Abstract
The purpose of this study was to investigate the oncolytic potential of the recombinant, granulocyte macrophage colony-stimulating factor (GM-CSF)-expressing vaccinia virus (VV) JX-594 in experimental malignant glioma (MGs) in vitro and in immunocompetent rodent models. We have found that JX-594 killed all MG cell lines tested in vitro. Intratumoral (i.t.) administration of JX-594 significantly inhibited tumor growth and prolonged survival in rats-bearing RG2 intracranial (i.c.) tumors and mice-bearing GL261 brain tumors. Combination therapy with JX-594 and rapamycin significantly increased viral replication and further prolonged survival in both immunocompetent i.c. MG models with several animals considered “cured” (three out of seven rats >120 days, terminated experiment). JX-594 infected and killed brain tumor-initiating cells (BTICs) from patient samples grown ex vivo, and did so more efficiently than other oncolytic viruses MYXV, Reovirus type-3, and VSVΔM51. Additional safety/toxicity studies in nontumor-bearing rodents treated with a supratherapeutic dose of JX-594 demonstrated GM-CSF-dependent inflammation and necrosis. These results suggest that i.c. administered JX-594 triggers a predictable GM-CSF-mediated inflammation in murine models. Before proceeding to clinical trials, JX-594 should be evaluated in the brains of nonhuman primates and optimized for the viral doses, delivery routes as well as the combination agents (e.g., mTOR inhibitors).
Introduction
Malignant glioma (MGs) are the most common primary intracranial (i.c.) malignancy, with survival times remaining relatively static over the past few decades. Oncolytic viruses (OVs) show promising treatment efficacy in models of MGs, with several OVs being tested in preclinical models of MGs1,2,3,4,5,6 and some evaluated in early clinical trials.7,8,9,10,11,12,13,14 These trials found that OV therapy is safe with no reachable maximum tolerated dose; however, only a few patients responded. Hence, more effective OVs must be found for the treatment of MGs.
Vaccinia virus (VV) is a double-stranded, enveloped, lytic DNA virus with a large capacity for foreign DNA. It has several advantages over other OVs, as it is easy to manipulate genetically, replication and spread are rapid, it is motile (actin tail-dependent), it does not integrate into host DNA, and it is safe in animal models and primates.15,16 There is extensive clinical experience with VV as a vaccine for smallpox. Several poxvirus-based cancer vaccination trials are also underway.17,18
Several strains of attenuated, replicating VVs have shown efficacy in preclinical MG models19,20 and other cancers.21,22,23,24,25 To address potential safety concerns of a replicating VV, a mutant “double-deleted” thymidine kinase (TK) deficient and vaccinia growth factor deficient version of the WR (Western Reserve) strain (vvDD) was created to enhance its safety. vvDD selectively targets several tumor types in murine models without significant toxicity.26 It is also nontoxic when administered intravenously in nonhuman primates27 and in rodent MG models20 suggesting a potential for systemic administration.
JX-594 is a targeted and transgene-armed oncolytic poxvirus modified by insertion of human granulocyte macrophage colony-stimulating factor (GM-CSF) and disruption of TK by insertion of LacZ genes into the viral TK gene.22 JX-594m expresses murine rather than human GM-CSF, and is used in rodent models because rodents do not respond to human GM-CSF. These VVs are designed to selectively replicate in cancer cells with cell-cycle abnormalities and epidermal growth factor receptor-ras pathway activation.22 JX-594 has efficacy in preclinical liver cancer models,28 and a phase I trial of intratumoral (i.t.) JX-594 in liver cancer found significant antitumor effects with viral replication and expression of the therapeutic transgene GM-CSF.29
The objectives of this study were to investigate the efficacy and safety/toxicity of JX-594 and JX-594m administered i.t. in immunocompetent rodent MGs and human brain tumor-initiating cells (BTICs).
Results
JX-594 and JX-594m productively infects and kills all tested glioma cell lines in vitro
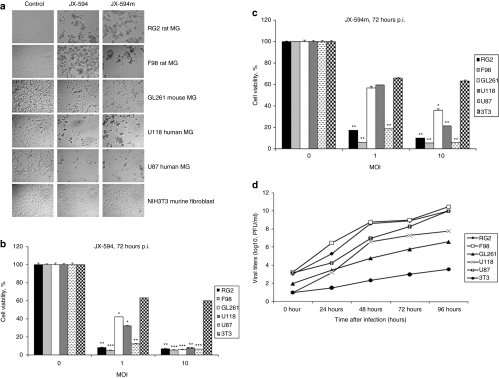
Five MG cell lines (GL261, F98, RG2, U87, and U118) all were permissive to infection and killed as demonstrated by cytopathic effect and MTT assay 72 hours after infection with JX-594/JX-594m (Figure 1a–c). NIH3T3 was poorly permissive (Figure 1a–c). Compared to other OVs (MYXV, VSVΔM51, and Reovirus type-3), we found JX-594/JX-594m had greater efficacy and a broader spectrum of activity; cell lines resistant to reovirus (U118), VSVΔM51, and MYXV (GL261) were susceptible to JX-594/JX-594m (Supplementary Figure S1). To determine whether viral infection occurred, viral titers were obtained after infection (Figure 1d). All cell lines were permissive, to different degrees with much higher titers than the NIH3T3 (Figure 1d). Longer time points (5 days) found all the cells dead after infection [multiplicity of infection (MOI) = 10] and we did not detect any live cells on the plates (Supplementary Figure S2).
Figure 1.
JX-594 and JX-594m infect and kills human and murine brain tumor cells in vitro. (a) CPE of MGs cells. Cells were plated at confluency and the next day infected with JX-594/JX-594m at an MOI of 10. Microscopy was performed 72 hours after viral infection (original magnification × 100). (b,c) MTT assay of MG cells compared to NIH3T3 controls 72 hours after (b) JX-594 and (c) JX-594m. (d) Viral titers were obtained in MGs and NIH3T3 cell lines after JX-594 infection. Viral titers were determined using a standard plaque titration assay on U2OS cells. Values represent mean PFUs ± SD from triplicate wells. *P < 0.05; **P < 0.01; ***P < 0.001 as analyzed by two-way ANOVA. ANOVA, analysis of variance; CPE, cytopathic effect; MG, malignant glioma; MOI, multiplicity of infection; p.i., postinfection.
