Abstract
Bones provide mechanical and protective function, while also serving as housing for marrow and a site for regulation of calcium ion homeostasis. The properties of bones do not remain constant with age; rather, they change throughout life, in some cases improving in function, but in others, function deteriorates. Here we review the modifications in the mechanical function and shape of bones, the bone cells, the matrix they produce, and the mineral that is deposited on this matrix, while presenting recent theories about the factors leading to these changes.
Keywords: bone properties, aging, senescence, bone cells, bone mineral, review
Introduction
The image of bone as a static “skeleton in the closet” has created the false impression that, once formed, bones do not change. The purpose of this review is to demonstrate how bones change with development and aging, from the whole bone level down to the specific components, and to suggest some mechanisms for the age-dependent modifications in bone structure, composition, and function.
Bone serves mechanical and homeostatic functions, protecting the internal organs, allowing for locomotion and load-bearing, and serving as a home for marrow, and as a reservoir for calcium homeostasis. With aging, these functions become impaired, bone becomes more fragile and less able to perform its mechanical functions, and the calcium stores are often depleted (Chan and Duque, 2002). To understand why this does not occur in all individuals and how the factors that control these processes change with age is a major area of investigation.
Bone is a composite structure, consisting of inorganic mineral crystals, an extracellular organic matrix, cells, lipids, and water. The mineral crystals are analogous to the geologic mineral, hydroxylapatite. Since bone mineral is made of OH-deficient nano-particles, we will refer to it here as hydroxyapatite (HA) (Boskey, 2007). Most of the mineral crystals contain impurities, mainly carbonate, magnesium, citrate, and other trace elements whose content depends on what the animal has ingested (Grynpas, 1993b). The organic matrix is mainly type I collagen, but other types of collagen and several non-collagenous proteins, reviewed elsewhere, are also present (Zhu et al., 2008). The cells, which produce, nurture, and remodel the mineralized extracellular matrix, also respond to mechanical and other signals, which determine the properties (morphology and function) of the bone. The relative composition of bone varies with health and disease, tissue site, and animal and tissue age. It is the variation with age that is the focus of this review.
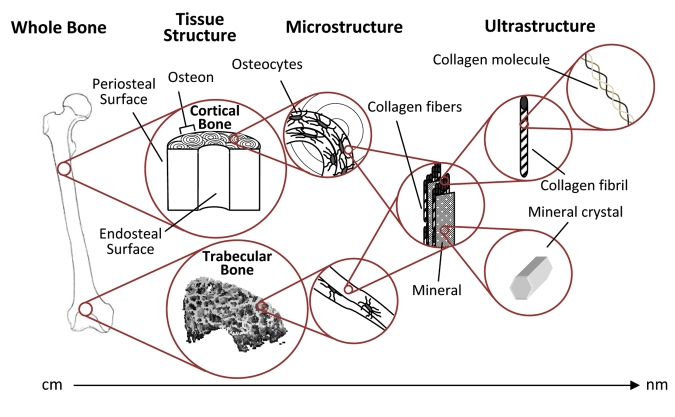
Independent of age, bone has a hierarchical structure: from the level of whole tissue, where there are different types of bones—long and short, flat and tubular—to the tissue level, where bone is arranged into cortical (compact) and trabecular (woven and lamellar) structures, to the microscopic level, where bone consists of cells, matrix, and mineral, and to the nanometer level, where the individual crystals and collagen fibrils can be seen (Fig. 1).
Figure 1.
The structural levels of bone. Cortical bone is made up of longitudinally oriented osteons, and the trabecular bone within the metaphyses is made up of connected struts and plates. In both bone types, the bone is laid down in layers (lamellae). Both tissue types contain identical components, and their properties are dependent on the amount, morphology, and interaction of these components at each level.
Bone Growth and Development
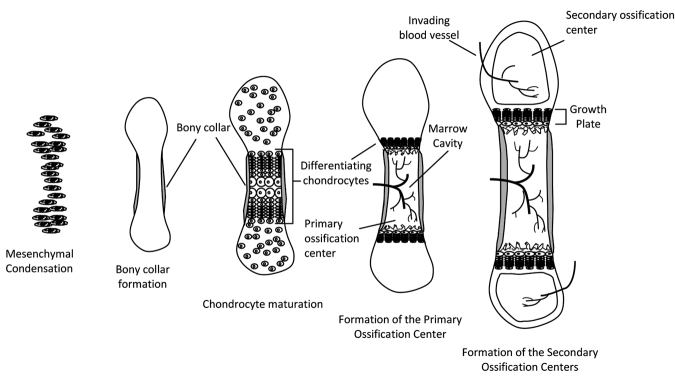
Bone development begins in the embryo with the formation of a cartilage anlagen that gradually becomes replaced with bone in a process known as endochondral ossification (Provot et al., 2008) (Fig. 2). As reviewed in detail elsewhere (Zuscik et al., 2008), during this process, developmental signals within the “growth plate”, cartilaginous tissue found at the distal and proximal ends of the developing long bone, regulate the differentiation and maturation of the resident cells (chondrocytes). Chondrocytes proliferate, deposit a matrix, and then become surrounded by a mineralized matrix, and subsequently undergo apoptosis (programmed cell death). Blood vessels then invade the tissue, forming a marrow cavity, and the calcified cartilage is replaced by bone, hence the bone grows in length. Bone grows in width (appositional bone growth) by periosteal expansion, with new bone forming on already existing surfaces. In most species, the growth plates close at puberty, and long bone growth essentially stops. Appositional bone growth continues, and this accounts for the changes in bone shape, and consequent mechanical changes (Rauch, 2005).
Figure 2.
Endochondral ossification of long bones. Condensation of mesenchymal cells forms the general shape of the long bone. Differentiation of these cells into chondrocytes begins the process of bone formation in the primary ossification center. In late differentiation, chondrocytes undergo apoptosis, leaving behind a mineralized scaffold onto which osteoblasts brought in by the invading vasculature lay down bone, lengthening the bone while forming the marrow cavity. As development continues, secondary ossification centers form in the epiphyses. Bone increases in width through deposition of bone on the periosteal side and, through endochondral ossification, continues to increase in length until the growth plates fuse.
The endochondral ossification process is recapitulated during fracture healing (Ferguson et al., 1999); thus, it is interesting to ask whether there are changes in fracture healing with age, although anecdotally one knows that, in general, children heal their fractured bones more rapidly than do adults. In rabbits, studied throughout their lifespan, the chondrogenic potential of the periosteum decreased with age (O’Driscoll et al., 2001). Age also affects the rate at which rats regain their mechanical competence after fracture (Meyer et al., 2001). In mice, the rate of cellular differentiation, and hence the rate of repair, decreases with age (Naik et al., 2009). The rate of revascularization also decreases with increasing age (Lu et al., 2008). No such comparative analysis of the rate of fracture healing in humans has been reported. These observations may explain some of the observed shifts in bone morphological and mechanical properties with age, with decreases in the rates of multiple processes such as cell proliferation and differentiation, as well as tissue revascularization, all being affected.
Age-Dependent Changes in Bone Mechanical Behavior
The components of bone are maintained in a balance to resist fracture while optimizing the weight of the skeleton. Stiffness (resistance to deformation) and strength (maximum stress to failure) are required to carry large loads, while toughness, or ductility, is required to absorb the energy from impact loads. A shift in the balance to a higher tissue mineral content will generally yield stiffer but more brittle bones. It is important to recognize that changes in collagen structure may also contribute to increased brittleness due to the shift in its cross-linking profile, which not only stiffens the organic matrix, but also affects the morphology of the mineral component, as will be discussed below. It is also important to realize that, although bone mineral density (BMD) decreases in some fragility diseases such as osteoporosis (Manolagas, 2010), it is increased in others such as osteopetrosis (Kaste et al., 2007). Thus, it is the tissue-level properties in combination with the bone geometry that determine fracture risk.
There are reports that older individuals may have a 10-fold-increased 10-year fracture risk compared with younger individuals with the same BMD (Kanis, 2002). Since many investigators and clinicians believe that BMD is a marker of fracture susceptibility, because it declines with age in both women and men, it is important to examine why there is an age-dependent increase in bone fragility or in brittleness of bone. Several studies have reported that cortical bone becomes more brittle and weaker with age (Currey and Butler, 1975; Burstein et al., 1976; McCalden et al., 1993; Tommasini et al., 2007). Similarly, the trabecular struts (see Fig. 1) also weaken with age (Nagaraja et al., 2007). In studies that extend to extremely old age (> 85 yrs), the incidence of fractures is reported to be 10-15 times as likely as in persons aged 60-65 yrs (Melton, 1996; Yates et al., 2007).
The strength of bone as a tissue is determined by the amount of mineral that is there (usually provided clinically as a two-dimensional BMD and a T- or Z-score comparing the value with that of healthy sex-matched 25-year-olds or with healthy age-matched control individuals, respectively), and the way that mineral is distributed relative to the forces applied to the bone (Ammann and Rizzoli, 2003). With aging, sex-related differences (not discussed here) in the distribution (geometry and morphology) become more pronounced, and these differences are believed to contribute to increased fracture incidence in the extremely elderly population (Yates et al., 2007). In a study of mice ranging in age from 3-77 wks, where the mice were grouped into age ranges of fewer than 12 wks, 13-50 wks, and 50-77 wks, bone density and elasticity decreased with age (Kavukcuoglu et al., 2007). Testing cortical bones of old and elderly specimens in tension, Nyman et al. (2007) also found that increasing age affected all parameters (including strength) except for the elastic stiffness. Age-dependent changes were associated with increases in cortical porosity, non-enzymatic collagen cross-links, and absolute collagen content, as will be discussed below.
