Abstract
Dendritic cells (DCs) are potent antigen-presenting cells that play a critical role in the activation of T cells. RNA interference (RNAi)-mediated silencing of negative immunoregulatory molecules expressed by DCs may provide a strategy to enhance the potency of DC-based vaccines and immunotherapy. Ablation of suppressor of cytokine signaling-1 (SOCS-1) in antigen-presenting cells has been shown to enhance cellular immune response in mice. Here, we used a previously reported DC-targeting approach to deliver small interfering RNA (siRNA) against SOCS-1 to human myeloid-derived DCs (MDDCs). SOCS1-silencing in MDDCs resulted in enhanced cytokine responses to lipopolysaccharide (LPS) and a strong mixed-lymphocyte reaction. Moreover, only DCs treated with SOCS-1 siRNA, and not controls, elicited strong primary in vitro responses to well-characterized HLA-A*0201-restricted Melan-A/MART-1 and human immunodeficiency virus (HIV) Gag epitopes in naive CD8+ T cells from healthy donors. Finally, stimulation of CD8+ T cells from HIV-seropositive subjects with SOCS1-silenced DCs resulted in an augmented polyfunctional cytotoxic T-lymphocyte (CTL) response, suggesting that SOCS-1 silencing can restore functionally compromised T cells in HIV infection. Collectively, these results demonstrate the feasibility of DC3-9dR-mediated manipulation of DC function to enhance DC immunogenicity for potential vaccine or immunotherapeutic applications.
Introduction
Cytotoxic T lymphocytes (CTL) are thought to play a central role in protection against human immunodeficiency virus (HIV) infection. This has been a major impetus for the design and testing of vaccines capable of eliciting broad and vigorous CTL response to prevent infection or, as a lesser goal, to ameliorate the disease course and viral transmission. However, the failure of the adenovirus serotype 5-based HIV vaccine used in the recent large-scale Merck STEP phase 2b trial to reduce infection rates or viral loads has been a major setback to this effort.1 Nevertheless, recent studies using a heterologous adenoviral prime-boost approach in the Simian immunodeficiency virus model have convincingly shown that T cell vaccines designed to maximize the breadth, magnitude, and polyfunctional attributes of the elicited responses are capable of exerting a durable, albeit partial, control of viral replication.2 Thus, with novel vaccine strategies, it may be possible to generate CTL responses that reach the threshold required for protection.
Optimal priming of naive T cells is greatly dependent on their effective stimulation by dendritic cells (DCs). DCs are potent antigen-presenting cells that shape the magnitude and duration of an immune response. Immature DCs endocytose exogenous antigens at sites of infection and migrate to lymphoid organs where they undergo maturation and present the processed antigens on the cell surface along with appropriate major histocompatibility complex molecules.3,4 Upon activation with antigen, DCs express several costimulatory molecules and produce high levels of cytokines, including interleukin (IL)-12 and type 1 interferons (IFNs), to orchestrate the induction of strong CD4+ Th1 and CD8+ CTL responses necessary for effective cell-mediated immunity to intracellular pathogens.4 At the same time, to prevent an excessive and prolonged response that could result in tissue destruction or autoimmunity, DCs are also equipped with negative feedback mechanisms that control their function and cytokine production. Suppressor of cytokine signaling-1 (SOCS-1), a member of the SOCS and cytokine-inducible SH2 family of intracellular proteins,5,6 has emerged as a critical inhibitory molecule for controlling the cytokine response and antigen presentation by DCs, thereby regulating the magnitude of adaptive immunity.7,8 SOCS-1 suppresses cytokine signaling by binding to Janus protein kinase/signal transducer and activator of transcription9 to prevent downstream signal transduction.10 DCs from SOCS-1 knockout mice are hypersensitive to lipopolysaccharide (LPS) stimulation and show a more mature phenotype than their wild-type littermates.11 Recently, a number of studies in mice have documented the important role of SOCS-1 in modulating the magnitude of immune stimulation by DCs,7,12,13,14 suggesting that blocking SOCS-1 may be a potentially useful strategy to enhance vaccine-induced immune responses.
The potency and selectivity of RNA interference (RNAi) to silence gene expression makes it a powerful strategy to modify the immunological characteristics of DCs. However, the major challenge of small interfering RNA (siRNA) delivery will have to be overcome if RNAi-based approaches are to be harnessed to modify the stimulatory potential of DCs. Previously, we used a fusion peptide consisting of a 12-amino-acid peptide (DC3), that specifically binds to a ligand expressed on DCs and nona-arginine (9dR) that binds nucleic acids by charge interaction for the specific delivery of siRNA to prevent dengue virus infection and to suppress virus-induced proinflammatory cytokine production in DCs.15 Here, we report that SOCS-1 ablation by siRNA delivered via the same DC-targeted chimeric peptide, DC3-9dR, resulted in enhanced induction of costimulatory molecules and cytokine production by DCs. As a consequence, the SOCS1-silenced DCs were able to stimulate robust primary CD8+ T cell responses to defined HLA-A*0201-restricted MelanA/MART-1 and HIV Gag antigens in vitro. SOCS1-silenced DCs not only stimulated numerically larger responses, but also enhanced the cytotoxic and cytokine secretion capabilities of the responding T cells. Additionally, DC-targeted delivery of SOCS-1 siRNA enhanced the HIV-specific cytokine response in CD8+ T cells from seropositive donors ex vivo, suggesting that SOCS-1 can rescue dysfunctional HIV-specific CD8+ T cells. Our findings provide proof of principle that siRNAs to negative modulatory molecules such as SOCS-1 can be targeted selectively to DCs by DC3-9dR and demonstrate that this approach holds potential as a molecular adjuvant strategy to boost immune responses to prophylactic and therapeutic HIV vaccines.
Results
DC3-9dR-mediated SOCS-1 silencing enhances cytokine production and stimulatory capacity of DCs
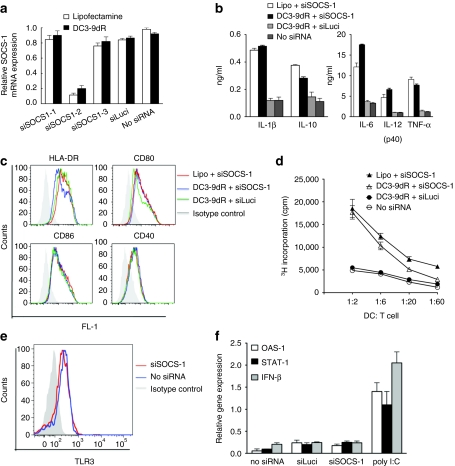
The ability of DC3-9dR-delivered siRNA to down-modulate SOCS-1 gene expression in myeloid-derived DCs (MDDCs) was compared with the conventional transfection reagent lipofectamine. Of the three siRNA sequences (SOCS1-1, -2, and -3) tested, one (SOCS1-2) was found to be effective at reducing SOCS-1 mRNA expression following LPS (100 ng/ml) stimulation as assessed by quantitative real-time PCR (qRT-PCR). DCs transfected with 400 pmol of this SOCS1-2 siRNA using either DC3-9dR or lipofectamine showed a comparable specific gene knockdown of about 75–85% whereas the irrelevant siRNA (siLuci) showed negligible effects (Figure 1a). This siRNA was used for all further experiments.