Efficacy of JX-594 and JX-594m when administered i.t. in immunocompetent racine and murine models of glioma
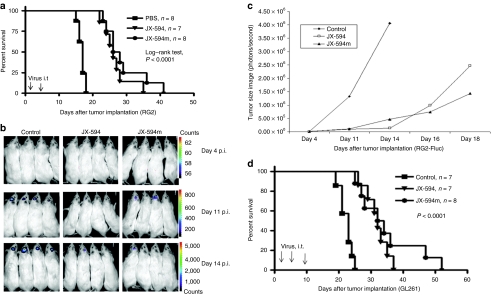
RG2-bearing rats were treated i.t. with multiple doses of JX-594 or JX-594m (at days 1 and 4). Treatment with virus prolonged survival (median survival 16 days for phosphate-buffered saline (PBS) control, 26 days for JX-594 and 27 days for JX-594m); some rats treated with JX-594 (one rat survived for 35 days) or JX-594m (two rats survived for 36 and 41 days, respectively) were “long-term” survivors (Figure 2a, long-rank test, P < 0.0001 PBS and JX-594 or JX-594m). Survival with JX-594 or JX-594m were not significantly different (log-rank test, P = 0.3288).
Figure 2.
i.t. administration of JX-594/JX-594m inhibited tumor growth and prolonged survival of immunocompetent animals-bearing intracranial glioma. (a) Kaplan–Meier survival of rats harboring intracranial RG2 tumor treated with PBS (n = 8) or i.t. administration of JX-594 (n = 7, 5 × 107 PFUs /rat) or i.t. administration of JX-594m (n = 8, 5 × 107/rat, at days 1 and 4). Arrows indicates virus administration. (b) Representative BLI obtained at days 4, 11, and 14 after tumor implantation of RG2-Fluc and treatment with JX-594, JX-594m, or PBS. (c) Quantification of the BLI. (d) Kaplan–Meier survival curves of C57/BL6 mice harboring GL261 tumor treated with control (PBS, n = 7), JX-594 (n = 7, 1 × 107 PFU/rat for three times, at days 1, 4, and 10) or JX-594m (n = 8). Arrows indicate the day of virus administration. BLI, bioluminescence image; i.t., intracranial; PBS, phosphate-buffered saline; PFU, plaque-forming unit; p.i., postinfection.
We next imaged a surrogate for tumor size in vivo using bioluminescence image (BLI) of RG2-Fluc tumors. BLI of control animals (n = 8) increased by day 4 after tumor implantation (8.12 × 103) and peaked on day 14 (4.06 × 106) (Figure 2b,c); JX-594- (n = 8) and JX-594m- (n = 8) treated rats had a BLI that slowly increased between day 4 (8.64 × 103, 8.12 × 103) and day 14 (1.35 × 105, 4.70 × 105), and still did not reach a peak level (control animals) by day 18 (2.47 × 106 and 1.43 × 106, termination of the experiment) (Figure 2c).
To determine whether JX-594/JX-594m i.t. prolongs survival in immuncompetent mice bearing a MG resistant to other OVs (resistant to MYXV, VSVΔM51, and reovirus in vitro), we treated mice on days 1, 4, and 10 after GL261 tumor inoculation and found survival was significantly prolonged(Figure 2d, long-rank test, P < 0.0001, PBS and JX-594 or JX-594m). Two out of eight mice (25%) treated with JX-594m were considered “long-term” survivors (>40 days). Interestingly, both JX-594 and JX-594m displayed similar survival patterns, despite the long-term survivors, suggesting that the addition of the GM-CSF cytokine in this model may not be necessary for survival benefit in this model.
Combination therapy with rapamycin promotes JX-594-mediated oncolysis in vitro and enhanced virus replication in vivo
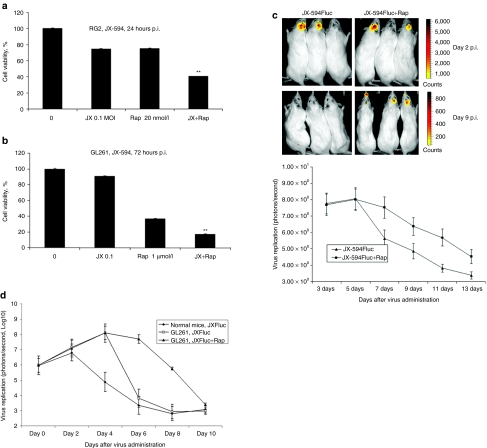
To determine whether combination therapy with rapamycin promotes JX-594 oncolysis in vitro, we assessed cell viability with or without pretreatment with rapamycin. Combination treatment resulted in greater cell killing than either treatment alone in both RG2 and GL261 glioma lines (Figure 3a,b).
Figure 3.
Combination therapy with rapamycin promotes oncolysis in vitro and enhanced viral replication in vivo. (a,b) Viability of rodents glioma cell lines (RG2, GL261) were measured using the MTT assay 24 and 72 hours postinfection with JX-594 (JX), rapamycin alone (Rap), or both (JX+Rap). **P < 0.05 as analyze by two-way ANOVA. (c) Representative viral replication BLI images (top) and quantification of BLI in JX-594Fluc alone (n = 3) or JX-594Fluc + rapamycin (n = 3) treated RG2 tumor-bearing rats (bottom). (d) Representative viral replication quantification of BLI in JX-594Fluc alone (n = 3) or JX-594Fluc + rapamycin (n = 3) treated GL261 tumor-bearing mice. BLI, bioluminescence image; p.i., postinfection.
We next determined whether rapamycin enhanced viral replication in vivo using BLI in the RG2 rat model. In the first 5 days, BLI virus imaging (yellow: virus image) was similar (JX-594Fluc, 7.76–8.05; JX-594 + Rap, 7.69–8.0) (Figure 3c, bottom). After 5 days, BLI declined for the JX-594Fluc-treated rats (8.05–5.63) but not for combination treated rats (8.0–7.53) (Figure 3c, bottom). Nine days after treatment, BLI virus image was almost undetectable in the JX-Fluc alone group when compared to the combination group (Figure 3c, top). We repeated this experiment and found similar results (Supplementary Figure S3a) and nontumor-bearing rats had virus replication that was much lower and shorter than tumor-bearing mice (Supplementary Figure S3a). We found similar results in mice with GL261 tumors (Figure 3d).
Efficacy of JX-594 and JX-594m administered i.t. combined with rapamycin in immunocompetent racine or murine animal models of glioma
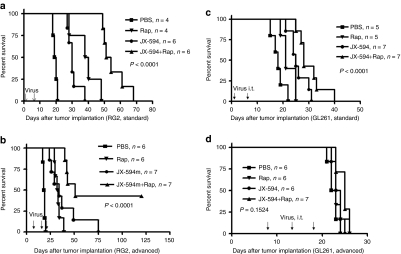
To determine whether combination therapy prolonged survival, we treated RG2-bearing rats with i.t. JX-594 combined with intraperitoneal (i.p.) rapamycin, with the treatment schedule described in methods. Treatment with JX-594 alone (Figure 4a, mean = 30.6 days, log-rank test, P = 0.0029) or rapamycin alone (mean = 38.5 days, P = 0.0069) significantly prolonged survival (control mean = 19.2 days). Rats treated with combination therapy had a marked increase in survival (mean = 55.3 days) compared to JX-594 alone (P = 0.0008), rapamycin alone (log-rank test, P = 0.0011) or PBS (Figure 4a; P = 0.0003, combination group compared to PBS).
