Abstract
Adoptive transfer of antigen-specific cytotoxic T lymphocytes has shown promise for the therapy of cancer. However, tumor-specific T cells are susceptible to diverse inhibitory signals from the tumor microenvironment. The Akt/protein kinase B plays a central role in T-cell proliferation, function, and survival and we hypothesized that expression of constitutively active Akt (caAkt) in T cells could provide resistance to many of these tumor-associated inhibitory mechanisms. caAkt expression in activated human T cells increased proliferation and cytokine production, a likely result of their sustained expression of nuclear factor-κB (NF-κB) and provided resistance to apoptosis by upregulating antiapoptotic molecules. caAkt expressing T cells (caAkt-T-cells) were also relatively resistant to suppression by and conversion into regulatory T cells (Tregs). These characteristics provided a survival advantage to T cells cocultured with tumor cells in vitro; CD3/28-stimulated T cells expressing a chimeric antigen receptor (CAR) specific for disialoganglioside (GD2) that redirected their activity to the immunosuppressive, GD2-expressing neuroblastoma cell line, LAN-1, resisted tumor-induced apoptosis when co-expressing transgenic caAkt. In conclusion, caAkt-transduced T cells showed resistance to several evasion strategies employed by tumors and may therefore enhance the antitumor activity of adoptively transferred T lymphocytes.
Introduction
T lymphocytes are major effector cells in adaptive immunity against pathogens and tumor cells. Their efficacy, however, is dampened by a wide range of immune evasion mechanisms that exist in a diversity of pathogenic circumstances including tumorigenesis, when the homeostatic balance between activation and suppression of immunity strongly favors immune suppression. This imbalance allows tumors to progress even in the presence of tumor-specific T cells.
Adoptive T-cell therapy is an effective treatment for virus-associated diseases and malignancies after hematopoietic stem cell transplantation1,2,3 and for lymphoma and melanoma in immunocompetent patients.4,5,6,7 T-cell therapy has high specificity and low toxicity compared with other conventional cancer therapies, but the survival and function of adoptively transferred T cells are limited by the same immune evasion mechanisms that impede endogenous antitumor immune responses, including ineffective presentation of tumor antigens, induction of effector T-cell apoptosis,8 production of T-cell inhibitory ligands such as transforming growth factor (TGF)-β and programmed death ligand-1, and recruitment of regulatory T cells (Tregs).9,10,11,12,13,14 The efficacy of adoptively transferred T cells therefore depends on their capacity to survive and function in the presence of multiple inhibitory signals.
Investigators are exploring strategies to enable tumor-specific T cells to survive and function in an adverse tumor environment in both preclinical and clinical systems. For example, resistance to apoptosis can be accomplished by downregulation of receptors for inhibitory ligands like Fas-ligand and TGF-β15,16 or by expression of immunostimulatory cytokines including interleukin-7 (IL-7) and IL-12.17,18,19 Although each strategy has effectively circumvented the individual suppressive mechanism for which it was designed, avoidance of a single immune evasion strategy may be insufficient to ensure T-cell function, survival, and proliferation in a complex, multifactorial, inhibitory environment. We reasoned that a strategy that could render tumor-specific T cells resistant to a broader range of inhibitory mechanisms might prove more effective.
The serine/threonine kinase Akt is a major component of the phosphatidylinositol 3-kinase (PI3K) family, and is critical for cell growth and survival.20 The PI3K/Akt pathway is central to T-cell activation, acting downstream of major T-cell-activating signals including the T-cell receptor, co-stimulatory receptors, and cytokine receptors.21 Ligation of the T-cell co-stimulatory molecules, CD28 and ICOS, upregulates Akt activity by activation of tyrosine kinases,22 leading to membrane recruitment of PI3K and Akt. Conversely, the Akt pathway is a central node downstream of multiple co-inhibitory receptors, including cytotoxic T lymphocyte antigen-4 and Programmed Death-1 that oppose the activation of Akt by counteracting tyrosine phosphatases.23 PI3K/Akt activation is reported to suppress several apoptotic mechanisms in T cells24,25 and the activation status of the PI3K/Akt pathway in T effector cells is a primary determinant of T effector sensitivity to a number of inhibitory factors, including TGF-β and Tregs.24,25,26 Thus, Akt targeting could both enhance activating stimuli and provide resistance to inhibitory signals.
Expression of a highly potent, constitutively active Akt (caAkt; originally called MF-ΔAkt) was previously shown to promote the survival of dendritic cells by upregulating Bcl-2 as well as promoting activation and maturation.23,27 Here, we show that caAkt increases nuclear factor-κB (NF-κB) activity in human T cells, and promotes their proliferation, survival, and resistance to multiple inhibitory factors commonly utilized by tumors. caAkt-transduced T cells were also resistant to suppression by Tregs and to conversion into Tregs by TGF-β. Finally, co-expression of caAkt with a chimeric antigen receptor (CAR) for disialoganglioside (GD2) expressed on neuroblastoma, enhanced T-cell activation, and proliferation in response to and cytotoxicity against this immunosuppressive tumor.
Results
Transduction of T cells with constitutively active Akt
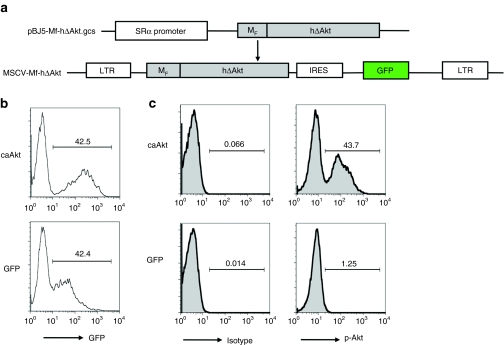
We transduced CD3/CD28 antibody–stimulated T cells with the retrovirus vector MSCV-MF-hΔAkt-IRES-GFP (caAkt), encoding constitutively active Akt as illustrated in Figure 1a. As determined by co-expression of GFP, 38.7% (34.2–45.7%, n = 7) cells were transduced with caAkt. Representative histograms of caAkt- and GFP control (control)–transduced T cells are shown in Figure 1b. Both CD4 and CD8 T cells were efficiently transduced (data not shown). To confirm the activation state of caAkt, we used intracellular fluorescence-activated cell-sorting analysis with an antibody specifically binding to the Akt phosphorylation site (S473). The percentage of T cells expressing phosphorylated Akt (pAkt) was consistent with GFP expression in caAkt-transduced T cells as 35.3–45% of the caAkt was phosphorylated, compared to ~1% controls (Figure 1c). These results indicated that the transduced caAkt gene was expressed in its active (phosphorylated) state in transduced T cells.
Figure 1.
caAkt can be expressed in activated T cell after retroviral transduction. (a) The schematics of retroviral vector expressing ΔAkt (MSCV.MF-hΔAkt.I.GFP). MF-hΔAkt fragment was cloned into an MSCV retroviral vector co-expressing GFP. (b) Human peripheral blood mononuclear cells (n = 7) were stimulated with CD3/CD28 antibodies and transduced with retrovirus expressing caAkt or GFP control. Five days later, transduction efficiencies were detected by GFP co-expression. Numbers indicate the percentage of GFP+ cells. (c) Phospho Akt (S473) expressions were determined by intracellular fluorescence-activated cell-sorting analysis. caAkt, constitutively active Akt; GFP, green fluorescent protein; IRES, internal ribosome entry site; LTR, long terminal repeat; MSCV, murine stem cell virus; MF, Fyn myristoylation-targeting sequence; hΔAkt, human truncated Akt sequence.
Increased expansion and selection of caAkt-transduced T cells
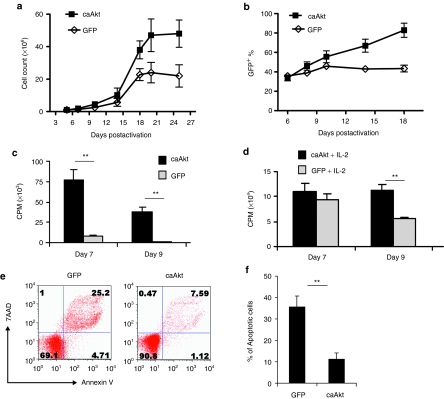
To determine whether caAkt influenced T-cell growth, we cultured caAkt-transduced T cells in the presence of IL-2 (50 U/ml) and measured cell numbers over time. T cells expressing caAkt demonstrated greater numerical expansion than control-transduced T cells after 3 weeks of culture (mean 47 × 106 versus 24 × 106 T cells from 1 × 106 starting T cells). Without additional stimulation, caAkt-T-cells did not proliferate further, but maintained their viability, whereas control-T-cell numbers and viability declined (Figure 2a).