Figure 1.
DC3-9dR-mediated SOCS-1 silencing enhances cytokine production and stimulatory capacity of DCs. (a) MDDCs were transfected with three different siRNAs (siSOCS1-1, -2, and -3) targeting different regions of SOCS-1 mRNA using lipofectamine 2,000 or DC3-9dR peptide and stimulated with LPS (100 ng/ml). Twenty-four hours later, SOCS-1 mRNA levels were measured by qRT-PCR. MDDCs treated with an irrelevant siRNA (siLuci) and mock-treated served as controls. (b,c) SOCS1-silenced MDDCs were stimulated with LPS; after 24 hours DC-specific cytokines were quantified from supernatants using a (b) multiplex human cytokine immunoassay (Millipore) and analyzed for costimulatory and MHC molecules by (c) flow cytometry. (d) MDDCs transfected with siSOCS-1 complexed with either lipofectamine or DC3-9dR (1:10 ratio) were cocultured with allogeneic T cells at the indicated ratios for 5 days and proliferation was assayed by 3H thymidine incorporation during the last 16 hours of the assay. (e,f) MDDCs were treated with siLuci or siSOCS-1, and 6 hours later expression of TLR3 was tested by flow cytometry (e) and the expression of the indicated IFN-responsive gene relative to β-actin was analyzed by qRT-PCR (f). Poly (I:C) was used as a positive control. DC, dendritic cell; IFN, interferon; LPS, lipopolysaccharide; MDDC, myeloid-derived DC; MHC, major histocompatibility complex; qRT-PCR, quantitative real-time PCR; siRNA, small interfering RNA; SOCS-1, suppressor of cytokine signaling-1.
We next tested the effect of SOCS-1 silencing on cytokine production and the expression of major histocompatibility complex and costimulatory molecules on DCs. Compared to controls, SOCS1-silenced DCs exhibited an enhanced production of proinflammatory cytokines such as tumor necrosis factor (TNF)-α, IL-1β, IL-6, and IL-12 in response to LPS stimulation as assessed by multiplex human cytokine immunoassay of supernatants collected 24 hours poststimulation (Figure 1b). Flow cytometric analysis revealed no differences in the expression of HLA-DR, CD80, CD86, and CD40 between SOCS1-silenced DCs versus controls (Figure 1c). To examine the immunostimulatory capability of gene-modulated DCs, we used these DCs to stimulate allogeneic T cells in an mixed-lymphocyte reaction assay. DCs treated with the DC3-9dR/siSOCS-1 complex had an augmented ability to stimulate T cell proliferation as compared to mock- or siLuci-treated DCs (Figure 1d). These results indicate that the DC3-9dR peptide can be used as a tool for RNAi-mediated manipulation of DC immunogenicity.
To determine whether SOCS1 siRNA stimulates the human innate immune system by engaging endosomally localized TLR,16 the treated MDDCs were assessed for TLR3 activation by flow cytometry. The siRNA did not induce TLR3 activation after 6 hours of treatment (Figure 1e). Additionally, we measured cellular mRNA level of type I IFN-β and the IFN-responsive molecules like 2′,5′-oligoadenylate synthetase-1 and signal transducer and activator of transcription-117 in MDDCs after siRNA exposure. Analysis by qRT-PCR showed that IFN-β, 2′,5′-oligoadenylate synthetase-1, and signal transducer and activator of transcription-1 expression were induced in positive control cultures stimulated with poly I:C, but not in siLuci or siSOCS-1 exposed cultures (Figure 1f), thereby indicating that neither of these siRNAs triggered nonspecific IFN responses.
DC3-9dR-mediated silencing of SOCS-1 induces strong DC-mediated priming of Melan-A/ MART-1-specific CD8+ T cell responses
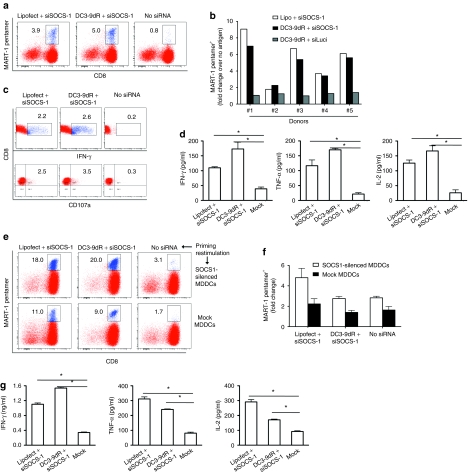
We sought to determine whether targeted silencing of SOCS-1 in DCs could induce an enhanced primary in vitro immune response to the well-characterized HLA-A*0201-restricted tumor-associated Melan-A/MART-1 epitope. Immature DCs were mock-transfected or transfected with SOCS-1 siRNA using lipofectamine or DC3-9dR, pulsed with Melan-A/MART-1 peptide (10 µg/ml), and then cocultured with autologous T cells in the presence of IL-7 (20 U/ml) and IL-15 (5 U/ml) for 1 week. DCs transfected with SOCS-1 siRNA, using either DC3-9dR or lipofectamine, demonstrated an enhanced induction of Melan-A/MART-1-specific CD8+ pentamer+ cells compared to untransfected DCs (5 and 3.9% versus 0.5%, Figure 2a). In all five HLA-A*0201+ donors tested, delivery of SOCS-1 siRNA by either lipofectamine or DC3-9dR enhanced the Melan-A/MART-1-specific CD8+ T cell response with a two- to ninefold increase in mean pentamer+ cell numbers over controls (Figure 2b). In one representative sample, we also analyzed mobilization of the degranulation marker CD107a and intracellular IFN-γ production by CD8+ T cells after restimulation with peptide-pulsed DCs on day 8. SOCS-1 siRNA-treated DCs elicited a higher number of IFNγ+CD8+ T cells compared to control DCs (2.2 and 2.6% versus 0.2%, Figure 2c, upper panel). CD8+ T cells stimulated with SOCS1-silenced DCs also degranulated to a greater extent compared to controls as assessed by surface mobilization of CD107a (2.5 and 3.5% versus 0.3%, Figure 2c, lower panel). In addition, we analyzed T cell cytokine production in a Luminex assay using supernatants collected 24 hours after restimulation with peptide-pulsed DCs. The results revealed that SOCS-1 siRNA-treated DCs elicited a polyfunctional (IFN-γ, TNF-α, IL-2) T cell response that was three- to fourfold higher than controls (Figure 2d).
Figure 2.
DC3-9dR-mediated silencing of SOCS-1 induces strong priming of Melan-A/MART-1-specific CD8+ T cell responses in vitro. (a,b) MDDCs from healthy HLA-A*0201+ donors were untreated or treated with SOCS-1 siRNA (complexed to DC3-9dR or lipofectamine), pulsed with Melan-A/MART-1 peptide (10 µg/ml) and cocultured with autologous CD8+ T cells for 7 days in the presence of IL-7 (20 U/ml) and IL-15 (5 U/ml). Representative flow cytometric analysis of Melan-A/MART-1 CD8+pentamer+ cells from one donor and (b) data from all five donors are shown. (c,d) Cognate T cells in a were restimulated with peptide-pulsed DCs and tested for cytokine production by (c) intracellular staining or by (d) Luminex assay. (e–g) Cultures from (a) were restimulated with Melan-A/MART-1 peptide-pulsed untreated- or SOCS1 siRNA-treated DCs, and after 7 days assayed for CD8+pentamer+ cell frequencies by (e) flow cytometry; representative data, (f) cumulative data from three donors, and (g) cytokine production by Luminex assay. DC, dendritic cell; IFN-γ, interferon-γ IL, interleukin; MDDC, myeloid-derived DC; siRNA, small interfering RNA; SOCS-1, suppressor of cytokine signaling-1.
