Abstract
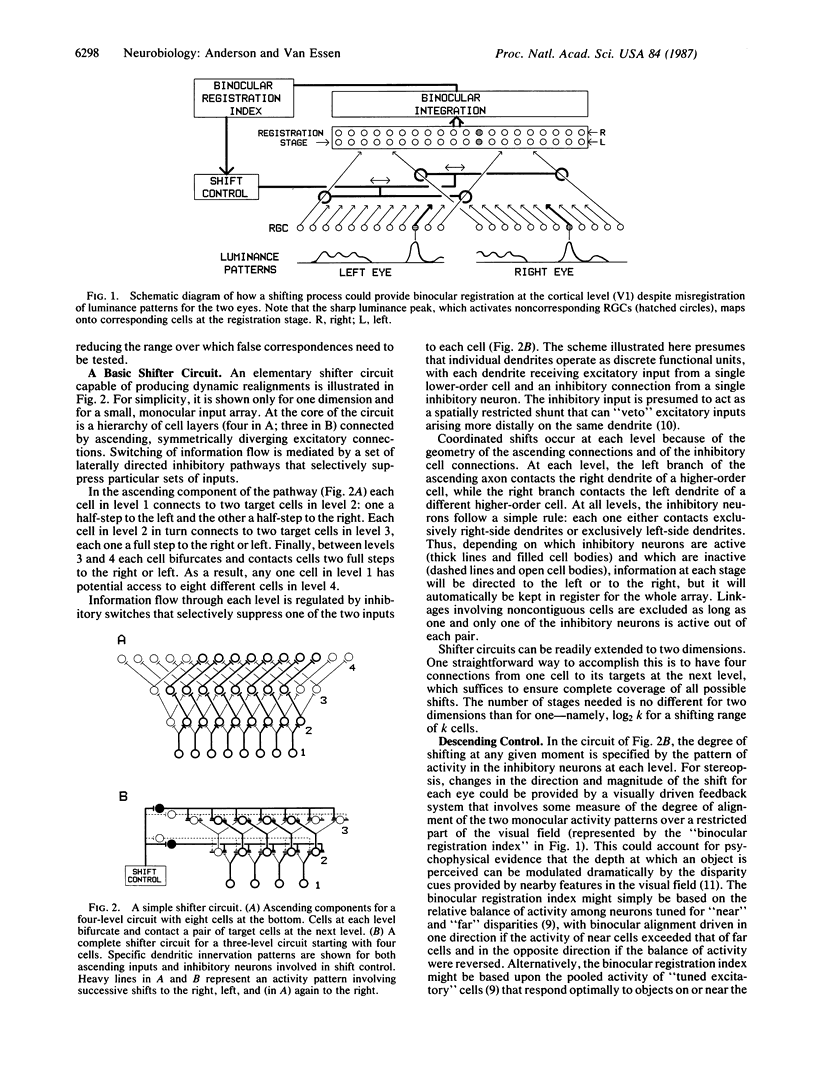
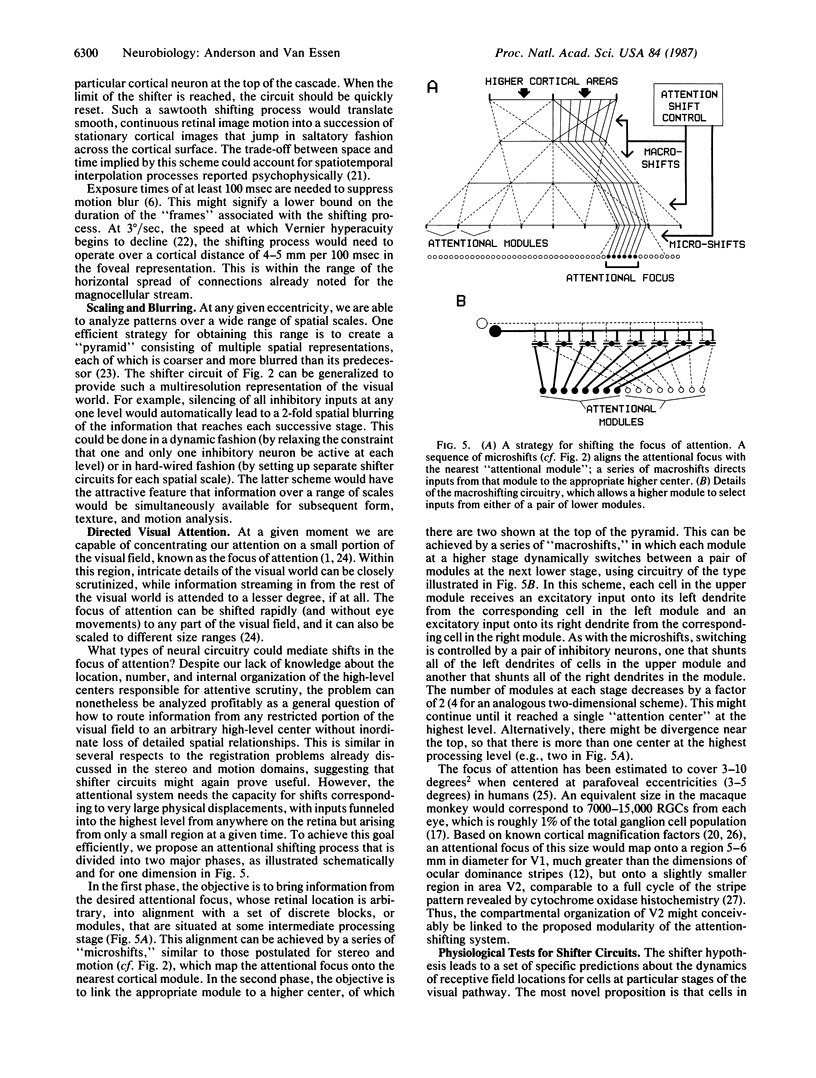
We propose a general strategy for dynamic control of information flow between arrays of neurons at different levels of the visual pathway, starting in the lateral geniculate nucleus and the geniculorecipient layers of cortical area V1. This strategy can be used for resolving computational problems arising in the domains of stereopsis, directed visual attention, and the perception of moving images. In each of these situations, some means of dynamically controlling how retinal outputs map onto higher-level targets is desirable--in order to achieve binocular fusion, to allow shifts of the focus of attention, and to prevent blurring of moving images. The proposed solution involves what we term "shifter circuits," which allow for dynamic shifts in the relative alignment of input and output arrays without loss of local spatial relationships. The shifts are produced in increments along a succession of relay stages that are linked by diverging excitatory inputs. The direction of shift is controlled at each stage by inhibitory neurons that selectively suppress appropriate sets of ascending inputs. The shifter hypothesis is consistent with available anatomical and physiological evidence on the organization of the primate visual pathway, and it offers a sensible explanation for a variety of otherwise puzzling facts, such as the plethora of cells in the geniculorecipient layers of V1.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barlow H. B. Reconstructing the visual image in space and time. Nature. 1979 May 17;279(5710):189–190. doi: 10.1038/279189a0. [DOI] [PubMed] [Google Scholar]
- Bergen J. R., Julesz B. Parallel versus serial processing in rapid pattern discrimination. Nature. 1983 Jun 23;303(5919):696–698. doi: 10.1038/303696a0. [DOI] [PubMed] [Google Scholar]
- Blasdel G. G., Fitzpatrick D. Physiological organization of layer 4 in macaque striate cortex. J Neurosci. 1984 Mar;4(3):880–895. doi: 10.1523/JNEUROSCI.04-03-00880.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasdel G. G., Lund J. S., Fitzpatrick D. Intrinsic connections of macaque striate cortex: axonal projections of cells outside lamina 4C. J Neurosci. 1985 Dec;5(12):3350–3369. doi: 10.1523/JNEUROSCI.05-12-03350.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crick F. Function of the thalamic reticular complex: the searchlight hypothesis. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4586–4590. doi: 10.1073/pnas.81.14.4586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Valois R. L., Albrecht D. G., Thorell L. G. Spatial frequency selectivity of cells in macaque visual cortex. Vision Res. 1982;22(5):545–559. doi: 10.1016/0042-6989(82)90113-4. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick D., Lund J. S., Blasdel G. G. Intrinsic connections of macaque striate cortex: afferent and efferent connections of lamina 4C. J Neurosci. 1985 Dec;5(12):3329–3349. doi: 10.1523/JNEUROSCI.05-12-03329.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattass R., Gross C. G., Sandell J. H. Visual topography of V2 in the macaque. J Comp Neurol. 1981 Oct 1;201(4):519–539. doi: 10.1002/cne.902010405. [DOI] [PubMed] [Google Scholar]
- Hendrickson A. E., Hunt S. P., Wu J. Y. Immunocytochemical localization of glutamic acid decarboxylase in monkey striate cortex. Nature. 1981 Aug 13;292(5824):605–607. doi: 10.1038/292605a0. [DOI] [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N. Ferrier lecture. Functional architecture of macaque monkey visual cortex. Proc R Soc Lond B Biol Sci. 1977 Jul 28;198(1130):1–59. doi: 10.1098/rspb.1977.0085. [DOI] [PubMed] [Google Scholar]
- Jay M. F., Sparks D. L. Auditory receptive fields in primate superior colliculus shift with changes in eye position. Nature. 1984 May 24;309(5966):345–347. doi: 10.1038/309345a0. [DOI] [PubMed] [Google Scholar]
- Kaas J. H., Merzenich M. M., Killackey H. P. The reorganization of somatosensory cortex following peripheral nerve damage in adult and developing mammals. Annu Rev Neurosci. 1983;6:325–356. doi: 10.1146/annurev.ne.06.030183.001545. [DOI] [PubMed] [Google Scholar]
- Koch C., Poggio T., Torre V. Nonlinear interactions in a dendritic tree: localization, timing, and role in information processing. Proc Natl Acad Sci U S A. 1983 May;80(9):2799–2802. doi: 10.1073/pnas.80.9.2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch C., Ullman S. Shifts in selective visual attention: towards the underlying neural circuitry. Hum Neurobiol. 1985;4(4):219–227. [PubMed] [Google Scholar]
- Mitchison G. J., McKee S. P. Interpolation in stereoscopic matching. 1985 May 30-Jun 5Nature. 315(6018):402–404. doi: 10.1038/315402a0. [DOI] [PubMed] [Google Scholar]
- Moran J., Desimone R. Selective attention gates visual processing in the extrastriate cortex. Science. 1985 Aug 23;229(4715):782–784. doi: 10.1126/science.4023713. [DOI] [PubMed] [Google Scholar]
- Motter B. C., Poggio G. F. Binocular fixation in the rhesus monkey: spatial and temporal characteristics. Exp Brain Res. 1984;54(2):304–314. doi: 10.1007/BF00236231. [DOI] [PubMed] [Google Scholar]
