Abstract
Tryptophan hydroxylase-2 (TPH2) synthesizes neuronal serotonin and is linked to numerous behavioral traits. We have previously characterized the functionality of polymorphisms (especially 2051A>C) in 3′-UTR of rhesus monkey TPH2 (rhTPH2). This study further assessed the functionality of additional polymorphisms (−1605T>C, −1491Tn, −1485(AT)n, −1454A>G, −1325In>Del and −363T>G) in rhTPH2 5′-FR, and evaluated the effects of rhTPH2 5′ and 3′ genotypes on central serotonin turnover, hypothalamic-pituitary-adrenal (HPA) axis function and self-injurious behavior in 32 unrelated adult male monkeys of Indian origin. Haplotypes of the rhTPH2 5′-FR polymorphisms exert a significant, cell-dependent effect on reporter gene expression, primarily conferred by −1485(AT)n. The −1485(AT)n and 2051A>C polymorphisms interact to influence CSF 5-HIAA and plasma ACTH in the afternoon. While −1485(AT)n exerts significant main effects on the afternoon cortisol level and nocturnal HPA negative feedback, 2051A>C has significant main effects on the morning cortisol level and cortisol response to ACTH challenge, as well as marginally significant main effects on the daytime HPA negative feedback and self-biting rate. In addition, the genotype/allele frequency of the 5′-FR-1325Ins>Del differed significantly between the self-wounders and non-wounders, whereas 3′-UTR 2128S>L polymorphism differed significantly in genotype/allele frequency between the high- and low-frequency biters. This study demonstrates the functionality of rhTPH2 5′-FR polymorphisms, and provides evidence for the differential association of rhTPH2 5′-FR and 3′-UTR polymorphisms with HPA axis function and SIB. Our findings shed light on the role of TPH2 gene variance in physiology and behavioral traits, and also contribute to the understanding of the pathophysiology and genetics of SIB.
Keywords: Tryptophan hydroxylase-2, regulatory polymorphism, gene expression, serotonin, HPA axis, self-injurious behavior, self-wounding, self-biting, rhesus monkey
Introduction
Serotonin (5-HT) is a major neurotransmitter involved in the stress response as well as many other brain functions. The synthesis of 5-HT is initiated by the hydroxylation of tryptophan, the first and rate-limiting step catalyzed by tryptophan hydroxylase (TPH). Recently, a second, brain-specific TPH isoform named TPH2 was identified (Cote et al. 2003; Walther & Bader 2003; Walther et al. 2003). Accordingly, TPH2 is a critical factor modulating 5-HT neurotransmission, and genetic or epigenetic factors affecting TPH2 expression or activity may alter 5-HT-related brain functions and behavioral traits. In fact, genetic variance in human TPH2 (hTPH2) has been linked to numerous endophenotypes and behavioral traits, such as amygdala reactivity (Brown et al. 2005; Furmark et al. 2008), major depression (Van den Bogaert et al. 2006; Zhou et al. 2005; Zill et al. 2004a) and suicidality (de Lara et al. 2007; Jollant et al. 2007; Ke et al. 2006; Lopez et al. 2007; Zill et al. 2004b), as well as the antidepressant response to selective serotonin reuptake inhibitors (SSRIs) (Peters et al. 2004; Tzvetkov et al. 2008). Similarly, mouse Tph2 genetic variance also differentiates aggressive behavior and antidepressant response to SSRIs (Cervo et al. 2005; Kulikov et al. 2005).
The hypothalamic-pituitary-adrenal (HPA) axis is a critical neuroendocrine system that responds to stress, and it is implicated in many neuropsychiatric disorders. It has been established that there are reciprocal interactions between 5-HT and the HPA axis (Dinan 1996; Lowry 2002). In support of this notion, TPH2 gene expression exhibits a circadian rhythm induced by the daily corticoid surge (Malek et al. 2007), and is regulated by a number of hormones and stressors (Brown et al. 2006; Clark et al. 2005; Gardner et al. 2009; Hiroi et al. 2006; Malek et al. 2005; Mueller & Bale 2008; Rahman & Thomas 2009; Sanchez et al. 2005; Paul et al. 2007). Thus, TPH2 gene expression is frequently modulated under specific physiological and stress conditions, presumably representing a mechanism underlying the activation and/or feedback of the HPA axis. Accordingly, genetic variants affecting TPH2 expression may alter HPA axis reactivity and thereby differentiate the vulnerability to stress-related disorders, i.e. HPA axis reactivity may mediate the association between TPH2 genetic variance and neuropsychiatric disorders, just like the case for the 5-HT transporter (5-HTT) (Gotlib et al. 2008), another serotonergic gene.
Self-injurious behavior, which may be defined as any self-directed act that results in tissue damage, is a significant health problem afflicting a diversity of clinical and nonclinical populations (Janis & Nock 2008; Yates 2004). SIB is also seen in a small percentage of nonhuman primates that injure themselves through self-directed biting. In rhesus monkeys, SIB is quite variable in expression and wounding is relatively rare; however, monkeys with a wounding history typically engage in some form of self-directed biting on a daily basis (Novak 2003). It has been documented that dysfunctions of 5-HT (McCloskey et al. 2009; Pies & Popli 1995; Sivam 1996), and the HPA axis (Novak 2003; Tiefenbacher et al. 2004) are implicated in the etiology of SIB, and manipulation of 5-HT neurotransmission by the SSRI fluoxetine and supplemental tryptophan is efficacious in reducing SIB (Fontenot et al. 2009; Fontenot et al. 2005; Luiselli et al. 2001; Weld et al. 1998). Hence, TPH2 genetic variance may contribute to SIB vulnerability via the alteration in 5-HT and the HPA axis.
Rhesus monkeys share genetic, physiological and behavioral similarities with humans, and harbor functionally orthologous, though often non-identical, polymorphisms that mimic human genetic variants in effect. We have previously identified a constellation of polymorphisms in the 5′- and 3′-regulatory regions of rhesus monkey TPH2 (rhTPH2), of which the 3′-UTR polymorphisms have a significant impact on gene expression and HPA axis function (Chen et al. 2006); however, the functionality of rhTPH2 5′-FR polymorphisms, as well as the potential interaction between the 5′ and 3′ polymorphisms, are yet to be determined. There is a distance of about 95 kb between the rhTPH2 5′ and 3′ polymorphisms, and they are likely transmitted independently due to lack of linkage (Chen et al. 2006), making interactions between rhTPH2 5′ and 3′ polymorphisms resemble gene by gene interactions. In the present study, we set out to assess the functionality of rhTPH2 5′-FR polymorphisms, as well as to comprehensively evaluate the effect of rhTPH2 genotype on the central 5-HT and HPA axis functions, and SIB.
Methods
Haplotypes of rhTPH2 5′-FR and construction of reporter vectors
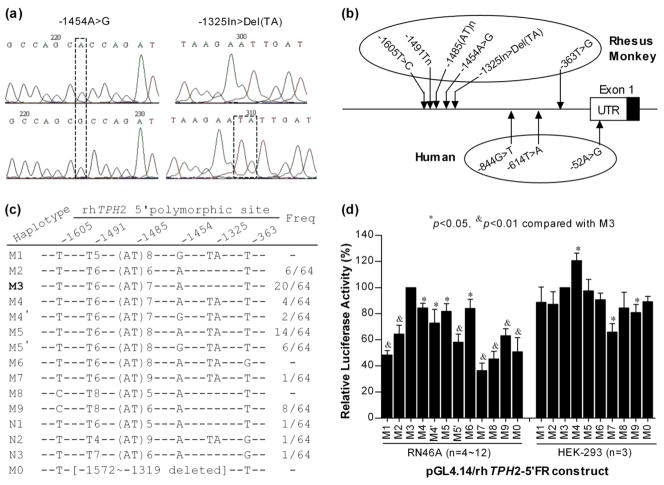
Cloning primers were designed on the basis of rhTPH2 5′-FR sequence (GenBank accession no. GQ337711). For clarity, we referred to the first nucleotide of the open reading frame as nucleotide 1, with the 5′-UTR beginning at-1 and proceeding in the negative direction. Besides the four polymorphisms (−1605T>C, −1491Tn, −1485(AT)n and −363T>G) reported previously (Chen et al. 2006), we identified another two polymorphisms (−1454A>G and −1325In>Del, Fig.1a) during the process of cloning. All six polymorphisms are illustrated in Fig.1b.
Figure 1.
Haplotypes of the rhTPH2 5′-FR and their effects on reporter gene expression. (a) Trace chromatograms of −1454A>G and −1325In>Del(TA) polymorphisms; (b) Illustration of 5′-FR polymorphisms in rhTPH2 and hTPH2; (c) rhTPH2 5′-FR haplotypes and their frequencies in the cohort of 32 monkeys; (d) Luciferase activity driven by rhTPH2 5′-FR haplotypes in RN46A and HEK-293 cells. Except for the deletion construct (M0), all haplotypes occur naturally for the loci between −1605 and −1325. M3 has the dominant prevalence in the population and is thus regarded as the wild-type haplotype. M4′/M5′ differs from M4/M5 only at the −1454 locus, with the former followed by a prime (') indicating a “G” substitution at −1454. Data are shown as Mean ± SEM.