Bone that is repetitively loaded (by normal activities of daily life, by extreme exercise, or in ex vivo experimental situations) develops cracks, initially at the sub-micron level, but eventually these cracks become visible, and if they are not repaired by the bone remodeling process, they can lead to failure (O’Brien et al., 2005). The cause of the initial damage is hypothesized (Mohsin et al., 2006) to be either disruption of bone mineral crystallites, debonding at the mineral organic interface, or disruption of collagen fibrils, or some combination of all three. Disruption of the structure of the bone cells that are embedded in mineral, the osteocytes, also can contribute (Verborgt et al., 2000). For example, in an animal model of early premature aging, the klotho mouse, osteocytes die and their interaction with the matrix is altered (Suzuki et al., 2005). The extent of this micro-damage increases exponentially with age in humans (Schaffler et al., 1995), as the micro-crack densities and lengths also increase (Vashishth, 2007). It is likely that both the inability to repair the cracks (Hirano et al., 2000) and their increasing propagation with age, contribute to the reduced toughness of both cortical and trabecular bone (Currey et al., 1996).
Changes in Bone Morphology with Age
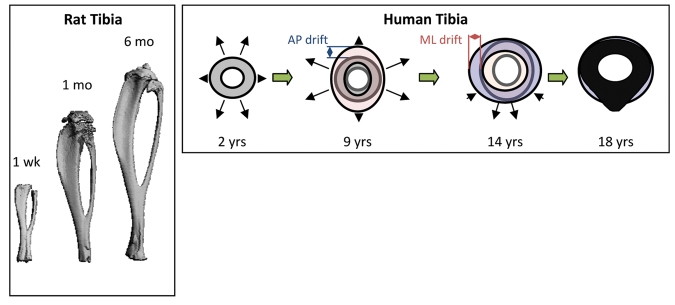
Morphology describes the shapes (geometry) of bones, in terms of whether they are long bones (such as the femur and tibia), short bones (such as the bones of the feet and hands), or flat bones (such as the calvaria or breast bones). The morphological traits that determine strength are the sizes and the shapes of the bones (Jepsen, 2009). There are compact areas (cortices) and spongy areas (trabecular) found in the ends of all long bones and in the central region of other bones (see Fig. 1). Bones change in shape to facilitate their mechanical functions—being strong enough to withstand large forces and streamlined enough to minimize energy demands (Seeman, 2003; Wang and Seeman, 2008; Seeman, 2009, Jepsen, 2009) (Fig. 3).
Figure 3.
Long bone geometrical changes with age. Left: micro-CT 3D renderings of rat tibias with increasing age. Right: Cortical drift in the tibias of human bones with age. Changes in periosteal and endosteal remodeling around the bone are non-uniform throughout life. Note that the AP drift is larger from 2 to 9 yrs, and that the ML drift is greater from 9 to 14 yrs. Right panel adapted from Goldman et al. (2009). AP: anterior-posterior direction. ML: medial-lateral direction.
During development and aging, bone shape changes in response to load, as postulated in Wolff’s Law and demonstrated in exercise experiments in various animals (Woo et al., 1981; Wallace et al., 2007; Chen et al., 2009), as well as hormonal and growth factor signals. As the shape changes (lengthening, narrowing walls, shifting the center of gravity), the bone’s functional ability may also be modified. This can be seen in animal models, as well as in humans. In mice, for example, cortical bone size, trabecular bone volume, and bone strength decline with age after 3 mos corresponding to achievement of peak bone mass in this species (Ramanadham et al., 2008), as the bones become more slender (Kawashima et al., 2009). In mature rats (from 8-36 mos of age), the only change reported in bone structure is an increase in the cross-sectional moment of inertia (distribution of the bone around the central axis), due to the expansion of the outer diameter (periosteal deposition) of their bones, with a thinning of the cortical walls (endosteal resorption) (LaMothe et al., 2003; Westerbeek et al., 2008). However, in a more recent study, when the trabecular bones in proximal tibias of 23-month-old and 5-month-old rats were compared, mineral density, bone volume fraction, and trabecular number were significantly reduced in the aged rats compared with the younger rats (Pietschmann et al., 2007). Serum markers of bone formation were also reduced in the older rats. In bovine samples (Nagaraja et al., 2007), the trabecular struts decreased in thickness with age, and the degree of anisotropy was decreased in the older specimens, as shown by micro-computed tomography.
Human samples, although more difficult to assess in such numbers, show similar patterns of morphological changes with age. In necropsied tibias from males ranging in age from 17 to 46 yrs, significant changes in mechanical properties were correlated with the size (slenderness) of the tibia in an age-independent fashion, with increased mechanical weakening, and more brittle behavior observed with increasing age (Tommasini et al., 2005, 2007).
The shape of long bones changes during development (Fig. 3). Beginning early in life, the cross-sectional geometry of long bones undergoes modification, beginning with a more uniform outer wall thickness and progressing to a more ellipsoidal shape through a process known as “cortical drift” (Goldman et al., 2009). Cortical drift occurs when formation is decreased and resorption increased on the endosteal surface, while bone is deposited on the periosteal surface. This leads to an increase in bone diameter and, in the case where endosteal resorption is greater than formation, cortical thinning. Cortical drift occurs rapidly during pre-pubertal growth, levels off after closure of the epiphyseal plate, and increases again in the elderly, often resulting in weaker bone with a wider diameter and significantly thinner cortices. Cortical drift does not occur uniformly around the bone diameter. Like many of the transformations we will discuss, it is the response to mechanical demands and biological signaling that results in the alteration in bone geometry throughout life (Goldman et al., 2009).
In humans, there is some debate (Seeman, 2003) as to whether the patterns of resorption and formation are similar in men and women, but this is beyond the scope of this review. It must be noted, however, that within trabecular bone, the age-related loss in men is predominantly due to thinning of the individual struts, while in women the loss is due to a decrease in connectivity (Aaron et al., 1987). Thus, in most animals studied, as well as in humans, bone gets stiffer with age, and the cross-sectional area (trabecular surface over which mechanical load is distributed) decreases. The impact of this is that stress (load/unit area) increases and bone deformation increases, and in fragile bone, this may lead to fracture.
In the healthy individual, bone formation and resorption are in a state of balance. The variations in bone morphology are related to the changes in this balance between bone formation and bone remodeling. While these changes do not affect all bones equally, the general trends are similar. For example, in terms of hip structure, men and women older than 85 yrs of age have been reported to have the most “unfavorable” hip geometry, narrower cortices, and decreased resistance to bending/buckling (Yates et al., 2007). Similar “unfavorable” properties also exist in tibias (Tommasini et al., 2005), and perhaps in other bones, but this might not be detected in bones that are loaded to a lesser extent than tibias and femurs. The reason for these morphological changes is related to genetics, the loading of the bones, and the activity of the cells.
The Bone Cells and How they Age
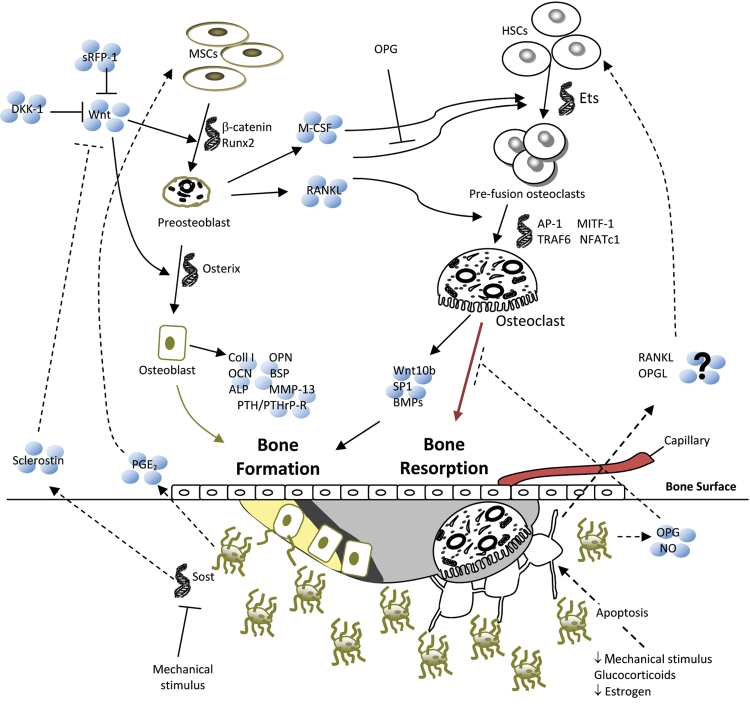
There are several types of cells in bone (Fig. 4) (for review, see Blair et al., 2007; Bonewald, 2007; Bonewald and Johnson, 2008; Lian and Stein, 2008; Boyce et al., 2009; Chen et al., 2009). The majority of the bone cells are of mesenchymal cell origin: chondrocytes, which are responsible for the deposition of the growth plate and its subsequent remodeling (Fig. 2); osteoblasts, which synthesize the bone matrix and facilitate the mineralization process; and osteocytes, which respond to load and regulate bone resorption and formation (Adachi et al., 2009).
