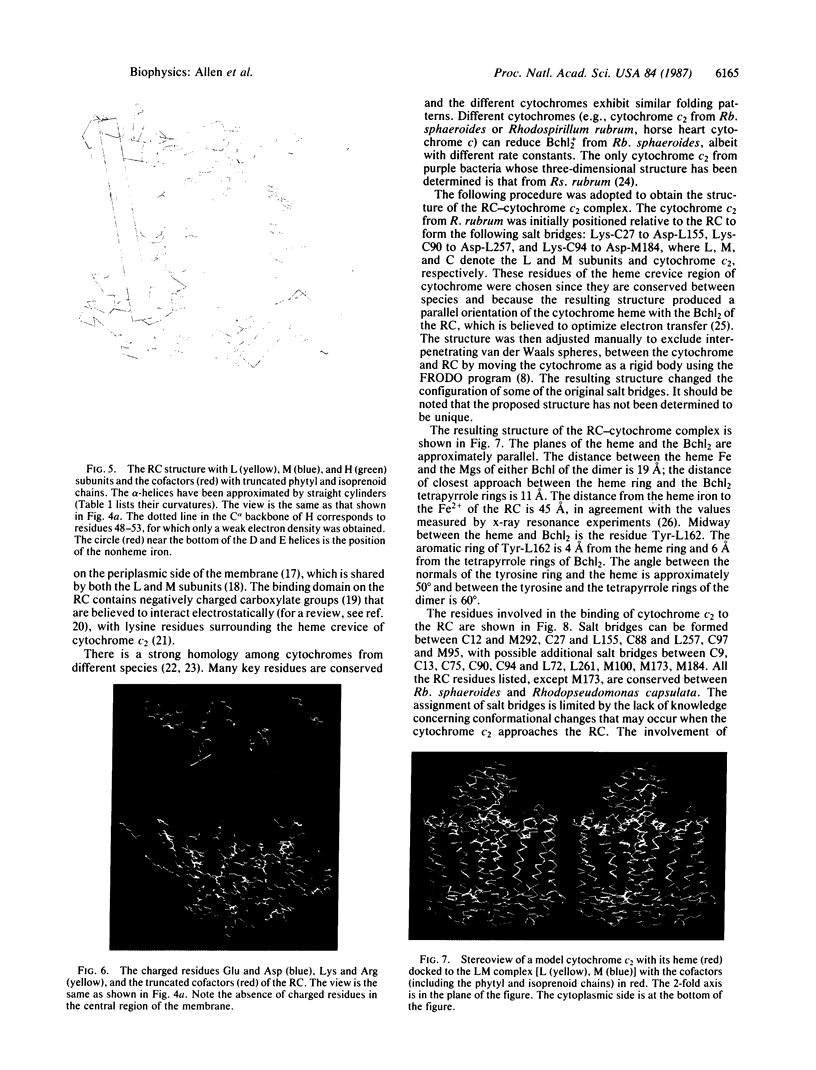
The three-dimensional structure of the protein subunits of the reaction center (RC) of Rhodobacter sphaeroides has been determined by x-ray diffraction at a resolution of 2.8 A with an R factor of 26%. The L and M subunits each contain five transmembrane helices and several helices that do not span the membrane. The L and M subunits are related to each other by a 2-fold rotational symmetry axis that is approximately the same as that determined for the cofactors. The H subunit has one transmembrane helix and a globular domain on the cytoplasmic side, which contains a helix that does not span the membrane and several beta-sheets. The structural homology with RCs from other purple bacteria is discussed. A structure of the complex formed between the water soluble cytochrome c2 and the RC from Rb. sphaeroides is proposed.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen J. P., Feher G., Yeates T. O., Komiya H., Rees D. C. Structure of the reaction center from Rhodobacter sphaeroides R-26: the cofactors. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5730–5734. doi: 10.1073/pnas.84.16.5730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blundell T., Barlow D., Borkakoti N., Thornton J. Solvent-induced distortions and the curvature of alpha-helices. Nature. 1983 Nov 17;306(5940):281–283. doi: 10.1038/306281a0. [DOI] [PubMed] [Google Scholar]
- Bosshard H. R., Snozzi M., Bachofen R. Interaction of horse cytochrome c with the photosynthetic reaction center of Rhodospirillum rubrum. J Bioenerg Biomembr. 1987 Aug;19(4):375–382. doi: 10.1007/BF00768540. [DOI] [PubMed] [Google Scholar]
- Dickerson R. E. Cytochrome c and the evolution of energy metabolism. Sci Am. 1980 Mar;242(3):137–153. [PubMed] [Google Scholar]
- Hall J., Zha X. H., Durham B., O'Brien P., Vieira B., Davis D., Okamura M., Millett F. Reaction of cytochromes c and c2 with the Rhodobacter sphaeroides reaction center involves the heme crevice domain. Biochemistry. 1987 Jul 14;26(14):4494–4500. doi: 10.1021/bi00388a048. [DOI] [PubMed] [Google Scholar]
- Jones T. A. Diffraction methods for biological macromolecules. Interactive computer graphics: FRODO. Methods Enzymol. 1985;115:157–171. doi: 10.1016/0076-6879(85)15014-7. [DOI] [PubMed] [Google Scholar]
- Lesk A. M., Hardman K. D. Computer-generated schematic diagrams of protein structures. Science. 1982 Apr 30;216(4545):539–540. doi: 10.1126/science.7071602. [DOI] [PubMed] [Google Scholar]
- Meyer T. E., Kamen M. D. New perspectives on c-type cytochromes. Adv Protein Chem. 1982;35:105–212. doi: 10.1016/s0065-3233(08)60469-6. [DOI] [PubMed] [Google Scholar]