Figure 2.
Increased expansion and selection of caAkt-transduced T cells are due to enhanced proliferation and decreased apoptosis. (a) caAkt- and control-transduced T cells were cultured in the presence of IL-2 (50 U/ml), and cell numbers were counted at different time points as indicated; P = 0.04. (b) The percentages of transduced T cells were determined by fluorescence-activated cell-sorting analysis based on GFP expression; P = 0.01. (c,d) Activated T cells were transduced with caAkt or GFP control vector. At 7 and 9 days after CD3/CD28 antibody activation, cell proliferation was measured by 3H-thymidine incorporation in (c) absence or (d) presence of IL-2. (e) At 4 weeks after transduction, cell apoptosis was determined by staining with annexin V and 7AAD. Percentages of cells are indicated in each quadrant. (f) The percentages of annexin V+ caAkt or GFP control cells are shown as mean percent ± SD (n = 5), **P < 0.01. caAkt, constitutively active Akt; CPM, counts per minute; GFP, green fluorescent protein; IL-2, interleukin-2; 7AAD, 7-aminoactinomycin D.
To examine whether caAkt gene expression was stable over time, we analyzed the frequency of GFP-expressing cells and found selective expansion of caAkt-transduced T cells from 35% (range 31–39%) to 83% (range 76–91%) over the first 2 weeks of culture, while there was no selection of GFP+ cells in control cultures (Figure 2b, P = 0.01). Hence, caAkt-transduced T cells had a selective growth/survival advantage in culture.
Proliferation is enhanced and apoptosis is reduced in caAkt-transduced T cells
The selective expansion of caAkt-T-cells may be due to an increased rate of T-cell division and/or reduced apoptosis. We measured proliferation and apoptosis of transduced T cells at early (7–14 days) and late (4–5 weeks) time points. Early on, there were few apoptotic cells in either group, while caAkt-T-cells demonstrated a significantly higher rate of cell division in the absence or presence of IL-2 as measured using tritiated thymidine uptake (Figure 2c,d). The difference was even more marked in the cultures without IL-2 supplementation (Figure 2c). Four to five weeks later, the proliferation of both groups of transduced T cells dropped to basal levels (data not shown) but caAkt-T-cells had superior survival. Apoptosis as determined by surface staining with 7-aminoactinomycin D and annexin V was markedly higher in control-T-cells than in caAkt-T-cells (35.6 ± 5.2% versus 11.1 ± 2.9%, n = 5, P < 0.01; Figure 2e,f). In addition to proliferation and apoptosis, we evaluated telomere length in caAkt-T-cells using the Q-FISH and qPCR methods (Supplementary Materials and Methods) and found that telomeres were longer than in control-T-cells (Supplementary Figure S1a,b). Consistent with increased telomere length, caAkt-T-cells possessed stronger telomerase activity than control-T-cells (Supplementary Figure S1c). In summary, caAkt-T-cells had increased proliferation shortly after activation, maintained cell survival for significantly longer than control-T-cells but did not proliferate autonomously in the absence of prosurvival cytokines (Supplementary Figure S2).
caAkt expression upregulates antiapoptotic molecules
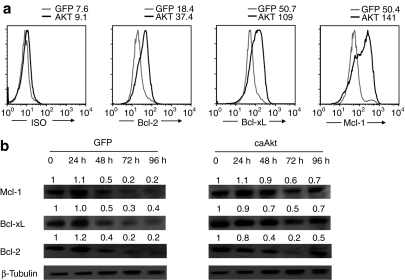
To determine the mechanism underlying decreased apoptosis in caAkt-expressing T cells, we examined the expression of antiapoptotic members of the Bcl-2 family that act downstream of Akt. T cells were harvested 3–4 weeks after transduction and cultured without IL-2 for 5 days. As shown before, the percentage of apoptotic cells was significantly higher in control-T-cells than in caAkt-T-cells. Increased levels of Bcl-2, Bcl-xL, and Mcl-1 expression were detected by intracellular staining (Figure 3a), and confirmed by western blot analysis at multiple time points (Figure 3b). The antiapoptotic molecules (especially Mcl-1 and Bcl-xL) were maintained at higher levels in caAkt-T-cells than in control-T-cells. The upregulation of these antiapoptotic molecules likely contributes to the longevity of T cells transduced with caAkt.
Figure 3.
caAkt-transduced T cells upregulated antiapoptotic molecules. Four weeks after transduction, caAkt- or GFP-control-transduced T cells were harvested and cultured in interleukin-2 (IL-2)–depleted medium for 5 days. (a) Intracellular expression of Bcl-2, Bcl-xL, Mcl-1 in caAkt-T-cells (black line) and GFP control (gray line) was determined by flow cytometry. Isotype controls are shown in parallel. (b) caAkt- or GFP-control-transduced T cells were cultured in absence of IL-2 for the indicated hours and analyzed for expression of Bcl-2, Bcl-xL, Mcl-1 by western blot. Data are representative of three experiments. caAkt, constitutively active Akt; GFP, green fluorescent protein.
caAkt-expressing T cells secreted larger amounts of IL-2 and IFN-γ and maintained higher NF-κB activity
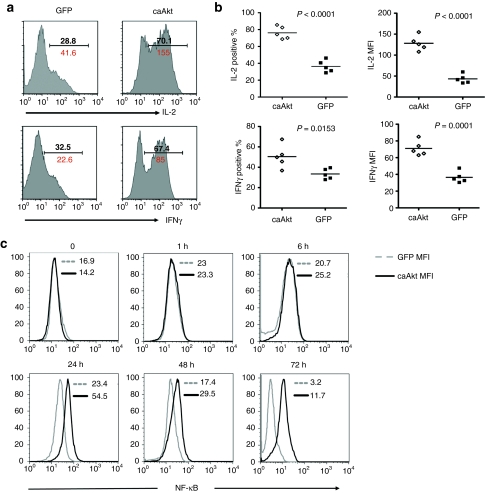
To evaluate the effects of caAkt on cytokine production, we measured production of various cytokines in response to T-cell receptor stimulation. Figure 4a,b shows that 2 weeks after transduction, caAkt-expressing T cells produced significantly more IL-2 and interferon-γ (IFN-γ) as measured by intracellular staining analysis. There were comparable low levels of IL-4, IL-10, IL-17 (<2%) in both caAkt-T-cells and control-T-cells (data not shown). Therefore, the production of Th1 cytokines dominates in caAkt-T-cells and should favor the cytotoxic function of Th1 cells.
Figure 4.
caAkt-transduced T cells have increased IL-2 and IFN-γ production and sustained NF-κB activity. (a) caAkt- and GFP-control-transduced T cells were cultured for 2 weeks and cytokine productions were detected by intracellular staining. A representative histogram is shown from five donors. (b) The percentages and MFIs of IL-2 and IFN-γ positive cells in caAkt-T-cells (open diamonds) and GFP control-T-cells (closed squares) are shown. (c) Intracelluar phospho NF-κB p65 (pS529) levels were determined in caAkt- (black lines) or GFP (gray lines)-transduced T cells at indicated hours. Data are representative of three donors. caAkt, constitutively active Akt; GFP, gree fluorescent protein; IFN-γ, interferon-γ IL-2, interleukin-2; MFI, mean fluorescence intensity; NF-κB, nuclear factor-κB.