Figure 4.
Bone remodeling pathways. Several factors can initiate bone remodeling, which, in a balanced system, begins with resorption by osteoclasts and ends with formation by osteoblasts. Signals from external sources (Wnts) and from within the bone (osteocyte apoptosis) contribute to osteoblast and osteoclast differentiation and activity, a selection of which is shown in this figure. Light gray = resorption pit, dark gray = osteoid, yellow = newly mineralized osteoid.
Osteoblastogenesis is regulated by numerous pathways, some of which are illustrated in Fig. 4. From 60 to 80% of osteoblasts recruited to a resorption pit die by apoptosis within 200 days (Jilka et al., 2007; Manolagas and Parfitt, 2010). Some of the osteoblasts become small, quiescent bone-lining cells along inactive surfaces. The remaining osteoblasts are surrounded by mineral and extend long processes (dendrites), which allow signaling and nutrition to pass from cell to cell through channels in the bone called canaliculi. These mineral-surrounded cells are known as osteocytes, and they make up approximately 90% of the cells in bone. They have a lifespan of 1 to 50 yrs (Manolagas and Parfitt, 2010), and they are the cells that are most responsive to mechanical signals (Matsuo, 2009; Rochefort et al., 2010). These cells maintain contact with other osteocytes and the surface through gap junctions. In fact, the most abundant gap junction protein in bone, connexin 43, is required for bone modeling and remodeling (Matsuo, 2009; Rochefort et al., 2010). Theoretically, mechanical loading results in fluid flow within the canalicular-lacunar network, resulting in cell-level shear forces, membrane deformation, and tension in elements connecting osteocytes with the canalicular walls, which results in the release of anti-apoptotic factors. Osteocyte apoptosis due to disruption of the canalicular network or lack of mechanical stimulus is hypothesized to regulate osteoclast and osteoblast function to induce the remodeling of the local bone tissue (Rochefort et al., 2010).
The other major bone cells, the osteoclasts, are of hematopoietic origin, and they are multinucleated giant cells responsible for removing bone (resorption) following signals from osteoblasts and osteocytes. In male rats and in all mice studied to date, osteoclast differentiation is impaired in old as compared with younger animals (Cao et al., 2005, 2007; Pietschmann et al., 2007), and the relative rates of bone removal far exceed the rates of new bone formation. Another interesting finding is that osteoclasts develop to a greater extent when they encounter older bone in vitro, so that older bone may be resorbed preferentially (Henriksen et al., 2007). Some of the factors involved in the differentiation of these cells are shown in Fig. 4. These factors and the way they control the development of the skeleton have been reviewed by Provot et al. (2008).
All normal cells, including osteoblasts, osteoclasts, and osteocytes, have a limited lifespan, which is controlled by the number of replication cycles and external factors (Fig. 5). The Hayflick limit of cell division indicates that cells can undergo only a limited number of divisions, and that the number of such divisions declines with age (Martin et al., 1970). In the cell nucleus, the lengths of the telomeres at the ends of genes are presumably the determinant of that number. A telomere is a repetitive length of DNA and associated proteins that provide stability to the ends of chromosomes. With each cell division, telomeres decrease in length due to the inability of the cell to fully replicate this region, and once the telomere length reaches some critical level, cell senescence and apoptosis are initiated (Calado, 2009). Self-renewing hematopoietic and mesenchymal stem cells have low levels of an enzyme, telomerase, which extends their life cycle significantly compared with that of mature cells. However, the activity of this enzyme is not indefinite and decreases with age (Calado, 2009). Damage to telomeres by UV light and oxidative stress may also accelerate telomere shortening, thereby curtailing the lifespan of cells (Muller, 2009).
Figure 5.
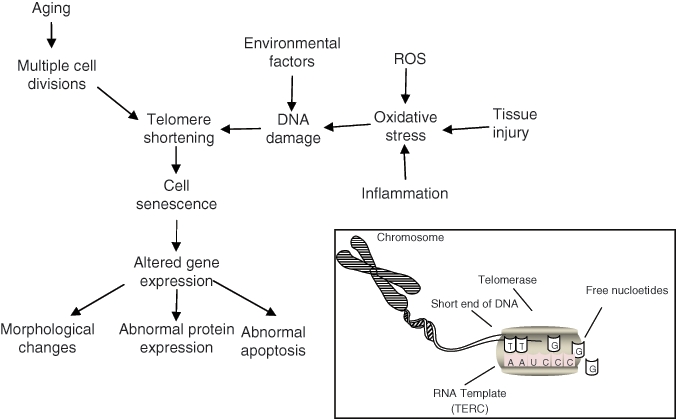
Factors affecting cell senescence. Cells reach senescence when regions at the ends of chromosomes, telomeres, reach a critical length due to the cells’ inability to replicate them properly. The inset shows the activity of an enzyme, telomerase, that allows stem cells to extend the length of the telomeres and therefore maintain an almost indefinite capacity for self-renewal. There are many factors that also contribute to telomere shortening, which may lead to abnormal cell behavior. The accumulation of damaged cells and the inability of a depleting stem cell population to replace these cells with aging may affect tissue function. Adapted from Muller (2009).
It has been noted in mice null for telomerase-specific genes that the first generation of the knock-out animals had no advanced aging phenotype, presumably due to telomere reserves that retained their chromosome stabilizing functions (Blasco et al., 1997). However, mice from the third generation and beyond exhibited systemic impaired organ function, tissue atrophy, and classic age-related disorders such as osteoporosis (Lee et al., 1998). Werner syndrome in humans results in the premature onset of age-associated diseases with accelerated telomere attrition after puberty (Martin, 2005). However, Wrn-null mice do not demonstrate any of the classic symptoms of the disease until the knock-out is developed in mice with telomerase-deficient backgrounds, further highlighting the role telomere maintenance in the onset of the premature aging phenotype (Chang et al., 2004). Studies in humans have found that telomere length was associated with decreased development of age-associated diseases; the connection between telomere length and longevity is still in question (Sahin and DePinho, 2010).
Cells often show changes in gene expression and activity with age, perhaps due to altered gene (mRNA) translation. A study by Kennedy and Kaeberlein (2009) found that treatments that decrease mRNA translation in animals increase their lifespan. Of particular importance to the cells in bone, although this appears to be true for all cells, aging is associated with the development of an inability to respond to forces [“mechanotransduction” (Chen et al., 2009)]. As recently reviewed (Wu et al., 2010), the ability of cells, in general, to sense, process, and respond to mechanical stimuli appears to be altered with aging, and these changes are associated with increased susceptibility to mechanical damage, increased apoptosis, alterations in intracellular signaling, and an impaired regulation of gene expression.
Among the key cell-based changes that occur with age, which in turn affect bone’s decline in mechanical function are modifications in the amount and rate of bone remodeling, the process by which osteoclasts remove existing bone (resorption), and osteoblasts then replace that bone (formation). With age, the amount of bone deposited with each cycle of remodeling decreases (Szulc and Seeman, 2009), possibly due to a reduction in the number of cell precursors of osteoblasts, a reduction in the number of stem cells from which these precursors are derived, or a reduction in the lifespan of osteoblasts. The signals that lead to differentiation of osteoblast precursors decrease with age (Lee et al., 2005), which may also contribute to the loss in osteoblast numbers. The number of hematopoietic cells, which are osteoclast precursors, declines with age in non-human primates (Lee et al., 2005), and there may also be a decrease in the amount of surface available for resorption. The net result is a decrease in the amount of bone with age, starting fairly early in life (Szulc and Seeman, 2009).
Few studies have examined whether human bone cell differentiation is age-dependent. In one study of 80 patients aged 14-79 yrs, bone marrow stromal cell gene expression was characterized for several markers of differentiation: Runt-related transcription factor 2 (Runx2) for osteoblasts, Peroxisome Proliferator-Activated Receptor gamma (PPAR-γ) for adipocytes, SRY-box containing gene 9 (sox9) for chondrocytes, and Receptor Activator for Nuclear Factor κB Ligand (RANKL) for osteoclasts were measured (Jiang et al., 2008). Markers of apoptosis were elevated with increasing age, and RANKL and PPAR-γ levels were positively correlated with age in female patients, but not in males. There was also a slight (not significant) decrease with increasing age in the osteoblast marker, Runx2. In another study (Zhou et al., 2008), the patients ranged in age from 17 to 90 yrs, and the markers examined were senescence-associated β-galactosidase, proliferation, apoptosis, p53 pathway genes, and osteoblast differentiation by alkaline phosphatase activity and osteoblast gene expression analysis. There were 4 times as many marrow stromal cells that were positive for senescence, and the doubling times for the older subjects’ samples were correlated with age. There was also an age-dependent decrease in marrow stromal cell proliferation and osteoblast differentiation. All of these indicate that cell differentiation and proliferation in human bone is age-dependent.