Figure 4.
Combination therapy with rapamycin promotes JX-594/JX-594m oncolysis in vitro and in vivo. (a) Kaplan–Meier survival of intracranial RG2-bearing rats after treatment with PBS (n = 4), JX-594 alone (n = 6), rapamycin alone (n = 4), or a combination of both JX-594 and rapamycin (n = 6, log-rank test, P < 0.0001 compared to PBS). (b) Kaplan–Meier survival of “advanced” intracranial RG2 tumor-bearing rats after treatment with PBS (n = 6), JX-594m alone (n = 7), rapamycin alone (n = 6), or a combination of both JX-594m and rapamycin (n = 7, log-rank test, P < 0.0001 compared to PBS). (c) Survival of intracranial GL261 tumor-bearing mice after treatment with PBS (n = 5), JX-594 alone (n = 7), rapamycin alone (n = 5), or a combination of both JX-594 and rapamycin (n = 7, log-rank test, P < 0.0001 compared to PBS). (d) Kaplan–Meier survival of “advanced” intracranial GL261 tumor-bearing mice after treatment with PBS (n = 6), JX-594m alone (n = 6), rapamycin alone (n = 6), or a combination of both JX-594m and rapamycin (n = 7, log-rank test, P = 0.1524 compared to PBS). PBS, phosphate-buffered saline; Rap, rapamycin.
We then repeated this combination experiment using an “advanced” RG2 model to mimic the clinical situation. We treated rats with JX-594m alone (mean = 36.1 days, log-rank test, P = 0.0003) or rapamycin alone (mean = 32.9 days, log-rank test, P = 0.0007), which prolonged survival (Figure 4b, control mean = 18.2 days). JX-594m + rapamycin further prolonged survival and was superior to either treatment alone (three out of seven rats were long-term survivors; JX-594m: log-rank test, P = 0.0248; rapamycin: log-rank test, P = 0.0003) (Figure 4b). We repeated this experiment and found similar results (Supplementary Figure S3b).
We then preformed these experiments in the Gl261-bearing mice and we found for the early time (started on day 2 after tumor implantation) treated model JX-594 alone (Figure 4c, log-rank test, P = 0.0025) significantly prolonged survival, a trend for rapamycin alone (log-rank test, P = 0.0551) to prolong survival, and combination treatment further prolonged survival [compared to JX-594 alone (log-rank test, P = 0.0191), rapamycin alone (log-rank test, P = 0.0018)] or PBS (Figure 4c; log-rank test, P < 0.0003, combination group compared to PBS). But for the “advanced” GL261 model, JX-594 alone, none of the treatments prolonged survival (Figure 4d; log-rank test, P = 0.1524).
Safety profile of the i.c. administration of JX-594 and JX-594m in nontumor-bearing immunocompetent rodents
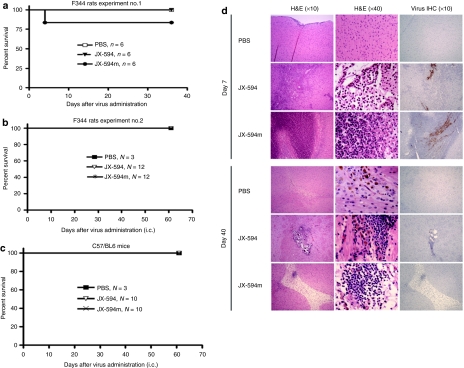
Nontumor-bearing rats were injected directly into normal brain with a supratherapeutic dose [4 × 108 plaque-forming unit (PFU)/kg, about 6–7 × 107 PFU/rat] of JX-594 or JX-594m. i.c. administration of JX-594 was well tolerated; rats exhibited a slight weight loss for 7 days and subsequently recovered (Supplementary Figure S4a). Animals appeared normal and none died (Figure 5a). In contrast, JX-594m was not well tolerated. The rats exhibited poorer grooming and were less active 3–5 days after i.c. administration. One rat could not ambulate normally and was sacrificed at day 5 (Figure 5a). Surviving animals (treated with either virus) then gradually gained weight (Supplementary Figure S4a) and appeared healthy; all animals were sacrificed at 40 days. Largely because of the death of the single rat treated with JX-594m, we repeated the above rat experiments twice (n = 12/group, n = 5/group) using the same doses/schedule of each virus and followed the animals for 60 and 40 days (Figure 5b, Supplementary Figure S4b). When we repeated the experiments all the JX-594/JX-594m-treated rats had similar transient weight loss (Supplementary Figure S4b) but remained healthy and none died (Figure 5b, Supplementary Figure S4b). C57BL/6 mice tolerated i.c. administration of JX-594/JX-594m well, had transient weight loss (Supplementary Figure S4d), but appeared well and none died (Figure 5c).
Figure 5.
Safety/toxicity evaluation of JX-594 and JX-594m when i.c. in immunocompetent rodents. (a,b) Survival of nontumor-bearing normal rats administered JX-594 or JX-594m i.t. in two separate experiments. (c) Survival of nontumor-bearing C57/BL6 mice administrated JX-594 or JX-594m i.t. (d) Representative images of the brains in rats treated with the i.c. inoculation of JX-594 and JX-594m (4 × 108 PFU/kg, about 6–7 × 107 PFU/rat) at 7 and 40 days after injection (H&E, left and middle columns; IHC for viral antigen, right column). Magnification with ×10 objective left and right columns; ×40 objective in middle column. H&E, hematoxylin and eosin; i.c., intracranial; IHC, immunohistochemically; PBS, phosphate-buffered saline; PFU, plaque-forming unit.
Neuropathological changes following the i.c. administration of JX-594 and JX-594m in nontumor-bearing immunocompetent rodents
Histological examination of the racine brains at 3 days following administration of JX-594 showed focal striatal and cerebral cortical necrosis with moderate meningoencephalitis (data not shown). The findings were similar 7 days postinfection but the density of inflammatory infiltrates and extent of necrosis was increased (Figure 5d, top). Pathologic changes were largely limited to the injected hemisphere. At 40 days after JX-594 administration a small area of chronic inflammation (along the needle track), residual focal cortical and striatal necrosis and areas of calcification were detected (Figure 5d, bottom). Most brains also had mild dilation of the ventricles.
In contrast, rats injected with JX-594m had a severe necrotizing meningoencephalitis at 3 days characterized by larger areas of focal full thickness cortical necrosis, striatal necrosis and more extensive meningeal inflammation (Figure 5d). Inflammatory changes were found in the meninges and superficial cortex of the contralateral hemisphere as well as the hemisphere of injection (Figure 5d). At 40 days postinfection, rats had a more extensive residual cortical or striatal tissue loss (Figure 5d) and most had some nonobstructive hydrocephalus.