To characterize events downstream of Akt activation, we utilized Phosflow to detect the kinetics of NF-κB activation (Figure 4c). Both control-T-cells and caAkt-T-cells upregulated p65-NF-κB to comparable levels at 15 minutes to 6 hours after phorbol myristate acetate stimulation (from 15 minutes to 6 hours). However, p65-NF-κB expression was greater in caAkt-T-cells after 24 hours; and by 72 hours while there was no detectable p65-NF-κB activity in control-T-cells in the absence of cytokines, caAkt-T-cells maintained NF-κB levels. Thus, caAkt induced and maintained NF-κB activity, which likely contributes to T-cell survival and cytokine production.
caAkt renders effector T cells resistant to suppression by Tregs
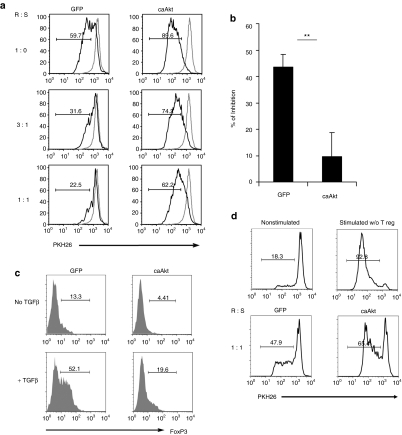
Tregs infiltrate most tumors and add their inhibitory effects to those of the tumor cells themselves. Because Tregs inhibit T effector cells through Akt-regulated pathways,28 we determined whether T cells expressing caAkt could resist Treg inhibition. We transduced peripheral blood mononuclear cells (PBMCs) from four healthy donors with caAkt or GFP, as described previously.15 CD4+CD25+ Tregs were isolated from autologous fresh PBMC using a Treg selection kit (Miltenyi Biotec, Bisley, UK). At 10 days after transduction, caAkt- and control-T-cells were labeled with the lipophilic dye, PKH26 Red, to allow cell division to be measured by flow cytometry, and cultured with or without autologous CD4+CD25+ Treg cells at different ratios in the presence of allogeneic PBMC and OKT3 MAb (500 ng/ml). Five days later, the proliferation of responder T cells was assessed from their PKH dye intensity. Compared with GFP-T-cells, caAkt-T-cells were resistant to Treg-mediated suppression and continued to proliferate as illustrated in Figure 5a. At a 3:1 ratio of responder T cells to Tregs, GFP-T-cells were inhibited by 43.6% (range 42.2–49.1%), compared to only 9.6% (range 0–18.3%) in caAkt-T-cells (Figure 5b, P < 0.01). These data directly illustrate the ability of caAkt to control the sensitivity of effector T cells to Treg suppression.
Figure 5.
caAkt rendered effector T cells more resistant to the suppression by and conversion to T regulatory cell. (a) CD4+CD25+ T regulatory cells were isolated from fresh peripheral blood mononuclear cell (PBMC). Suppression assays were performed by using PKH26-labeled-transduced T cells as responder cells. 1 × 106/ml PKH26-labeled responders and suppressor cells were plated at different ratios as indicated in the presence of 2 × 106/ml allogeneic 30 Gy irradiated PBMCs and 500 ng/ml OKT3. A representative histogram is shown. (b) The percentages of inhibition are shown at the ratio of responder to suppressor as 3:1, n = 4, **P < 0.01. (c) caAkt- or GFP-control-transduced T cells were restimulated with anti-CD3/CD28 in presence or absence of 2.5 ng/ml TGFβ. FoxP3 staining was performed 14 days after restimulation. FoxP3 expressions are shown in histogram. (d) Suppression assays were performed using PKH26-labeled autologous PBMC as responders and TGFβ-treated caAkt or GFP control-T-cells as suppressors. Responder cells were cocultured with suppressors at a 1:1 ratio. Data are representative of four donors. Cell division is illustrated by the density of PKH26 dye. caAkt, constitutively active Akt; GFP, green fluorescent protein; TGFβ, transforming growth factor-β.
caAkt provides resistance to TGF-β-mediated Treg conversion
In addition to their susceptibility to suppression by Tregs, effector T cells are also susceptible to conversion into Treg cells at tumor sites by mechanisms involving TGF-β.29 The Akt-mTOR axis has been reported to regulate FoxP3, the key transcription factor of Tregs.30,31 We therefore investigated the effect of caAkt activity on the induction of Tregs by TGF-β. Transduced CD3/28-stimulated T cells were restimulated on day 14 with anti-CD3/CD28 in the presence of TGF-β (2.5 ng/ml) and IL-2 (50 U/ml). Two weeks after reactivation, we observed significant elevations of FoxP3+ T cells in both caAkt- and control-T-cells cultured with TGF-β. However, the frequency of FoxP3+ T cells was markedly lower in caAkt-T-cells than in control-T-cells (4.8 ± 2.1% versus 14.5 ± 4.3% without TGF-β and 17 ± 6.5% versus 46.9 ± 8.6% with TGF-β, n = 4, P < 0.01). A representative histogram is shown in Figure 5c. To determine whether the induced FoxP3+ T cells were suppressive, we used TGF-β-treated T cells as suppressor cells (as there is no appropriate selection marker for these FoxP3+ T cells) and autologous PBMC as responders. TGF-β-activated caAkt-T-cells showed decreased suppressive capacity relative to TGF-β-activated control-T-cells (Figure 5d), which is consistent with their reduced expression of FoxP3. These data indicate that caAkt provides resistance to FoxP3+ Treg induction/conversion so that TGF-β-cultured caAkt-T-cells are less suppressive.
We also determined whether Akt overexpression inhibits natural Treg function and FoxP3 expression; CD4+CD25+ T cells were purified from buffy coats and stimulated with anti-CD3 plus allogeneic PBMCs, then transduced with MSCV-MF-hΔAkt-IRES-GFP or control vector MSCV-IRES-GFP. caAkt-T-cells expressed less FoxP3 (35.2 ± 9.4% versus 61.5 ± 10.7%, n = 4, P < 0.05) and elevated levels of IL-2 (68.3 ± 5.8% versus 41.6 ± 8.1%, P < 0.01) compared to control-T-cells. Representative data are shown in Supplementary Figures S3 and S4a. Consistent with these findings, caAkt-transduced Tregs lost their suppressive capacity (Supplementary Figure S4b). Thus, transgenic caAkt can both enhance effector T cells and suppress the function of pre-existing Tregs.
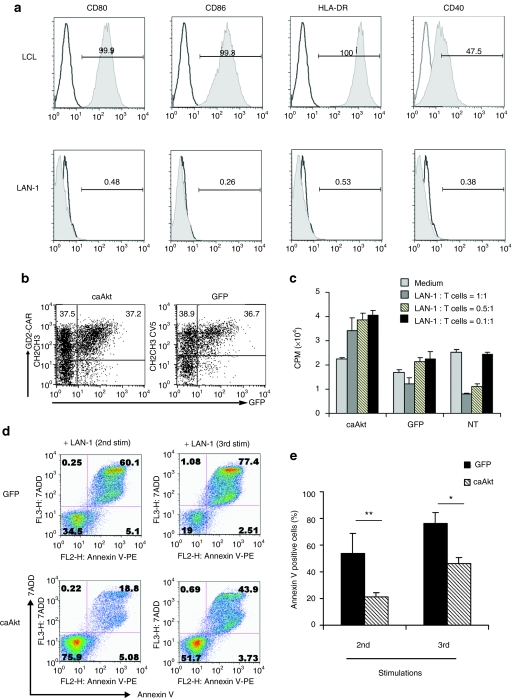
caAkt increases the survival and proliferation of tumor-specific T cell on exposure to tumor
To determine whether constitutive Akt expression increases survival of tumor-specific T cells in a tumor model, we redirected the antigen specificity of T cells to the GD2 that is expressed on neuroblastoma cells by expression of a CAR. The GD2-CAR was generated by joining the heavy- and light-chain variable regions of a GD2-specific monoclonal antibody expressed as a single-chain Fv molecule, to the cytoplasmic CD3-ζ of the T-cell receptor signaling domain.32 We used the LAN-1 neuroblastoma cell line that highly expresses GD2, but lacks co-stimulatory and MHC class II molecules (Figure 6a) and actively inhibits the proliferation of GD2-CAR expressing T cells.33 We cotransduced caAkt or control GFP-vector with the GD2-CAR into T cells and cocultured them with irradiated LAN-1 neuroblastoma cells. As shown in Figure 6b, GD2-CAR and caAkt (or GFP control) could be co-expressed in T cells at comparable levels. Coculture with LAN-1 cells at different ratios for 48 hours suppressed control-T-cell proliferation in a dose-dependent manner as measured by tritiated thymidine assays. This is likely due to stimulation through the ζ chain of the TCR in the absence of co-stimulation. Compared with control-T-cells, caAkt/GD2-CAR-cotransduced T cells showed greater proliferation and resisted LAN-1-mediated suppression (Figure 6c). To begin to understand the mechanism of T-cell suppression by LAN-1 and the resistance provided by caAkt, we measured GD2-CAR transduced T-cell apoptosis by 7-aminoactinomycin D and annexin V staining after weekly stimulation by LAN-1. caAkt GD2-CAR-cotransduced T cells had greater resistance to multiple LAN-1 stimulations than GFP/GD2-CAR-cotransduced T cells (21 ± 3.3% versus 54 ± 14.5% apoptosis after the 2nd stimulation, 46 ± 4.5% versus 77 ± 7.6% after 3rd stimulation, n = 5, P < 0.05) as illustrated in Figure 6d,e. These data indicate that co-expression of caAkt supports the survival of tumor-antigen-specific T cells in the presence of LAN-1 neuroblastoma cells.