Apoptosis is a regulatory mechanism in most tissues and plays a role in normal tissue maintenance (Carrington, 2005). The dysregulation of apoptosis contributes to the imbalance between bone resorption and formation, as well as changes in local tissue mechanical properties (Carrington, 2005). It has been noted that osteocyte apoptosis increases with tissue age, which contributes to bone weakening independent of BMD through at least two mechanisms: (i) the formation of areas of micropetrosis due to mineralization of empty lacunae; and (ii) disruption of the canalicular system, thereby decreasing repair of microcracks (Jilka et al., 2007; Manolagas and Parfitt, 2010). Studies have also shown that many of the factors that regulate cell function in bone remodeling, such as steroids and hormones (glucocorticoids and estrogen), local autocrine and paracrine factors (inflammatory cytokines, Wnts, and the TGF-β superfamily), and mechanical stimuli also regulate apoptosis of osteocytes and osteoblasts (Almeida et al., 2007b). Cell senescence may alter the responses of cells to apoptotic signals (see Muller, 2009, for review), although whether this is the case in bone cells has yet to be investigated.
Genetic, Molecular, and Other Changes in Bone Cell Responses with Aging
There are multiple pathways that control differentiation and metabolism by the bone cells. These were recently reviewed in detail elsewhere (Provot et al., 2008; Chau et al., 2009), and here we will consider only those that are known both to be affected by age and to result in age-dependent changes in bone properties.
Signaling by a family of genes called wingless-type MMTV integration site (Wnt) (Williams and Insogna, 2009) is believed to regulate cellular aging in all cells, although the mechanism through which this occurs has not been completely accepted (Brack et al., 2007; DeCarolis et al., 2008) (Fig. 6). A study comparing the expression levels of all genes in the Wnt pathway in bones of different ages, and in cells isolated from young and old bones, found that all Wnt-associated genes were decreased in adult and old mice compared with younger mice, with many being significantly decreased (Rauner et al., 2008). Of note, transgenic overexpression of one of the Wnt genes (Wnt 10b) prevented bone mass loss in aged mice, again demonstrating the importance of the Wnt pathway in aging osteoblasts (Bennett et al., 2005). Studies in an animal model of early senescence, the senescence-accelerated mouse strain P6 (SAMP6), reported that a soluble frizzled related protein, sFRP-4, a protein that increases osteoblast differentiation, is negatively associated with peak bone mass (Nakanishi et al., 2006). In the canonical Wnt pathway, Wnt proteins bind to a Frizzled family member transmembrane receptor to initiate the signaling cascade by activating the Disheveled protein (Dsh) by excessive phosphorylation. This in turn prevents phosphorylation of β-catenin by the complex (GSK-3, APC, and Axin) (Fig. 6). If not proteolytically degraded, β-catenin accumulates exterior to the cell nucleus, where stable β-catenin interacts with lymphoid enhancer factor/T-cell factor (Lef/Tcf) and is translocated into the nucleus as a complex of β-catenin/Lef/Tcf to stimulate target gene transcription (Novak and Dedhar, 1999). When Wnt signals are not present, β-catenin is kept in low concentrations via the degradation complex that consists of 4 proteins (GSK-3, Axin, APC, and β-TrCP/Slimb) and by the proteolytic pathway. In the degradation complex, Axin and APC are the scaffolds that bind to both β-catenin and GSK-3 to facilitate the phosphorylation of β-catenin’s amino terminus by GSK-3, facilitating its ubiquitination and degradation.
Figure 6.
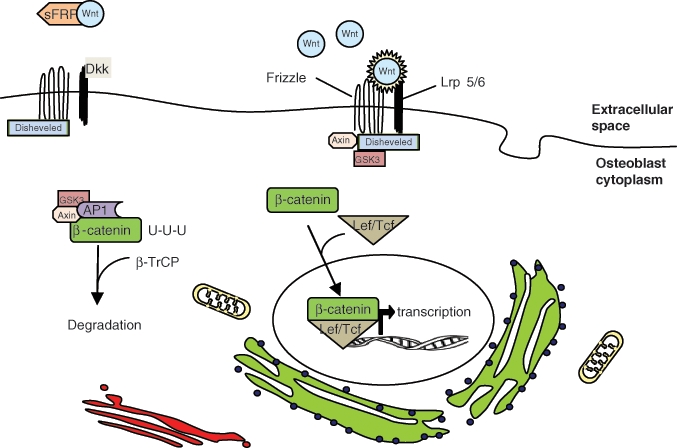
Wnt/β-catenin pathway of osteoblast differentiation. The translation of genes that stimulate osteoblast differentiation require the translocation of β-catenin from the cytoplasm to the nucleus of mesenchymal stem cells. β-catenin is targeted for degradation by the GSK3/AP1/Axin complex. Wnt binding to frizzled and Lrp 5/6 interrupts this process, allowing β-catenin to complex with Lef/Tcf. Dkk inhibits Wnt binding by interacting with Lrp 5/6. sFRP also inhibits this pathway by sequestering Wnt.
Recently, oxidative stress has been suggested to be an additional factor contributing to bone cell aging. This again occurs through the Wnt pathway, albeit indirectly, as reactive oxygen species (ROS) activates FoxOs [a series of transcription factors reviewed elsewhere (Salih and Brunet, 2008), which in turn ties up β-catenin, minimizing its effective concentration in the cells and decreasing the formation of osteoblasts (Almeida et al., 2007a). A decrease in Wnt signaling by the activation of PPAR-γ via ligands generated from lipid oxidation also contributes to the age-dependent decrease in osteoblast formation (Manolagas, 2010).
Bone Protein Changes with Age
The organic matrix of bone consists of collagen (mainly type I) and approximately 5% (by weight) non-collagenous proteins. The collagen provides the flexibility (toughness) to the bone structure, which provides resistance to impact loading, and serves as a template for the oriented deposition of mineral crystals. Collagen is secreted from the cell as triple-helical fibrils which self-associate to form larger fibrils and then fibers. Extensive post-translational modifications (hydroxylation, glycation) occur before the fibrils associate within the cell. Once extruded from the cell, globular domains that help keep the fibrils soluble in the cell are cleaved. These fibrils are then stabilized and modified extracellularly by the formation of cross-links, based both on reduction of Schiff-bases and aldol condensation products within and between the fibrils, and by the addition of sugars to the collagen fibrils (advanced glycation end-products) [for review, see Saito and Marumo (2010). It is the cross-linking of the collagen fibrils that has the greatest impact on the strength of collagen fibrils.
There are two different categories of collagen cross-links, and they vary differently with age (Fig. 7). The cross-links that are formed enzymatically by lysyl hydroxylase and lysyloxidase (enzymatic cross-links) connect the N- or C-terminus of one collagen molecule to the helical region of another. They then mature, with age, to trivalent pyridinoline (PYD) and pyrrole (PYL) cross-links, which connect two terminal regions and a helical region, thereby increasing the stiffness of the collagen (Vashishth et al., 2001). Those formed by glycation- or oxidation-induced non-enzymatic processes, advanced glycation end-products (AGEs), such as glucosepane and pentosidine, increase in formation as the collagen persists for longer times in the tissue. Limited numbers of non-enzymatic cross-links were found to be structurally related to the morphology of the trabecular bone (Banse et al., 2002). In a study of autopsy samples from individuals 54-95 yrs old, Viguet-Carrin et al. (2010) reported that the enzymatic cross-links were not correlated with micro-architecture, while there was a strong correlation with the non-enzymatic glycation cross-link, pentosidine, in vertebral trabecular bone. The study also reported an increase in the amount of pyridinoline but not the other cross-links, a finding noted earlier in iliac crest trabecular bone (Bailey et al., 1999). Age-dependent increases in both pentosidine and lysyl pyridinoline cross-links, but not in hydroxylysyl-pyridinoline cross-links, were found in cortical bone osteons (Nyman et al., 2006). This finding led the authors to speculate that the rate of non-enzymatic cross-linking increases with age, while formation of mature enzymatic cross-links may decrease. Such changes would result in decreased strength and toughness of the bone and, ultimately, decreased resistance to crack propagation. Similar findings were also reported for bovine bone (Tang et al., 2007). Thus, cross-links based on non-enzymatic glycation occur to a greater extent with age than do enzymatic cross-links.
Figure 7.
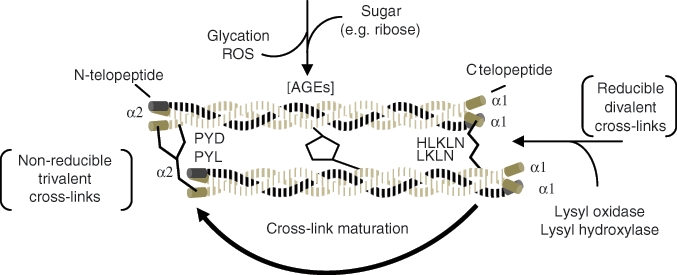
Chronological formation of collagen cross-links. As collagen matures, reducible cross-links become non-reducible. Advanced glycation end-products (AGEs) also accumulate between the helical parts of the molecules as the collagen persists in the tissue. Both contribute to the stiffening of the collagen matrix with age, which may contribute to bone tissue properties. Adapted from Leeming et al. (2009) and Saito and Marumo (2010).
