Abstract
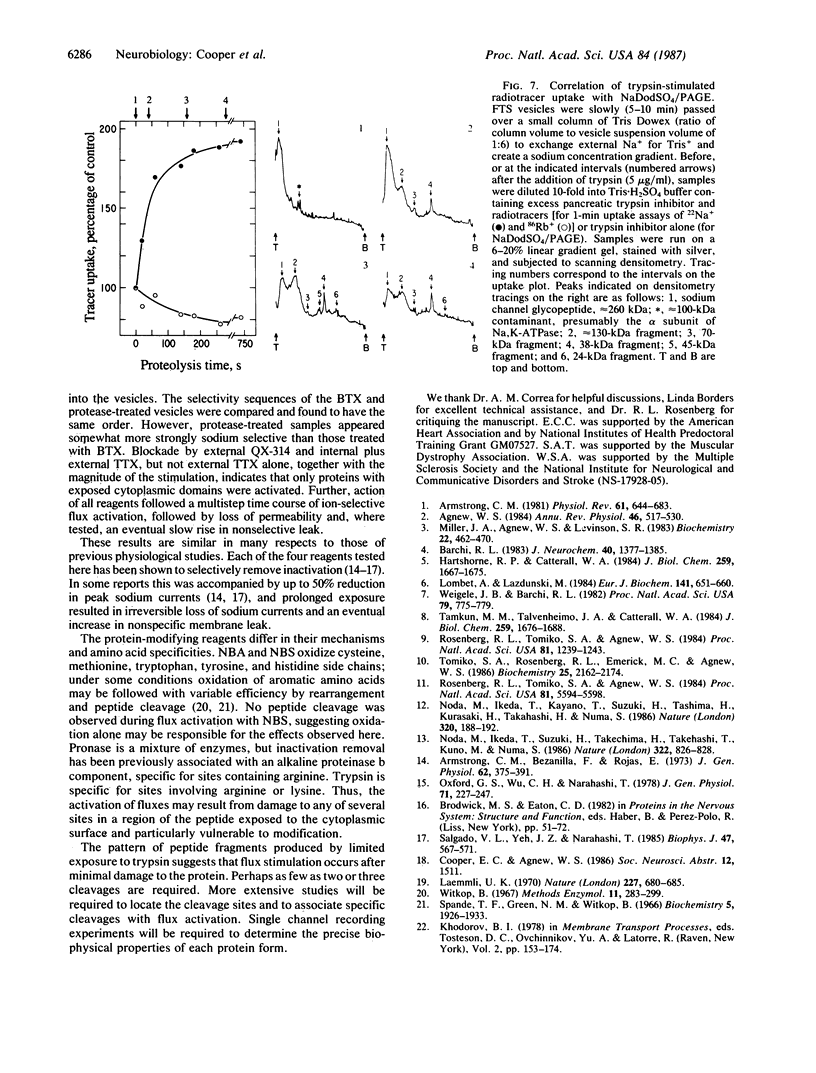
At equilibrium, voltage-sensitive sodium channels normally are closed at all potentials. They open transiently in response to changes in membrane voltage or chronically under the influence of certain neurotoxins. Covalent modifications that result in chronic opening may help identify molecular domains involved in conductance regulation. Here, the purified sodium channel from electric eel electroplax, reconstituted in artificial liposomes, has been used to screen for such modifications. When the liposomes were treated with the alkaloid neurotoxin batrachotoxin, sodium-selective ion fluxes were produced, with permeability ratios PNa greater than PTl greater than PK greater than PRb greater than PCs. When the liposomes were treated with either of two oxidizing reagents (N-bromoacetamide or N-bromosuccinimide), or with Pronase or trypsin, ion-selective fluxes also were stimulated. These were blocked by tetrodotoxin and the anesthetic QX-314 in a manner suggesting that only modification of the cytoplasmic protein surface resulted in stimulation. Limited exposure to trypsin resulted in strong flux activation, with the concomitant appearance of peptide fragments with masses of approximately equal to 130, 70, and 38 kDa and fragments with masses of 45 and 24 kDa appearing later. We propose that characterization of these fragments may allow identification of channel domains important for inactivation gating.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agnew W. S. Voltage-regulated sodium channel molecules. Annu Rev Physiol. 1984;46:517–530. doi: 10.1146/annurev.ph.46.030184.002505. [DOI] [PubMed] [Google Scholar]
- Armstrong C. M., Bezanilla F., Rojas E. Destruction of sodium conductance inactivation in squid axons perfused with pronase. J Gen Physiol. 1973 Oct;62(4):375–391. doi: 10.1085/jgp.62.4.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong C. M. Sodium channels and gating currents. Physiol Rev. 1981 Jul;61(3):644–683. doi: 10.1152/physrev.1981.61.3.644. [DOI] [PubMed] [Google Scholar]
- Barchi R. L. Protein components of the purified sodium channel from rat skeletal muscle sarcolemma. J Neurochem. 1983 May;40(5):1377–1385. doi: 10.1111/j.1471-4159.1983.tb13580.x. [DOI] [PubMed] [Google Scholar]
- Hartshorne R. P., Catterall W. A. The sodium channel from rat brain. Purification and subunit composition. J Biol Chem. 1984 Feb 10;259(3):1667–1675. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lombet A., Lazdunski M. Characterization, solubilization, affinity labeling and purification of the cardiac Na+ channel using Tityus toxin gamma. Eur J Biochem. 1984 Jun 15;141(3):651–660. doi: 10.1111/j.1432-1033.1984.tb08241.x. [DOI] [PubMed] [Google Scholar]
