Abstract
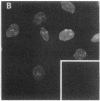
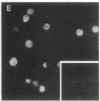
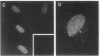
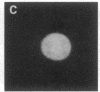
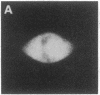
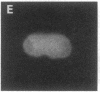
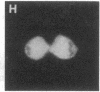
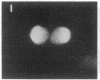
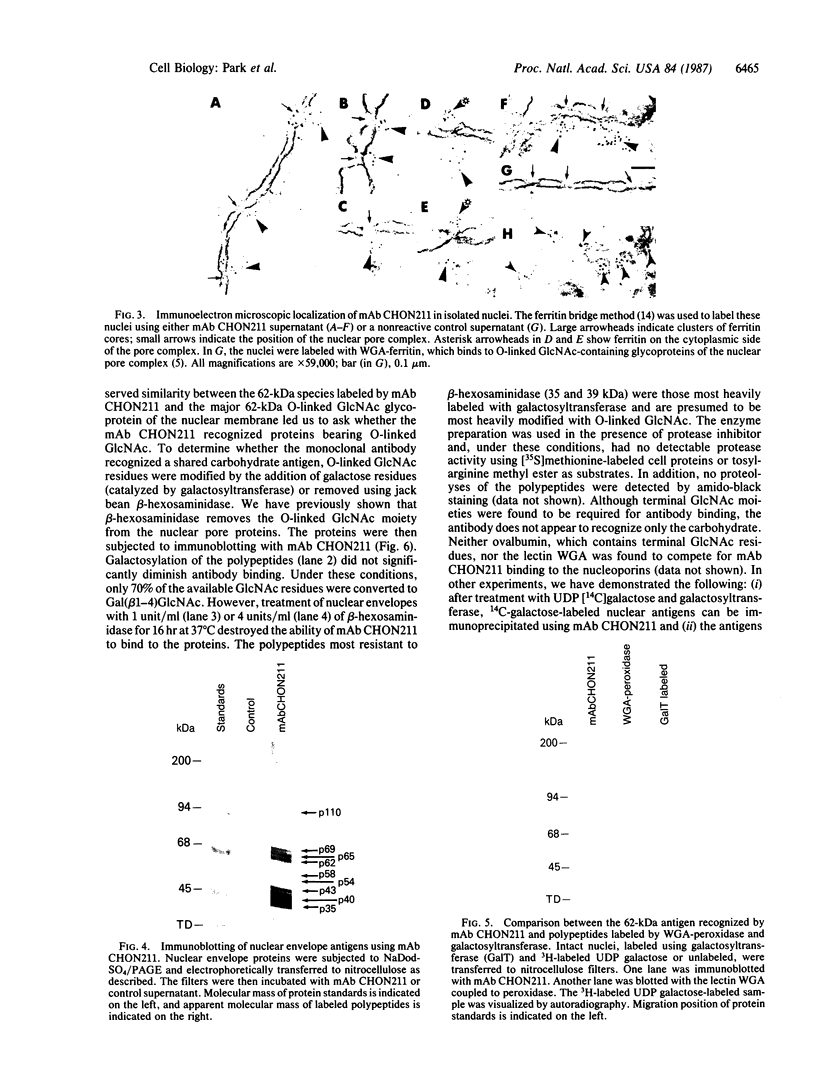
Using nuclear envelopes from Chinese hamster ovary (CHO) cells as an antigen, a mouse monoclonal antibody (IgM; designated mAb CHON211) that specifically binds to components of the nuclear pore complex (nucleoporins) was isolated. Immunofluorescence localization of the antigen recognized by mAb CHON211 revealed a punctate pattern restricted to the nuclear envelope; this pattern changed dramatically during the cell cycle. When examined by electron microscopy, the antigen was largely restricted to the nucleoplasmic and cytoplasmic faces of the nuclear pore complex. Immunoblots showed that mAb CHON211 bound to a series of polypeptides enriched in the nuclear envelope fraction with apparent molecular masses ranging from 110 to 35 kDa. Using galactosyltransferase and the lectin wheat germ agglutinin as probes, we have previously shown that proteins bearing O-linked N-acetylglucosamine (GlcNAc) are restricted to the cytoplasmic and nucleoplasmic faces of the nuclear pore complex. The nuclear membrane proteins recognized by mAb CHON211 had properties very similar to those identified by galactosyltransferase and wheat germ agglutinin labeling. Removal of O-linked GlcNAc residues with beta-hexosaminidase greatly reduced the binding of mAb CHON211, strongly suggesting that O-linked GlcNAc moieties are part of the immunodeterminant. This monoclonal antibody defines a new family of antigens that are restricted to the nuclear pore and bear a common modification: O-linked GlcNAc. mAb CHON211 will be useful for defining the role of the nucleoporins in nucleo-cytoplasmic exchange.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baglia F. A., Maul G. G. Nuclear ribonucleoprotein release and nucleoside triphosphatase activity are inhibited by antibodies directed against one nuclear matrix glycoprotein. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2285–2289. doi: 10.1073/pnas.80.8.2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G., Potter V. R. Nuclei from rat liver: isolation method that combines purity with high yield. Science. 1966 Dec 30;154(3757):1662–1665. doi: 10.1126/science.154.3757.1662. [DOI] [PubMed] [Google Scholar]
- Conner G. E., Noonan N. E., Noonan K. D. Nuclear envelope of Chinese hamster ovary cells. Re-formation of the nuclear envelope following mitosis. Biochemistry. 1980 Jan 22;19(2):277–289. doi: 10.1021/bi00543a005. [DOI] [PubMed] [Google Scholar]