The inflammatory infiltrates at days 3 and 7 (as detected by in situ hybridization) with both JX-594 and JX-594m consisted of neutrophils (data not show), CD68+ macrophages, activated microglia (Supplementary Figure S5a) and CD8+ T cells (Supplementary Figure S5b). There was no significant infiltration of CD4+ T cells immunohistochemically (data not shown). Rare CD20+ B cells were found (data not shown). At 40 days, scattered macrophages, activated microglia, and CD8+ T cells persisted (data not show). No CD4+ T cells or CD20 B+ cells were found (data not shown). In order to determine a correlation between the area of inflammation and the site of virus injection, immunohistochemical analysis detecting virus proteins was performed. Evaluation of viral distribution within the brain by immunohistochemical analysis at 3 and 7 days after viral administration showed that focal positivity for viral antigens was limited to areas of the most severe inflammation in the leptomeninges and striatum along the site of the viral injection (Figure 5d, right column); no residual virus was detected at 40 days. There was no evidence of an extensive viral dissemination or of a demyelinating process. Control brains showed only a localized reactive glial scar and scattered hemosiderin-laden macrophages with little or no granulocytic or lymphocytic infiltration.
BTICs are susceptible to JX-594 infection and killing ex vivo
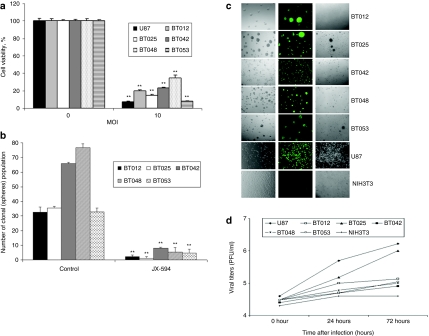
Five established BTICs were tested for susceptibility to JX-594GFP using the cell viability, self-renewal, expression of viral gene [green fluorescent protein (GFP)], and the viral replication assay. Cell viability assays showed all BTICs were killed by JX-594GFP (Figure 6a), which was confirmed by self-renewal assays (Figure 6b). All BTICs, but not NIH3T3, were permissive to infection as measured by GFP expression and cytopathic effect (Figure 6c). Finally, viral titers (Figure 6d) showed a minor productive infection occurred in most BTICs, save BT025. In all cases, viral titers were less than what was seen in the U87 cell line which is the most susceptible MG cell line to OVs in our hands.
Figure 6.
Effect of JX-594GFP on human BTICs ex vivo. (a) Cell viability assay using JX-594GFP in different BTICs (MOI = 10, 5 days postinfection). U87 was a positive control. **P < 0.001 as compared by Student's t-test to the control. (b) Self-renewal of BTICs was inhibited after JX-594GFP infection. BTICs were infected with JX-594GFP (MOI = 3); 5 days after infection replanted and then after 10 days the number of spheres was counted. **P < 0.001 as compared by Student's t-test to the control. (c) All five BTICs were permissive to infection, as demonstrated by the expression of viral GFP 72 hours after infection with JX-594GFP (MOI = 1). (d) The viral replication assay showed that viral titers varied between BTICs and mirrored their susceptibility. BTIC, brain tumor-initiating cell; GFP, green fluorescent protein; MOI, multiplicity of infection; PFU, plaque-forming unit.
Discussion
We found that VV JX-594/JX-594m, engineered to express GM-CSF, infects and kills MGs in vitro and in vivo. In vitro, JX-594/JX-594m infected all MG cell lines tested (including ones resistant to other OVs) and our panel of BTICs (a.k.a. brain tumor stem cells). In vivo, JX-594/JX-594m prolonged survival in two immunocompetent rodent MG models and this was significantly enhanced by rapamycin. JX-594 administered at a supratherapeutic dose into normal brain of nontumor-bearing rodents resulted in inflammation and necrosis limited to the injected hemisphere. In contrast, JX-594-expressing GM-CSF which is active in rodents (JX-594m) caused a more diffuse and bilateral inflammatory response. This finding suggests that the enhanced inflammatory response may be a result of GM-CSF expression rather than being a direct result of virus replication; however, there was no difference in survival outcome of JX-594 or JX-594m, suggesting that GM-CSF does not change VV OV efficacy.
The consideration of JX-594 for evaluation in a clinical trial with MGs is not straightforward because its promising characteristics must be balanced by its toxicities in rodent models. There are many reasons to consider this as an attractive OV in MGs. First, there is a long history of safety with VV as 100s of millions have been vaccinated with it. Second, it shows significant activity and little toxicity in patients with liver cancer.29 Third, compared to other OVs we have used, JX-594 has greater efficacy and a broader spectrum of activity. For example, in immunocompetent animal models, it infects/kills GL261 that is resistant to other OVs and persists seven times longer than other OVs (reovirus and MYXV persist for 48 (30) and 24 hours31); this may allow several rounds of lytic infection in patients. Fourth, similar to the VV with deletions in vaccinia growth factor and TK,20 survival was much more prolonged with JX-594 when combined with rapamycin, some rats were long-term survivors. This marks the first time we have seen using OVs in combination with rapamycin or cyclophosphamide.20,31,32 Fifth, it's oncolytic effect has not been shown to be dependent on specific cellular receptors or genetic alterations within tumor cells, giving it potentially broad applicability. Finally, JX-594 is able to stop proliferation and possibly kill BTICs, and in some instances replicate to similar levels seen in U87 (the most susceptible MG cell line we have tested). Hence, JX-594 may overcome treatment resistance in BTICs to chemotherapy/radiotherapy,33,34 given resistance is based on these persisting cell types. Others have found that OVs are able to infect and kill BTICs.35,36
The promising antitumoral activity of JX-594 versus MGs observed in rodent models provide rationale for clinical testing of JX-594 in patients with advanced MGs. However, injection of a supratherapeutic dose of JX-594 into the normal rodent brain was associated with diffuse inflammation. Therefore, care must be taken to avoid inadvertent injection of JX-594 into adjacent normal brain tissue and subsequent inflammation. Furthermore, careful dose-escalation should be undertaken in this patient population. Indeed, JX-594 infection of tumors has resulted in acute necrosis and edema formation in patients with extracranial tumors, which may be deleterious in patients with cranial tumors. However, as many MG patients are treated with corticosteroids, inflammation and edema formation may be mitigated.
In spite of these promising characteristics, its toxicity in nontumor-bearing rodents needs to be considered carefully. Both JX-594 and JX-594m produced marked inflammation and focal tissue necrosis. Logically, these changes are more severe with JX-594m than JX-594, and were more severe and diffuse than other OVs3,5,20,30,31,37,38,39,40,41 (except for i.c. VSVΔM51 which causes a severe/fatal meningoencephalitis6). Would these inflammatory changes be harmful or beneficial to MG patients? MG patients with inflammation42 or i.c. infections have longer survival, and are sometimes even cured.43,44 OVs tested in clinical trials produce only microscopic inflammatory responses/necrosis but their clinical results are also disappointing.7,8,9,10,11,12,13,14 Responses are uncommon, short lived and only one or two patients in each trial have very long survivals, or rarely, an absence of tumor at autopsy.11,12,13 The oncological aphorism that effective therapy always has some toxicity might also apply to OVs. The direct test of the significance of JX-594-induced inflammation in MGs awaits testing in nonhuman primates and ultimately in patients. The finding that oncolysis of JX-594 was enhanced with the addition of rapamycin suggests that rapamycin (or another rapalogue) might be used in the clinic but would first require a detailed evaluation of the potential toxicities of the combination therapy.