- Miller J. A., Agnew W. S., Levinson S. R. Principal glycopeptide of the tetrodotoxin/saxitoxin binding protein from Electrophorus electricus: isolation and partial chemical and physical characterization. Biochemistry. 1983 Jan 18;22(2):462–470. doi: 10.1021/bi00271a032. [DOI] [PubMed] [Google Scholar]
- Noda M., Ikeda T., Kayano T., Suzuki H., Takeshima H., Kurasaki M., Takahashi H., Numa S. Existence of distinct sodium channel messenger RNAs in rat brain. Nature. 1986 Mar 13;320(6058):188–192. doi: 10.1038/320188a0. [DOI] [PubMed] [Google Scholar]
- Noda M., Ikeda T., Suzuki H., Takeshima H., Takahashi T., Kuno M., Numa S. Expression of functional sodium channels from cloned cDNA. 1986 Aug 28-Sep 3Nature. 322(6082):826–828. doi: 10.1038/322826a0. [DOI] [PubMed] [Google Scholar]
- Oxford G. S., Wu C. H., Narahashi T. Removal of sodium channel inactivation in squid giant axons by n-bromoacetamide. J Gen Physiol. 1978 Mar;71(3):227–247. doi: 10.1085/jgp.71.3.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg R. L., Tomiko S. A., Agnew W. S. Reconstitution of neurotoxin-modulated ion transport by the voltage-regulated sodium channel isolated from the electroplax of Electrophorus electricus. Proc Natl Acad Sci U S A. 1984 Feb;81(4):1239–1243. doi: 10.1073/pnas.81.4.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg R. L., Tomiko S. A., Agnew W. S. Single-channel properties of the reconstituted voltage-regulated Na channel isolated from the electroplax of Electrophorus electricus. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5594–5598. doi: 10.1073/pnas.81.17.5594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salgado V. L., Yeh J. Z., Narahashi T. Voltage-dependent removal of sodium inactivation by N-bromoacetamide and pronase. Biophys J. 1985 Apr;47(4):567–571. doi: 10.1016/S0006-3495(85)83952-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spande T. F., Green N. M., Witkop B. The reactivity toward N-bromosuccinimide of tryptophan in enzymes, zymogens, and inhibited enzymes. Biochemistry. 1966 Jun;5(6):1926–1933. doi: 10.1021/bi00870a020. [DOI] [PubMed] [Google Scholar]
- Tamkun M. M., Talvenheimo J. A., Catterall W. A. The sodium channel from rat brain. Reconstitution of neurotoxin-activated ion flux and scorpion toxin binding from purified components. J Biol Chem. 1984 Feb 10;259(3):1676–1688. [PubMed] [Google Scholar]
- Tomiko S. A., Rosenberg R. L., Emerick M. C., Agnew W. S. Fluorescence assay for neurotoxin-modulated ion transport by the reconstituted voltage-activated sodium channel isolated from eel electric organ. Biochemistry. 1986 Apr 22;25(8):2162–2174. doi: 10.1021/bi00356a047. [DOI] [PubMed] [Google Scholar]