- Davis L. I., Blobel G. Identification and characterization of a nuclear pore complex protein. Cell. 1986 Jun 6;45(5):699–709. doi: 10.1016/0092-8674(86)90784-1. [DOI] [PubMed] [Google Scholar]
- Deutscher S. L., Hirschberg C. B. Mechanism of galactosylation in the Golgi apparatus. A Chinese hamster ovary cell mutant deficient in translocation of UDP-galactose across Golgi vesicle membranes. J Biol Chem. 1986 Jan 5;261(1):96–100. [PubMed] [Google Scholar]
- Dwyer N., Blobel G. A modified procedure for the isolation of a pore complex-lamina fraction from rat liver nuclei. J Cell Biol. 1976 Sep;70(3):581–591. doi: 10.1083/jcb.70.3.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay D. R., Newmeyer D. D., Price T. M., Forbes D. J. Inhibition of in vitro nuclear transport by a lectin that binds to nuclear pores. J Cell Biol. 1987 Feb;104(2):189–200. doi: 10.1083/jcb.104.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glew R. H., Kayman S. C., Kuhlenschmidt M. S. Studies on the binding of concanavalin A to rat liver mitochondria. J Biol Chem. 1973 May 10;248(9):3137–3145. [PubMed] [Google Scholar]
- Hanover J. A., Cohen C. K., Willingham M. C., Park M. K. O-linked N-acetylglucosamine is attached to proteins of the nuclear pore. Evidence for cytoplasmic and nucleoplasmic glycoproteins. J Biol Chem. 1987 Jul 15;262(20):9887–9894. [PubMed] [Google Scholar]
- Hanover J. A., Lennarz W. J. N-Linked glycoprotein assembly. Evidence that oligosaccharide attachment occurs within the lumen of the endoplasmic reticulum. J Biol Chem. 1980 Apr 25;255(8):3600–3604. [PubMed] [Google Scholar]
- Hanover J. A., Lennarz W. J. The topological orientation of N,N'-diacetylchitobiosylpyrophosphoryldolichol in artificial and natural membranes. J Biol Chem. 1979 Sep 25;254(18):9237–9246. [PubMed] [Google Scholar]
- Holt G. D., Hart G. W. The subcellular distribution of terminal N-acetylglucosamine moieties. Localization of a novel protein-saccharide linkage, O-linked GlcNAc. J Biol Chem. 1986 Jun 15;261(17):8049–8057. [PubMed] [Google Scholar]
- Jarvis D. L., Butel J. S. Modification of simian virus 40 large tumor antigen by glycosylation. Virology. 1985 Mar;141(2):173–189. doi: 10.1016/0042-6822(85)90250-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan F. W., da Silva P. P. Preferential association of glycoproteins to the euchromatin regions of cross-fractured nuclei is revealed by fracture-label. J Cell Biol. 1986 Feb;102(2):576–586. doi: 10.1083/jcb.102.2.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko I., Sato H., Ukita T. Binding of radioactively labeled concanavalin A and Ricinus communis agglutinin to rat liver- and rat ascites hepatoma-nuclei. Biochem Biophys Res Commun. 1972 Sep 26;48(6):1504–1510. doi: 10.1016/0006-291x(72)90884-4. [DOI] [PubMed] [Google Scholar]