Figure 6.
Cotransduction of caAkt increases tumor-specific T-cell proliferation and reduces apoptosis in a neuroblastoma model. (a) Surface staining of neuroblastoma LAN-1 cells with CD80, CD86, HLA-DR, and CD40. Lymphoblastoid cell line (LCL) was used as a positive control. (b) At 5 days after cotransduction, the expressions of GD2-CAR and caAkt/GFP were detected by flow cytometry. (c) Cotransduced T cells were cultured with LAN-1 cells at indicated ratios. Proliferation was determined by 3H-thymidine incorporation. (d) Cotransduced T cells were stimulated weekly with 80 Gy irradiated LAN-1 cells. After 2nd and 3rd stimulation, cell apoptosis was determined by staining with annexin V and 7AAD on gated T cells. Percentages of cells are indicated in each quadrant. (e) The percentages of annexin V+ caAkt- or GFP-control-cotransduced cells are shown as mean percent ± SD, n = 5, *P < 0.05, **P < 0.01. caAkt, constitutively active Akt; GFP, green fluorescent protein; 7AAD, 7-aminoactinomycin D.
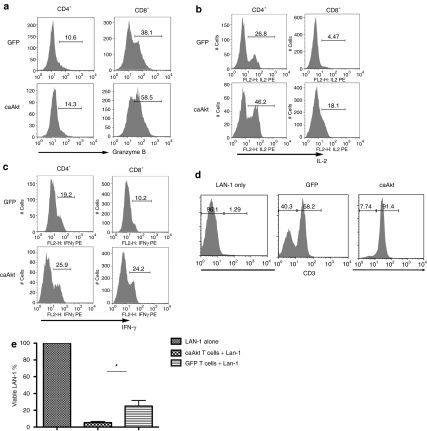
caAkt-GD2-CAR-cotransduced T cells produce more Th1 cytokines and granzyme B and have increased cytotoxic activity against LAN-1 neuroblastoma cells
As caAkt-cotransduced GD2-specific T cells maintained higher viability during their encounters with tumor cells, we next investigated their effector functions after these tumor cocultures. We detected higher granzyme B production in caAkt-cotransduced T cells, particularly in the CD8+ subset (Figure 7a), as well as higher levels of IL-2 and IFN-γ (Figure 7b,c). To evaluate the cytotoxicity of caAkt-transduced T cells, we cocultured them with LAN-1. In control-T-cell cultures, LAN-1 cells represented over 40% of the total cell number after 5 days, but were only 8% in cocultures with caAkt-cotransduced T cells (Figure 7d). caAkt-cotransduced T cells eliminated LAN-1 cells more efficiently than GFP control cells (5.33 ± 1.4% versus 25.03 ± 6.7% LAN-1 remaining in the culture, n = 4, P < 0.05; Figure 7e). Altogether, caAkt-cotransduced tumor-specific T cells retained higher activity than control-transduced T cells on exposure to tumor.
Figure 7.
caAkt-cotransduced T cells produce more Th1 cytokines and granzyme B and increase cytotoxic function to LAN-1 neuroblastoma cells. T cells were cotransduced with GD2-CAR and caAkt (or GFP control) vectors. Transduced cells were cocultured with LAN-1 cells for 7 days. The expressions of (a) granzyme B and (b,c) cytokines were detected by intracellular staining. (d) caAkt/GD2-CAR- or GFP/GD2-CAR-transducted cells were sorted by GFP expression. Sorted GFP+ cells were cocultured with LAN-1 cells at a 5:1 ratio for 4–5 days. The percentages of live LAN-1 and T cells were detected by CD3 staining. LAN-1 cells were CD3− and T cells were CD3+. (e) Remaining live LAN-1 cells were normalized to the cell numbers in the culture with LAN-1 cell alone. The data are shown as mean percent ± SD, n = 4, *P < 0.05. caAkt, constitutively active Akt; GFP, green fluorescent protein.
Discussion
We have demonstrated that a constitutively active Akt provides T cells with resistance to multiple distinct tumor immune inhibitory strategies. Both proliferation and survival of caAkt-expressing T cells were increased, an effect that was particularly evident under conditions of cytokine deprivation, and on exposure to TGF-β and Tregs. These characteristics could be explained by increased NF-κB activity, which upregulated the antiapoptotic molecules, Bcl-2, Bcl-xL, and Mcl-1 and the Th1 cytokines IL-2 and IFN-γ after stimulation. Finally, T cells co-expressing caAkt with a chimeric tumor antigen receptor had superior survival and function in the presence of an immunosuppressive neuroblastoma cell line. Despite their enhanced proliferation and effector functions, T cells expressing caAkt did not grow autonomously, but required continued stimulation with cytokines and antigen. However, to ensure the safety of this strategy, it would be possible to co-express suicide genes34,35 or to use an inducible caAkt construct that is expressed only in presence of 4-hydroxytamoxifen (data not shown). Thus, forced expression of constitutively active Akt may replace a multiplicity of other manipulations of tumor-specific T cells intended as countermeasures for the broad range of immune evasion strategies employed by human tumors.
Potentially immunogenic tumors have evolved an array of immune evasion strategies that may present tumor antigens in an inhibitory context or directly or indirectly modulate effector T-cell induction and function. Several strategies to overcome specific tumor-mediated effects have been developed, such as expression of dominant-negative receptors for inhibitory molecules like TGF-β, short-hairpin RNA for the apoptotic receptor Fas and prosurvival cytokines to maintain T-cell proliferation and disrupt the inhibitory tumor environment.15,16,17 Because most tumors use multiple T-cell evasion strategies, single genetic modifications might have limited overall effect. For example, Hodgkin lymphoma cells express the death-inducing molecules, RCAS-1 and Fas-ligand,36 secrete TGF-β, and produce TARC and IL-1337,38 that, respectively, recruit and promote the growth of Th2 and Tregs.39 Downstream substrates of the PI3K/Akt pathway regulate diverse signaling including T-cell growth, proliferation, and survival, and Akt activation levels determined the fate of T cells in the presence of Treg cells and TGF-β. Thus, increased Akt activity would be expected to improve T-cell resistance to multiple inhibitory mechanisms. To test this hypothesis, we used an optimized caAkt construct that had been previously used to transduce both mouse and human dendritic cells, that gained enhanced longevity and increased T-cell stimulatory capacity.27
caAkt-expressing T cells had both an increased growth rate and reduced apoptosis, consistent with the established roles of Akt activation in cell proliferation and survival. Akt is a central node in downstream signaling by cytokine receptors, co-stimulatory molecules, and the T-cell receptor itself. Akt can stimulate proliferation through multiple downstream targets including p27kip1, p21, GSK3, and mTORC1 that affect cell cycle regulation.20 One of its best-conserved functions is its ability to promote cell survival by blocking the function of proapoptotic proteins, including BAD, which binds to and inactivates prosurvival Bcl-2 family members.20,25 Akt activation upregulated Bcl-2 family members in primary dendritic cells, the Jurkat T-cell line and in tumor cell lines. In this study, we showed that the expression of Bcl-2 family members (Bcl-2, Bcl-xL, and Mcl-1) was upregulated in caAkt-transduced primary human T cells, providing significant survival benefits. caAkt also blocks FOXO-mediated transcription of target genes that promote apoptosis and cell cycle arrest. However, Akt-mediated phosphorylation of FOXO proteins occurs in the nucleus, so this mechanism is unlikely to explain the activity of our Fyn-myristoylated caAkt construct, which is targeted to lipid rafts.27 Akt is also responsible for phosphorylation of the enzyme telomerase that is responsible for increasing telomere length and cell longevity.40 We evaluated telomere length in caAkt-transduced T cells and found that they were indeed longer than those of control-transduced T cells, which could also explain their prolonged survival (Supplementary Figure S1). Of note, the preferential expansion of caAkt-expressing T cells was not observed under conditions in which potent antigen presentation and co-stimulation were supplied, for example by Epstein–Barr virus–transformed B-cell lines (data not shown). Hence, caAkt activity can compensate for suboptimal T-cell activation signals (such as in the tumor microenvironment) but did not improve the function of T cells receiving adequate stimulation. GFP control/GD2-CAR–cotransduced T cells were suppressed on receiving stimulation through the CAR by LAN-1 neuroblastoma cells in the absence of co-stimulation, whereas caAkt/GD2-CAR-cotransduced T cell activity could overcome this lack of co-stimulation, likely through activation of signals downstream of co-stimulatory and cytokine receptors and by upregulation of antiapoptotic molecules.