Next, we tested whether the stimulatory effect of SOCS-1 silencing in DCs occurs only during the initial priming phase or continues during secondary stimulation. Antigen-specific CD8+ T cells generated using SOCS1-silenced DCs were restimulated on day 8 with antigen-pulsed DCs transfected with control or SOCS-1 siRNA. Without SOCS-1 siRNA during secondary stimulation, Melan-A/MART-1 CD8+pentamer+ cells increased modestly from 3.9 to 11% and from 5.0 to 9.0%, respectively, in cultures primed with DCs transfected with SOCS-1 siRNA using either lipofectamine or DC3-9dR (Figure 2e, lower panel). A more dramatic increase in Melan-A/MART-1 CD8+pentamer+ cell numbers was observed when SOCS1-silenced DCs were used to prime as well as to restimulate the same cultures (from 3.9 to 18% using lipofectamine and from 5.0 to 20.0% using DC3-9dR to deliver the siRNA; Figure 2e, upper panel). The use of SOCS1-silenced DCs during restimulation alone increased the CD8+pentamer+ cell population from 0.5 to 3.1%; a lesser increase (from 0.5 to 1.7%) was observed with control untreated DCs (Figure 2e). Cumulative data from three donors is plotted in Figure 2f. The increase in antigen-specific CD8+ T cells was accompanied by a corresponding increase in cytokine secretion (IFN-γ, TNF-α, IL-2) in culture supernatants upon restimulation (Figure 2g). In sum, these data demonstrate that antigen presentation by SOCS1-silenced DCs can be used as a strategy not only to enhance naive CD8+ T cell priming, but also to increase the magnitude of recall responses.
SOCS1-silenced DCs induce potent primary HIV Gag-specific CD8+ T cell responses
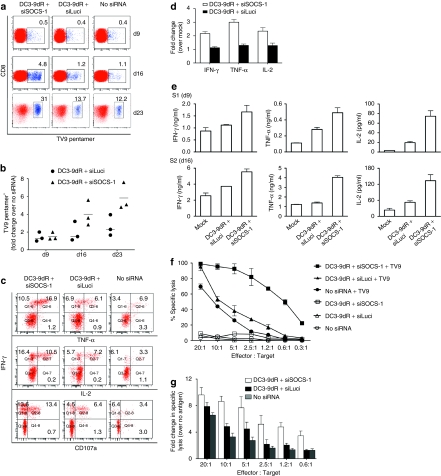
As Melan-A/MART-1 is expressed in normal cells of the melanocytic lineage in skin, uveal tract, and retina, the generation of a T cell response to this self-antigen may not be representative of typical naive T cell priming.18 To evaluate the effect of SOCS-1 silencing in DCs on the induction of primary in vitro CTL responses more stringently, we tested the ability of such DCs to prime HIV Gag-specific CD8+ T cells from seronegative donors. DCs that were untransfected or transfected with either SOCS-1 or luciferase siRNA using DC3-9dR were pulsed with the HLA-A*0201-restricted HIV Gag TV9 peptide (20 µg/ml) and cocultured with autologous CD8+ T cells. A low but identifiable population of antigen-specific CD8+pentamer+ T cells could be detected by day 9 in all cultures. However, with subsequent rounds of restimulation the numbers increased substantially with SOCS-1-silenced DCs compared to parallel control cultures stimulated with either siLuci-transfected or mock-transfected DCs. A representative flow cytometric analysis is shown in Figure 3a; cumulative data are shown in Figure 3b. Increased CD8+pentamer+ T cell frequencies were also associated with increased intracellular production of IFN-γ, TNF-α, and IL-2 in cultures generated with SOCS1-silenced DCs compared to controls; representative flow cytometric and cumulative data are shown in Figure 3c and d, respectively. Similar increases were observed in CD107a mobilization (Figure 3c, lower panel). The intracellular cytokine data shown in Figure 3c and d were further verified by measuring the levels of the same cytokines in supernatants harvested after overnight stimulation on day 9 (S1) and day 16 (S2) (Figure 3e). In addition, we directly tested the cytotoxic capabilities of these polyfunctional CD8+pentamer+ T cells in a 51Cr release assay with peptide-pulsed C1R-A2 targets. Peptide-specific lysis was found to be two- to threefold higher in cultures generated with SOCS1-silenced DCs at all effector:target ratios tested; representative and cumulative data are shown in Figure 3f and g, respectively. Taken together, these results suggest that silencing SOCS-1 in DCs enables the generation and expansion of potent primary CD8+ T cell responses.
Figure 3.
SOCS1-silenced DCs induce potent primary HIV Gag-specific CD8+ T cell responses. (a,b) SOCS1-silenced or control MDDCs from HIV-seronegative HLA-A*0201+ individuals were pulsed with the HLA-A*0201-restricted HIV Gag TV9 (TLNAWVKVV) peptide epitope (20 µg/ml) and cultured with autologous CD8+ T cells for 9 days in presence of IL-2 (5 U/ml) and IL-7 (20 U/ml); restimulations were conducted under identical conditions for each individual culture at day 16 and day 23. CD8+pentamer+ cells were quantified just before restimulation (d9, d16, d23). Representative (a) flow cytometry plots and (b) cumulative data from three donors are shown. (c,d) HIV Gag TV9 cultures (d23) from (a) were restimulated as described in methods and assayed for cytokine production (IFN-γ, TNF-α, IL-2) by intracellular staining and degranulation (CD107a mobilization) by flow cytometric analysis. (c) Representative results and (d) cumulative data from three donors are shown. (e) Culture supernatants were tested for T cell-specific cytokines at day 9 (S1) and day 16 (S2) by Luminex assay. (f,g) HIV Gag TV9 cultures (day 23) were incubated with 51Cr-labeled, peptide-pulsed C1R-A2 target cells at the indicated E:T ratios for 4 hours and percent cytotoxicity was determined. (f) Representative results and (g) cumulative data from three donors are shown. DC, dendritic cell; HIV, human immunodeficiency virus; IFN-γ, interferon-γ IL, interleukin; MDDC, myeloid-derived DC; siRNA, small interfering RNA; SOCS-1, suppressor of cytokine signaling-1; TNF, tumor necrosis factor.