- Michel H., Weyer K. A., Gruenberg H., Dunger I., Oesterhelt D., Lottspeich F. The 'light' and 'medium' subunits of the photosynthetic reaction centre from Rhodopseudomonas viridis: isolation of the genes, nucleotide and amino acid sequence. EMBO J. 1986 Jun;5(6):1149–1158. doi: 10.1002/j.1460-2075.1986.tb04340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel H., Weyer K. A., Gruenberg H., Lottspeich F. The ;heavy' subunit of the photosynthetic reaction centre from Rhodopseudomonas viridis: isolation of the gene, nucleotide and amino acid sequence. EMBO J. 1985 Jul;4(7):1667–1672. doi: 10.1002/j.1460-2075.1985.tb03835.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince R. C., Baccarini-Melandri A., Hauska G. A., Melandri B. A., Crofts A. R. Asymmetry of an energy transducing membrane the location of cytochrome c2 in Rhodopseudomonas spheroides and Rhodopseudomonas capsulata. Biochim Biophys Acta. 1975 May 15;387(2):212–227. doi: 10.1016/0005-2728(75)90104-8. [DOI] [PubMed] [Google Scholar]
- Rosen D., Okamura M. Y., Abresch E. C., Valkirs G. E., Feher G. Interaction of cytochrome c with reaction centers of Rhodopseudomonas sphaeroides R-26: localization of the binding site by chemical cross-linking and immunochemical studies. Biochemistry. 1983 Jan 18;22(2):335–341. doi: 10.1021/bi00271a016. [DOI] [PubMed] [Google Scholar]
- Rosen D., Okamura M. Y., Abresch E. C., Valkirs G. E., Feher G. Interaction of cytochrome c with reaction centers of Rhodopseudomonas sphaeroides R-26: localization of the binding site by chemical cross-linking and immunochemical studies. Biochemistry. 1983 Jan 18;22(2):335–341. doi: 10.1021/bi00271a016. [DOI] [PubMed] [Google Scholar]
- Salemme F. R. An hypothetical structure for an intermolecular electron transfer complex of cytochromes c and b5. J Mol Biol. 1976 Apr 15;102(3):563–568. doi: 10.1016/0022-2836(76)90334-x. [DOI] [PubMed] [Google Scholar]
- Salemme F. R., Freer S. T., Xuong N. H., Alden R. A., Kraut J. The structure of oxidized cytochrome c 2 of Rhodospirillum rubrum. J Biol Chem. 1973 Jun 10;248(11):3910–3921. doi: 10.2210/pdb1c2c/pdb. [DOI] [PubMed] [Google Scholar]
- Valkirs G. E., Feher G. Topography of reaction center subunits in the membrane of the photosynthetic bacterium, rhodopseudomonas sphaeroides. J Cell Biol. 1982 Oct;95(1):179–188. doi: 10.1083/jcb.95.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. C., Steiner L. A., Feher G. Primary structure of the reaction center from Rhodopseudomonas sphaeroides. Proteins. 1986 Dec;1(4):312–325. doi: 10.1002/prot.340010405. [DOI] [PubMed] [Google Scholar]
- Williams J. C., Steiner L. A., Feher G., Simon M. I. Primary structure of the L subunit of the reaction center from Rhodopseudomonas sphaeroides. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7303–7307. doi: 10.1073/pnas.81.23.7303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. C., Steiner L. A., Ogden R. C., Simon M. I., Feher G. Primary structure of the M subunit of the reaction center from Rhodopseudomonas sphaeroides. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6505–6509. doi: 10.1073/pnas.80.21.6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youvan D. C., Bylina E. J., Alberti M., Begusch H., Hearst J. E. Nucleotide and deduced polypeptide sequences of the photosynthetic reaction-center, B870 antenna, and flanking polypeptides from R. capsulata. Cell. 1984 Jul;37(3):949–957. doi: 10.1016/0092-8674(84)90429-x. [DOI] [PubMed] [Google Scholar]