We have previously cloned segment −1857~−974 of rhTPH2 5′-FR into the pGEM-T vector to identify polymorphisms (Chen et al. 2006). To clone different haplotypes of rhTPH2 5′-FR into the reporter vector, a 1633-bp fragment (-1633~-1) was amplified from a −363T/G heterozygote by using primers KpnI-rhTPH2(−1633)F (5′-gcctGGTACCtgattagagctgacctttacaga-3′) and XhoI-rhTPH2(−1)R (5′-ctggCTCGAGggatcccagtgtaatattctttc-3′) that have incorporated restriction sites. Following sequential digestion by KpnI and XhoI, the PCR product was cloned into pGL4.14 (Promega), with two constructs (−363T and G) being generated. The −363T construct was then used as the template to generate other pGL4.14/rhTPH2-5FR constructs. Briefly, haplotypes of 5′-FR loci (except −363) in pGEM-T were amplified using primers KpnI-rhTPH2(−1633)F and rhTPH2(−974)R (5′-ttgatttagccacagggagttt-3′). Following sequential digestion by KpnI and SnaBI (cuts rhTPH2 5′-FR at −997), PCR products were subcloned into the template pGL4.14/rhTPH2-5FR construct by replacing the corresponding sequence. Altogether, we subcloned nine previously identified haplotypes (M1~M9) and two novel haplotypes (M4′ and M5′) into the reporter vector (shown in Fig.1c). In addition, a deletion construct (M0) without the highly polymorphic region was generated by AseI digestion of the pGL4.14/rhTPH2-5FR construct, followed by re-ligation of the recovered long fragment. AseI cuts rhTPH2 5′-FR twice at −1572 and −1319, respectively, spanning most of the loci. All constructs were sequence-verified and confirmed for correct orientation.
Cell lines, transient transfection and luciferase assay
To examine whether the rhTPH2 5′ haplotypes exert cell-dependent effects on gene expression, this study employed RN46A and HEK-293 cells, among which RN46A expresses TPH2 mRNA while HEK-293 does not (data not shown). RN46A is a serotonergic cell line derived from embryonic day 13 rat medullary raphe cells, while HEK-293 is derived from primary cultures of human embryonic kidney cells. Both cell lines were cultured as previously described (Chen et al. 2008).
The day before transfection, cells were seeded into 24-well plates at approximately 2×105 cells/well. The TransFectin™ Lipid Reagent (Bio-Rad) and ProFection® Mammalian Transfection System (Promega) were used to perform transfection for RN46A and HEK-293 cells, respectively. For each well, 0.8 μg of the construct was transfected along with 0.2 μg of pRL-TK Renilla reporter vector as the control. Cells were harvested 24h after transfection, and the firefly and Renilla luciferase activities were measured using the Dual Luciferase Reporter Assay System (Promega) on a Victor3 V Multilabel Counter (Wallac-PerkinElmer). Transfections and assays were performed in triplicate on at least three occasions, with different DNA preparations to ensure validity. The normalized luciferase data (firefly/Renilla) was used to perform statistics and are expressed relative to the predominant M3 haplotype.
Animals
32 unrelated adult male rhesus monkeys of Indian origin that had been behaviorally and physiologically characterized between 1994 and 2004 were involved in this study. All these monkeys were maintained in accordance with the guidelines of the Committee on Animals of the Harvard Medical School and the Guide for Care and Use of Laboratory Animals of the Institute of Laboratory Animal Resources, National Research Council, Department of Health and Human Services, publication No. (NIH) 85–23 revised 1985. All the animals were individually housed during the period of study. The early rearing history was available for 28 monkeys (Fig. S1), among which 16 were mother-reared (MR) in social groups for at least the first 6 months, while the other twelve were reared without adults, instead with constant access to monkeys of similar age in a “peer-only reared” (PR) condition.
Phenotyping
The central 5-HT turnover was evaluated by the cerebrospinal fluid (CSF) level of 5-hydroxyindole-3-acetic acid (5-HIAA, a major metabolite of 5-HT and therefore an index of 5-HT turnover), while the HPA axis activity was assessed by the plasma levels of cortisol and ACTH, cortisol response to ACTH challenge and dexamethasone (DEX) suppression. The detailed phenotying procedures have been described elsewhere (Tiefenbacher et al. 2000; Tiefenbacher et al. 2004) and the involved monkeys are indicated in Fig. S1. Briefly, cisternal CSF samples were withdrawn from 21 monkeys between 1000–1200h in 1997, following an overnight fast and Telazol (5 mg/kg, i.m.) anesthetization, and the CSF samples were stored at −80°C for no longer than 2 months before assayed for 5–HIAA by the isocratic high-performance liquid chromatography with electrochemical detection (HPLC–EC). In December 1997, blood samples were collected twice for 22 monkey following ketamine (10mg/kg, i.m.) anesthetization, once between 08:30 and 09:00 h (AM sample) and again between 15:30 and 16:00 h (PM sample). At least 5 days elapsed between the AM and PM drawings for a given subject. Approximately half of the animals were sampled first in the morning, and the remaining animals were sampled first in the afternoon. Following ketamine anesthetization, 10 ml of femoral blood was drawn from each monkey into two 5-ml EDTA-containing vacutainer tubes. The blood samples were chilled and centrifuged at 6°C for 10 min at 10,000 rpm, and plasma was stored at −40°C until analyzed in duplicate for cortisol and ACTH in our laboratory using commercially available radioimmunoassay (RIA) kits from ICN Biomedicals (Costa Mesa, CA, USA).
In the spring of 1999, HPA negative feedback sensitivity was examined in 19 monkeys. Urine samples were collected twice each day (at 08:00 h and 16:00 h, respectively) over a 3-day baseline period (BSL1-3) to estimate basal 24-h urinary free cortisol excretion. Upon completion of baseline sampling at 16:00 h on BSL3, a low dose of DEX (12 μg/kg, i.m.) was administered. Urine samples were stored at −20°C for no more than 2 months before assayed for cortisol using RIA kit. DEX-induced cortisol suppression was compared in two additional urine fractions collected at 08:00 h (DEX-N) and at 16:00 h (DEX-D) after the DEX injection. In the summer of 2000, 24 monkeys were subjected to a combined DEX/ACTH challenge. After an overnight fast, a high dose of DEX (0.5 mg/kg, i.m.) was administered to each subject at 08:00 h to suppress endogenous HPA axis activity. At 13:00 h, 5 h post-injection, subjects were anesthetized with ketamine (15 mg/kg, i.m.) and a catheter was inserted into the right saphenous vein for both ACTH administration and serial blood sampling. Upon collection of an initial baseline blood sample, 10 ng/kg of synthetic ACTH (Cortrosyn®, Organon Inc., West Orange, NJ) was infused i.v. over a 60 s period and the cortisol response was monitored in two additional blood samples collected at 15 and 30 min post-ACTH. Additional ketamine (7.5 mg/kg, i.m.) was administered as necessary to maintain adequate sedation. Blood samples were processed, stored and assayed for cortisol as mentioned above.
Based on an assessment of veterinary records spanning a period from 1990 to 2004, 20 of the monkeys were identified as self-wounder that experienced one or more instances of self-inflicted wounding serious enough to require veterinary treatment, while 11 animals were non-wounder (NW) and the remaining one had no sufficient record to determine the history of self-wounding. The self-biting rates were determined in 1999 and 2000 by systematical observation using a modified frequency scoring system described elsewhere (Novak et al. 1998; Novak et al. 1992), in which a 5-min sampling period was subdivided into 20 15s-intervals. The presence or absence of self-biting was scored by observers who were trained to identify behaviors from an established ethogram of rhesus monkey behavior. An average of 334 five-minute observations were made on each monkey, and each of the observers met an inter-observer reliability criterion of 90% agreement. Biting rate was defined as the mean number of intervals per 5-min sample in which “self-bite” occurred (Tiefenbacher et al. 2000). Based on the median split of the self-biting distribution in 2000, 12 subjects were identified as high-frequency biters (HFB) while another 12 subjects were low-frequency biters (LFB) (same animals as described in Tiefenbacher et al. 2004). The self-injurious group for each subject is shown in Fig. S1.
Genotyping
The −363T>G and 3′-UTR polymorphisms have been previously genotyped (Chen et al. 2006). To genotype other 5′-FR polymorphisms, we amplified a DNA fragment spanning all these loci using primers KpnI-rhTPH2(−1633)F and rhTPH2(−974)R, followed by PCR product purification and DNA sequencing, as described previously (Chen et al. 2006). However, messy trace chromatograms were observed due to the repeat and indel polymorphisms, and sequencing of PCR products did not resolve haplotype phase. So, PCR products were then cloned into pGEM-T vector, followed by plasmid DNA isolation and sequencing with a vector primer T7f (5′-taatacgactcactataggg-3′) or PCR primers. For each sample, 3 positive clones were initially selected for plasmid DNA isolation and sequencing, with more clones being added until both haplotypes were established (haplotype heterozygosity was determined by the sequencing trace chromatogram of PCR products). Both strands were sequenced as necessary.
Data analysis
Statistics were performed using the SAS Software Version 9.1 (SAS Institute Inc., USA). Because the polymorphisms/haplotypes of rhTPH2 5′-FR are rather complicated, Spearman’s rank correlation were initially performed to assess the relationship between specific genotypes and phenotypic variables, so as to screen the specific rhTPH2 5′-FR locus/haplotype that is most likely linked to alteration in phenotypic variables. The Spearman’s rank correlation is performed without specific hypothesis, and the outcomes were then examined for their compliance with the reporter gene expression data. If a locus/haplotype shows a significant correlation with phenotypic variables and exerts a significant effect on reporter gene expression, it is presumed to be functionally significant. To perform genotype-phenotype correlation correlation, the incidence of the potentially functional polymorphism/haplotype will also be considered because a rare polymorphism/haplotype will not yield enough sample size for specific genotype group. The selected rhTPH2 5′-FR locus, together with the 3′-UTR genotypes, were treated as two independent variables for two-way analysis of variance using the GLM procedure and Type III Sums-of-Squares (SS), followed by multiple comparisons of Tukey-Kramer least-squares means, so as to evaluate their independent and interactive effects on phenotypic variables. Departed by ~95 kb, the rhTPH2 5′ and 3′ polymorphisms showed no LD and are expected to transmit independently, just like the case for two loci residing on two different chromosomes. So, it is rational for us to treat the rhTPH2 5′ and 3′ polymorphisms as two independent variables. For variables (HPA axis and self-biting rate) measured on two different occasions, repeated measures ANOVA (RANOVA) were also carried out. Comparisons of the genotype/allele distribution between SIB groups were performed by appropriate chi-square test. Hardy-Weinberg Equilibrium (HWE) and linkage disequilibrium (LD) were assessed by the SAS Allele procedure.