Tregs suppress T effector cells by attenuation of the PI3K/Akt pathway in antigen-primed T cells,28 and the activation status of the PI3K/Akt pathway in T effector cells is a primary determinant of sensitivity to Tregs.26 In several genetically engineered murine models (cbl-b−/−, Traf6−/−, dominant-negative TGF-β receptor) of spontaneous autoimmunity, CD4+CD25− T effector cells are resistant to Treg-mediated suppression, even though Tregs are functionally normal.26 A characteristic common to all of these models is their increased activity of the PI3K/Akt pathway, a concept that is confirmed in murine Akt transgenic T cells26 and in our study by directly expressing caAkt in human T cells. Therefore, caAkt-expressing tumor-specific T cells may maintain their activity in tumors despite the presence of infiltrating Tregs.
Inducible Tregs are generated by T-cell stimulation in the presence of TGF-β, and this cytokine severely affects the efficacy of tumor-specific effector T cells. TGFβ is produced by Tregs and by most tumors and plays a role in T-cell homeostasis by limiting immune responses to antigens and inducing tolerance.41,42 Haxhinasto et al. identified Akt as a strong repressor of entry into the Treg phenotype in vitro and in vivo.31 Likewise, pharmacological inhibitors of the PI3K/Akt pathway inhibited mTOR, a downstream target of Akt signaling increased FoxP3 induction in TCR-primed T cells,30 and enhanced Treg expansion.43 Our own data support these observations, as Treg induction by TGF-β was significantly lower in caAkt-transduced T-cells. caAkt also inhibits FoxP3 expression and the suppressive function of natural Tregs (freshly isolated CD4+CD25+ T cells), which is consistent with a previous study using an inducibly active form of Akt.44 Of note, these results are in apparent conflict with a recent report, which showed that Akt signals enhanced FoxP3 induction and the acquisition of Treg phenotypes in a murine transgenic model.45 The difference might be due to the level and localization of Akt activity in the Akt transgenic model versus our retrovirally transduced dual-acylated, lipid-raft-targeted Akt in human T cells.
These findings have implications for adoptive T-cell therapy for malignancy. First, tumor-antigen-specific T cells receive limited co-stimulatory signals from tumor cells that might be compensated by the enforced expression of caAkt. Second, multiple inhibitory factors utilized by tumor immune evasion negatively regulate PI3K/Akt signaling pathway, and enforced constitutively active Akt expression will confer effector T-cell resistance. Additionally, adoptively transferred T cells should resist conversion into Tregs in situ, an important tumor evasion mechanism.46 Third, PI3K inhibitors have been developed for the treatment of some tumors. The nonselective nature of this treatment would also inhibit the immune system, negating any immune-mediated antitumor effects from therapy. Adoptive transfer of PI3K inhibitor–resistant, tumor-specific T cells in combination with PI3K inhibitors could be complementary. Thus, our findings support exploration of caAkt transduction to safely and broadly enhance the antitumor activity of adoptively transferred, tumor-directed, T lymphocytes.
Materials and Methods
Plasmids and retrovirus vectors. Preparation of the expression plasmid, pBJ5/MF-ΔhAkt, encoding human caAkt has been described previously.27 caAkt was created by fusing myristoylation-targeting sequences from Fyn kinase to human Akt in which the pleckstrin homology domain was truncated to improve its activity (denoted “ΔAkt”). A Fyn myristoylation-targeting sequence (MF) was used to target Akt to lipid rafts, where much of its effector function is realized.47 MF sequence showed the most efficient lipid-raft localization, NF-κB induction, and Akt-S473 phosphorylation in the membrane-targeting sequences tested.27 MF-hΔAkt was cloned into the SacII and EcoRI sites of the retroviral vector murine stem cell virus (MSCV), encoding the internal ribosome entry site (IRES) followed by the enhanced green fluorescence protein sequence (Figure 1a) to produce MSCV-MF-hΔAkt-IRES-GFP (caAkt). The retrovirus vector MSCV-IRES-GFP (GFP) was used as a control. The generation of GD2-CAR retroviral vector has been described previously.33 Briefly, the ectodomain is a single-chain variable fragment derived from a GD2)-binding monoclonal antibody, 14g2a. The endodomain transmits intracellular signals through the T-cell receptor ζ chain.
Cell lines and cell culture. Donor blood was obtained either from the Gulf Coast Blood Center, Houston, Texas or from healthy volunteers with informed consent from the Baylor College of Medicine institutional review board. After centrifugation on Ficoll-Hypaque density gradients (Sigma-Aldrich, St Louis, MO), PBMCs were cultured in complete media (45% RPMI 1640; Hyclone, Logan, UT, 45% EHAA; Irvine Scientific, Santa Ana, CA) supplemented with 2 mmol/l -glutamine (GlutaMAX-I; Invitrogen, Carlsbad, CA) and 10% fetal bovine serum (Hyclone). 293T and LAN-1 cells were obtained from the American Type Culture Collection (Rockville, MD) and maintained in culture with Dulbecco's modified Eagle's medium (BioWhittaker, Walkersville, MD) supplemented with 10% fetal bovine serum, 2 mmol/l -glutamine.
Production of retrovirus and transduction of T cells. Transient retroviral vector supernatant was produced by co-transfection of 293T cells with the gag/pol expression plasmid PegPam3(-env), the RD114 env expression plasmid RDF and MSCV vectors at a ratio of 2:3:3, respectively, using GeneJuice transfection reagent (Calbiochem, Gibbstown, NJ) as previously described.32 PBMCs were stimulated on non-tissue-culture-treated 24-well plates coated with 1 µg/ml OKT3 (Ortho Biotech, Bridgewater, NJ) and 1 µg/ml anti-CD28 (Becton Dickinson, Franklin Lakes, NJ) antibodies in the presence of recombinant human IL-2 (50 IU/ml, Proleukin; Chiron, Emeryville, CA) for 2 days. For T-cell transduction, 24-well plates were coated with RetroNectin (TaKaRa Shuzo, Shiga, Japan) according to the manufacturer's protocol, and stored at 4 °C. The following day, the plates were coated twice with 0.5 ml retroviral vector supernatant, each added for 30 minutes at 37 °C. A total of 5 × 105 OKT3-stimulated T cells in 0.5 ml medium were mixed with 1.5 ml retroviral vector supernatant and transferred to the precoated wells. Recombinant IL-2 (100 U/ml) was added and the plates were incubated at 37 °C for 2 days. Then, T cells were transferred to a tissue culture–treated plate. IL-2 (50 U/ml) was added twice weekly.
Flow cytometry. Transduced or nontransduced T cells were stained with phycoerythrin (PE)-, peridinin chlorophyll protein-, or allophycocyanin-conjugated CD3, CD4, CD8, CD16, CD19, CD27, CD28, CD45RO, and CCR7 monoclonal antibodies. All monoclonal antibodies were purchased from Becton Dickinson. Control samples labeled with an appropriate isotype-matched antibody were included in each experiment. To detect FoxP3 protein expression, the surface-stained cells were further subjected to intracellular staining with PE-conjugated monoclonal antibodies to human FoxP3 (clone PCH101; eBioscience, San Diego, CA) using staining buffers and conditions specified by the manufacturer. For intracellular cytokine staining, cells were stimulated for 6 hours in the presence of 50 ng/ml phorbol myristate acetate, 500 ng/ml ionomycin (Sigma-Aldrich), and 2 µmol/l monensin (GolgiStop; BD Biosciences, San Jose, CA). and incubated with peridinin chlorophyll protein–conjugated anti-CD4 and allophycocyanin-conjugated anti-CD8 (RPA-T8) antibodies for surface staining followed by intracellular staining using PE-conjugated anti-mouse IFN-γ (4S.B3) or PE-conjugated anti-mouse IL-2 (MQ1-17H12) antibodies and FIX/PERM buffers (BD Pharmingen, San Diego, CA) according to the manufacturer's instructions. For pAkt and pNF-κB (p-P65) intracellular staining, cells were first fixed with 1% prewarmed paraformaldehyde for 10 minutes at 37 °C and then permeabilized with 90% prechilled methanol for 30 minutes on ice; finally the cells were stained with Alexa Fluor(R) 647-conjugated anti-Akt (Ser473) antibodies (Cell Signaling Technology, Danvers, MA) or PE-conjugated anti-P65 and the matched isotype control (BD Pharmingen) for 30 minutes at room temperature. Cells were analyzed by a FACSCalibur equipped with the filter set for four fluorescence signals using Cell Quest software (Becton Dickinson).