Formation of cross-links affects both the way the collagen mineralizes and the way micro-damage is propagated. There is also evidence to suggest that the accumulation of AGEs within bone tissue can be removed only by bone resorption, and their presence increases osteoclast activity while decreasing formation by osteoblasts, thereby contributing to the fragility of bone with age (Viguet-Carrin et al., 2010).
Important features of the bone collagen network include the orientation of the collagen fibrils and the co-alignment of mineral crystals with the fiber axis of the collagen. Collagen orientation increases with tissue age, as is seen in images of osteons obtained by second-harmonic generation microscopy (Fig. 8), where the intensity is proportional to the orientation (Campagnola and Loew, 2003). Similar to the accumulation of non-enzymatic cross-links, orientation is an age-dependent feature.
Figure 8.
Collagen orientation of osteons obtained by second-harmonic generation microscopy, where the intensity is proportional to the orientation of the collagen. The orientation increases with tissue age, as is apparent in the outer, older rings of the osteon, which have a brighter intensity. Image from a 6-year-old baboon courtesy of Jayme Burket.
There are also age-dependent changes in the expression and relative presence of the non-collagenous proteins (Ikeda et al., 1995). Such proteins, reviewed in detail elsewhere (Zhu et al., 2008), are for the most part multifunctional proteins important for regulating cell-matrix and mineral-matrix interactions, as regulators of mineralization, and for playing a role in signaling. Much of their multifunctionality is related to the extensive post-translations they undergo (fragmentation, glycosylation/ de-glycosylation, phosphorylation/dephosphorylation). Thus, it is important to note that not only do their distributions change with age, but also the extent of their post-translational modification decreases with increasing age (Plantalech et al., 1991; Grzesik et al., 2002).
Decreased protein production with age was reported by Grynpas et al. (1994), where a comparison of trabecular bone from human femoral necks showed that younger individuals (ages 18-37 yrs) had more extracellular bone matrix proteins than individuals aged 51-79 yrs, and that there were increased bone matrix protein fragments in the older group. This was also shown in cultures of undifferentiated osteoblasts (pre-osteoblasts) obtained from human trabecular bone from the embryo to age 60 yrs (Fedarko et al., 1992). Cell proliferation was greatest in the 16-18th wks of gestation, declining after birth and then continuously with donor age, so that the rate of proliferation in the 30-year-old age group was ¼ that of the fetal samples; the rates of total protein synthesis mirrored the age-dependent changes in proliferation. The relative distribution of the proteins expressed in culture was not constant, however, indicating that synthesis rates do vary. Of the proteins studied, small proteoglycan content followed total protein content, but osteonectin, a cell-binding protein, increased in the teen years until puberty, and then steadily decreased thereafter. Similarly, in the klotho knockout mouse, a model of early senescence discussed later in this review, comparison of the distribution of proteins in 6-month-old mutant and wild-type mice of the same sex, background, and age showed increased histochemical staining for dentin matrix protein 1 (DMP1) and osteopontin in the mutant mice. DMP1 is a protein relatively specific for osteocytes; osteopontin is made by a multitude of cells (Suzuki et al., 2005) and functions in cell signaling and control of mineralization, among other things. Analysis of the data stresses the alteration in protein expression with age in the klotho mice, although the effect of genetic manipulation on the expression of these proteins is not known.
Mineral Changes with Age
The mineral content of bone (also referred to as “mineralization” or “ash content”) increases with age, and classic studies have shown that the breaking stress of bone increases exponentially with ash content, while the toughness of bone (resistance to fracture, or the inverse of brittleness) declines as the ash content reaches a maximum (Currey, 1969). This is seen not only in ash weight determination but also in the areal BMD, which is more frequently measured. BMD measurements have been compared by a variety of techniques for a variety of species, demonstrating the increase in mineral content during growth and development, and the decline with later aging (Fig. 9).
Figure 9.
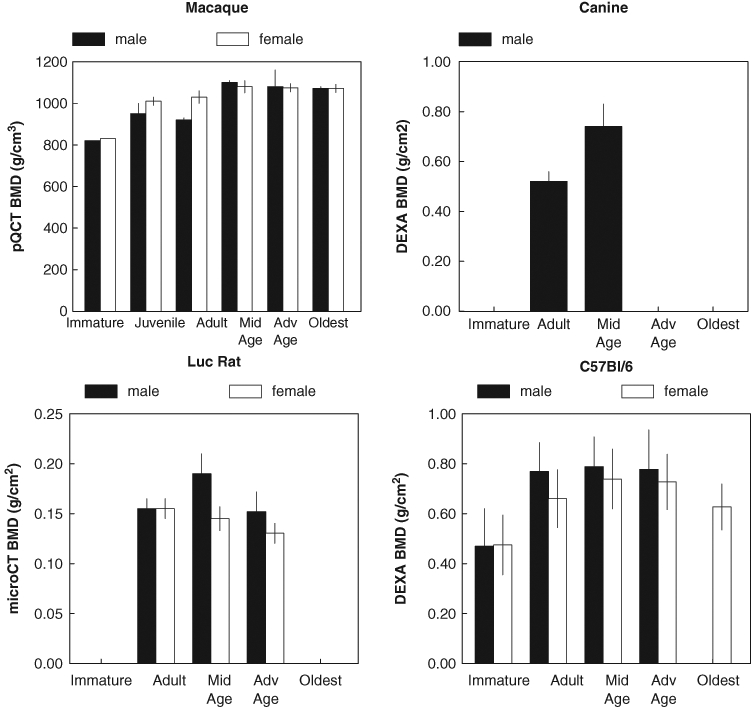
Change in BMD of various species with age. All of the plotted species show an increase in BMD with age. Male increases faster and female decreases earlier, but both sexes decline with age. Adapted from the following: macaque – Cerroni et al. (2000); canine – Schneider et al. (2004); Luc rat – Duque et al. (2009); C57Bl/6 – Somerville et al. (2004).
The mineral found in bone is an analogue of the natural occurring mineral, hydroxyapatite [Ca10(PO4)6(OH)2]. Bone mineral crystals are nano-crystals of approximate dimensions (1-1.5 nm thick x 5-25 nm wide x 8-40 nm long) (Eppell et al., 2001; Tong et al., 2003; Burger et al., 2008). They also contain a variety of inclusions and substitutions that also vary with age. Prevalent among these substituents is carbonate, which substitutes for hydroxyl and phosphate within the apatite surface and the crystal lattice (LeGeros, 2002). The most comprehensive report describing how normal human bone mineral changes in composition and crystal size as a function of age was based on x-ray diffraction analyses by Hanschin and Stern (1995), who examined 117 homogenized iliac crest biopsies from patients aged 0-95 yrs. They found that the bone mineral crystal size and perfection increased during the first 25-30 yrs and then decreased thereafter, slightly increasing in the oldest individuals. Maturation of the mineral, as shown by the line-broadening of the x-ray diffraction data, was later also noted in human embryonic vertebrae as a function of age (Meneghini et al., 2003). While in some of these cases cortical and trabecular bone tissues were examined separately, there was no attempt to distinguish locations in the bone (surface vs. internal) or male and female differences, which are known to exist (Tosi et al., 2005).
The same 117 homogenized biopsy samples were analyzed by wavelength-dispersive x-ray fluorescence to quantify the carbonate substitution in the hydroxyapatite mineral as a function of age. Although the changes observed in carbonate substitution were relatively slight (at most 10%), there was a general increase from 0-90 yrs that is distinct from the absence of a change in crystallinity after age 30 in these samples. The increase in carbonate content with age has also been reported based on Raman (Tarnowski et al., 2002; Yerramshetty et al., 2006; Donnelly et al., 2009) and infrared spectroscopy (Miller et al., 2007; Kuhn et al., 2008; Gourion-Arsiquaud et al., 2009b), as well as x-ray diffraction and chemical analyses (Pellegrino and Biltz, 1972; Burnell et al., 1980; Rey et al., 1991a,b) of bones from a variety of animal species.
Combining x-ray diffraction, Fourier transform infrared spectroscopy (FTIR), and chemical analysis, Kuhn and co-workers analyzed mineral crystals in bovine bones at two different ages, and reported that the crystals mature with age with greater increases of crystal size and Ca/P ratio for the cortical as compared with the trabecular bone of younger (1- to 3-month-old calves) vs. 4- to 5-year-old animals. The Ca/[P + CO3] was relatively constant within a given bone type and in both bone types in the older animals. Analysis of the FTIR data showed that the labile ions decreased with age, to a greater extent in cortical in contrast to trabecular bone, but in general, similar patterns of maturation were seen for both bone types when old and young bones were compared (Kuhn et al., 2008). It is important to note that all the x-ray diffraction data and most of the spectroscopic data came from homogenized bone.