Results
The effects of rhTPH2 5′-FR haplotypes on reporter gene expression
Luciferase expression driven by the rhTPH2 5′ haplotypes is shown in Fig.1d. In RN46A cells, M3 and M7 haplotypes showed the highest and lowest expression, respectively, while other haplotypes showed various levels of expression in between. The deletion construct M0 showed reduced expression, suggesting positive cis-element(s) may exist in the highly polymorphic region. The effect of individual polymorphisms on gene expression was interpreted by comparison between haplotypes that differ at the specific locus. Noticeable was the effect imparted by the −1485 locus. The most common haplotype M3 (31% in the population) that harbors 7 AT repeats at −1485 showed a significantly higher expression compared to all other haplotypes, and either an increase or decrease in the number of repeats lowered expression. The −1325 and −1454 loci also exerted significant effects on gene expression, as inferred by M3 vs M4 and M5 vs M5′, respectively. In HEK-293 cells, M4 and M7 haplotypes showed the highest and lowest expression, respectively. Thus, we postulate that rhTPH2 5′-FR polymorphisms may differentiate TPH2 gene expression in vivo.
Distribution of rhTPH2 5′-FR polymorphisms in the cohort of 32 rhesus monkey
The rhTPH2 5′-FR and 3′-UTR genotypes for each monkey are shown in Fig. S1. All of the polymorphisms are common, and with the exception of −1485(AT)n and −1454A>G, they conform to HWE. As shown in Fig.1c, besides the previously identified haplotypes (M1–M9), 5 novel haplotypes (M4′, M5′ and N1–N3) were identified experimentally by cloning. Similar to our previous findings (Chen et al. 2006), LD existed between polymorphisms in the same 5′ or 3′ region, but not between 5′ and 3′ regions (data not shown). The increased number of haplotypes for rhTPH2 5′-FR seems to be driven by a high mutation rate at locus −1485 that has resulted in alleles identical by state, but not descent, which may explain partially why this polymorphism is not in HWE.
Association of rhTPH2 5′ and 3′ genotypes with CSF 5-HIAA and HPA axis function
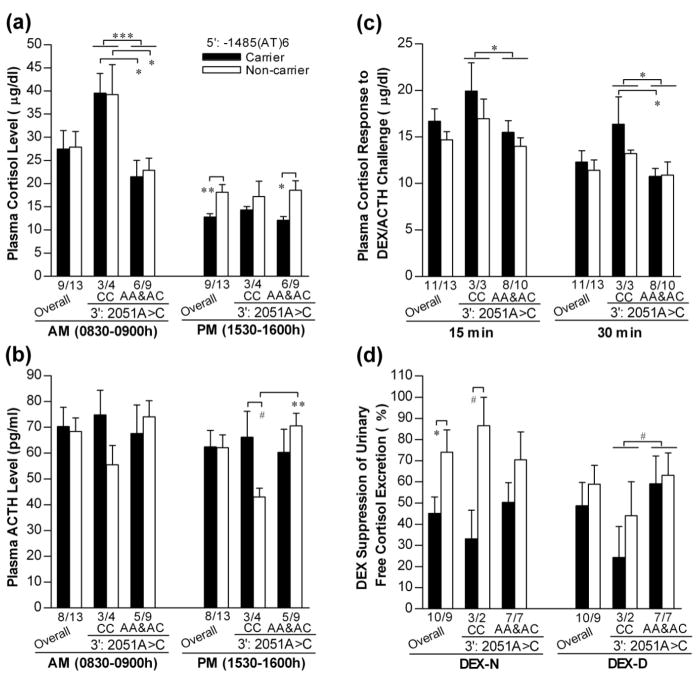
We have previously reported the association of rhTPH2 3′-UTR polymorphisms with gene expression and HPA axis function, with 2051CC homozygotes showing strikingly higher morning cortisol level than 2051AA&AC (Chen et al. 2006). However, polymorphisms in 5′-and 3′-regulatory regions may interact to influence TPH2 expression and HPA axis function, making it possible that the association for a specific locus may be affected by another. In addition, it is unknown whether 2051A>C associates with SIB or not. For these considerations, 2051A>C was also integrated for data analysis. Due to the complexity of rhTPH2 5′-FR, we first performed a Spearman’s rank correlation analysis to screen for polymorphisms or haplotypes potentially linked to phenotypic variables. Based on the correlation coefficient (Table S1), as well as the frequency distribution and reporter assay data, the −1485(AT)6 allele was of particular interest for genotype-phenotype correlation. The −1485(AT)6 allele, which is always accompanied by the −1454A/−1325del allele, is carried by the common M2 and M9 haplotypes that share a similar reduced gene expression, and by a rare N3 haplotype that was not tested for reporter expression. Accordingly, each subject was grouped as “carrier” or “non-carrier” of (AT)6 allele according to the −1485(AT)n genotype, and as “CC” or “AA&AC” according to the 2051A>C genotype for further analyses.
Comparisons of the CSF 5-HIAA between rhTPH2 genotypes are shown in Fig.2. Both −1485(AT)n and 2051A>C had a significant main effect on CSF 5-HIAA (F(1,20)=4.49, p=0.049 for −1485(AT)n; F(1,20)=5.39, p=0.033 for 2051A>C), and interacted to influence CSF 5-HIAA (F(1,20)=12.74, p=0.0024). 2051CC showed significantly higher CSF 5-HIAA than AA&AC in −1485(AT)6 non-carriers (t=3.02, p=0.029) while no such difference was observed in (AT)6 carriers, and −1485(AT)6 carriers showed lower CSF 5-HIAA than non-carriers in 2051CC group (t=4.91, p=0.0005) but not in AA&AC group.
Figure 2.
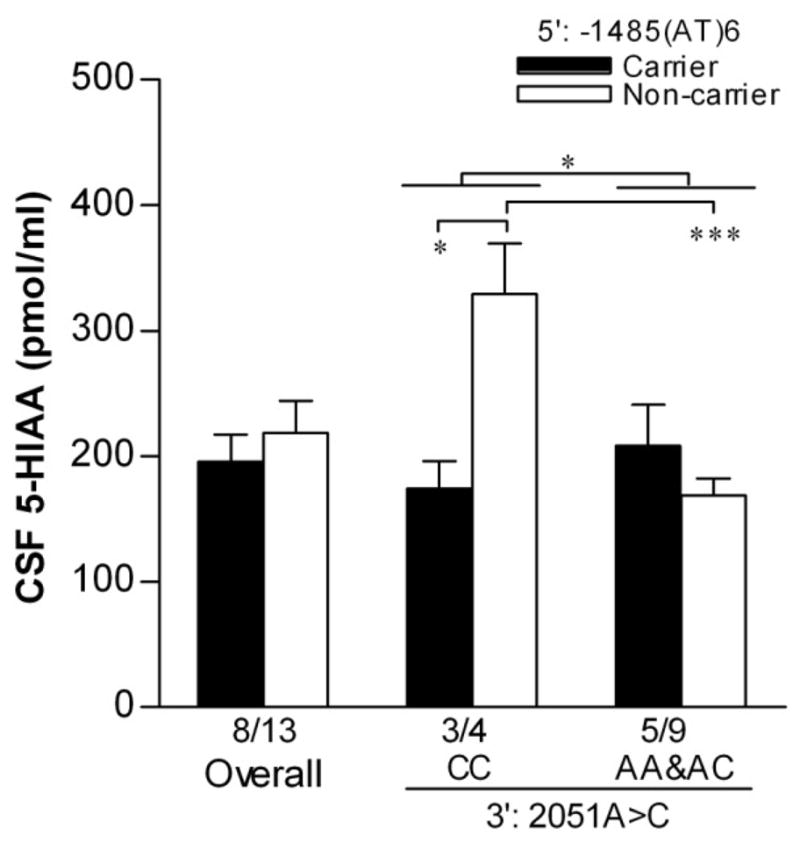
Comparisons of CSF level of 5-HIAA (1997 sample) between rhTPH2 genotypes. Subjects were grouped as −1485(AT)6 carriers or non-carriers, and stratified by 2051A>C genotype (CC or AA&AC). Data are shown as Mean±SEM and sample size (n) for each group is indicated. *p<0.05, **p<0.01, ***p<0.001 for comparisons between indicated groups.
Comparisons of plasma cortisol level between rhTPH2 groups are shown in Fig.3a. Plasma cortisol level differed significantly between the AM and PM samples (RANOVA: F(1,18)=41.04, p<0.0001). In consistence with our previous findings (Chen et al. 2006), 2051A>C exerted a significant main effect on cortisol level in the morning (ANOVA: F(1,21)=16.49, p=0.0007), but not in the afternoon. In contrast, the −1485(AT)n showed no main effect on AM cortisol level but a main effect on PM cortisol level (ANOVA: F(1,21)=5.93, p=0.026; carriers<non-carriers), which was pronounced in 2051AA&AC animals. Fig.3b shows the comparisons of plasma ACTH level between rhTPH2 groups. Both 2051A>C and −1485(AT)n showed no significant main effect on ACTH level, but a significant interaction was observed for the PM sample (ANOVA: F(1,20)=5.21, p=0.036). In 2051CC homozygotes, −1485(AT)6 non-carriers tended to have lower ACTH level than carriers (RANOVA: F(1,5)=4.48, p=0.088), while in −1485(AT)6 non-carriers, 2051CC homozygotes showed significantly lower ACTH level than AA&AC (RANOVA: F(1,11)=7.81, p=0.017). Accordingly, animals with 2051CC and −1485(AT)6 non-carrier genotypes showed the lowest ACTH level.