The expression of antiapoptotic molecules was detected by intracellular staining. Cells were harvested and counted. A density of 2 × 106 cells were washed with fluorescence-activated cell-sorting buffer and resuspended in 200 µl 4% paraformaldehyde on ice to for 10 minutes for fixation. Cells were washed and permeabilized in 1% saponin for 30 minutes on ice. Then, primary antibodies Bcl-2, Bcl-xL (Cell Signaling Technology), and Mcl-1 (Santa Cruz, Santa Cruz, CA) were added and incubated on ice for 30 minutes. Cells were washed with 0.1% saponin and incubated with secondary antibody conjugated with PE for 30 minutes at 4 °C in the dark. Then, cells were analyzed by flow cytometry.
Cell selection. CD4+CD25+ Treg cells were selected from fresh PBMCs using a Treg selection kit (Miltenyi Biotec). CD4+ T cells were negatively selected using Miltenyi Mini-MACS column according to the manufacture's instructions. CD4+ T cells were then incubated with CD25-microbeads for 15 minutes at 4 °C. CD4+CD25+ T cells were positively selected, then cultured in 24-well plate precoated with antiCD3/CD28 antibodies and supplemented with IL-2 (100 U/ml).
Proliferation assay. Transduced T cells were collected from 24-well culture plates then incubated at 1 × 105/well in 96-well U-bottomed plates with or without IL-2 (50 U/ml). Wells were pulsed with 0.037 Mbq (1 µCi)/well of [3H]thymidine (Amersham Pharmacia Biotech, Uppsala, Sweden) for 18 hours, and the samples were harvested onto glass fiber filter paper for β-scintillation counting (TriCarb 2500 TR; Packard BioScience, Waltham, MA).
Suppression assay. To analyze cell division, responder T cells were labeled with 2 µmol/l of the lipophilic dye, PKH26 (Sigma-Aldrich), for 5 minutes at room temperature in phosphate-buffered saline with 0.1% bovine serum albumin. The reaction was quenched with RPMI 1640 medium with 10% fetal bovine serum for 10 minutes at 37 °C. A density of 1 × 106/ml PKH26 labeled responders and suppressor cells at different ratios as indicated were plated into 96-well plates in the presence of 2 × 106/ml allogeneic 30 Gy irradiated PBMCs and 500 ng/ml OKT3 in T-cell complete medium (total volume is 0.2 ml/well). After 5 days, the cells were harvested, and analysis of cell division was performed by flow cytometry.
Western blot analysis. Transduced T cells were cultured in medium without IL-2 for the indicated time points. The cells were harvested and lysed. The lysates were collected and quantified for protein content. Equal amounts of proteins (40 µg) were electrophoretically fractionated in 8% sodium dodecyl sulfate–polyacrylamide gels, and subjected to immunoblot analysis with specific antibodies against Bcl-2, Bcl-xL (Cell Signaling), and Mcl-1 (Santa Cruz) and β-tubulin (Sigma-Aldrich). Autoradiography of the membranes was performed using Amersham ECL Western blotting detection reagents (Amersham Biosciences, Piscataway, NJ). The densities of the protein bands compared to the β-tubulin protein control were measured with Imaging Analysis software (ImageQuant Tool, version 5.2; Amersham Biosciences, Piscataway, NJ).
Apoptosis assay. The T-cell apoptosis assay was performed with the annexin V/7-aminoactinomycin D staining kit (BD Pharmingen). Transduced T cells were cultured for 4–5 weeks under different culture conditions. The cells were harvested and washed twice with ice-cold phosphate-buffered saline and resuspended in 1× binding buffer (BD Pharmingen) at a concentration of 1 × 106 cells/ml. Next, PE-conjugated annexin V and 7-aminoactinomycin D were added, the cells were incubated for 20 minutes at 25 °C in the dark, and CD3+ T-cell apoptosis was analyzed by flow cytometry within 1 hour.
Statistical analysis. All data are presented as mean ± 1 SD. The Student's t-test was used to determine the statistical significance of differences between samples, and P values <0.05 were accepted as indicating a significant difference.
SUPPLEMENTARY MATERIAL Figure S1. caAkt-transduced T cells maintained longer telomere length and increased telomerase activity in culture. (a). Human PBMCs were stimulated with CD3/CD28 Abs and transduced with retrovirus expressing caAkt or GFP control. Five weeks after transduction, T cells were FACS-sorted by GFP expression. Telomere length was analyzed by quantitative FISH performed by molecular cytogenetics core facility in MD Anderson Cancer Center. Quantitative fluorescence in situ hybridization (Q-FISH) was performed with Cy-3–labeled (CCCTAA)3 PNA probe and subsequent quantitative analysis of digital images. Individual telomere length was quantified by the level fluorescence intensity of each telomere spot, expressed in telomere fluorescence units (TFUs). A fluorescent image and related TFU value were shown. (b). Five weeks afer transduction, genomic DNA was extracted from caAkt-T-cells and control-T-cells. Using quantitative real time PCR, relative telomere length (RTL) was calculated from the telomere repeat to single gene copy number ratio. RTL values are shown as mean + SD. n=3, *p < 0.05 (c). Transduced T cells as described in (b) and autologous PBMC were collected and lysed to detect telomerase activity using the telomeric repeat amplification protocol. Each sample has a heat-inactivated control. Data are representative of three experiments. Figure S2. caAkt-T-cells did not proliferate autonomously in the absence of prosurvival cytokines. After transduction, caAkt and control T cells were cultured in complete medium in the absence of cytokines. Cell numbers were counted at different time points as indicated. Figure S3. FoxP3 expression was reduced in caAkt-transduced CD4+CD25+ T regulatory cell. CD4+CD25+ Tregs were selected from fresh PBMC and stimulated with anti-CD3/CD28 in presence of 100 U/ml IL-2. On day 2, stimulated Tregs were transduced with caAkt or GFP control vector. Intracellular staining of FoxP3, GATA-3 and T bet was performed 5 days later. Data represent 4 donors. Figure S4. Suppressive function was abrogated in caAkt transduced CD4+CD25+ T regulatory cells. (a). Intracellular levels of IL-2 and IFN-γ were determined in caAkt/GFP-transduced Tregs by flow cytometry. Representative histograms are shown from 4 donors. (b). Suppression assays were performed by culturing T responder cells and suppressor cells at different ratios for 5 days and 3H was added to the culture for last 18 hours. Responder cell: Autologous PBMC; Suppressor cell: caAkt/GFP control-transduced Treg. Materials and Methods.
Acknowledgments
We thank Awateef Akrabi for assistance with flow cytometry and Christopher Williams, Sharon Lam for assistance in telomere measurements. This work was supported partially by NIH-NCI 2 P01 CA094237 and the Center for Cell and Gene therapy of Baylor College of Medicine. No potential conflicts of interest were disclosed.
Supplementary Material
caAkt-transduced T cells maintained longer telomere length and increased telomerase activity in culture. (a). Human PBMCs were stimulated with CD3/CD28 Abs and transduced with retrovirus expressing caAkt or GFP control. Five weeks after transduction, T cells were FACS-sorted by GFP expression. Telomere length was analyzed by quantitative FISH performed by molecular cytogenetics core facility in MD Anderson Cancer Center. Quantitative fluorescence in situ hybridization (Q-FISH) was performed with Cy-3–labeled (CCCTAA)3 PNA probe and subsequent quantitative analysis of digital images. Individual telomere length was quantified by the level fluorescence intensity of each telomere spot, expressed in telomere fluorescence units (TFUs). A fluorescent image and related TFU value were shown. (b). Five weeks afer transduction, genomic DNA was extracted from caAkt-T-cells and control-T-cells. Using quantitative real time PCR, relative telomere length (RTL) was calculated from the telomere repeat to single gene copy number ratio. RTL values are shown as mean + SD. n=3, *p < 0.05 (c). Transduced T cells as described in (b) and autologous PBMC were collected and lysed to detect telomerase activity using the telomeric repeat amplification protocol. Each sample has a heat-inactivated control. Data are representative of three experiments.
caAkt-T-cells did not proliferate autonomously in the absence of prosurvival cytokines. After transduction, caAkt and control T cells were cultured in complete medium in the absence of cytokines. Cell numbers were counted at different time points as indicated.