Enhanced induction and expansion of primary Melan-A/MART-1 and HIV-specific CD8+ T cell populations by SOCS1-silenced DCs is associated with distinct patterns of clonotype recruitment
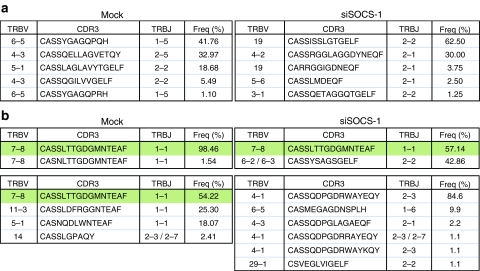
To gain further insight into the mechanistic basis for enhanced priming of antigen-specific CD8+ T cell responses by SOCS1-silenced DCs, we conducted a comparative and unbiased molecular analysis of all expressed TRB gene products in the CD8+pentamer+ T cell populations specific for Melan-A/MART-1 and HIV Gag TV9 depicted in Figures 2 and 3. At day 9, the Melan-A/MART-1-specific CD8+ T cell populations primed with mock-transfected or SOCS1-silenced DCs exhibited oligoclonal clonotypic architectures with skewed hierarchies (Figure 4a). The constituent clonotypes displayed diverse TRBV gene usage and glycine-rich CDR3s, consistent with previously published data.19,20 Notably, there was no clonotypic overlap between the two populations, which presumably reflects the stochastic nature of in vitro amplification from a small number of cells and the large available pool of naive precursors.21 In contrast, the corresponding HIV Gag TV9-specific CD8+ T cell populations were even more oligoclonal at day 9 and shared a highly dominant clonotype (Figure 4b, upper panels). At day 23, after two further rounds of in vitro restimulation with mock-transfected DCs in the control culture, this TRBV7-8/CASSLTTGDGMNTEAF/TRBJ1-1 clonotype persisted as the dominant species in the marginally larger HIV Gag TV9-specific CD8+ T cell population (Figure 4b, lower left panel). In contrast, the corresponding and dramatically expanded HIV Gag TV9-specific CD8+ T cell population that was primed and restimulated with SOCS1-silenced DCs exhibited a more polyclonal and entirely distinct repertoire at day 23 (Figure 4b, lower right panel). Thus, the initially dominant clonotype was superseded by the expansion of late emergent clonotypes to the extent that it was no longer observed within the sampled repertoire. These data suggest that the ability of SOCS1-silenced DCs to amplify foreign antigen-specific CD8+ T cell populations in vitro is related, at least in part, to the enhanced recruitment of additional cognate clonotypes.
Figure 4.
Clonotypic analysis of CD8+ T cells generated with mock-transfected and SOCS1-silenced DCs. CD8+ T cells from HLA-A*0201+ HIV-seronegative donors were primed and expanded with MDDCs that were either mock-transfected or treated with SOCS-1 siRNA. CD8+pentamer+ T cells were sorted by flow cytometry to >98% purity and all the expressed TRB gene products were analyzed using an unbiased template-switch anchored RT-PCR. CDR3 amino acid sequences, TRBV and TCRBJ usage, and the relative frequency of CD8+ T cell clonotypes specific for the (a) Melan-A/MART-1 and (b) HIV Gag TV9 epitopes are shown. Analyses were conducted on day 9 in (a) or on days 9 and day 23 in (b); top and bottom rows, respectively. Shaded boxes indicate common clonotypes. The IMGT nomenclature is used.48 DC, dendritic cell; HIV, human immunodeficiency virus; IFN-γ, interferon-γ MDDC, myeloid-derived DC; RT-PCR, real-time PCR; SOCS-1, suppressor of cytokine signaling-1; TCR, T-cell receptor.
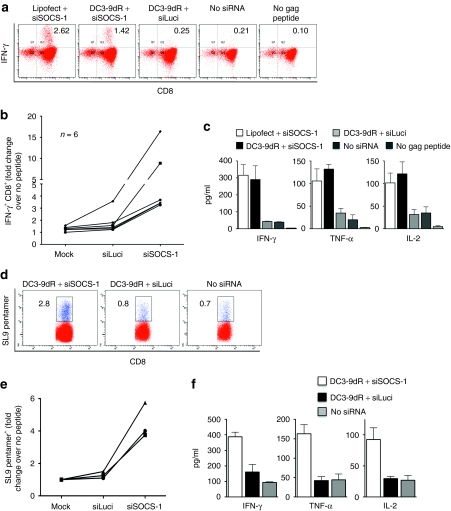
DC3-9dR-mediated delivery of SOCS-1 siRNA to DCs from HIV-seropositive subjects improves HIV-specific CD8+ T cell cytokine secretion
With disease progression, HIV-1-specific CD8+ T cells are functionally compromised with a characteristic loss of proliferative capacity and cytokine production; these defects correlate with the progressive loss of CD4+ T helper cells.22,23,24,25 As stimulation with SOCS1-silenced DCs also enhanced secondary CD8+ T cell responses, we tested whether the same approach could restore the functional capability of HIV-specific CD8+ T cells from infected subjects. Immunomagnetically isolated CD8+ T cells from HIV-seropositive subjects were cocultured with autologous DCs transfected with SOCS-1 or luciferase siRNA complexed to DC3-9dR. DCs transfected with SOCS-1 siRNA using lipofectamine served as a positive control and mock-transfected DCs pulsed with or without a pool of Gag peptides served as negative controls. After 6 days, cultures were restimulated with DCs pulsed with a Gag peptide pool in the presence of brefeldin A (5 µg/ml) and intracellular IFN-γ production by CD8+ T cells was measured by flow cytometry 4 hours later. Stimulation of CD8+ T cells with SOCS1-silenced DCs resulted in a threefold increase in cell numbers by day 6 (data not shown). Moreover, SOCS-1 silencing in DCs, using either lipofectamine or DC3-9dR, resulted in increased Gag-specific IFN-γ production by CD8+ T cells (1.42 and 2.62%, respectively) compared to mock-transfected DCs (0.21%) and irrelevant siRNA-treated DCs (0.25%) used as controls. Representative data are shown in Figure 5a; cumulative data are shown in Figure 5b. Furthermore, CD8+ T cells generated by stimulation with SOCS1-silenced MDDCs secreted large amounts of IFN-γ, TNF-α, and IL-2 compared to control MDDC-stimulated CD8+ T cells (Figure 5c) indicating that these dysfunctional CTLs are capable of producing multiple cytokines upon appropriate stimulation.
Figure 5.
Stimulation of PBMCs from HIV seropositive subjects with SOCS1-silenced DCs increases the frequency of polyfunctional Gag-specific CD8+ T cells. (a–c) MDDCs from HIV-seropositive donors were transfected with siSOCS-1 or siLuci using DC3-dR, pulsed with a pool of Gag peptides (2 µg/ml for each individual constituent peptide), and cultured with autologous CD8+ T cells. After 5 days, cells were restimulated with peptide-pulsed MDDCs for 4 hours in the presence of brefeldin A (5 µg/ml) and intracellular IFN-γ production was measured by flow cytometry. (a) Representative results and (b) cumulative data from six subjects are depicted. (c) Supernatants of cocultures from six donors were assayed for the indicated cytokines by multiplex human cytokine immunoassay. (d–f) MDDCs from HLA.A*0201+ HIV-infected subjects were transfected with siSOCS-1 or siLuci by DC3-9dR and pulsed with HIV Gag SL9 peptide (10 µg/ml) for 2 hours, and cultured with autologous CD8+ T cells for 5 days. (d) Representative SL9-specific CD8+ pentamer+ analysis and (e) cumulative data from three donors are shown; (f) cytokine quantification of culture supernatants from all three donors. DC, dendritic cell; HIV, human immunodeficiency virus; IFN-γ, interferon-γ MDDC, myeloid-derived DC; siRNA, small interfering RNA; SOCS-1, suppressor of cytokine signaling-1; TNF, tumor necrosis factor.