In the homogenized bulk bone samples of all species examined, parallel to the age-dependent increase in carbonate content, there is a decrease in acid phosphate content (Rey et al., 1991a,b) and an increase in the hydroxyl content (Rey et al., 1995a,b; Loong et al., 2000; Wu et al., 2002). Bone apatite is non-stoichiometric, and is both hydroxyl- and calcium-deficient (Cho et al., 2003), but these deficiencies decrease with age. Whether this is because the crystals are actually perfecting with time (which argues against their incorporating carbonate ions) or whether, as suggested by in vitro studies showing that osteoclasts preferentially resorb older bone (Henriksen et al., 2007), the accelerated remodeling of bone leaves behind only the least soluble, more perfect crystals, has not been fully established.
To measure actual crystal size, rather than estimating the sizes of particles causing the broadening of the x-ray diffraction pattern of powdered bone mineral, investigators have used transmission electron microscopy and atomic force microscopy. These studies confirm the sizes suggested by x-ray diffraction, but, to date, perhaps because of the amount of work required for each sample, and the site-to-site variation that occurs within the bony tissues (Weiner and Traub, 1989), there are few reports of the age-dependent variation in actual mineral crystal size. However, Eppell’s group studied immature and mature bovine bone, from which the changes in crystal size suggested by the x-ray diffraction discussed above were confirmed (Eppell et al., 2001; Tong et al., 2003). There is a TEM study in which crystal sizes in lamellar bone of “mature” and “senior” individuals were compared by TEM, and, similar to the findings above, the length and the width of crystal aggregates were significantly higher in senile age as compared with the younger “mature” group (Denisov-Nikolskii et al., 2002)
A half-century ago, backscattered electron imaging suggested that there was a gradient of mineral density around osteons (Jowsey, 1966; Barer and Jowsey, 1967), with the youngest bone, closest to the blood vessels (Haversian canals), being the least dense. Within cortical bone, a series of almost concentric circles surrounds the blood vessel, cutting a cone through the bone (see Fig. 1). With the application of infrared microspectroscopy and microspectroscopic imaging to bone (for review, see Carden and Morris, 2000; Boskey and Pleshko Camacho, 2007), this pattern was extended to mineral crystallinity, carbonate content, and acid phosphate content, as well as to the maturity of the collagen in osteons (Fig. 10) (Paschalis et al., 1996; Fuchs et al., 2008; Gourion-Arsiquaud et al., 2009b). Other studies scanning across cortical bone, or going from the formative surface to the resorptive surface of cortical or trabecular bone in rodents (Boskey et al., 2003, 2005b; Ling et al., 2005), canines (Gourion-Arsiquaud et al., 2009a), and humans (Paschalis et al., 1997, 2003; Boskey et al., 2005a), demonstrated that the changes noted as a function of animal age also changed as a function of tissue age. The wide range of values (and large coefficients of variation) in the whole bone samples noted above could thus be partly explained by the relative contributions of younger and older bone, or by the relative numbers of new and old osteons examined. Similar spatial variations were noted by Raman microspectroscopy (Akkus et al., 2003). Thus, as summarized in the Table, there are parallel tissue-age- and animal-age-dependent changes in bone mineral composition.
Figure 10.
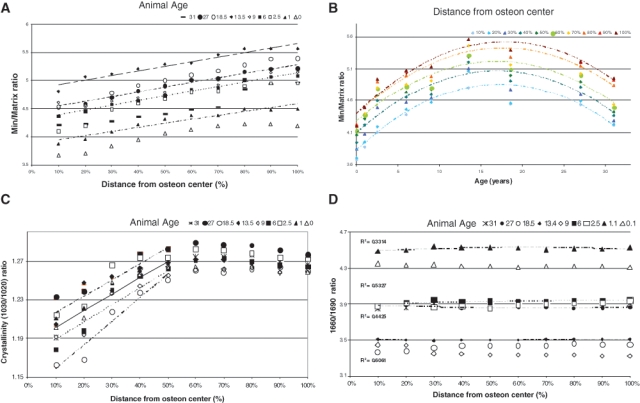
Heterogeneity of FTIR parameters in baboon osteonal bone. (A) Mineral/matrix ratio as a function of tissue age (distance from osteon center) in baboons of ages ranging from 0 to 37 yrs (R2 ~ 0.92-0.99). (B) Mineral/matrix ratio plotted as a function of baboon age for each section along the line from the center of the osteon (R2 ~ 0.83-0.89). (C) Crystallinity for each baboon sample as a function of tissue age (distance from the osteon center) (0.84 < R2 < 0.94). (D) Collagen cross-link ratio (maturity) for each baboon sample (0.1 < R2 < 0.5) as a function of tissue age (distance from the osteon center). Modified from Gourion-Arsiquaud et al. (2009b).
Table.
Age-dependent Changes in Bone Mineral Composition with Increasing Animal or Tissue Age
| Increasing mineral content |
| Increasing carbonate substitution |
| Decreasing acid phosphate substitution |
| Increasing hydroxyl content |
| Increasing Ca/P molar ratio |
| Increasing crystal size and perfection |
Animal Models of Aging Bone
Much of the information available on age-dependent changes in bone has come from animal studies and in particular from models where there is early senescence. The SAMP6 mouse (Jilka et al., 1996), along with the 11 related senescence-accelerated mouse strains and the Klotho mouse (Kuro-o et al., 1997; Kuro-o, 2009), are examples of such premature aging models. A mutation in the klotho gene (co-receptor for FGF-23) leads the “klotho” mice to show decreased bone formation and increased bone turnover, associated with kidney abnormalities, at an early age (Negri, 2005). Knockout of the klotho gene, which is predominantly expressed in the kidney, leads to premature aging starting at about 4 wks of age, while overexpression extends the mouse lifetime (Masuda et al., 2005). Overexpression can rescue the premature aging changes noted in bones and teeth; hence this is an excellent model for studying age-related changes over a short time frame. There are also other rodents that undergo premature aging, such as the Louvain rat (Luc/c) (Duque et al., 2009). In this model, rats avoid serious systemic disorders while demonstrating low-turnover bone loss, which may allow investigators to study bone fragility independent of the effects of decreased estrogen levels of OVX rats.
Almeida et al. suggested a potential mechanism for the aging effects seen in bone. In a study where female and male C57BL/6 mice were compared as a function of age, the authors found that the expected age-related loss of strength in the spine and hind limbs was detectable 9 mos earlier than the loss of BMD (Almeida et al., 2007b). Suggestive of the mechanism was the finding that aging in C57BL/6 mice was associated with an increase in adrenal production of glucocorticoids, as well as bone expression of 11β-hydroxysteroid dehydrogenase (11β-HSD) type 1, the enzyme that activates glucocorticoids. Aging also decreased the volume of the bone vasculature and bone fluid transport. These changes could be reproduced by pharmacologic hyperglucocorticoidism or by preventing cell-specific transgenic expression of the enzyme that inactivates glucocorticoids (11β-HSD 2). Glucocorticoids suppressed angiogenesis in fetal metatarsals and hypoxia-inducible factor 1α transcription and VEGF production in osteoblasts and osteocytes. The mechanism suggested for increased skeletal fragility in old age is via similar effects associated with the increases in endogenous glucocorticoids that are known to occur with aging in humans (Van Cauter et al., 1996; Dennison et al., 1999; Purnell et al., 2004; Reynolds et al., 2005). Identical to the way excess glucocorticoids affected the mouse, it is suggested that the age-associated increase in endogenous glucocorticoids decreases the lifespan of osteoblasts and osteocytes, the size of the vascular bed in bone, and solute transport through the lacunar-canalicular network. These results revealed that endogenous glucocorticoids increase skeletal fragility in old age as a result of cell-autonomous effects on osteoblasts and osteocytes, leading to interconnected decrements in bone angiogenesis, vasculature volume, and osteocyte-fluid flow.
There are also other mechanisms that may account for the age-dependent changes in bone properties, since aging mice show reduced cell proliferation, apoptosis, and loss of IGF-I-induced signaling at the receptor level and at key regulatory sites along the MAPK (ERK1/2) and PI3K (AKT) pathways (Cao et al., 2007). These pathways have been reviewed elsewhere (Provot et al., 2008).
Non-human primates have been studied as aging models, since they closely resemble changes in humans (Grynpas, 1993a; Jayo et al., 1994; Colman et al., 1999; Cerroni et al., 2000; Black and Lane, 2002). As discussed earlier, the standard clinical measurement for the assessment of human bone mineral changes is dual-photon absorptiometry (DEXA). While this method is not sensitive to drug-induced changes, it does show developmental changes in the two-dimensional bone mineral densities measured. For example, BMD in male and female rhesus macaques differs with age (Cerroni et al., 2000) (Fig. 9). Females show an initial increase with age, with peak bone density occurring around age 9.5 yrs, and remaining constant until 17.2 yrs, after which there is a steady decline in BMD. Males acquire bone mass at a faster rate, showing a higher peak BMD at an earlier age than females, and BMD remains constant between ages 7 and 19 yrs. Older males were not included in that study.
Based on analyses in changes in osteonal mineral and matrix properties in baboons of different ages (Havill, 2004), our laboratory (Gourion-Arsiquaud et al., 2009b) characterized the mineral and matrix changes in these osteons as a function of animal age and tissue age from newborn to 33 yrs of age in female animals. We found that there was an increase in mineral content, based on FTIR analyses of mineral/matrix ratio from age 0 to age 13 yrs, in agreement with the data reported by Cerroni et al. (2000), and then there was a decrease in mineral content, although the slope of the change as a proportion of the osteonal radius (tissue age) was similar (Fig. 10). Crystal size and perfection and collagen maturity, assessed by FTIR imaging, showed similar patterns, with an age-dependent increase up to age 13 yrs, a decrease in the global average after that, but similar increases in the carbonate/phosphate ratio in all bones analyzed.