FoxP3 expression was reduced in caAkt-transduced CD4+CD25+ T regulatory cell. CD4+CD25+ Tregs were selected from fresh PBMC and stimulated with anti-CD3/CD28 in presence of 100 U/ml IL-2. On day 2, stimulated Tregs were transduced with caAkt or GFP control vector. Intracellular staining of FoxP3, GATA-3 and T bet was performed 5 days later. Data represent 4 donors.
Suppressive function was abrogated in caAkt transduced CD4+CD25+ T regulatory cells. (a). Intracellular levels of IL-2 and IFN-γ were determined in caAkt/GFP-transduced Tregs by flow cytometry. Representative histograms are shown from 4 donors. (b). Suppression assays were performed by culturing T responder cells and suppressor cells at different ratios for 5 days and 3H was added to the culture for last 18 hours. Responder cell: Autologous PBMC; Suppressor cell: caAkt/GFP control-transduced Treg.
REFERENCES
- Rooney CM, Smith CA, Ng CY, Loftin S, Li C, Krance RA, et al. Use of gene-modified virus-specific T lymphocytes to control Epstein-Barr-virus-related lymphoproliferation. Lancet. 1995;345:9–13. doi: 10.1016/s0140-6736(95)91150-2. [DOI] [PubMed] [Google Scholar]
- Riddell SR, Walter BA, Gilbert MJ., and, Greenberg PD. Selective reconstitution of CD8+ cytotoxic T lymphocyte responses in immunodeficient bone marrow transplant recipients by the adoptive transfer of T cell clones. Bone Marrow Transplant. 1994;14 Suppl 4:S78–S84. [PubMed] [Google Scholar]
- Rooney CM, Smith CA, Ng CY, Loftin SK, Sixbey JW, Gan Y, et al. Infusion of cytotoxic T cells for the prevention and treatment of Epstein-Barr virus-induced lymphoma in allogeneic transplant recipients. Blood. 1998;92:1549–1555. [PubMed] [Google Scholar]
- Straathof KC, Bollard CM, Popat U, Huls MH, Lopez T, Morriss MC, et al. Treatment of nasopharyngeal carcinoma with Epstein-Barr virus–specific T lymphocytes. Blood. 2005;105:1898–1904. doi: 10.1182/blood-2004-07-2975. [DOI] [PubMed] [Google Scholar]
- Dudley ME, Wunderlich JR, Yang JC, Sherry RM, Topalian SL, Restifo NP, et al. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol. 2005;23:2346–2357. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan RA, Dudley ME, Wunderlich JR, Hughes MS, Yang JC, Sherry RM, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126–129. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee C, Thompson JA, Byrd D, Riddell SR, Roche P, Celis E, et al. Adoptive T cell therapy using antigen-specific CD8+ T cell clones for the treatment of patients with metastatic melanoma: in vivo persistence, migration, and antitumor effect of transferred T cells. Proc Natl Acad Sci USA. 2002;99:16168–16173. doi: 10.1073/pnas.242600099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahne M, Rimoldi D, Schröter M, Romero P, Schreier M, French LE, et al. Melanoma cell expression of Fas(Apo-1/CD95) ligand: implications for tumor immune escape. Science. 1996;274:1363–1366. doi: 10.1126/science.274.5291.1363. [DOI] [PubMed] [Google Scholar]
- Thomas DA., and, Massagué J. TGF-beta directly targets cytotoxic T cell functions during tumor evasion of immune surveillance. Cancer Cell. 2005;8:369–380. doi: 10.1016/j.ccr.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Gorelik L., and, Flavell RA. Immune-mediated eradication of tumors through the blockade of transforming growth factor-beta signaling in T cells. Nat Med. 2001;7:1118–1122. doi: 10.1038/nm1001-1118. [DOI] [PubMed] [Google Scholar]
- Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T., and, Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci USA. 2002;99:12293–12297. doi: 10.1073/pnas.192461099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- Yu P, Lee Y, Liu W, Krausz T, Chong A, Schreiber H, et al. Intratumor depletion of CD4+ cells unmasks tumor immunogenicity leading to the rejection of late-stage tumors. J Exp Med. 2005;201:779–791. doi: 10.1084/jem.20041684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotti G, Savoldo B, Pule M, Straathof KC, Biagi E, Yvon E, et al. Human cytotoxic T lymphocytes with reduced sensitivity to Fas-induced apoptosis. Blood. 2005;105:4677–4684. doi: 10.1182/blood-2004-08-3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollard CM, Rössig C, Calonge MJ, Huls MH, Wagner HJ, Massague J, et al. Adapting a transforming growth factor beta-related tumor protection strategy to enhance antitumor immunity. Blood. 2002;99:3179–3187. doi: 10.1182/blood.v99.9.3179. [DOI] [PubMed] [Google Scholar]
- Vera JF, Hoyos V, Savoldo B, Quintarelli C, Giordano Attianese GM, Leen AM, et al. Genetic manipulation of tumor-specific cytotoxic T lymphocytes to restore responsiveness to IL-7. Mol Ther. 2009;17:880–888. doi: 10.1038/mt.2009.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehrotra PT, Grant AJ., and, Siegel JP. Synergistic effects of IL-7 and IL-12 on human T cell activation. J Immunol. 1995;154:5093–5102. [PubMed] [Google Scholar]
- Foster AE, Leen AM, Lee T, Okamura T, Lu A, Vera J, et al. Autologous designer antigen-presenting cells by gene modification of T lymphocyte blasts with IL-7 and IL-12. J Immunother. 2007;30:506–516. doi: 10.1097/CJI.0b013e318046f3b1. [DOI] [PubMed] [Google Scholar]
- Manning BD., and, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J, Lei FT, Xiong X., and, Haque R. Intracellular signals of T cell costimulation. Cell Mol Immunol. 2008;5:239–247. doi: 10.1038/cmi.2008.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LX, La Rose J, Chen L, Neale C, Mak T, Okkenhaug K, et al. CD28 regulates the translation of Bcl-xL via the phosphatidylinositol 3-kinase/mammalian target of rapamycin pathway. J Immunol. 2005;174:180–194. doi: 10.4049/jimmunol.174.1.180. [DOI] [PubMed] [Google Scholar]
- Schneider H, Prasad KV, Shoelson SE., and, Rudd CE. CTLA-4 binding to the lipid kinase phosphatidylinositol 3-kinase in T cells. J Exp Med. 1995;181:351–355. doi: 10.1084/jem.181.1.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su CC, Lin YP, Cheng YJ, Huang JY, Chuang WJ, Shan YS, et al. Phosphatidylinositol 3-kinase/Akt activation by integrin-tumor matrix interaction suppresses Fas-mediated apoptosis in T cells. J Immunol. 2007;179:4589–4597. doi: 10.4049/jimmunol.179.7.4589. [DOI] [PubMed] [Google Scholar]
- Hähnel PS, Thaler S, Antunes E, Huber C, Theobald M., and, Schuler M. Targeting AKT signaling sensitizes cancer to cellular immunotherapy. Cancer Res. 2008;68:3899–3906. doi: 10.1158/0008-5472.CAN-07-6286. [DOI] [PubMed] [Google Scholar]
- Wohlfert EA., and, Clark RB. ‘Vive la Résistance!'–the PI3K-Akt pathway can determine target sensitivity to regulatory T cell suppression. Trends Immunol. 2007;28:154–160. doi: 10.1016/j.it.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Park D, Lapteva N, Seethammagari M, Slawin KM., and, Spencer DM. An essential role for Akt1 in dendritic cell function and tumor immunotherapy. Nat Biotechnol. 2006;24:1581–1590. doi: 10.1038/nbt1262. [DOI] [PubMed] [Google Scholar]
- Kojima H, Kanno Y, Hase H., and, Kobata T. CD4+CD25+ regulatory T cells attenuate the phosphatidylinositol 3-kinase/Akt pathway in antigen-primed immature CD8+ CTLs during functional maturation. J Immunol. 2005;174:5959–5967. doi: 10.4049/jimmunol.174.10.5959. [DOI] [PubMed] [Google Scholar]