There are several limitations of our study. First, experiments were biased to produce an efficacy because most animals were treated only 1 day after tumor inoculation but most patients present with large, established tumors. Second, since a dose–response effect for the toxicity of JX-594 was not evaluated, instead using the highest dose we can prepared was used for administration in the brain. Lower doses may retain efficacy without toxicity. The relevant next step is to determine toxicology in nonhuman primates to find a phase I dosing regimen. Third, since the precise mechanism that rapamycin uses to enhance oncolysis in vivo is unknown, improved results are possible with the use of other rapalogues or selective mTORC inhibitors.
Materials and Methods
Cell lines. Murine NIH3T3 fibroblasts and racine GBM cell lines RG2, F98, and human U87, U118 are from the American Type Culture Collection (Manassas, VA). GL261 was provided by Dr Luc Vallieres, Laval University, Montreal, QC, Canada. The firefly luciferase gene plasmid (pGL3 enhancer vector: Promega, Madison, WI) was cotransfected into RG2 cells (RG2-Fluc, ref. 45). All cells were grown as described previously20,30 and routinely test myoplasma before use them.
Viruses and replication assay. The recombinant VV JX-594 clone#1 (20080624KH) and JX-594m GM-CSF (20080624KH) are from the Ottawa Hospital Research Institute (Dr John C. Bell). JX-594 was modified by insertion of human GM-CSF and LacZ genes into the viral TK region, whereas JX-594m contains murine GM-CSF. JX-594GFP expresses EGFP. JX-594Fluc expresses firefly luciferase. All viruses were propagated and tittered on UO2S cells.22
For in vitro viral replication assays, cells were infected with JX-594 at an MOI of 0.1, after 24, 48, 72, and 96 hours incubating, cells lysates with medium underwent three cycles of freeze/thawing, then serial dilutions of supernatants/lysates were tittered.22
Cell viability and cytopathic effect assays. Cells were infected with JX-594/JX-594m (MOI = 0, 1, and 10) then incubated at 37 °C. Cell viability was measured 72 or 120 hours postinfection by MTT.5,20,30 We compared the results with MYXV (Dr Grant McFadden, University of Florida, Gainesville, FL), VSVΔM51 (Dr John C. Bell, Ottawa, ON, Canada) and Reovirus type-3 (Dr Peter Forsyth, Calgary, AB, Canada). For combination therapy with rapamycin (LC Laboratories, Toronto, Ontario, Canada, 20 nmol/l–1 µmol/l) was added 2 hours before viral treatment. In vitro cytopathic effect were visualized/photographed with a Zeiss inverted microscope (Axiovert 200M) and a Carl Zeiss camera (AxioCam MRc).
Immunohistochemistry. Paraffin-embedded sections were deparaffinized and rehydrated, blocked, and incubated with primary antibody. Antibodies used were the murine monoclonal VV antibody (1:1500; Abcam, Cambridge, MA), mouse anti-rat CD68 (1:500; Serotec, Raleigh, NC), CD8 and CD4 (1:300; Serotec); CD20 (1:300; Secotec) used overnight at 4 °C. Biotinylated donkey anti-mouse immunoglobulin G or anti-rabbit immunoglobulin G (1:2,000; Vector Laboratories, Burlington, Ontario, Canada) was used as the secondary antibody. Sections were incubated with avidin conjugated to horseradish peroxidase (Vectastain ABC immunohistochemistry kit; Vector Laboratories), and staining was visualized by the addition of 3,3́-diaminodbenzidine substrate with hematoxylin counterstaining.
Efficacy studies of orthotopic glioma models in immunocompetent hosts. To investigate efficacy, female Fischer 344 rats or C57/BL6 mice were inoculated with 1 × 104 RG2 (or GL261) cells under anesthesia as described previously.5,30 Animals were treated with JX-594/JX-594m (5 × 107 PFU/rat, 1 × 107 PFU/mouse) i.t. 1, 4, and 10 days (GL261) after tumor implantation; control animals were treated with PBS, animals were followed daily for survival. The times of i.t. inoculation were selected to favor efficacy when the tumors were expected to be both established and very small.
In vivo monitoring tumor growth/inhibition using BLI. RG2-Fluc-bearing rats [5 days after tumor implantation (2 × 104/rat)] were treated with i.t. JX-594 or JX-594m (5 × 107 PFU/rat) as a single dose. On days 4, 11, 14, 16, and 18 days after tumor implantation they were imaged with the Xenogen IVIS 200 System (Xenogen, Alameda, CA) to record BLI emitted from tumors. Data were analyzed based on total photon flux emission (photons/second) in the region of interest.46
Combination therapy with rapamycin. RG2 or GL261 cells were treated ± rapamycin (20 nmol/l or 1 µmol/l) 2 hours before infection, then infected with JX-594 (MOI = 0.1) and MTT performed after 24 or 72 hours incubation. For viral replication in vivo, RG2- or GL261-bearing animals were treated with JX-594Fluc alone (i.t. 5 × 106 PFU/animal at day 8 after tumor implantation) or JX-594Fluc + rapamycin. Rapamycin at a dose of 5 mg/kg/rat (2.5 mg/kg/mouse) was given i.p. every day for 10 days started same day with virus. Every other day after virus administration, animals were imaged using Xenogen IVIS 200 System (Xenogen).
To evaluate the in vivo effects of combination therapy i.c. animals were divided into the following four groups (n = 4–7 animals), 2 days after tumor implantation: (i) PBS control, (ii) rapamycin alone (i.p. administration: rapamycin 5 mg/kg/every other day for 3 weeks), (iii) JX-594 [1 × 107 PFU/rat (5 × 106 PFU/mouse), administered at days 2 and 8] alone, and (iv) JX-594 + rapamycin.
To determine the effects on an “advanced” or “late-stage” tumor model RG2- (or GL261) bearing animals were divided into four groups (n = 6–7 animals), 8 days after tumor implantation: (i) PBS control, (ii) rapamycin alone [i.p. administration: rapamycin 5 mg/kg/day for 4 weeks (2.5 mg/kg/mouse), starting at day 8], (iii) JX-594m [1 × 107 PFU/rat (JX-594, 5 × 106 PFU/mouse), administered at days 8, 13, and 18] alone, and (iv) JX-594m (JX-594) + rapamycin.