Is Osteoporosis a Disease of Aging?
Osteoporosis, also known as the “silent disease”, occurs in women and men, and is associated with a loss of bone mass and increased risk of fracture. As has been pointed out throughout this review, with aging there is a loss of bone mass and bone strength. But does that mean that osteoporosis is a disease characteristic of aging, or that its incidence increases in old age? It is our contention that these are not the same, and for reasons discussed below, osteoporosis is not necessarily a disease of aging (Carrington, 2005; Blair and Carrington, 2006).
Osteoporotic bone, by definition, is bone that fractures easily. Both trabeculae and cortices are often thinner, the mineral content per area of tissue is often increased, and the mean crystal size, collagen maturity, and carbonate contents are increased relative to those of age-matched controls (Gourion-Arsiquaud et al., 2010). Further, there is a less broad distribution of the pixel histograms describing each of these properties (Boskey et al., 2009). The general characteristics are also true, as discussed above, of bones in older animals. However, although the incidence of fragility fractures and decreased bone strength (Banks et al., 2009) increases exponentially with age in males and females (Nieves et al., 2009), we suggest that the answer to the question above is a definite “no”: Osteoporosis is not a disease of aging; it is only age-associated. There are several reasons for this conclusion. First, and most obvious, is that not all the elderly, here referring to people over age 85 yrs, show signs of skeletal fragility or have fractures (Mellibovsky et al., 2007). Second, while there are specific genes associated with bone loss in rodents and humans (Richards et al., 2009), their presence or absence does not offer specific protection to any age group, and finally, because osteoporosis does occur in the very young—for example, in idiopathic juvenile osteoporosis, a disease of skeletal fragility in teenagers (Rauch et al., 2002; Płudowski et al., 2006). To argue against osteoporosis being a disease of aging, as opposed to a disease which has a greater prevalence in older individuals, we point to the case of two centenarians and their relatives (one being 113 years old) who had no fractures and no genetic abnormalities that could be linked to their younger-appearing bone properties (Mellibovsky et al., 2007).
Osteoporosis and Oral Bone Loss
As demonstrated by the expression of the intramembranous bone-specific marker periostin (Kashima et al., 2009), alveolar bone forms through the process of intramembranous ossification, i.e., without forming a cartilage model, it too undergoes age-dependent bone loss. Interestingly, this loss occurs primarily in the mandibular bone and not in the maxilla (Sarajlic et al., 2009), although changes in the maxilla have also been reported.
In human studies, significant, moderate correlations were found between hip or vertebral BMD and some measure of alveolar bone density or quality in most studies (Von Wowern and Stoltze, 1978; Kribbs et al., 1989; Krall et al., 1996; Taguchi et al., 1999; White and Rudolph, 1999; Jonasson et al., 2006). However, no other association between osteoporosis and oral bone loss in humans has been reported in previous investigations (Daniell, 1983; Mohajery and Brooks, 1992; Mohammad et al., 1997; Earnshaw et al., 1998; Southard et al., 2000). These variable findings can be attributed to small sample sizes, use of different methods of analysis, analysis of different sites within the jaw, and failure to account for mechanical factors such as missing teeth. Oral hygiene, often not reported, also appeared to be a critical factor in predicting oral bone loss (Streckfus et al., 1999). Additionally, Sanfilippo and Bianchi (2003) reported that oral bone loss was due more to alterations in maxillary function, which in turn changed the mechanical environment.
Rodent experiments, such as those in ovariectomized rats, have similarly reported both positive (Tanaka et al., 2002, 2003) and negative (Moriya et al., 1998) associations between osteoporosis in long bones and spine and effects in the jawbone. Recently, in a study of male mice of different ages, not specifically treated with any pathogens, there was significant oral bone loss from 9 mos to 18 mos, with markers of periodontitis significantly elevated in the gingivae but not the spleen of these animals, suggesting that mice are subject to age-related bone loss (Liang et al., 2010). It is difficult, however, to separate which came first, periodontal disease or bone loss. Periodontitis is characterized by inflammation of the tissues that support the teeth, and it causes resorption of the alveolar bone and the loss of the soft tissue attachment to the tooth, hence causing tooth and bone loss. The effect of inflammation on oral bone loss, and on osteoporosis, has been reviewed previously (Chung et al., 2009).
A recent study that compared lumbar and mandibular bone in ovariectomized monkeys also reported a direct correlation between bone BMD and jaw BMD. The monkeys were more than 9 yrs old, adults but not elderly, and were examined 76 wks after ovariectomy, relative to sham-operated controls of the same age (Binte Anwar et al., 2007). Periodontal disease and jaw mechanics per se were not noted.
As in the case of osteoporosis, oral bone loss may be age-associated because of poorer nutrition (Ritchie et al., 2002), alterations in dental hygiene, and hormonal changes. However, it is thought that aging alone does not cause the loss of periodontal attachment in the healthy elderly (Huttner et al., 2009). Thus, because there is only an association, rather than causality, we maintain that while oral bone loss may be accelerated in aging, in both animals and humans, the loss of bone is not directly attributable to aging.
Conclusions
The composition of bone and its mechanical properties vary as a function of age. New understanding of the factors controlling the aging of the cell in general, and the aging of osteoblasts, osteocytes, and osteoclasts in particular, is pinpointing the pathways that could be targeted to delay these age-dependent changes. But since not all individuals’ bones age similarly, whom to target and which specific pathway should be selected may become the greatest challenge.
Acknowledgments
The authors’ work was supported by NIH DE04141, AR037661, AR041325 (ALB), and F32AR057295-01 (RMC). Images from the authors’ laboratory were created in the NIH-sponsored Core Center for Musculoskeletal Repair and Regeneration, AR046121, at the Hospital for Special Surgery.
References
- Aaron JE, Makins NB, Sagreiya K. (1987). The microanatomy of trabecular bone loss in normal aging men and women. Clin Orthop Relat Res 215:260-271 [PubMed] [Google Scholar]
- Adachi T, Aonuma Y, Ito S, Tanaka M, Hojo M, Takano-Yamamoto T, et al. (2009). Osteocyte calcium signaling response to bone matrix deformation. J Biomech 42:2507-2512 [DOI] [PubMed] [Google Scholar]
- Akkus O, Polyakova-Akkus A, Adar F, Schaffler MB. (2003). Aging of microstructural compartments in human compact bone. J Bone Miner Res 18:1012-1019 [DOI] [PubMed] [Google Scholar]
- Almeida M, Han L, Martin-Millan M, Plotkin LI, Stewart SA, Roberson PK, et al. (2007a). Skeletal involution by age-associated oxidative stress and its acceleration by loss of sex steroids. J Biol Chem 282:27285-27297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida M, Han L, Martin-Millan M, O’Brien CA, Manolagas SC. (2007b). Oxidative stress antagonizes Wnt signaling in osteoblast precursors by diverting beta-catenin from T cell factor- to forkhead box O-mediated transcription. J Biol Chem 282:27298-27305 [DOI] [PubMed] [Google Scholar]
- Ammann P, Rizzoli R. (2003). Bone strength and its determinants. Osteoporos Int 14(Suppl 3):13-18 [DOI] [PubMed] [Google Scholar]
- Bailey AJ, Sims TJ, Ebbesen EN, Mansell JP, Thomsen JS, Mosekilde L. (1999). Age-related changes in the biochemical properties of human cancellous bone collagen: relationship to bone strength. Calcif Tissue Int 65:203-210 [DOI] [PubMed] [Google Scholar]
- Banks E, Reeves GK, Beral V, Balkwill A, Liu B, Roddam A. (Million Women Study Collaborators) (2009). Hip fracture incidence in relation to age, menopausal status, and age at menopause: prospective analysis. PLoS Med 6:e1000181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banse X, Devogelaer JP, Lafosse A, Sims TJ, Grynpas M, Bailey AJ. (2002). Cross-link profile of bone collagen correlates with structural organization of trabeculae. Bone 31:70-76 [DOI] [PubMed] [Google Scholar]