Figure 3.
Comparisons of the plasma levels of cortisol (a) and ACTH (b), plasma cortisol response to ACTH stimulation (c), and DEX suppression of urinary cortisol excretion (d) between rhTPH2 genotypes. Subjects are grouped as −1485(AT)6 carriers or non-carriers, and stratified by 2051A>C genotype (CC or AA&AC). *p<0.05, **p<0.01, ***p<0.001, #0.05≥p<0.10 for comparisons between indicated groups.
Comparisons of the cortisol response to ACTH stimulation between rhTPH2 groups are shown in Fig.3c. Just like the case for the morning cortisol level, 2051A>C exerted a significant main effect (CC>AA&AC; RANOVA: F(1,20)=6.08, p=0.023) on cortisol response to ACTH challenge, while −1485(AT)n did not. As shown in Fig.3d, −1485(AT)n showed a significant main effect on DEX suppression of cortisol excretion during the nighttime (carrier<non-carrier; ANOVA: F(1,18)=5.86, p=0.029) but not daytime, while 2051CC tended to have lower DEX suppression than 2051AA&AC (ANOVA: F(1,18)=3.39, p=0.085) during the daytime.
Association of rhTPH2 5′ and 3′ genotypes with SIB
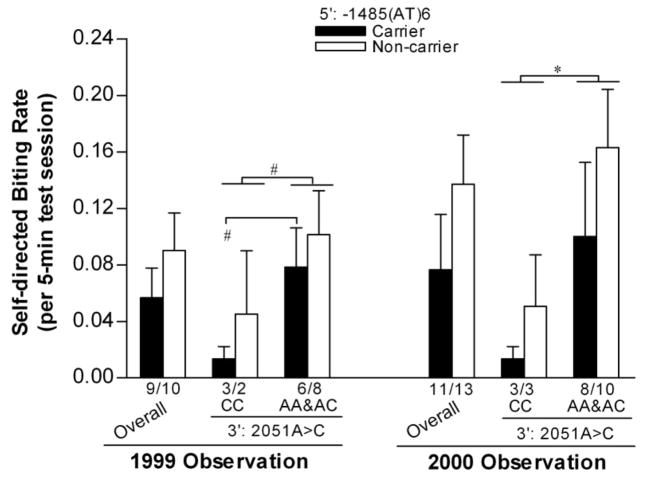
As shown in Fig.4, 2051A>C exerted a marginally significant main effect on self-biting rate (CC<AA&AC; RANOVA: F(1,16)=3.88, p=0.066; t test: t=1.77, p=0.095 and t=2.75, p=0.012 for the 1999 and 2000 observations, respectively), while −1485(AT)n did not.
Figure 4.
Comparisons of self-directed biting rate between rhTPH2 genotypes. Animals are grouped as −1485(AT)6 carriers or non-carriers, and stratified by 2051A>C genotype (CC or AA&AC). *p<0.05, #0.05≥p<0.10 for the comparisons between designated groups.
We then compared rhTPH2 genotype/allele distribution between SIB groups. As shown in Table 1, both genotype and allele frequencies of −1325Ins>Del differed significantly between SW and NW, but not between HFB and LFB. There are a total of eight −1325Ins/Ins homozygotes in the cohort of monkeys, and with the exception of one subject whose veterinary record was unavailable, all of them had a history of self-wounding. The −1325Ins allele frequency was 0.55 (22/40) in SW and 0.18 (4/22) in NW (χ2=6.644, p=0.005), with an odds ratio of 5.5 (95% CL: 1.576~19.192). In contrast, the genotype and allele frequencies of 2128S>L differed significantly between HFB and LFB (L allele: 9/24 for HFB and 3/24 for LFB, χ2=4.000, p=0.047), with an odds ratio of 0.2381 (95% CL: 0.055~1.030) for the 2128S allele. Consistently, distribution of rhTPH2 5′-FR haplotypes differed significantly between SW and NW, while distribution of rhTPH2 3′-UTR haplotypes differed significantly between HFB and LFB (Table S2).
Table 1.
Comparisons of the genotypic and allelic distribution of the rhTPH2 5′-FR and 3′-UTR polymorphisms between SIB groups
| Polymorphism | Genotype/Allele | Self-injury history |
Biting frequency |
||||
|---|---|---|---|---|---|---|---|
| SW(n=20) | NW(n=11) | *p value | HFB(n=12) | LFB(n=12) | *p value | ||
| −1605T>C | TT | 14 | 9 | 9 | 9 | ||
| TC | 6 | 2 | 0.6757 | 3 | 3 | 1.0000 | |
| C/T | 6/34 | 2/20 | 0.7003 | 3/21 | 3/21 | 1.0000 | |
| −1491Tn | T(6,4) | 1 | - | - | - | ||
| T(6,6) | 13 | 8 | 9 | 8 | |||
| T(6,7) | - | 1 | - | 1 | |||
| T(6,8) | 6 | 2 | 0.7837 | 3 | 3 | 0.8173 | |
| T: 4/6/7/8 | 1/33/-/6 | -/19/1/2 | 0.7972 | -/21/-/3 | -/20/1/3 | 0.8309 | |
| −1485(AT)n | AT(5,7) | 1 | - | 1 | - | ||
| AT(6,6) | - | 1 | 1 | - | |||
| AT(6,7) | 4 | 6 | 3 | 5 | |||
| AT(6,8) | 3 | - | - | 2 | |||
| AT(7,8) | 10 | 4 | 7 | 5 | |||
| AT(8,9) | 2 | - | 0.1194 | - | - | 0.8646 | |
| AT: 5/6/7/8/9 | 1/7/15/15/2 | -/8/10/4/- | 0.0594 | 1/5/11/7/- | -/7/10/7/- | 1.0000 | |
| −1454A>G | AA | 15 | 10 | 10 | 11 | ||
| AG | 3 | 1 | 2 | - | |||
| GG | 2 | - | 0.2304 | - | 1 | 1.0000 | |
| A/G | 7/33 | 1/21 | 0.1441 | 2/22 | 2/22 | 1.0000 | |
| −1325In>Del (TA>--) | DelDel | 5 | 7 | 5 | 5 | ||
| InsDel | 8 | 4 | 5 | 5 | |||
| InsIns | 7 | - | 0.0118 | 2 | 2 | 1.0000 | |
| Ins/Del | 22/18 | 4/18 | 0.0047 | 9/15 | 9/15 | 1.0000 | |
| −363T>G | TT | 6 | 6 | 4 | 5 | ||
| TG | 10 | 5 | 6 | 7 | |||
| GG | 4 | - | 0.0817 | 2 | - | 0.3265 | |
| T/G | 22/18 | 17/5 | 0.0702 | 10/14 | 7/17 | 0.5469 | |
| 2051A>C | AA | 6 | 3 | 4 | 3 | ||
| AC | 10 | 5 | 7 | 4 | |||
| CC | 4 | 3 | 0.7142 | 1 | 5 | 0.1739 | |
| A/C | 22/18 | 11/11 | 0.7081 | 9/15 | 14/10 | 0.1238 | |
| 2128S>L (1503A>G) | SS (AA) | 10 | 6 | 4 | 9 | ||
| SL (AG) | 10 | 4 | 7 | 3 | |||
| LL (GG) | - | 1 | 0.8317 | 1 | - | 0.0378 | |
| S/L (A/G) | 30/10 | 16/6 | 1.0000 | 15/9 | 21/3 | 0.0467 | |
Statistics for the 2×2 and R×C contingency tables were evaluated by the Fisher’s exact test and Mantel-Haenszel Chi-Square test, respectively. SW: self-wounder; NW: non-wounder; HFB: high-frequency biter; LFB: low-frequency biter. The 1503A>G and 2128S>L polymorphisms in rhTPH2 3′-UTR are completely linked.
Comparison of phenotypic variables between SIB groups
In support of the notion that monkeys with a self-wounding history typically engage in some forms of self-directed biting (Novak 2003), 10 of the 12 HFB monkeys were SW, while 7 of the 12 LFB monkeys were NW (Fig. S1; χ2=4.444, p=0.045), indicating that HFB is associated with a higher risk of developing self-wounding. However, it is yet to be determined whether self-biters and self-wounders are two separate populations, or whether self-biters eventually wound themselves (Lutz et al. 2007). Due to the incomplete overlap between the self-wounders and self-biters, as well as the differential association of rhTPH2 5′ and 3′ genotypes with self-wounding and self-biting, we presume that distinct physiology may underlie the two types of SIB groups. The phenotypic data have been previously compared between SIB groups (Tiefenbacher et al. 2000; Tiefenbacher et al. 2004), but the comparisons did not consider the confounding effect between the two types of SIB groups. Also, the self-wounding group has been updated since then, with additional 3 and 2 animals identified as SW and NW (shown in Fig. S1), respectively. Accordingly, we performed an updated analysis for the correlation between SIB and physiology.