Safety/toxicity of i.c. administration of JX-594/JX-594m in nontumor-bearing normal F344 rats or C57/BL6 mice. Fischer 344 rats or C57/BL6 mice were injected i.c. with JX-594 and JX-594m (4 × 108 PFU/kg, about 6–7 × 107 PFU/rat) or PBS (in 5–10 µl of volume) under anesthesia (80 mg/kg ketamine and 8 mg/kg xylazine i.p.).5,30 Animals were followed for 40 (rats) or 60 (mice) days. Animals losing ≥20% body weight or developing other unaccepted symptoms were sacrificed (Animal Care Guidelines). To evaluate the histology and viral distribution experiments, animals were sacrificed at 3, 7, and 40 days after viral administration. Because one rat died after i.c. administration the toxicity experiments were performed three times.
Oncolytic effect of JX-594GFP against BTICs ex vivo. All BTICs provided by Dr Samuel Weiss and Dr Gregory Cairncross lab (University of Calgary, Calgary, AB, Canada). We cultured BTICs as described previously47,48 and placed BT012, BT025, BT042, BT048, and BT053 in 96-well plates at 2 × 104 cells/well in 100 µl serum free medium, then infected with JX-594GFP (MOI = 10). Cell viability was measured 5 days postinfection using Alamar Blue assay. The permissive U87 was used as positive controls.
To evaluate self-renewal capability, we performed the secondary neurosphere formation assay 5 days after viral infection (MOI = 3). Then live/viable cells were resuspended in fresh BTIC medium and seeded into 96-well plates at 100 cells/well. Ten days later, the number of wells containing neurospheres was recorded.
To assess viral replication, we used viral gene expression and viral titers. BTICs were seeded at 2 × 104 cells/well in 24-well plates infected with JX-594GFP (MOI = 0.1). The plates were then subjected to three rounds of freezing/thawing to titer virus. Viral titers were determined and repeated thrice.
Statistics. Statistical Analysis Software (SAS Institute) and GraphPad Prism (version 4; GraphPad Software, La Jolla, CA) were used for statistical analyses. Survival curves were generated by the Kaplan–Meier method. The multiple group average data were analyzed with the two-way analysis of variance. All reported P values were two-sided and were considered to be statistically significant at P < 0.05.
SUPPLEMENTARY MATERIAL Figure S1. JX-594 and JX-594m infects and kills glioma cell lines that are resistant to other oncolytic viruses in vitro. Figure S2. Five days MTT, CPE, and crystal violet staining results after JX-594 and JX-594m-infected glioma cell lines. Figure S3. Combination therapy with rapamycin enhanced JX-594Fluc replication and JX-594m combined rapamycin treatment further prolonged survival in rat RG2 model. Figure S4. More data about JX-594 and JX-594m safety/toxicity studies in immunocompetent animals. Figure S5. Representative CD68/CD8 infiltration in the brain after JX-594/JX-594m intracranial administration.
Acknowledgments
This work was funded by the Canadian Cancer Society Research Institute (P.A.F. and D.L.S.), a Program Project Grant from the Terry Fox Foundation (J.C.B. is P.I.; P.A.F., D.L.S.), the Clark Smith Brain Tumor Center (P.A.F.), and Jennerex Biotherapeutics of USA (T.-C.L., D.K., and J.C.B.). About the conflict of interest, see attached addition information for Molecular Therapy. The other authors declared no conflict of interest. We thank Franz Zemp for reviewing and editing this paper.
Supplementary Material
JX-594 and JX-594m infects and kills glioma cell lines that are resistant to other oncolytic viruses in vitro.
Five days MTT, CPE, and crystal violet staining results after JX-594 and JX-594m-infected glioma cell lines.
Combination therapy with rapamycin enhanced JX-594Fluc replication and JX-594m combined rapamycin treatment further prolonged survival in rat RG2 model.
More data about JX-594 and JX-594m safety/toxicity studies in immunocompetent animals.
Representative CD68/CD8 infiltration in the brain after JX-594/JX-594m intracranial administration.
REFERENCES
- Martuza RL, Malick A, Markert JM, Ruffner KL., and, Coen DM. Experimental therapy of human glioma by means of a genetically engineered virus mutant. Science. 1991;252:854–856. doi: 10.1126/science.1851332. [DOI] [PubMed] [Google Scholar]
- Fueyo J, Gomez-Manzano C, Alemany R, Lee PS, McDonnell TJ, Mitlianga P, et al. A mutant oncolytic adenovirus targeting the Rb pathway produces anti-glioma effect in vivo. Oncogene. 2000;19:2–12. doi: 10.1038/sj.onc.1203251. [DOI] [PubMed] [Google Scholar]
- Wilcox ME, Yang W, Senger D, Rewcastle NB, Morris DG, Brasher PM, et al. Reovirus as an oncolytic agent against experimental human malignant gliomas. J Natl Cancer Inst. 2001;93:903–912. doi: 10.1093/jnci/93.12.903. [DOI] [PubMed] [Google Scholar]
- Gromeier M, Lachmann S, Rosenfeld MR, Gutin PH., and, Wimmer E. Intergeneric poliovirus recombinants for the treatment of malignant glioma. Proc Natl Acad Sci USA. 2000;97:6803–6808. doi: 10.1073/pnas.97.12.6803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lun X, Yang W, Alain T, Shi ZQ, Muzik H, Barrett JW, et al. Myxoma virus is a novel oncolytic virus with significant antitumor activity against experimental human gliomas. Cancer Res. 2005;65:9982–9990. doi: 10.1158/0008-5472.CAN-05-1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lun X, Senger DL, Alain T, Oprea A, Parato K, Stojdl D, et al. Effects of intravenously administered recombinant vesicular stomatitis virus (VSV(ΔM51)) on multifocal and invasive gliomas. J Natl Cancer Inst. 2006;98:1546–1557. doi: 10.1093/jnci/djj413. [DOI] [PubMed] [Google Scholar]
- Markert JM, Medlock MD, Rabkin SD, Gillespie GY, Todo T, Hunter WD, et al. Conditionally replicating herpes simplex virus mutant, G207 for the treatment of malignant glioma: results of a phase I trial. Gene Ther. 2000;7:867–874. doi: 10.1038/sj.gt.3301205. [DOI] [PubMed] [Google Scholar]