The HLA-A*0201-restricted HIV Gag SL9 epitope is recognized by majority of chronically infected individuals expressing this allele. We analyzed the SL9-specific CD8+ T cell response in three chronically infected subjects after stimulation with SOCS1-silenced autologous DCs. In all cases, stimulation with SOCS1-silenced MDDCs resulted in increased numbers of SL9-specific CD8+ pentamer+ cells after 5 days of coculture compared to controls. Representative data are shown in Figure 5d; cumulative data from all three subjects are plotted in Figure 5e. Furthermore, SL9 specific CD8+ T cells stimulated with SOCS1-silenced DCs also produced higher levels of IFN-γ, TNF-α, and IL-2 compared to controls (Figure 5f). Thus, SOCS1-silencing in DCs can augment the numbers and polyfunctional status of HIV-specific CD8+ T cells.
Discussion
In this study, we demonstrate that a targeted siRNA delivery approach can be used to silence negative regulatory molecules in DCs. Our data show that reversing the immunoattenuation mediated by SOCS-1 activates DCs and increases their production of proinflammatory cytokines, such as IL-12, which is critical for the induction of efficacious T cell-mediated immunity. We further demonstrate that downregulation of SOCS-1 expression in DCs during in vitro priming significantly enhances the potency of primary CD8+ T cell responses to HIV Gag and Melan-A/MART-1 antigens. Moreover, stimulation with SOCS1-ablated DCs increased the magnitude of polyfunctional CTLs that were cytotoxic and capable of producing multiple cytokines, which are considered to be important correlates of protective CD8+ T cell responses. These results suggest that targeted modulation of SOCS-1 expression in DCs could be exploited as a novel molecular adjuvant strategy to improve the potency of vaccine-induced CD8+ T cell responses to pathogens and tumors.
DCs are the most potent antigen-presenting cells and DC-based approaches are being intensively investigated as potential preventive/therapeutic vaccination strategies for several diseases including HIV. In addition to the expression of costimulatory molecules that facilitate an immune response, DCs also express negative immunoregulatory molecules that normally function to prevent harmful inflammation or autoimmune diseases. Inactivation of such negative regulatory molecules provides a way to enhance the efficacy of DC-based vaccines. SOCS-1 has emerged as a critical inhibitory molecule for controlling antigen presentation by DCs and thereby the magnitude of the adaptive immune response.7,26 In mice, immunization with DCs in which SOCS-1 was silenced using lentivirally-expressed short hairpin RNA led to the induction of potent CTL responses to tumor and HIV antigens.26,27 However, lentiviral approaches may not be appropriate to enhance immunity in humans because: (i) their effects are irreversible, which could lead to harmful consequences in vivo; (ii) they do not target-specific cell types; (iii) very high titer virus is needed for in vivo use; and, (iv) there are associated toxicities and bystander issues, including insertional mutagenesis and vector-induced immune responses.28 On the other hand, siRNA provides a drug-like approach that transiently silences genes in specific cell types and may be particularly suited for the silencing of immunomodulatory genes on a time-limited basis. However, because siRNAs are relatively large and carry a net negative charge, the efficiency with which synthetic siRNAs cross the plasma membrane and enter the cytoplasm is usually very low unless appropriate carriers, such as transfection reagents, are used. Although lipid reagents can transfect siRNA into different cell types in vitro, they cannot be used for in vivo siRNA delivery. Thus, to apply RNAi technology for the modulation of immune responses in clinical settings, it will be important to develop methods that induce siRNA uptake in primary immune cells in vivo. Recently, novel delivery approaches have been used to target-specific antigens to DCs. For example, monoclonal antibodies or peptides that recognize the antigen uptake receptors have been used as ligand mimics to deliver vaccine antigens to DCs or their subsets in vivo.29,30,31 In human DCs, antigen delivered as a fusion antibody to DEC-205 has been shown to induce responses to numerous HIV Gag epitopes in a spectrum of human HLA haplotypes.32 Although these reagents effectively target DCs, they do not bind siRNA and thus cannot be used to induce RNAi. In previous studies, we have shown that conjugation of a siRNA-binding moiety to a cell targeting peptide or antibody fragment can effectively bind and deliver siRNA to specific cell types in vivo.33,34 In a similar strategy, we fused a nona-arginine peptide that binds to siRNA by charge interaction to a DC-targeting DC3 peptide and reported that this reagent targets heterogeneous subsets of human DCs.15
Our results agree with murine studies in which SOCS-1 silencing dramatically enhanced antigen-specific CD8+ T cell responses.7,26,27 In fact, one-to-two rounds of stimulation with SOCS1-silenced DCs was enough to generate a robust primary response in vitro, a task that normally requires multiple stimulations extended over several weeks.35,36 In addition, our study also shows that SOCS-1 silencing stimulates a polyfunctional T cell response that is known to be an important correlate of protective immunity. Numerous studies have shown that, in chronic infections such as HIV, the polyfunctional capabilities of CTLs with respect to proliferation, multiple cytokine secretion, and expression of cytolytic effector molecules like perforin and granzyme is more predictive of protective capability than the mere presence of high frequency virus-specific CD8+ T cells capable only of secreting IFN-γ.37,38 Our finding that SOCS1-silenced autologous DCs from HIV-infected individuals were also able to enhance the CD8+ T cell response is particularly significant considering that CD8+ T cells are often dysfunctional in infected subjects.22,24,25 Moreover, since DC function is also compromised in HIV-infected patients with common defects in the production of proinflammatory cytokines,39,40 our results also suggests that restoration of the cytokine production defect in DCs with SOCS-1 silencing may be the key to restoring CTL functionality. These data suggest that silencing negative modulators could be potentially useful as a therapeutic approach to rescue the function of exhausted virus-specific T cells.
Taken together, our results may have implications for the design of a new generation of preventive/therapeutic HIV vaccines that induce more optimal T cell responses than those elicited with current approaches, such as the adenovirus serotype 5 vaccine used in the recent Merck STEP phase 2b trial.1 Even with a more potent heterologous prime-boost adenovirus vaccine tested in monkeys, only partial control of viral replication was achieved;2 this highlights the need to further improve the magnitude and quality of vaccine-elicited T cell immunity. Manipulation of DC function using targeted delivery of siRNA may provide one way to achieve this goal.
SOCS-1 knockout mice develop a complicated disease with multiple defects and succumb within 3–4 weeks after birth, suggesting that long-term silencing of SOCS-1 (for example, with lentivirally expressed short hairpin RNA) could be potentially dangerous.41,42 In contrast, siRNA effects are short-lived and would therefore be ideal to transiently suppress important immune modulatory molecules like SOCS-1 during priming and/or boosting of a vaccine-induced response. Moreover, targeting delivery specifically to DCs could further reduce the potentially harmful effects of SOCS-1 silencing in other organs and cell types. DC3-9R appears to provide a novel tool to achieve this. Apart from SOCS-1, enhancement of T cell responses may also be possible with DC-specific siRNA delivery targeting other immunosuppressive molecules in DCs such as IL-10 or PD-L1/L2 ligands that bind programmed death-1 on T cells.43 Programmed death-1 expression has been recognized as a functional marker of exhausted virus-specific CD8+ T cells in chronic infections, particularly in HIV progressors.44,45 Thus, DC3-9R mediated siRNA delivery could also potentially be used to suppress these inhibitory pathways.