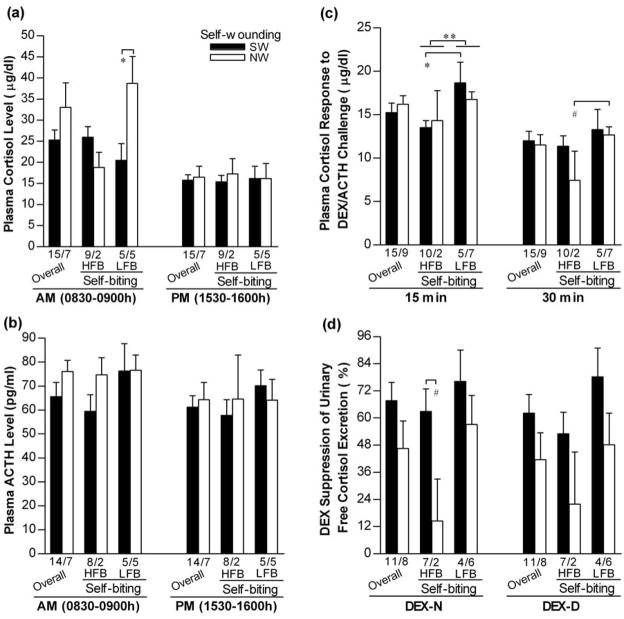
Comparisons of HPA axis function between SIB groups after stratification were showed in Fig.5. While there was self-wounding by self-biting interaction for the AM cortisol level (ANOVA: F(1,20)=6.78, p=0.019), SIB groups showed no differences in the PM cortisol and plasma ACTH levels (Fig. 5a and 5b). HFB exhibited lower cortisol response to ACTH challenge (Fig.5c; ANOVA: F(1,23)=5.27, p=0.033 for 15-min and F(1,23)=3.59, p=0.073 for 30-min; RANOVA: F(1,20)=4.84, p=0.040), and tended to have lower HPA negative feedback than LFB (Fig.5d; RANOVA: F(1,15)=3.96, p=0.065), while SW had significant higher HPA negative feedback (especially during the night) than NW (Fig.5d; ANOVA: F(1,18)=5.32, p=0.036 for DEX-N and F(1,18)=4.17, p=0.059 for DEX-D; RANOVA: F(1,15)=5.66, p=0.031).
Figure 5.
Comparison of plasma levels of cortisol (a), ACTH (b), cortisol response to ACTH challenge (c), and DEX suppression of urinary cortisol excretion (d) between SIB groups. *p<0.05, **p<0.01, #0.05≥p<0.10 for comparisons between designated groups.
Discussion
By using the reporter assay, we demonstrated that rhTPH2 5′-FR haplotypes exert a significant, cell-dependent effect on gene expression in vitro. In the 5-HT-positive RN46A cells, the predominant M3 haplotype showed the highest expression amongst the examined haplotypes, and deletion of the highly polymorphic region down-regulated gene expression, suggesting positive cis-element(s) may exist in this region and its binding with trans-factors may be affected by genetic variants. While the reporter assay suggests that the −1485, −1454 and −1325 loci in high linkage exert considerable effects on gene expression, genotype-phenotype correlation revealed that −1485(AT)n and −1325Ind/Del polymorphisms are linked to HPA axis function and susceptibility to self-wounding, respectively. Accordingly, specific polymorphisms in rhTPH2 5′-FR are functionally significant.
This study revealed that rhTPH2 5′- and 3′-regulatory polymorphisms interact to influence central 5-HT function (presumably resulting from altered TPH2 expression), which in turn may alter HPA axis function and behavioral traits. While the 3′-UTR 2051A>C exerted a significant effect on AM cortisol level, cortisol response to ACTH stimulation and daytime HPA negative feedback, the 5′-FR −1485(AT)n significantly influenced the PM cortisol level and nighttime HPA negative feedback. These findings strongly suggest that 2051A>C differentiates HPA axis activity upon its activation, while −1485(AT)n moderates the basal activity of HPA axis. It is noteworthy that unlike cortisol, ACTH showed no remarkable AM-PM fluctuation, and the morning ACTH level did not differ between 2051A>C groups, suggesting the disassociation of ACTH and cortisol, a phenomenon observed under many physiological and pathophysiological conditions (Bornstein et al. 2008). It has been recognized that the pituitary and adrenal gland activation are differentially regulated. For example, the rhythm of glucocorticoids is regulated by a gating mechanism residing in the adrenal cortical clock, which determines the adrenal response to ACTH stimulation (Oster et al. 2006; Torres-Farfan et al. 2009). In addition, increasing evidence indicates that ACTH-independent mechanisms may have an important role in fine-tuning and modulating the highly sensitive adrenal stress system to adapt its response appropriately to physiological needs (Bornstein et al. 2008; Dickmeis 2009). In the present study, 2051A>C differentiates both morning cortisol level and cortisol response to ACTH, but not the ACTH level, suggesting that the genotype-dependent cortisol release upon HPA axis activation is primarily conferred by altered adrenal response to ACTH or ACTH-independent mechanisms, but not due to the variation in ACTH level. Our findings, in line with the documented association of 5-HTT and MAOA gene polymorphisms with the HPA axis in rhesus monkeys (Barr et al. 2004b; Capitanio et al. 2008; Karere et al. 2009; McCormack et al. 2009), reinforce the role of 5-HT in the regulation of HPA axis function.
It has been documented that anesthetic agents including ketamine and telazol can affect cortisol and ACTH levels, despite the discrepancy between literatures (Bentson et al. 2003; Broadbear et al. 2004; Powers & Wood 2007). In rhesus macaques, Broadbear et al. (2004) reported inhibition of the cortisol response after administration of ketamine, while Bentson et al. (2003) found that telazol but not ketamine reduced morning cortisol level. In baboons, however, ketamine but not telazol increased cortisol level (Bentson et al. 2003). These findings suggest that the effect of anesthetic agents on the HPA axis may depend on different species of animals, the time of drug administration to sample collection, stress experience, and other environmental factors. As for the cohort of monkeys in our present study, Tiefenbacher et al. (2000 and 2004) have previously reported that the plasma ACTH and cortisol levels were unrelated to the time needed for sedation or blood drawing, or the time intervals involving ketamine administration and sample collection. Although the possibility that ketamine affects cortisol and ACTH levels in our present study could not be excluded due to the lack of control, we speculate that variation in cortisol and ACTH levels in our present study should primarily result from genetic variance rather than from the injection of ketamine, because all the involved monkeys received the identical treatment and blood samples were drawn immediately upon sedation. In other words, it is unlikely that the effect of genetic variance on the HPA axis will be obscured by administration of ketamine or other anesthetic agents. In support of this notion, a marginally significant main effect of 2051A>C on cortisol (ANOVA: F(1,21)=3.70, p=0.070; Fig. S2a), as well as a significant interaction of −1485(AT)n and 2051A>C on ACTH (ANOVA: F(1,21)=6.41, p=0.021; Fig. S2b), was observed for another set of blood samples collected in conjunction with CSF samples in the late morning (1000–1200h) following telazol (5mg/kg, i.m.) anesthetization in the same cohort of monkeys (detail procedures are available in Tiefenbacher et al. 2000), consistent with the findings with ketamine anesthetization. Hence, it is expected that the genetic regulation of 5-HIAA and HPA axis would not be essentially obscured by an identical administration of ketamine or telazol when the experimental procedures are adequately controlled. Notably, like our present study, 3–5mg/kg of telazol was also employed by other groups to collect CSF samples for measuring monoamine metabolites (Winslow et al. 2003; Maestripieri et al. 2006; Felger et al. 2007), suggesting that the effect of telazol on monoamine metabolites is negligible. As for the combined DEX/ACTH challenge test, some animals received multiple doses of ketamine while the others were given a single dose during the experimental procedure; however, we found no significant effect of multiple ketamine injections on cortisol level (Fig. S3). In addition, the effect of 2051A>C on cortisol response to ACTH challenge is similar to that on the morning cortisol surge induced by the biological clock, suggesting that ketamine will not obscure the effect of rhTPH2 genetic variance on the cortisol response to stress.
Mechanism(s) underlying the differential association of rhTPH2 5′ and 3′ polymorphisms with HPA axis function are yet to be determined; however, the distinct role of 5′-FR and 3′-UTR in gene expression regulation, as well as the characteristics of TPH2 gene expression, might be contributable. While 5′-FR modulates gene transcription, 3′-UTR regulation of gene expression primarily involves post-transcriptional processes including mRNA stability, translocation and translation. Based on the rhythmic TPH2 expression (Liang et al. 2004; Malek et al. 2005) and 5′-UTR repression of TPH2 expression (Chen & Miller 2009; Chen et al. 2008; Patel et al. 2007), it is likely that in the absence of clock or stress stimulation, TPH2 exhibits a low level of constitutive expression which might be differentiated by specific polymorphisms in TPH2 5′-FR. However, when TPH2 expression is induced by clock or stress stimulation, the inductive TPH2 expression will dominate and therefore attenuate the effect of 5′-FR genotype on gene expression unless the 5′-FR polymorphism per se differentiates the induction, and 3′-UTR-associated post-transcriptional processes may then function as the major determinant of gene expression. In support of this presumption, our previous study showed that deletion of the repressive 5′-UTR eliminates the effect of hTPH2 5′-FR haplotype on gene expression in vitro (Chen et al. 2008). Meanwhile, another one of our previous studies showed that the 2051C allele exhibits significantly lower gene expression than 2051A (Chen et al. 2006), and new preliminary data shows that this allele-specific expression primarily results from an alteration in mRNA stability (Fig. S4). Consistently, the effect of −1485(AT)n on CSF 5-HIAA level was pronounced in the 2051CC group (low expression expected), while 2051A>C exerted a significant effect on 5-HIAA in −1485(AT)6 non-carriers (high expression expected), but not in −1485(AT)6 carriers (low expression expected) (Fig. S2). Thus, specific polymorphisms in the 5′-FR and 3′-UTR may preferentially function under the circumstance of low (or constitutive) and high (or inductive) TPH2 gene expression, respectively. Nevertheless, this presumption requires further verification.