- Chiocca EA, Abbed KM, Tatter S, Louis DN, Hochberg FH, Barker F, et al. A phase I open-label, dose-escalation, multi-institutional trial of injection with an E1B-Attenuated adenovirus, ONYX-015, into the peritumoral region of recurrent malignant gliomas, in the adjuvant setting. Mol Ther. 2004;10:958–966. doi: 10.1016/j.ymthe.2004.07.021. [DOI] [PubMed] [Google Scholar]
- Freeman AI, Zakay-Rones Z, Gomori JM, Linetsky E, Rasooly L, Greenbaum E, et al. Phase I/II trial of intravenous NDV-HUJ oncolytic virus in recurrent glioblastoma multiforme. Mol Ther. 2006;13:221–228. doi: 10.1016/j.ymthe.2005.08.016. [DOI] [PubMed] [Google Scholar]
- Forsyth P, Roldán G, George D, Wallace C, Palmer CA, Morris D, et al. A phase I trial of intratumoral administration of reovirus in patients with histologically confirmed recurrent malignant gliomas. Mol Ther. 2008;16:627–632. doi: 10.1038/sj.mt.6300403. [DOI] [PubMed] [Google Scholar]
- Harrow S, Papanastassiou V, Harland J, Mabbs R, Petty R, Fraser M, et al. HSV1716 injection into the brain adjacent to tumour following surgical resection of high-grade glioma: safety data and long-term survival. Gene Ther. 2004;11:1648–1658. doi: 10.1038/sj.gt.3302289. [DOI] [PubMed] [Google Scholar]
- Papanastassiou V, Rampling R, Fraser M, Petty R, Hadley D, Nicoll J, et al. The potential for efficacy of the modified (ICP 34.5(-)) herpes simplex virus HSV1716 following intratumoural injection into human malignant glioma: a proof of principle study. Gene Ther. 2002;9:398–406. doi: 10.1038/sj.gt.3301664. [DOI] [PubMed] [Google Scholar]
- Aghi MK., and, Chiocca EA. Phase ib trial of oncolytic herpes virus G207 shows safety of multiple injections and documents viral replication. Mol Ther. 2009;17:8–9. doi: 10.1038/mt.2008.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markert JM, Liechty PG, Wang W, Gaston S, Braz E, Karrasch M, et al. Phase Ib trial of mutant herpes simplex virus G207 inoculated pre-and post-tumor resection for recurrent GBM. Mol Ther. 2009;17:199–207. doi: 10.1038/mt.2008.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge JW, Schlom J, Donohue SJ, Tomaszewski JE, Wheeler CW, Levine BS, et al. A recombinant vaccinia virus expressing human prostate-specific antigen (PSA): safety and immunogenicity in a non-human primate. Int J Cancer. 1995;63:231–237. doi: 10.1002/ijc.2910630215. [DOI] [PubMed] [Google Scholar]
- Fulginiti VA, Papier A, Lane JM, Neff JM., and, Henderson DA. Smallpox vaccination: a review, part I. Background, vaccination technique, normal vaccination and revaccination, and expected normal reactions. Clin Infect Dis. 2003;37:241–250. doi: 10.1086/375824. [DOI] [PubMed] [Google Scholar]
- Madan RA, Arlen PM., and, Gulley JL. PANVAC-VF: poxviral-based vaccine therapy targeting CEA and MUC1 in carcinoma. Expert Opin Biol Ther. 2007;7:543–554. doi: 10.1517/14712598.7.4.543. [DOI] [PubMed] [Google Scholar]
- Park BH, Hwang T, Liu TC, Sze DY, Kim JS, Kwon HC, et al. Use of a targeted oncolytic poxvirus, JX-594, in patients with refractory primary or metastatic liver cancer: a phase I trial. Lancet Oncol. 2008;9:533–542. doi: 10.1016/S1470-2045(08)70107-4. [DOI] [PubMed] [Google Scholar]
- Timiryasova TM, Li J, Chen B, Chong D, Langridge WH, Gridley DS, et al. Antitumor effect of vaccinia virus in glioma model. Oncol Res. 1999;11:133–144. [PubMed] [Google Scholar]
- Lun XQ, Jang JH, Tang N, Deng H, Head R, Bell JC, et al. Efficacy of systemically administered oncolytic vaccinia virotherapy for malignant gliomas is enhanced by combination therapy with rapamycin or cyclophosphamide. Clin Cancer Res. 2009;15:2777–2788. doi: 10.1158/1078-0432.CCR-08-2342. [DOI] [PubMed] [Google Scholar]
- McCart JA, Puhlmann M, Lee J, Hu Y, Libutti SK, Alexander HR, et al. Complex interactions between the replicating oncolytic effect and the enzyme/prodrug effect of vaccinia-mediated tumor regression. Gene Ther. 2000;7:1217–1223. doi: 10.1038/sj.gt.3301237. [DOI] [PubMed] [Google Scholar]
- Kim JH, Oh JY, Park BH, Lee DE, Kim JS, Park HE, et al. Systemic armed oncolytic and immunologic therapy for cancer with JX-594, a targeted poxvirus expressing GM-CSF. Mol Ther. 2006;14:361–370. doi: 10.1016/j.ymthe.2006.05.008. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Yu YA, Wang E, Chen N, Danner RL, Munson PJ, et al. Eradication of solid human breast tumors in nude mice with an intravenously injected light-emitting oncolytic vaccinia virus. Cancer Res. 2007;67:10038–10046. doi: 10.1158/0008-5472.CAN-07-0146. [DOI] [PubMed] [Google Scholar]
- Hung CF, Tsai YC, He L, Coukos G, Fodor I, Qin L, et al. Vaccinia virus preferentially infects and controls human and murine ovarian tumors in mice. Gene Ther. 2007;14:20–29. doi: 10.1038/sj.gt.3302840. [DOI] [PubMed] [Google Scholar]
- Deng H, Tang N, Stief AE, Mehta N, Baig E, Head R, et al. Oncolytic virotherapy for multiple myeloma using a tumour-specific double-deleted vaccinia virus. Leukemia. 2008;22:2261–2264. doi: 10.1038/leu.2008.120. [DOI] [PubMed] [Google Scholar]
- McCart JA, Ward JM, Lee J, Hu Y, Alexander HR, Libutti SK, et al. Systemic cancer therapy with a tumor-selective vaccinia virus mutant lacking thymidine kinase and vaccinia growth factor genes. Cancer Res. 2001;61:8751–8757. [PubMed] [Google Scholar]
- Naik AM, Chalikonda S, McCart JA, Xu H, Guo ZS, Langham G, et al. Intravenous and isolated limb perfusion delivery of wild type and a tumor-selective replicating mutant vaccinia virus in nonhuman primates. Hum Gene Ther. 2006;17:31–45. doi: 10.1089/hum.2006.17.31. [DOI] [PubMed] [Google Scholar]
- Liu TC, Hwang T, Park BH, Bell J., and, Kirn DH. The targeted oncolytic poxvirus JX-594 demonstrates antitumoral, antivascular, and anti-HBV activities in patients with hepatocellular carcinoma. Mol Ther. 2008;16:1637–1642. doi: 10.1038/mt.2008.143. [DOI] [PubMed] [Google Scholar]
- Park BH, Hwang T, Liu TC, Sze DY, Kim JS, Kwon HC, et al. Use of a targeted oncolytic poxvirus, JX-594, in patients with refractory primary or metastatic liver cancer: a phase I trial. Lancet Oncol. 2008;9:533–542. doi: 10.1016/S1470-2045(08)70107-4. [DOI] [PubMed] [Google Scholar]