In summary, we have shown that a targeted siRNA delivery approach can be used to silence immunosuppressive molecules in DC and induce strong human T cell immune responses. Although we have confined our studies to the effect of DC-specific SOCS-1 ablation by this approach to potentiate primary and secondary CD8+ T cell responses to HIV, studies of the role of this molecule in mice suggest that a broader enhancing effect on antibody and T helper responses can also be expected.7 With further refinement, use of the reagent for simultaneous delivery of immunogenic antigens and siRNA can be foreseen as paving the way for a new class of DC-targeted molecular adjuvant and vaccine combination that could provide reliable protection against HIV or other pathogens. In addition, since siRNAs could easily be designed to suppress a plethora of immunostimulatory as well as immunosuppressive molecules produced by DCs, this delivery reagent would potentially be useful not only to enhance a vaccine response, but also to suppress overzealous responses that characterize autoimmune diseases.
Materials and Methods
Healthy donors and HIV-seropositive subjects. Buffy coats from healthy seronegative HLA-A*0201+ donors and HIV-seropositive subjects were either purchased from Research Blood Components (Boston, MA) or were obtained from volunteers in the El Paso, TX area. All human participants gave written informed consent and appropriate institutional review board approval was obtained.
Peptide DC3-9dR and siRNA. DC3-9dR (FYPSYHSTPQRPGGGGSRR RRRRRRR with arginine residues as -isomers) was synthesized and purified at the Tufts University Core Facility (Boston, MA). The sense strand sequences of siRNA designed to target the human SOCS-1 gene, siSOCS1-1 (5′-CACTTCCGCACATTCCGTT-3′), siSOCS1-2 (5′-CTGGGATGCCGTGTTATTT-3′) and siSOCS1-3 (5′-ACTACCTGAGCTCCTTCCCCT-3′), and firefly luciferase (siLuci, 5′-TCGAAGTACTCAGCGTAAG-3′), were synthesized by Dharmacon (Lafayette, CO).
Peptides and HLA-A*0201 pentamers. The Melan-A/MART-1 peptide (ELAGIGILTV, Melan-A/MART-126–35) and Melan-A/MART-1 pentamer, HIV Gag TV9 peptide (TLNAWVKVV, Gag p2419–27) and TV9 pentamer and HIV Gag SL9 peptide (SYLNTVATYL, Gag p1777–85) and SL9 pentamer were obtained from Proimmune (Oxford, UK).
Gene silencing experiments and qRT-PCR. For gene silencing experiments, lipofectamine or DC3-9dR peptide complexed with 400 pmol of siSOCS-1 (molar ratio 10:1) was added to 4 × 105 MDDCs, which were then stimulated with LPS for 16 hours. SOCS-1 mRNA levels were assessed 24 hours after LPS stimulation by qRT-PCR as described previously (Subramanya et al.15) using the following primers: SOCS-1 (forward, 5′-CAGTCTCCACAGCAGCAGAGC-3′ reverse, 5′-TCCCGAGGCCATCTTCACG-3′) and β-actin (forward, 5′-TGAGTCTGACGTGGACATC-3′ reverse, 5′-ACTCGTCATACTCCTGCTTG-3′). For detection of IFN-regulated genes, indicated siRNAs was added to MDDC cultures and mRNA extracted after 8 hours. RT-PCR for detection of 2′,5′-oligoadenylate synthetase-1, signal transducer and activator of transcription-1, and IFN-β mRNA expression was performed as described in ref. 17. Relative mRNA expression was calculated using the ΔCt method.
Ex vivo priming of CD8+ T cells with MDDC. Peripheral blood mononuclear cells were isolated using Ficoll–Paque (GE Healthcare, Piscataway, NJ) according to standardized protocols. Monocytes were immunomagnetically separated using CD14 microbeads (Miltenyi Biotech, Bergisch Gladbach, Germany) and cultured in RPMI 1640 medium containing 10% human AB serum supplemented with GM-CSF (1,000 U/ml; R&D Systems) and IL-4 (500 U/ml; R&D Systems, Minneapolis, MN). For HIV Gag stimulations, maturation was induced by overnight exposure of DCs to LPS (1 µg/ml). CD8+ T cells were positively selected with Dynabeads (Dynal Biotech, Oslo, Norway) and primed with irradiated (4,000 cGy), peptide-pulsed DCs at a T cell:DC ratio of 5:1 in 48- or 96-well plates. Cells were cultured in complete medium containing IL-7 (20 U/ml; R&D Systems) and restimulated with SOCS1-silenced or unsilenced, peptide-pulsed autologous DCs at weekly intervals thereafter. IL-2 (20 U/ml; R&D Systems) or IL-15 (5 U/ml; R&D Systems) was added at day 1 or day 4 poststimulation to lines specific for HIV Gag or Melan-A/MART-1, respectively.
Chromium release assay. C1R-A2 target cells (C1R cells expressing full-length wild-type HLA-A*0201) were labeled with 51Cr (50 µCi) for 1 hour and washed twice in serum-free medium. Antigen-specific CD8+ T cells were cocultured with labeled target cells (4,000 cells/well) at different effector:target ratios. After an incubation period of 4 hours, supernatants (100 µl) were harvested, mixed with scintillation fluid (Optiphase SuperMix; PerkinElmer-Wallac, Gaithersburg, MD) and counted in a MicroBeta counter (PerkinElmer-Wallac). Unpulsed C1R-A2 cells were used as negative controls. Percent-specific lysis was calculated using the following formula: (experimental release − spontaneous release)/(total release – spontaneous release) × 100.
Cytokine mutliplex array. Supernatants were collected from cocultures overnight after each restimulation (day 16 or day 23) and the indicated cytokines were assayed using the multiplex human cytokine immunoassay (Millipore, Billerica, MA) according to the manufacturer's instructions.
Flow cytometry and intracellular cytokine staining. Pentamer and TLR3 staining was performed according to the manufacturer's protocol (Proimmune and BD Biosciences, San Jose, CA, respectively). For intracellular staining, CD8+ T cells were cocultured with peptide-pulsed (2 µg/ml) or unpulsed C1R-A2 cells and treated with brefeldin A (5 µg/ml) for 4 hours before intracellular staining with monoclonal antibodies (mAbs) specific for IFN-γ, IL-2, and TNF-α as described previously.46 For CD107a staining, the monoclonal antibody was added at the time of coculture.
Analysis of CD8+ T cell clonotypes. Live CD3+CD8+pentamer+ cells at >98% purity were sorted into 1.5-ml microfuge tubes containing 100 µl RNAlater (Applied Biosystems) using a FACSAria II flow cytometer (BD Biosciences). Molecular analysis of all expressed TRB gene products was conducted as described previously using an unbiased template-switch anchored RT-PCR with minor modifications.47 The MART-1 data comprise a total of 171 sequences and the TV9 data comprise a total of 246 sequences.
Acknowledgments
We thank Joseph Steigner (Immune Disease Institute) and Zach Brower (University of Texas at El Paso) for technical assistance with blood collection and PBMC isolation. This work was supported by NIH grant AI071882 to P.S. D.A.P. is a Medical Research Council (UK) Senior Clinical Fellow. The authors declared no conflict of interest.