- O'Kusky J., Colonnier M. A laminar analysis of the number of neurons, glia, and synapses in the adult cortex (area 17) of adult macaque monkeys. J Comp Neurol. 1982 Sep 20;210(3):278–290. doi: 10.1002/cne.902100307. [DOI] [PubMed] [Google Scholar]
- Parker A., Hawken M. Capabilities of monkey cortical cells in spatial-resolution tasks. J Opt Soc Am A. 1985 Jul;2(7):1101–1114. doi: 10.1364/josaa.2.001101. [DOI] [PubMed] [Google Scholar]
- Perry V. H., Cowey A. The ganglion cell and cone distributions in the monkey's retina: implications for central magnification factors. Vision Res. 1985;25(12):1795–1810. doi: 10.1016/0042-6989(85)90004-5. [DOI] [PubMed] [Google Scholar]
- Poggio G. F., Poggio T. The analysis of stereopsis. Annu Rev Neurosci. 1984;7:379–412. doi: 10.1146/annurev.ne.07.030184.002115. [DOI] [PubMed] [Google Scholar]
- Poggio G. F., Talbot W. H. Mechanisms of static and dynamic stereopsis in foveal cortex of the rhesus monkey. J Physiol. 1981 Jun;315:469–492. doi: 10.1113/jphysiol.1981.sp013759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond B. J., Wurtz R. H., Sato T. Visual responses of inferior temporal neurons in awake rhesus monkey. J Neurophysiol. 1983 Dec;50(6):1415–1432. doi: 10.1152/jn.1983.50.6.1415. [DOI] [PubMed] [Google Scholar]
- Sagi D., Julesz B. Enhanced detection in the aperture of focal attention during simple discrimination tasks. Nature. 1986 Jun 12;321(6071):693–695. doi: 10.1038/321693a0. [DOI] [PubMed] [Google Scholar]
- Sherman S. M., Koch C. The control of retinogeniculate transmission in the mammalian lateral geniculate nucleus. Exp Brain Res. 1986;63(1):1–20. doi: 10.1007/BF00235642. [DOI] [PubMed] [Google Scholar]
- Steinman R. M., Cushman W. B., Martins A. J. The precision of gaze. A review. Hum Neurobiol. 1982;1(2):97–109. [PubMed] [Google Scholar]
- Tootell R. B., Silverman M. S., De Valois R. L., Jacobs G. H. Functional organization of the second cortical visual area in primates. Science. 1983 May 13;220(4598):737–739. doi: 10.1126/science.6301017. [DOI] [PubMed] [Google Scholar]
- Van Essen D. C., Newsome W. T., Maunsell J. H. The visual field representation in striate cortex of the macaque monkey: asymmetries, anisotropies, and individual variability. Vision Res. 1984;24(5):429–448. doi: 10.1016/0042-6989(84)90041-5. [DOI] [PubMed] [Google Scholar]
- Westheimer G., McKee S. P. Visual acuity in the presence of retinal-image motion. J Opt Soc Am. 1975 Jul;65(7):847–850. doi: 10.1364/josa.65.000847. [DOI] [PubMed] [Google Scholar]