SIB is a significant health problem for which there is still no effective treatment, and it is still unknown whether self-biters and self-wounders are two separate populations, or whether self-biters eventually wound themselves (Lutz et al. 2007). In our present study, both genotype-phenotype correlation and case-control analysis showed that rhTPH2 5′-FR and 3′-UTR polymorphisms are linked to self-wounding and self-biting, respectively, suggesting that the two types of SIB may involve distinct pathophysiology. Notably, HFB and LFB differed significantly in cortisol response to ACTH that is influenced by 2051A>C, while SW and NW differed in HPA negative feedback (especially during nighttime) that is modulated by −1485(AT)n. Hence, self-wounding and self-biting behaviors may be linked to distinct aspects of the HPA axis, and conversely, distinct aspects of the HPA axis may underlie distinct behavioral traits. In support of this assumption, it has been reported in rhesus monkeys that the AM and PM cortisol levels are differentially associated with the personality traits of confidence and excitability, respectively (Capitanio et al. 2004). Thus, like the case for the 5-HTT (Gotlib et al. 2008), HPA axis function may underlie the association between TPH2 and SIB. Consistently, the association of rhTPH2 2128S>L with SIB score was also observed in a larger population of rhesus monkeys (http://www.asp.org/asp2008/abstractDisplay.cfm?abstractID=2422&confEventID=2514).
This study focused on the functionality of rhTPH2 5′-FR polymorphisms, as well as its potential interaction with the rhTPH2 3′-UTR polymorphisms to influence 5-HT turnover, HPA axis function and SIB. However, previous studies in rhesus monkeys have demonstrated that 5-HTT and MAOA gene polymorphisms interact with rearing experience to modulate central 5-HT metabolism, HPA axis function and behavioral traits (Barr et al. 2004a; Barr et al. 2004b; Bennett et al. 2002; Karere et al. 2009; Newman et al. 2005). Likewise, TPH2 genetic variance may interact with rearing experience to modulate 5-HT turnover, HPA axis function and behavioral traits (Chen et al. 2009); however, rearing experience does not essentially change the majority of effects of the rhTPH2 5′ and 3′ polymorphisms on phenotypic variables (Table S3 and Chen et al. 2009). Interestingly, rearing experience had no main effect on self-biting rate (Table S3) and did not distribute differentially between HFB and LFB (Fig. S1; χ2=0.269, p=0.604), while MR monkeys tended to develop self-wounding more frequently than PR monkeys (Fig. S1; 13 of 16 MR and 6 of 12 PR were SW, χ2=3.07, p=0.080). This finding of higher incidence of SW in MR monkeys seems to be inconsistent with our previous report of no difference between SIB groups with respect to rearing condition in the same cohort of monkeys (Tiefenbacher et al. 2004); however, as mentioned in the Result section, the self-wounding groups have been updated since our previous report (3 and 2 animals were additionally identified as SW and NW, respectively), suggesting a change in the composition of the self-wounding groups. Thus, it is not surprising that a discrepancy exists between our present study and previous report, and our present study should provide more accurate information on the relationship between rearing experience and SIB. These findings provide additional support for the involvement of distinct pathophysiology in self-wounding and-biting behaviors. Notably, it has been established that maternal care leads to increased expression of glucocorticoid receptor, and therefore enhanced HPA negative feedback and reduced cortisol response to stress (Liu et al. 1997). Hence, our finding of a link between enhanced HPA negative feedback and self-wounding history, as well as the previous finding of reduced cortisol response to stress in monkeys with SIB (Tiefenbacher et al. 2000), suggest that the relatively higher incidence of SW in MR monkeys may result from enhanced HPA negative feedback and therefore blunted cortisol response to stress. However, despite the rationality for our findings, it should be pointed out that in our sample, MR monkeys entered individual cage housing at an earlier age than did PR animals (Tiefenbacher et al. 2000), while early onset of individual housing appears to be a major predictor of the development of SIB (Lutz et al. 2003). Thus, an apparent difference among monkeys with different early rearing experiences may not actually indicate a rearing effect. Nevertheless, this interesting and rational link between enhanced HPA negative feedback and risk for self-wounding warrants further verification.
In summary, this study demonstrates the functionality of rhTPH2 5′-FR polymorphisms, and provides evidence for the differential association of rhTPH2 5′- and 3′-regulatory polymorphisms with HPA axis function and SIB. Also, this study reveals that self-wounders and self-biters are linked to alteration in distinct aspects of HPA axis function, and that HPA axis reactivity may underlie the association between rhTPH2 and SIB. Our findings shed light on the role of TPH2 gene variance in physiological and behavioral traits, and also contribute to the understanding of the pathophysiology and genetics of SIB.
Supplementary Material
Acknowledgments
This study was supported by MH077995 (GMM), MH082507 (EJV), AA016194 (GMM), DA025697 (GMM), RR11122 (MAN) and RR00168 (NEPRC). We thank Mrs. Hong Yang for technician support. We also acknowledge the NEPRC Primate Genetics Core for supplying genomic DNA and for genotyping assistance.
Abbreviations
- TPH2
Tryptophan hydroxylase-2
- 5-HT
Serotonin
- HPA axis
hypothalamic-pituitary-adrenal axis
- 3′-UTR
3′-untranslated region
- 5′-FR
5′-flaning region
- SIB
self-injurious behavior
- ACTH
Adrenocorticotropic hormone
- DEX
dexamethasone
- ANOVA
analysis of variance
- RANOVA
repeated measurement ANOVA
- CSF
cerebrospinal fluid
- 5-HIAA
5-hydroxyindole-3-acetic acid
- MR
Mother-reared
- PR
Peer-reared
References
- Barr CS, Newman TK, Lindell S, Shannon C, Champoux M, Lesch KP, Suomi SJ, Goldman D, Higley JD. Interaction between serotonin transporter gene variation and rearing condition in alcohol preference and consumption in female primates. Arch Gen Psychiatry. 2004a;61:1146–1152. doi: 10.1001/archpsyc.61.11.1146. [DOI] [PubMed] [Google Scholar]
- Barr CS, Newman TK, Shannon C, Parker C, Dvoskin RL, Becker ML, Schwandt M, Champoux M, Lesch KP, Goldman D, Suomi SJ, Higley JD. Rearing condition and rh5-HTTLPR interact to influence limbic-hypothalamic-pituitary-adrenal axis response to stress in infant macaques. Biol Psychiatry. 2004b;55:733–738. doi: 10.1016/j.biopsych.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Bennett AJ, Lesch KP, Heils A, Long JC, Lorenz JG, Shoaf SE, Champoux M, Suomi SJ, Linnoila MV, Higley JD. Early experience and serotonin transporter gene variation interact to influence primate CNS function. Mol Psychiatry. 2002;7:118–122. doi: 10.1038/sj.mp.4000949. [DOI] [PubMed] [Google Scholar]
- Bentson KL, Capitanio JP, Mendoza SP. Cortisol responses to immobilization with Telazol or ketamine in baboons (Papio cynocephalus/anubis) and rhesus macaques (Macaca mulatta) J Med Primatol. 2003;32:148–160. doi: 10.1034/j.1600-0684.2003.00018.x. [DOI] [PubMed] [Google Scholar]
- Bornstein SR, Engeland WC, Ehrhart-Bornstein M, Herman JP. Dissociation of ACTH and glucocorticoids. Trends Endocrinol Metab. 2008;19:175–180. doi: 10.1016/j.tem.2008.01.009. [DOI] [PubMed] [Google Scholar]
- Broadbear JH, Winger G, Woods JH. Self-administration of fentanyl, cocaine and ketamine: effects on the pituitary-adrenal axis in rhesus monkeys. Psychopharmacology. 2004;176:398–406. doi: 10.1007/s00213-004-1891-x. [DOI] [PubMed] [Google Scholar]
- Brown HJ, Henderson LA, Keay KA. Hypotensive but not normotensive haemorrhage increases tryptophan hydroxylase-2 mRNA in caudal midline medulla. Neurosci Lett. 2006;398:314–318. doi: 10.1016/j.neulet.2006.01.019. [DOI] [PubMed] [Google Scholar]
- Brown SM, Peet E, Manuck SB, Williamson DE, Dahl RE, Ferrell RE, Hariri AR. A regulatory variant of the human tryptophan hydroxylase-2 gene biases amygdala reactivity. Mol Psychiatry. 2005;10:884–888. doi: 10.1038/sj.mp.4001716. [DOI] [PubMed] [Google Scholar]