REFERENCES
- Buchbinder SP, Mehrotra DV, Duerr A, Fitzgerald DW, Mogg R, Li D, Step Study Protocol Team et al. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet. 2008;372:1881–1893. doi: 10.1016/S0140-6736(08)61591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, O'Brien KL, Lynch DM, Simmons NL, La Porte A, Riggs AM, et al. Immune control of an SIV challenge by a T-cell-based vaccine in rhesus monkeys. Nature. 2009;457:87–91. doi: 10.1038/nature07469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellman I, Turley SJ., and, Steinman RM. Antigen processing for amateurs and professionals. Trends Cell Biol. 1998;8:231–237. doi: 10.1016/s0962-8924(98)01276-8. [DOI] [PubMed] [Google Scholar]
- Steinman RM. Dendritic cells: understanding immunogenicity. Eur J Immunol. 2007;37 Suppl 1:S53–S60. doi: 10.1002/eji.200737400. [DOI] [PubMed] [Google Scholar]
- Nicholson SE., and, Hilton DJ. The SOCS proteins: a new family of negative regulators of signal transduction. J Leukoc Biol. 1998;63:665–668. doi: 10.1002/jlb.63.6.665. [DOI] [PubMed] [Google Scholar]
- Alexander WS, Starr R, Metcalf D, Nicholson SE, Farley A, Elefanty AG, et al. Suppressors of cytokine signaling (SOCS): negative regulators of signal transduction. J Leukoc Biol. 1999;66:588–592. doi: 10.1002/jlb.66.4.588. [DOI] [PubMed] [Google Scholar]
- Shen L, Evel-Kabler K, Strube R., and, Chen SY. Silencing of SOCS1 enhances antigen presentation by dendritic cells and antigen-specific anti-tumor immunity. Nat Biotechnol. 2004;22:1546–1553. doi: 10.1038/nbt1035. [DOI] [PubMed] [Google Scholar]
- Zhou H, Zhang D, Wang Y, Dai M, Zhang L, Liu W, et al. Induction of CML28-specific cytotoxic T cell responses using co-transfected dendritic cells with CML28 DNA vaccine and SOCS1 small interfering RNA expression vector. Biochem Biophys Res Commun. 2006;347:200–207. doi: 10.1016/j.bbrc.2006.06.093. [DOI] [PubMed] [Google Scholar]
- Cooney RN. Suppressors of cytokine signaling (SOCS): inhibitors of the JAK/STAT pathway. Shock. 2002;17:83–90. doi: 10.1097/00024382-200202000-00001. [DOI] [PubMed] [Google Scholar]
- Zhang JG, Metcalf D, Rakar S, Asimakis M, Greenhalgh CJ, Willson TA, et al. The SOCS box of suppressor of cytokine signaling-1 is important for inhibition of cytokine action in vivo. Proc Natl Acad Sci USA. 2001;98:13261–13265. doi: 10.1073/pnas.231486498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanada T, Yoshida H, Kato S, Tanaka K, Masutani K, Tsukada J, et al. Suppressor of cytokine signaling-1 is essential for suppressing dendritic cell activation and systemic autoimmunity. Immunity. 2003;19:437–450. doi: 10.1016/s1074-7613(03)00240-1. [DOI] [PubMed] [Google Scholar]
- Jackson SH, Yu CR, Mahdi RM, Ebong S., and, Egwuagu CE. Dendritic cell maturation requires STAT1 and is under feedback regulation by suppressors of cytokine signaling. J Immunol. 2004;172:2307–2315. doi: 10.4049/jimmunol.172.4.2307. [DOI] [PubMed] [Google Scholar]
- Tsukada J, Ozaki A, Hanada T, Chinen T, Abe R, Yoshimura A, et al. The role of suppressor of cytokine signaling 1 as a negative regulator for aberrant expansion of CD8α+ dendritic cell subset. Int Immunol. 2005;17:1167–1178. doi: 10.1093/intimm/dxh294. [DOI] [PubMed] [Google Scholar]
- Bartz H, Avalos NM, Baetz A, Heeg K., and, Dalpke AH. Involvement of suppressors of cytokine signaling in toll-like receptor-mediated block of dendritic cell differentiation. Blood. 2006;108:4102–4108. doi: 10.1182/blood-2006-03-008946. [DOI] [PubMed] [Google Scholar]
- Subramanya S, Kim SS, Abraham S, Yao J, Kumar M, Kumar P, et al. Targeted delivery of small interfering RNA to human dendritic cells to suppress dengue virus infection and associated proinflammatory cytokine production. J Virol. 2010;84:2490–2501. doi: 10.1128/JVI.02105-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques JT., and, Williams BR. Activation of the mammalian immune system by siRNAs. Nat Biotechnol. 2005;23:1399–1405. doi: 10.1038/nbt1161. [DOI] [PubMed] [Google Scholar]
- Song E, Zhu P, Lee SK, Chowdhury D, Kussman S, Dykxhoorn DM, et al. Antibody mediated in vivo delivery of small interfering RNAs via cell-surface receptors. Nat Biotechnol. 2005;23:709–717. doi: 10.1038/nbt1101. [DOI] [PubMed] [Google Scholar]
- Marincola FM, Rivoltini L, Salgaller ML, Player M., and, Rosenberg SA. Differential anti-MART-1/MelanA CTL activity in peripheral blood of HLA-A2 melanoma patients in comparison to healthy donors: evidence of in vivo priming by tumor cells. J Immunother Emphasis Tumor Immunol. 1996;19:266–277. doi: 10.1097/00002371-199607000-00003. [DOI] [PubMed] [Google Scholar]
- Wieckowski S, Baumgaertner P, Corthesy P, Voelter V, Romero P, Speiser DE, et al. Fine structural variations of αβTCRs selected by vaccination with natural versus altered self-antigen in melanoma patients. J Immunol. 2009;183:5397–5406. doi: 10.4049/jimmunol.0901460. [DOI] [PubMed] [Google Scholar]
- Serana F, Sottini A, Caimi L, Palermo B, Natali PG, Nisticò P, et al. Identification of a public CDR3 motif and a biased utilization of T-cell receptor Vβ and Jβ chains in HLA-A2/Melan-A-specific T-cell clonotypes of melanoma patients. J Transl Med. 2009;7:21. doi: 10.1186/1479-5876-7-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zippelius A, Pittet MJ, Batard P, Rufer N, de Smedt M, Guillaume P, et al. Thymic selection generates a large T cell pool recognizing a self-peptide in humans. J Exp Med. 2002;195:485–494. doi: 10.1084/jem.20011658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Shankar P, Xu Z, Harnisch B, Chen G, Lange C, et al. Most antiviral CD8 T cells during chronic viral infection do not express high levels of perforin and are not directly cytotoxic. Blood. 2003;101:226–235. doi: 10.1182/blood-2002-03-0791. [DOI] [PubMed] [Google Scholar]
- Appay V, Nixon DF, Donahoe SM, Gillespie GM, Dong T, King A, et al. HIV-specific CD8(+) T cells produce antiviral cytokines but are impaired in cytolytic function. J Exp Med. 2000;192:63–75. doi: 10.1084/jem.192.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostense S, Vandenberghe K, Joling J, Van Baarle D, Nanlohy N, Manting E, et al. Persistent numbers of tetramer+ CD8(+) T cells, but loss of interferon-γ+ HIV-specific T cells during progression to AIDS. Blood. 2002;99:2505–2511. doi: 10.1182/blood.v99.7.2505. [DOI] [PubMed] [Google Scholar]