- Yang WQ, Lun X, Palmer CA, Wilcox ME, Muzik H, Shi ZQ, et al. Efficacy and safety evaluation of human reovirus type 3 in immunocompetent animals: racine and nonhuman primates. Clin Cancer Res. 2004;10:8561–8576. doi: 10.1158/1078-0432.CCR-04-0940. [DOI] [PubMed] [Google Scholar]
- Lun X, Alain T, Zemp FJ, Zhou H, Rahman MM, Hamilton MG, et al. Myxoma virus virotherapy for glioma in immunocompetent animal models: optimizing administration routes and synergy with rapamycin. Cancer Res. 2010;70:598–608. doi: 10.1158/0008-5472.CAN-09-1510. [DOI] [PubMed] [Google Scholar]
- Alain, T, Lun XQ, Martineau, Y, Sean, P, Pulendran, B, Petroulakis, E, et al. Vesicular stomatitis virus oncolysis is potentiated by impairing mTORC1-dependent type I IFN production. Proc Natl Acad Sci USA. 2010;107:1576–1581. doi: 10.1073/pnas.0912344107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbertson RJ., and, Rich JN. Making a tumour's bed: glioblastoma stem cells and the vascular niche. Nat Rev Cancer. 2007;7:733–736. doi: 10.1038/nrc2246. [DOI] [PubMed] [Google Scholar]
- Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- Jiang, H, Gomez-Manzano, C, Aoki, H, Alonso, MM, Kondo, S, McCormick, F, et al. Examination of the therapeutic potential of Δ-24-RGD in brain tumor stem cells: role of autophagic cell death. 2007. J Natl Cancer Inst. 99:1410–1414. doi: 10.1093/jnci/djm102. [DOI] [PubMed] [Google Scholar]
- Wakimoto H, Kesari S, Farrell CJ, Curry WT, Jr, Zaupa C, Aghi M, et al. Human glioblastoma-derived cancer stem cells: establishment of invasive glioma models and treatment with oncolytic herpes simplex virus vectors. Cancer Res. 2009;69:3472–3481. doi: 10.1158/0008-5472.CAN-08-3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currier MA, Gillespie RA, Sawtell NM, Mahller YY, Stroup G, Collins MH, et al. Efficacy and safety of the oncolytic herpes simplex virus rRp450 alone and combined with cyclophosphamide. Mol Ther. 2008;16:879–885. doi: 10.1038/mt.2008.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JG, Raper SE, Wheeldon EB, Hackney D, Judy K, Wilson JM, et al. Intracranial administration of adenovirus expressing HSV-TK in combination with ganciclovir produces a dose-dependent, self-limiting inflammatory response. Hum Gene Ther. 1997;8:943–954. doi: 10.1089/hum.1997.8.8-943. [DOI] [PubMed] [Google Scholar]
- Cello J, Toyoda H, Dejesus N, Dobrikova EY, Gromeier M., and, Wimmer E. Growth phenotypes and biosafety profiles in poliovirus-receptor transgenic mice of recombinant oncolytic polio/human rhinoviruses. J Med Virol. 2008;80:352–359. doi: 10.1002/jmv.21063. [DOI] [PubMed] [Google Scholar]
- Todo T, Feigenbaum F, Rabkin SD, Lakeman F, Newsome JT, Johnson PA, et al. Viral shedding and biodistribution of G207, a multimutated, conditionally replicating herpes simplex virus type 1, after intracerebral inoculation in aotus. Mol Ther. 2000;2:588–595. doi: 10.1006/mthe.2000.0200. [DOI] [PubMed] [Google Scholar]
- Myers R, Harvey M, Kaufmann TJ, Greiner SM, Krempski JW, Raffel C, et al. Toxicology study of repeat intracerebral administration of a measles virus derivative producing carcinoembryonic antigen in rhesus macaques in support of a phase I/II clinical trial for patients with recurrent gliomas. Hum Gene Ther. 2008;19:690–698. doi: 10.1089/hum.2008.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naganuma H, Sasaki A, Satoh E, Nagasaka M, Isoe S, Nakano S, et al. Long-term survival in a young patient with anaplastic glioma. Brain Tumor Pathol. 1997;14:71–74. doi: 10.1007/BF02478872. [DOI] [PubMed] [Google Scholar]
- Abou-Ghazal M, Yang DS, Qiao W, Reina-Ortiz C, Wei J, Kong LY, et al. The incidence, correlation with tumor-infiltrating inflammation, and prognosis of phosphorylated STAT3 expression in human gliomas. Clin Cancer Res. 2008;14:8228–8235. doi: 10.1158/1078-0432.CCR-08-1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal DT, Raizer JJ, Rosenblum MK, Bilsky MH, Hariharan S., and, Abrey LE. Primary intracranial neoplasms in patients with HIV. Neurology. 1999;52:1648–1651. doi: 10.1212/wnl.52.8.1648. [DOI] [PubMed] [Google Scholar]
- Moriyama EH, Bisland SK, Lilge L., and, Wilson BC. Bioluminescence imaging of the response of rat gliosarcoma to ALA-PpIX-mediated photodynamic therapy. Photochem Photobiol. 2004;80:242–249. doi: 10.1562/2004-02-20-RA-088. [DOI] [PubMed] [Google Scholar]
- Szentirmai O, Baker CH, Lin N, Szucs S, Takahashi M, Kiryu S, et al. Noninvasive bioluminescence imaging of luciferase expressing intracranial U87 xenografts: correlation with magnetic resonance imaging determined tumor volume and longitudinal use in assessing tumor growth and antiangiogenic treatment effect. Neurosurgery. 2006;58:365–372; discussion 365. doi: 10.1227/01.NEU.0000195114.24819.4F. [DOI] [PubMed] [Google Scholar]
- Kelly JJ, Stechishin O, Chojnacki A, Lun X, Sun B, Senger DL, et al. Proliferation of human glioblastoma stem cells occurs independently of exogenous mitogens. Stem Cells. 2009;27:1722–1733. doi: 10.1002/stem.98. [DOI] [PubMed] [Google Scholar]
- Wang L, Rahn JJ, Lun X, Sun B, Kelly JJ, Weiss S, et al. Gamma-secretase represents a therapeutic target for the treatment of invasive glioma mediated by the p75 neurotrophin receptor. PLoS Biol. 2008;6:e289. doi: 10.1371/journal.pbio.0060289. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
JX-594 and JX-594m infects and kills glioma cell lines that are resistant to other oncolytic viruses in vitro.
Five days MTT, CPE, and crystal violet staining results after JX-594 and JX-594m-infected glioma cell lines.
Combination therapy with rapamycin enhanced JX-594Fluc replication and JX-594m combined rapamycin treatment further prolonged survival in rat RG2 model.
More data about JX-594 and JX-594m safety/toxicity studies in immunocompetent animals.
Representative CD68/CD8 infiltration in the brain after JX-594/JX-594m intracranial administration.