- Capitanio JP, Abel K, Mendoza SP, Blozis SA, McChesney MB, Cole SW, Mason WA. Personality and serotonin transporter genotype interact with social context to affect immunity and viral set-point in simian immunodeficiency virus disease. Brain Behav Immun. 2008;22:676–689. doi: 10.1016/j.bbi.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capitanio JP, Mendoza SP, Bentson KL. Personality characteristics and basal cortisol concentrations in adult male rhesus macaques (Macaca mulatta) Psychoneuroendocrinology. 2004;29:1300–1308. doi: 10.1016/j.psyneuen.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Cervo L, Canetta A, Calcagno E, Burbassi S, Sacchetti G, Caccia S, Fracasso C, Albani D, Forloni G, Invernizzi RW. Genotype-dependent activity of tryptophan hydroxylase-2 determines the response to citalopram in a mouse model of depression. J Neurosci. 2005;25:8165–8172. doi: 10.1523/JNEUROSCI.1816-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen GL, Miller GM. 5′-Untranslated region of the tryptophan hydroxylase-2 gene harbors an asymmetric bidirectional promoter but not internal ribosome entry site in vitro. Gene. 2009;435:53–62. doi: 10.1016/j.gene.2008.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen GL, Novak MA, Hakim S, Xie Z, Miller GM. Tryptophan hydroxylase-2 gene polymorphisms in rhesus monkeys: association with hypothalamic-pituitary-adrenal axis function and in vitro gene expression. Mol Psychiatry. 2006;11:914–928. doi: 10.1038/sj.mp.4001870. [DOI] [PubMed] [Google Scholar]
- Chen GL, Novak MA, Meyer JS, Kelly BJ, Vallender EJ, Miller GM. The effect of rearing experience and TPH2 genotype on HPA axis function and aggression in rhesus monkeys: A retrospective analysis. Horm Behav. 2009 doi: 10.1016/j.yhbeh.2009.10.012. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen GL, Vallender EJ, Miller GM. Functional characterization of the human TPH2 5 ′ regulatory region: untranslated region and polymorphisms modulate gene expression in vitro. Hum Genet. 2008;122:645–657. doi: 10.1007/s00439-007-0443-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cichon S, Winge I, Mattheisen M, Georgi A, Karpushova A, Freudenberg J, Freudenberg-Hua Y, Babadjanova G, Van Den Bogaert A, Abramova LI, Kapiletti S, Knappskog PM, McKinney J, Maier W, Abou Jamra R, Schulze TG, Schumacher J, Propping P, Rietschel M, et al. Brain-specific tryptophan hydroxylase 2 (TPH2): a functional Pro206Ser substitution and variation in the 5′-region are associated with bipolar affective disorder. Hum Mol Genet. 2008;17:87–97. doi: 10.1093/hmg/ddm286. [DOI] [PubMed] [Google Scholar]
- Clark JA, Pai LY, Flick RB, Rohrer SP. Differential hormonal regulation of tryptophan hydroxylase-2 mRNA in the murine dorsal raphe nucleus. Biol Psychiatry. 2005;57:943–946. doi: 10.1016/j.biopsych.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Cote F, Thevenot E, Fligny C, Fromes Y, Darmon M, Ripoche MA, Bayard E, Hanoun N, Saurini F, Lechat P, Dandolo L, Hamon M, Mallet J, Vodjdani G. Disruption of the nonneuronal tph1 gene demonstrates the importance of peripheral serotonin in cardiac function. Proc Natl Acad Sci USA. 2003;100:13525–13530. doi: 10.1073/pnas.2233056100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lara CL, Brezo J, Rouleau G, Lesage A, Dumont M, Alda M, Benkelfat C, Turecki G. Effect of tryptophan hydroxylase-2 gene variants on suicide risk in major depression. Biol Psychiatry. 2007;62:72–80. doi: 10.1016/j.biopsych.2006.09.008. [DOI] [PubMed] [Google Scholar]
- Dickmeis T. Glucocorticoids and the circadian clock. J Endocrinol. 2009;200:3–22. doi: 10.1677/JOE-08-0415. [DOI] [PubMed] [Google Scholar]
- Dinan TG. Serotonin and the regulation of hypothalamic-pituitary-adrenal axis function. Life Sci. 1996;58:1683–1694. doi: 10.1016/0024-3205(96)00066-5. [DOI] [PubMed] [Google Scholar]
- Felger JC, Alagbe O, Hu F, Mook D, Freeman AA, Sanchez MM, Kalin NH, Ratti E, Nemeroff CB, Miller AH. Effects of interferon-alpha on rhesus monkeys: A nonhuman primate model of cytokine-induced depression. Biol Psychiatry. 2007;62:1324–1333. doi: 10.1016/j.biopsych.2007.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontenot MB, Musso MW, McFatter RM, Anderson GM. Dose-Finding Study of Fluoxetine and Venlafaxine for the Treatment of Self-Injurious and Stereotypic Behavior in Rhesus Macaques (Macaca mulatta) J Am Assoc Lab Anim Sci. 2009;48:176–184. [PMC free article] [PubMed] [Google Scholar]
- Fontenot MB, Padgett EE, Dupuy AM, Lynch CR, De Petrillo PB, Higley JD. The effects of fluoxetine and buspirone on self-injurious and stereotypic behavior in adult male rhesus macaques. Comp Med. 2005;55:67–74. [PubMed] [Google Scholar]
- Furmark T, Appel L, Henningsson S, Ahs F, Faria V, Linnman C, Pissiota A, Frans O, Bani M, Bettica P, Pich EM, Jacobsson E, Wahlstedt K, Oreland L, Langstrom B, Eriksson E, Fredrikson M. A Link between Serotonin-Related Gene Polymorphisms, Amygdala Activity, and Placebo-Induced Relief from Social Anxiety. J Neurosci. 2008;28:13066–13074. doi: 10.1523/JNEUROSCI.2534-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner KL, Hale MW, Oldfield S, Lightman SL, Plotsky PM, Lowry CA. Adverse experience during early life and adulthood interact to elevate tph2 mRNA expression in serotonergic neurons within the dorsal raphe nucleus. Neuroscience. 2009;163:991–1001. doi: 10.1016/j.neuroscience.2009.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotlib IH, Joormann J, Minor KL, Hallmayer J. HPA Axis Reactivity: A Mechanism Underlying the Associations Among 5-HTTLPR, Stress, and Depression. Biol Psychiatry. 2008;63:847–851. doi: 10.1016/j.biopsych.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiroi R, McDevitt RA, Neumaier JF. Estrogen selectively increases tryptophan hydroxylase-2 mRNA expression in distinct subregions of rat midbrain raphe nucleus: Association between gene expression and anxiety behavior in the open field. Biol Psychiatry. 2006;60:288–295. doi: 10.1016/j.biopsych.2005.10.019. [DOI] [PubMed] [Google Scholar]
- Janis IB, Nock MK. Behavioral forecasts do not improve the prediction of future behavior: a prospective study of self-injury. J Clin Psychol. 2008;64:1164–1174. doi: 10.1002/jclp.20509. [DOI] [PubMed] [Google Scholar]
- Jollant F, Buresi C, Guillaume S, Jaussent I, Bellivier F, Leboyer M, Castelnau D, Malafosse A, Courtet P. The influence of four serotonin-related genes on decision-making in suicide attempters. Am J Med Genet B Neuropsychiatr Genet. 2007;144B:615–624. doi: 10.1002/ajmg.b.30467. [DOI] [PubMed] [Google Scholar]
- Karere GM, Kinnally EL, Sanchez JN, Famula TR, Lyons LA, Capitanio JP. What is an “Adverse” Environment? Interactions of Rearing Experiences and MAOA Genotype in Rhesus Monkeys. Biol Psychiatry. 2009;65:770–777. doi: 10.1016/j.biopsych.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke L, Qi ZY, Ping Y, Ren CY. Effect of SNP at position 40237 in exon 7 of the TPH2 gene on susceptibility to suicide. Brain Res. 2006;1122:24–26. doi: 10.1016/j.brainres.2006.09.007. [DOI] [PubMed] [Google Scholar]
- Kulikov AV, Osipova DV, Naumenko VS, Popova NK. Association between Tph2 gene polymorphism, brain tryptophan hydroxylase activity and aggressiveness in mouse strains. Genes Brain Behav. 2005;4:482–485. doi: 10.1111/j.1601-183X.2005.00145.x. [DOI] [PubMed] [Google Scholar]
- Liang J, Wessel JH, Iuvone PM, Tosini G, Fukuhara C. Diurnal rhythms of tryptophan hydroxylase 1 and 2 mRNA expression in the rat retina. Neuroreport. 2004;15:1497–1500. doi: 10.1097/01.wnr.0000131007.59315.66. [DOI] [PubMed] [Google Scholar]
- Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, Sharma S, Pearson D, Plotsky PM, Meaney MJ. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science. 1997;277:1659–1662. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- Lopez VA, Detera-Wadleigh S, Cardona I, Kassem L, McMahon FJ. Nested association between genetic variation in tryptophan hydroxylase II, bipolar affective disorder, and suicide attempts. Biol Psychiatry. 2007;61:181–186. doi: 10.1016/j.biopsych.2006.03.028. [DOI] [PubMed] [Google Scholar]
- Lowry CA. Functional subsets of serotonergic neurones: Implications for control of the hypothalamic-pituitary-adrenal axis. J Neuroendocrinol. 2002;14:911–923. doi: 10.1046/j.1365-2826.2002.00861.x. [DOI] [PubMed] [Google Scholar]
- Luiselli JK, Blew P, Thibadeau S. Therapeutic effects and long-term efficacy of antidepressant medication for persons with developmental disabilities- Behavioral assessment in two cases of treatment-resistant aggression and self-injury. Behav Modif. 2001;25:62–78. doi: 10.1177/0145445501251004. [DOI] [PubMed] [Google Scholar]