- Lichterfeld M, Kaufmann DE, Yu XG, Mui SK, Addo MM, Johnston MN, et al. Loss of HIV-1-specific CD8+ T cell proliferation after acute HIV-1 infection and restoration by vaccine-induced HIV-1-specific CD4+ T cells. J Exp Med. 2004;200:701–712. doi: 10.1084/jem.20041270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song XT, Evel-Kabler K, Rollins L, Aldrich M, Gao F, Huang XF, et al. An alternative and effective HIV vaccination approach based on inhibition of antigen presentation attenuators in dendritic cells. PLoS Med. 2006;3:e11. doi: 10.1371/journal.pmed.0030011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evel-Kabler K, Song XT, Aldrich M, Huang XF., and, Chen SY. SOCS1 restricts dendritic cells' ability to break self tolerance and induce antitumor immunity by regulating IL-12 production and signaling. J Clin Invest. 2006;116:90–100. doi: 10.1172/JCI26169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manjunath N, Wu H, Subramanya S., and, Shankar P. Lentiviral delivery of short hairpin RNAs. Adv Drug Deliv Rev. 2009;61:732–745. doi: 10.1016/j.addr.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson TS, Mahnke K, Storn V, Schönfeld K, Ring S, Nettelbeck DM, et al. Inhibition of melanoma growth by targeting of antigen to dendritic cells via an anti-DEC-205 single-chain fragment variable molecule. Clin Cancer Res. 2008;14:8169–8177. doi: 10.1158/1078-0432.CCR-08-1474. [DOI] [PubMed] [Google Scholar]
- Tacken PJ, Torensma R., and, Figdor CG. Targeting antigens to dendritic cells in vivo. Immunobiology. 2006;211:599–608. doi: 10.1016/j.imbio.2006.05.021. [DOI] [PubMed] [Google Scholar]
- Altin JG, van Broekhoven CL., and, Parish CR. Targeting dendritic cells with antigen-containing liposomes: antitumour immunity. Expert Opin Biol Ther. 2004;4:1735–1747. doi: 10.1517/14712598.4.11.1735. [DOI] [PubMed] [Google Scholar]
- Bozzacco L, Trumpfheller C, Siegal FP, Mehandru S, Markowitz M, Carrington M, et al. DEC-205 receptor on dendritic cells mediates presentation of HIV gag protein to CD8+ T cells in a spectrum of human MHC I haplotypes. Proc Natl Acad Sci USA. 2007;104:1289–1294. doi: 10.1073/pnas.0610383104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P, Wu H, McBride JL, Jung KE, Kim MH, Davidson BL, et al. Transvascular delivery of small interfering RNA to the central nervous system. Nature. 2007;448:39–43. doi: 10.1038/nature05901. [DOI] [PubMed] [Google Scholar]
- Kumar P, Ban HS, Kim SS, Wu H, Pearson T, Greiner DL, et al. T cell-specific siRNA delivery suppresses HIV-1 infection in humanized mice. Cell. 2008;134:577–586. doi: 10.1016/j.cell.2008.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman D, Ni H, Scales D, Dude A, Capodici J, McGibney K, et al. HIV gag mRNA transfection of dendritic cells (DC) delivers encoded antigen to MHC class I and II molecules, causes DC maturation, and induces a potent human in vitro primary immune response. J Immunol. 2000;165:4710–4717. doi: 10.4049/jimmunol.165.8.4710. [DOI] [PubMed] [Google Scholar]
- Gruber A, Kan-Mitchell J, Kuhen KL, Mukai T., and, Wong-Staal F. Dendritic cells transduced by multiply deleted HIV-1 vectors exhibit normal phenotypes and functions and elicit an HIV-specific cytotoxic T-lymphocyte response in vitro. Blood. 2000;96:1327–1333. [PubMed] [Google Scholar]
- Betts MR, Nason MC, West SM, De Rosa SC, Migueles SA, Abraham J, et al. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood. 2006;107:4781–4789. doi: 10.1182/blood-2005-12-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migueles SA, Osborne CM, Royce C, Compton AA, Joshi RP, Weeks KA, et al. Lytic granule loading of CD8+ T cells is required for HIV-infected cell elimination associated with immune control. Immunity. 2008;29:1009–1021. doi: 10.1016/j.immuni.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo J, Chen H, Kraus T, Hirsch D, Polyak S, George I, et al. Altered cytokine production and accessory cell function after HIV-1 infection. J Immunol. 1996;157:1313–1320. [PubMed] [Google Scholar]
- Grassi F, Hosmalin A, McIlroy D, Calvez V, Debré P., and, Autran B. Depletion in blood CD11c-positive dendritic cells from HIV-infected patients. AIDS. 1999;13:759–766. doi: 10.1097/00002030-199905070-00004. [DOI] [PubMed] [Google Scholar]
- Marine JC, Topham DJ, McKay C, Wang D, Parganas E, Stravopodis D, et al. SOCS1 deficiency causes a lymphocyte-dependent perinatal lethality. Cell. 1999;98:609–616. doi: 10.1016/s0092-8674(00)80048-3. [DOI] [PubMed] [Google Scholar]
- Starr R, Metcalf D, Elefanty AG, Brysha M, Willson TA, Nicola NA, et al. Liver degeneration and lymphoid deficiencies in mice lacking suppressor of cytokine signaling-1. Proc Natl Acad Sci USA. 1998;95:14395–14399. doi: 10.1073/pnas.95.24.14395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson SM, Macaulay R, Kiani-Alikhan S., and, Akbar AN. The use of the inhibitory receptors for modulating the immune responses. Curr Pharm Des. 2008;14:2643–2650. doi: 10.2174/138161208786264124. [DOI] [PubMed] [Google Scholar]
- Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- Petrovas C, Casazza JP, Brenchley JM, Price DA, Gostick E, Adams WC, et al. PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J Exp Med. 2006;203:2281–2292. doi: 10.1084/jem.20061496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaubert KL, Price DA, Frahm N, Li J, Ng HL, Joseph A, et al. Availability of a diversely avid CD8+ T cell repertoire specific for the subdominant HLA-A2-restricted HIV-1 Gag p2419-27 epitope. J Immunol. 2007;178:7756–7766. doi: 10.4049/jimmunol.178.12.7756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price DA, Brenchley JM, Ruff LE, Betts MR, Hill BJ, Roederer M, et al. Avidity for antigen shapes clonal dominance in CD8+ T cell populations specific for persistent DNA viruses. J Exp Med. 2005;202:1349–1361. doi: 10.1084/jem.20051357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefranc MP, Pommié C, Ruiz M, Giudicelli V, Foulquier E, Truong L, et al. IMGT unique numbering for immunoglobulin and T cell receptor variable domains and Ig superfamily V-like domains. Dev Comp Immunol. 2003;27:55–77. doi: 10.1016/s0145-305x(02)00039-3. [DOI] [PubMed] [Google Scholar]