- Liu VC, Wong LY, Jang T, Shah AH, Park I, Yang X, et al. Tumor evasion of the immune system by converting CD4+CD25- T cells into CD4+CD25+ T regulatory cells: role of tumor-derived TGF-beta. J Immunol. 2007;178:2883–2892. doi: 10.4049/jimmunol.178.5.2883. [DOI] [PubMed] [Google Scholar]
- Sauer S, Bruno L, Hertweck A, Finlay D, Leleu M, Spivakov M, et al. T cell receptor signaling controls Foxp3 expression via PI3K, Akt, and mTOR. Proc Natl Acad Sci USA. 2008;105:7797–7802. doi: 10.1073/pnas.0800928105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haxhinasto S, Mathis D., and, Benoist C. The AKT-mTOR axis regulates de novo differentiation of CD4+Foxp3+ cells. J Exp Med. 2008;205:565–574. doi: 10.1084/jem.20071477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossig C, Bollard CM, Nuchtern JG, Merchant DA., and, Brenner MK. Targeting of G(D2)-positive tumor cells by human T lymphocytes engineered to express chimeric T-cell receptor genes. Int J Cancer. 2001;94:228–236. doi: 10.1002/ijc.1457. [DOI] [PubMed] [Google Scholar]
- Pulè MA, Straathof KC, Dotti G, Heslop HE, Rooney CM., and, Brenner MK. A chimeric T cell antigen receptor that augments cytokine release and supports clonal expansion of primary human T cells. Mol Ther. 2005;12:933–941. doi: 10.1016/j.ymthe.2005.04.016. [DOI] [PubMed] [Google Scholar]
- Straathof KC, Spencer DM, Sutton RE., and, Rooney CM. Suicide genes as safety switches in T lymphocytes. Cytotherapy. 2003;5:227–230. doi: 10.1080/14653240310001497. [DOI] [PubMed] [Google Scholar]
- Ramos CA, Asgari Z, Liu E, Yvon E, Heslop HE, Rooney CM, et al. An inducible caspase 9 suicide gene to improve the safety of mesenchymal stromal cell therapies. Stem Cells. 2010;28:1107–1115. doi: 10.1002/stem.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strand S, Hofmann WJ, Hug H, Müller M, Otto G, Strand D, et al. Lymphocyte apoptosis induced by CD95 (APO-1/Fas) ligand-expressing tumor cells–a mechanism of immune evasion. Nat Med. 1996;2:1361–1366. doi: 10.1038/nm1296-1361. [DOI] [PubMed] [Google Scholar]
- Poppema S, Potters M, Visser L., and, van den Berg AM. Immune escape mechanisms in Hodgkin's disease. Ann Oncol. 1998;9 Suppl 5:S21–S24. doi: 10.1093/annonc/9.suppl_5.s21. [DOI] [PubMed] [Google Scholar]
- Rooney CM, Bollard C, Huls MH, Gahn B, Gottschalk S, Wagner HJ, et al. Immunotherapy for Hodgkin's disease. Ann Hematol. 2002;81 Suppl 2:S39–S42. [PubMed] [Google Scholar]
- Piccirillo CA., and, Shevach EM. Cutting edge: control of CD8+ T cell activation by CD4+CD25+ immunoregulatory cells. J Immunol. 2001;167:1137–1140. doi: 10.4049/jimmunol.167.3.1137. [DOI] [PubMed] [Google Scholar]
- Plunkett FJ, Franzese O, Finney HM, Fletcher JM, Belaramani LL, Salmon M, et al. The loss of telomerase activity in highly differentiated CD8+CD28-CD27- T cells is associated with decreased Akt (Ser473) phosphorylation. J Immunol. 2007;178:7710–7719. doi: 10.4049/jimmunol.178.12.7710. [DOI] [PubMed] [Google Scholar]
- Remy I, Montmarquette A., and, Michnick SW. PKB/Akt modulates TGF-beta signalling through a direct interaction with Smad3. Nat Cell Biol. 2004;6:358–365. doi: 10.1038/ncb1113. [DOI] [PubMed] [Google Scholar]
- Lin SJ, Chang C, Ng AK, Wang SH, Li JJ., and, Hu CP. Prevention of TGF-beta-induced apoptosis by interlukin-4 through Akt activation and p70S6K survival signaling pathways. Apoptosis. 2007;12:1659–1670. doi: 10.1007/s10495-007-0085-5. [DOI] [PubMed] [Google Scholar]
- Battaglia M, Stabilini A., and, Roncarolo MG. Rapamycin selectively expands CD4+CD25+FoxP3+ regulatory T cells. Blood. 2005;105:4743–4748. doi: 10.1182/blood-2004-10-3932. [DOI] [PubMed] [Google Scholar]
- Crellin NK, Garcia RV., and, Levings MK. Altered activation of AKT is required for the suppressive function of human CD4+CD25+ T regulatory cells. Blood. 2007;109:2014–2022. doi: 10.1182/blood-2006-07-035279. [DOI] [PubMed] [Google Scholar]
- Pierau M, Engelmann S, Reinhold D, Lapp T, Schraven B., and, Bommhardt UH. Protein kinase B/Akt signals impair Th17 differentiation and support natural regulatory T cell function and induced regulatory T cell formation. J Immunol. 2009;183:6124–6134. doi: 10.4049/jimmunol.0900246. [DOI] [PubMed] [Google Scholar]
- Greenberg PD. Adoptive T cell therapy of tumors: mechanisms operative in the recognition and elimination of tumor cells. Adv Immunol. 1991;49:281–355. doi: 10.1016/s0065-2776(08)60778-6. [DOI] [PubMed] [Google Scholar]
- Fujio Y., and, Walsh K. Akt mediates cytoprotection of endothelial cells by vascular endothelial growth factor in an anchorage-dependent manner. J Biol Chem. 1999;274:16349–16354. doi: 10.1074/jbc.274.23.16349. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
caAkt-transduced T cells maintained longer telomere length and increased telomerase activity in culture. (a). Human PBMCs were stimulated with CD3/CD28 Abs and transduced with retrovirus expressing caAkt or GFP control. Five weeks after transduction, T cells were FACS-sorted by GFP expression. Telomere length was analyzed by quantitative FISH performed by molecular cytogenetics core facility in MD Anderson Cancer Center. Quantitative fluorescence in situ hybridization (Q-FISH) was performed with Cy-3–labeled (CCCTAA)3 PNA probe and subsequent quantitative analysis of digital images. Individual telomere length was quantified by the level fluorescence intensity of each telomere spot, expressed in telomere fluorescence units (TFUs). A fluorescent image and related TFU value were shown. (b). Five weeks afer transduction, genomic DNA was extracted from caAkt-T-cells and control-T-cells. Using quantitative real time PCR, relative telomere length (RTL) was calculated from the telomere repeat to single gene copy number ratio. RTL values are shown as mean + SD. n=3, *p < 0.05 (c). Transduced T cells as described in (b) and autologous PBMC were collected and lysed to detect telomerase activity using the telomeric repeat amplification protocol. Each sample has a heat-inactivated control. Data are representative of three experiments.
caAkt-T-cells did not proliferate autonomously in the absence of prosurvival cytokines. After transduction, caAkt and control T cells were cultured in complete medium in the absence of cytokines. Cell numbers were counted at different time points as indicated.
FoxP3 expression was reduced in caAkt-transduced CD4+CD25+ T regulatory cell. CD4+CD25+ Tregs were selected from fresh PBMC and stimulated with anti-CD3/CD28 in presence of 100 U/ml IL-2. On day 2, stimulated Tregs were transduced with caAkt or GFP control vector. Intracellular staining of FoxP3, GATA-3 and T bet was performed 5 days later. Data represent 4 donors.
Suppressive function was abrogated in caAkt transduced CD4+CD25+ T regulatory cells. (a). Intracellular levels of IL-2 and IFN-γ were determined in caAkt/GFP-transduced Tregs by flow cytometry. Representative histograms are shown from 4 donors. (b). Suppression assays were performed by culturing T responder cells and suppressor cells at different ratios for 5 days and 3H was added to the culture for last 18 hours. Responder cell: Autologous PBMC; Suppressor cell: caAkt/GFP control-transduced Treg.