- Maestripieri D, McCormack K, Lindell SG, Higley JD, Sanchez MM. Influence of parenting style on the offspring’s behaviour and CSF monoamine metabolite levels in crossfostered and noncrossfostered female rhesus macaques. Behav Brain Res. 2006;175:90–95. doi: 10.1016/j.bbr.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Malek ZS, Dardente H, Pevet P, Raison S. Tissue-specific expression of tryptophan hydroxylase mRNAs in the rat midbrain: anatomical evidence and daily profiles. Eur J Neurosci. 2005;22:895–901. doi: 10.1111/j.1460-9568.2005.04264.x. [DOI] [PubMed] [Google Scholar]
- Malek ZS, Sage D, Pevet P, Raison S. Daily rhythm of tryptophan hydroxylase-2 messenger ribonucleic acid within raphe neurons is induced by corticoid daily surge and modulated by enhanced locomotor activity. Endocrinology. 2007;148:5165–5172. doi: 10.1210/en.2007-0526. [DOI] [PubMed] [Google Scholar]
- McCloskey M, Ben-Zeev D, Lee R, Berman M, Coccaro E. Acute tryptophan depletion and self-injurious behavior in aggressive patients and healthy volunteers. Psychopharmacology (Berl) 2009;203:53–61. doi: 10.1007/s00213-008-1374-6. [DOI] [PubMed] [Google Scholar]
- McCormack K, Newman TK, Higley JD, Maestripieri D, Sanchez MM. Serotonin transporter gene variation, infant abuse, and responsiveness to stress in rhesus macaque mothers and infants. Horm Behav. 2009;55:538–547. doi: 10.1016/j.yhbeh.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller BR, Bale TL. Sex-Specific Programming of Offspring Emotionality after Stress Early in Pregnancy. J Neurosci. 2008;28:9055–9065. doi: 10.1523/JNEUROSCI.1424-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman TK, Syagailo YV, Barr CS, Wendland JR, Champoux M, Graessle M, Suomi SJ, Higley JD, Lesch KP. Monoamine oxidase A gene promoter variation and rearing experience influences aggressive behavior in rhesus monkeys. Biol Psychiatry. 2005;57:167–172. doi: 10.1016/j.biopsych.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Novak MA. Self-injurious behavior in rhesus monkeys: New insights into its etiology, physiology, and treatment. Am J Primatol. 2003;59:3–19. doi: 10.1002/ajp.10063. [DOI] [PubMed] [Google Scholar]
- Novak MA, Kinsey JH, Jorgensen MJ, Hazen TJ. Effects of puzzle feeders on pathological behavior in individually housed rhesus monkeys. Am J Primatol. 1998;46:213–227. doi: 10.1002/(SICI)1098-2345(1998)46:3<213::AID-AJP3>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Novak MA, Oneill P, Suomi SJ. Adjustments and Adaptations to Indoor and Outdoor Environments- Continuity and Change in Young-Adult Rhesus-Monkeys. Am J Primatol. 1992;28:125–138. doi: 10.1002/ajp.1350280205. [DOI] [PubMed] [Google Scholar]
- Oster H, Damerow S, Kiessling S, Jakubcakova V, Abraham D, Tian J, Hoffmann MW, Eichele G. The circadian rhythm of glucocorticoids is regulated by a gating mechanism residing in the adrenal cortical clock. Cell Metab. 2006;4:163–173. doi: 10.1016/j.cmet.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Patel PD, Bochar DA, Turner DL, Meng F, Mueller HM, Pontrello CG. Regulation of tryptophan hydroxylase-2 gene expression by a bipartite RE-1 silencer of Transcription/Neuron restrictive silencing factor (REST/NRSF) binding motif. J Biol Chem. 2007;282:26717–26724. doi: 10.1074/jbc.M705120200. [DOI] [PubMed] [Google Scholar]
- Paul IA, Maciag D, Swilley S, Austin MC, Lin RCS, Simpson KL, Weaver K. Early life SSRI exposure in rats results in a lasting reduction in the expression of TPH2 mRNA in the dorsal raphe nucleus. Biol Psychiatry. 2007;61:308. [Google Scholar]
- Peters EJ, Slager SL, McGrath PJ, Knowles JA, Hamilton SP. Investigation of serotonin-related genes in antidepressant response. Mol Psychiatry. 2004;9:879–889. doi: 10.1038/sj.mp.4001502. [DOI] [PubMed] [Google Scholar]
- Pies RW, Popli AP. Self-injurious behavior: Pathophysiology and implications for treatment. J Clin Psychiatry. 1995;56:580–588. [PubMed] [Google Scholar]
- Powers MJ, Wood CE. Ketamine inhibits fetal ACTH responses to cerebral hypoperfusion. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1542–1549. doi: 10.1152/ajpregu.00300.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman MS, Thomas P. Molecular Cloning, Characterization and Expression of Two Tryptophan Hydroxylase (Tph-1 and Tph-2) Genes in the Hypothalamus of Atlantic Croaker: Down-Regulation after Chronic Exposure to Hypoxia. Neuroscience. 2009;158:751–765. doi: 10.1016/j.neuroscience.2008.10.029. [DOI] [PubMed] [Google Scholar]
- Sanchez RL, Reddy AP, Centeno ML, Henderson JA, Bethea CL. A second tryptophan hydroxylase isoform, TPH-2 mRNA, is increased by ovarian steroids in the raphe region of macaques. Brain Res Mol Brain Res. 2005;135:194–203. doi: 10.1016/j.molbrainres.2004.12.011. [DOI] [PubMed] [Google Scholar]
- Sivam SP. Dopamine, serotonin and tachykinin in self-injurious behavior. Life Sci. 1996;58:2367–2375. doi: 10.1016/0024-3205(96)00121-x. [DOI] [PubMed] [Google Scholar]
- Tiefenbacher S, Novak MA, Jorgensen MJ, Meyer JS. Physiological correlates of self-injurious behavior in captive, socially-reared rhesus monkeys. Psychoneuroendocrinology. 2000;25:799–817. doi: 10.1016/s0306-4530(00)00027-5. [DOI] [PubMed] [Google Scholar]
- Tiefenbacher S, Novak MA, Marinus LM, Chase WK, Miller JA, Meyer JS. Altered hypothalamic-pituitary-adrenocortical function in rhesus monkeys (Macaca mulatta) with self-injurious behavior. Psychoneuroendocrinology. 2004;29:501–515. doi: 10.1016/s0306-4530(03)00068-4. [DOI] [PubMed] [Google Scholar]
- Torres-Farfan C, Abarzua-Catalan L, Valenzuela FJ, Mendez N, Richter HG, Valenzuela GJ, Seron-Ferre M. Cryptochrome 2 Expression Level Is Critical for Adrenocorticotropin Stimulation of Cortisol Production in the Capuchin Monkey Adrenal. Endocrinology. 2009;150:2717–2722. doi: 10.1210/en.2008-1683. [DOI] [PubMed] [Google Scholar]
- Tzvetkov MV, Brockmoller J, Roots I, Kirchheiner J. Common genetic variations in human brain-specific tryptophan hydroxylase-2 and response to antidepressant treatment. Pharmacogenet Genomics. 2008;18:495–506. doi: 10.1097/FPC.0b013e3282fb02cb. [DOI] [PubMed] [Google Scholar]
- Van den Bogaert A, Sleegers K, De Zutter S, Heyrman L, Norrback KF, Adolfsson R, Van Broeckhoven C, Del-Favero J. Association of brain-specific tryptophan hydroxylase, TPH2, with unipolar and bipolar disorder in a Northern Swedish, isolated population. Arch Gen Psychiatry. 2006;63:1103–1110. doi: 10.1001/archpsyc.63.10.1103. [DOI] [PubMed] [Google Scholar]
- Walther DJ, Bader M. A unique central tryptophan hydroxylase isoform. Biochem Pharmacol. 2003;66:1673–1680. doi: 10.1016/s0006-2952(03)00556-2. [DOI] [PubMed] [Google Scholar]
- Walther DJ, Peter JU, Bashammakh S, Hortnagl H, Voits M, Fink H, Bader M. Synthesis of serotonin by a second tryptophan hydroxylase isoform. Science. 2003;299:76–76. doi: 10.1126/science.1078197. [DOI] [PubMed] [Google Scholar]
- Weld KP, Mench JA, Woodward RA, Bolesta MS, Suomi SJ, Higley JD. Effect of tryptophan treatment on self-biting and central nervous system serotonin metabolism in rhesus monkeys (Macaca mulatta) Neuropsychopharmacology. 1998;19:314–321. doi: 10.1016/S0893-133X(98)00026-8. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Noble PL, Lyons CK, Sterk SM, Insel TR. Rearing Effects on Cerebrospinal Fluid Oxytocin Concentration and Social Buffering in Rhesus Monkeys. Neuropsychopharmacology. 2003;28:910–918. doi: 10.1038/sj.npp.1300128. [DOI] [PubMed] [Google Scholar]
- Yates TM. The developmental psychopathology of self-injurious behavior: Compensatory regulation in posttraumatic adaptation. Clin Psychol Rev. 2004;24:35–74. doi: 10.1016/j.cpr.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Zhou ZF, Roy A, Lipsky R, Kuchipudi K, Zhu GS, Taubman J, Enoch MA, Virkkunen M, Goldman D. Haplotype-based linkage of tryptophan hydroxylase 2 to suicide attempt, major depression, and cerebrospinal fluid 5-hydroxyindoleacetic acid in 4 populations. Arch Gen Psychiatry. 2005;62:1109–1118. doi: 10.1001/archpsyc.62.10.1109. [DOI] [PubMed] [Google Scholar]
- Zill P, Baghai TC, Zwanzger P, Schule C, Eser D, Rupprecht R, Moller HJ, Bondy B, Ackenheil M. SNP and haplotype analysis of a novel tryptophan hydroxylase isoform (TPH2) gene provide evidence for association with major depression. Mol Psychiatry. 2004a;9:1030–1036. doi: 10.1038/sj.mp.4001525. [DOI] [PubMed] [Google Scholar]
- Zill P, Buttner A, Eisenmenger W, Moller HJ, Bondy B, Ackenheil M. Single nucleotide polymorphism and haplotype analysis of a novel tryptophan hyrdroxylase isoform (TPH2) gene in suicide victims. Biol Psychiatry. 2004b;56:581–586. doi: 10.1016/j.biopsych.2004.07.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.