- Maul G. G. The nuclear and the cytoplasmic pore complex: structure, dynamics, distribution, and evolution. Int Rev Cytol Suppl. 1977;(6):75–186. [PubMed] [Google Scholar]
- Meyer D. I., Burger M. M. The chromaffin granule surface. Localization of carbohydrate on the cytoplasmic surface of an intracellular organelle. Biochim Biophys Acta. 1976 Sep 7;443(3):428–436. doi: 10.1016/0005-2736(76)90462-4. [DOI] [PubMed] [Google Scholar]
- Park M. K., Wakabayashi K. Preparation of a monoclonal antibody to common amino acid sequence of LHRH and its application. Endocrinol Jpn. 1986 Apr;33(2):257–272. doi: 10.1507/endocrj1954.33.257. [DOI] [PubMed] [Google Scholar]
- Perez M., Hirschberg C. B. Topography of glycosylation reactions in the rough endoplasmic reticulum membrane. J Biol Chem. 1986 May 25;261(15):6822–6830. [PubMed] [Google Scholar]
- Snider M. D., Robbins P. W. Transmembrane organization of protein glycosylation. Mature oligosaccharide-lipid is located on the luminal side of microsomes from Chinese hamster ovary cells. J Biol Chem. 1982 Jun 25;257(12):6796–6801. [PubMed] [Google Scholar]
- Snider M. D., Rogers O. C. Transmembrane movement of oligosaccharide-lipids during glycoprotein synthesis. Cell. 1984 Mar;36(3):753–761. doi: 10.1016/0092-8674(84)90355-6. [DOI] [PubMed] [Google Scholar]
- Snow C. M., Senior A., Gerace L. Monoclonal antibodies identify a group of nuclear pore complex glycoproteins. J Cell Biol. 1987 May;104(5):1143–1156. doi: 10.1083/jcb.104.5.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres C. R., Hart G. W. Topography and polypeptide distribution of terminal N-acetylglucosamine residues on the surfaces of intact lymphocytes. Evidence for O-linked GlcNAc. J Biol Chem. 1984 Mar 10;259(5):3308–3317. [PubMed] [Google Scholar]
- Unwin P. N., Milligan R. A. A large particle associated with the perimeter of the nuclear pore complex. J Cell Biol. 1982 Apr;93(1):63–75. doi: 10.1083/jcb.93.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virtanen I., Wartiovaara J. Distribution of lectin binding sites on rat liver cell nuclei: comparison of fluorescein- and ferritin-labelling methods. Cell Mol Biol Incl Cyto Enzymol. 1978;23(1):73–79. [PubMed] [Google Scholar]
- Willingham M. C. Electron microscopic immunocytochemical localization of intracellular antigens in cultured cells: the EGS and ferritin bridge procedures. Histochem J. 1980 Jul;12(4):419–434. doi: 10.1007/BF01011958. [DOI] [PubMed] [Google Scholar]