- Barer M, Jowsey J. (1967). Bone formation and resorption in normal human rib. A study of persons from 11 to 88 years of age. Clin Orthop Relat Res 52:241-247 [DOI] [PubMed] [Google Scholar]
- Bennett CN, Longo KA, Wright WS, Suva LJ, Lane TF, Hankenson KD, et al. (2005). Regulation of osteoblastogenesis and bone mass by Wnt10b. Proc Natl Acad Sci USA 102:3324-3329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binte Anwar R, Tanaka M, Kohno S, Ikegame M, Watanabe N, Nowazesh Ali M, et al. (2007). Relationship between porotic changes in alveolar bone and spinal osteoporosis. J Dent Res 86:52-57 [DOI] [PubMed] [Google Scholar]
- Black A, Lane MA. (2002). Nonhuman primate models of skeletal and reproductive aging. Gerontology 48:72-80 [DOI] [PubMed] [Google Scholar]
- Blair HC, Carrington JL. (2006). Bone cell precursors and the pathophysiology of bone loss. Ann NY Acad Sci 1068:244-249 [DOI] [PubMed] [Google Scholar]
- Blair HC, Simonet S, Lacey DL, Zaidi M. (2007). Osteoclast biology. In: Osteoporosis. 3rd ed. Marcus R, Feldman D, Nelson D, Rosen CJ, editors. New York, NY: Academic Press, Chapter 7, pp. 151-168 [Google Scholar]
- Blasco MA, Lee HW, Hande MP, Samper E, Lansdorp PM, DePinho RA, et al. (1997). Telomere shortening and tumor formation by mouse cells lacking telomerase RNA. Cell 91:25-34 [DOI] [PubMed] [Google Scholar]
- Bonewald LF. (2007). Osteocytes. In: Osteoporosis. 3rd ed. Marcus R, Feldman D, Nelson D, Rosen CJ, editors. New York, NY: Academic Press, Chapter 8, pp. 169-190 [Google Scholar]
- Bonewald LF, Johnson ML. (2008). Osteocytes, mechanosensing and Wnt signaling. Bone 42:606-615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boskey A. (2007). Mineralization of bones and teeth. Elements Magazine 3:385-391 [Google Scholar]
- Boskey A, Pleshko Camacho N. (2007). FT-IR imaging of native and tissue-engineered bone and cartilage. Biomaterials 28:2465-2478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boskey AL, Moore DJ, Amling M, Canalis E, Delany AM. (2003). Infrared analysis of the mineral and matrix in bones of osteonectin-null mice and their wildtype controls. J Bone Miner Res 18:1005-1011 [DOI] [PubMed] [Google Scholar]
- Boskey AL, DiCarlo E, Paschalis E, West P, Mendelsohn R. (2005a). Comparison of mineral quality and quantity in iliac crest biopsies from high- and low-turnover osteoporosis: an FT-IR microspectroscopic investigation. Osteoporos Int 16:2031-2038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boskey AL, Young MF, Kilts T, Verdelis K. (2005b). Variation in mineral properties in normal and mutant bones and teeth. Cells Tissues Organs 181:144-153 [DOI] [PubMed] [Google Scholar]
- Boskey AL, Spevak L, Weinstein RS. (2009). Spectroscopic markers of bone quality in alendronate-treated postmenopausal women. Osteoporos Int 20:793-800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce BF, Yao Z, Xing L. (2009). Osteoclasts have multiple roles in bone in addition to bone resorption. Crit Rev Eukaryot Gene Expr 19:171-180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brack AS, Conboy MJ, Roy S, Lee M, Kuo CJ, Keller C, et al. (2007). Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science 317:807-810 [DOI] [PubMed] [Google Scholar]
- Burger C, Zhou HW, Wang H, Sics I, Hsiao BS, Chu B, et al. (2008). Lateral packing of mineral crystals in bone collagen fibrils. Biophys J 95:1985-1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnell JM, Teubner EJ, Miller AG. (1980). Normal maturational changes in bone matrix, mineral, and crystal size in the rat. Calcif Tissue Int 31:13-19 [DOI] [PubMed] [Google Scholar]
- Burstein AH, Reilly DT, Martens M. (1976). Aging of bone tissue: mechanical properties. J Bone Joint Surg Am 58:82-86 [PubMed] [Google Scholar]
- Calado RT. (2009). Telomeres and marrow failure. Hematology Am Soc Hematol Educ Program 2009:338-343 [DOI] [PubMed] [Google Scholar]
- Campagnola PJ, Loew LM. (2003). Second-harmonic imaging microscopy for visualizing biomolecular arrays in cells, tissues and organisms. Nat Biotechnol 21:1356-1360 [DOI] [PubMed] [Google Scholar]
- Cao JJ, Wronski TJ, Iwaniec U, Phleger L, Kurimoto P, Boudignon B, et al. (2005). Aging increases stromal/osteoblastic cell-induced osteoclastogenesis and alters the osteoclast precursor pool in the mouse. J Bone Miner Res 20:1659-1668 [DOI] [PubMed] [Google Scholar]
- Cao JJ, Kurimoto P, Boudignon B, Rosen C, Lima F, Halloran BP. (2007). Aging impairs IGF-I receptor activation and induces skeletal resistance to IGF-I. J Bone Miner Res 22:1271-1279 [DOI] [PubMed] [Google Scholar]
- Carden A, Morris MD. (2000). Application of vibrational spectroscopy to the study of mineralized tissues. J Biomed Opt 5:259-268 [DOI] [PubMed] [Google Scholar]
- Carrington JL. (2005). Aging bone and cartilage: cross-cutting issues. Biochem Biophys Res Commun 328:700-708 [DOI] [PubMed] [Google Scholar]
- Cerroni AM, Tomlinson GA, Turnquist JE, Grynpas MD. (2000). Bone mineral density, osteopenia, and osteoporosis in the rhesus macaques of Cayo Santiago. Am J Phys Anthropol 113:389-410 [DOI] [PubMed] [Google Scholar]
- Chan GK, Duque G. (2002). Age-related bone loss: old bone, new facts. Gerontology 48:62-71 [DOI] [PubMed] [Google Scholar]
- Chang S, Multani AS, Cabrera NG, Naylor ML, Laud P, Lombard D, et al. (2004). Essential role of limiting telomeres in the pathogenesis of Werner syndrome. Nat Genet 36:877-882 [DOI] [PubMed] [Google Scholar]
- Chau JF, Leong WF, Li B. (2009). Signaling pathways governing osteoblast proliferation, differentiation and function. Histol Histopathol 24:1593-1606 [DOI] [PubMed] [Google Scholar]
- Chen JH, Liu C, You L, Simmons CA. (2009). Boning up on Wolff’s Law: mechanical regulation of the cells that make and maintain bone. J Biomech 43:108-118 [DOI] [PubMed] [Google Scholar]
- Cho G, Wu Y, Ackerman JL. (2003). Detection of hydroxyl ions in bone mineral by solid-state NMR spectroscopy. Science 300:1123-1127 [DOI] [PubMed] [Google Scholar]
- Chung HY, Cesari M, Anton S, Marzetti E, Giovanni S, Seo AY, et al. (2009). Molecular inflammation: underpinnings of aging and age-related diseases. Ageing Res Rev 8:18-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman RJ, Lane MA, Binkley N, Wegner FH, Kemnitz JW. (1999). Skeletal effects of aging in male rhesus monkeys. Bone 24:17-23 [DOI] [PubMed] [Google Scholar]
- Currey JD. (1969). The relationship between the stiffness and the mineral content of bone. J Biomech 2:477-480 [DOI] [PubMed] [Google Scholar]
- Currey JD, Butler G. (1975). The mechanical properties of bone tissue in children. J Bone Joint Surg Am 57:810-814 [PubMed] [Google Scholar]
- Currey JD, Brear K, Zioupos P. (1996). The effects of ageing and changes in mineral content in degrading the toughness of human femora. J Biomech 29:257-262; erratum in J Biomech 30:1001, 1997 [DOI] [PubMed] [Google Scholar]
- Daniell HW. (1983). Postmenopausal tooth loss. Contributions to edentulism by osteoporosis and cigarette smoking. Arch Intern Med 143:1678-1682 [DOI] [PubMed] [Google Scholar]
- DeCarolis NA, Wharton KA, Jr, Eisch AJ. (2008). Which way does the Wnt blow? Exploring the duality of canonical Wnt signaling on cellular aging. Bioessays 30:102-106 [DOI] [PubMed] [Google Scholar]
- Denisov-Nikolskii YI, Zhilkin BA, Doktorov AA, Matveĭchuk IV. (2002). (Ultrastructural organization of the human lamellar bone tissue mineral component in aged and elderly). Morfologiia 122:79-83 [in Russian]. [PubMed] [Google Scholar]
- Dennison E, Hindmarsh P, Fall C, Kellingray S, Barker D, Phillips D, et al. (1999). Profiles of endogenous circulating cortisol and bone mineral density in healthy elderly men. J Clin Endocrinol Metab 84:3058-3063 [DOI] [PubMed] [Google Scholar]
- Donnelly E, Boskey AL, Baker SP, van der Meulen MC. (2009). Effects of tissue age on bone tissue material composition and nanomechanical properties in the rat cortex. J Biomed Mater Res A 92:1048-1056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque G, Rivas D, Li W, Li A, Henderson JE, Ferland G, et al. (2009). Age-related bone loss in the LOU/c rat model of healthy ageing. Exp Gerontol 44:183-189 [DOI] [PubMed] [Google Scholar]
- Earnshaw SA, Keating N, Hosking DJ, Chilvers CE, Ravn P, McClung M, et al. (1998). Tooth counts do not predict bone mineral density in early postmenopausal Caucasian women. EPIC study group. Int J Epidemiol 27:479-483 [DOI] [PubMed] [Google Scholar]
- Eppell SJ, Tong W, Katz JL, Kuhn L, Glimcher MJ. (2001). Shape and size of isolated bone mineralites measured using atomic force microscopy. J Orthop Res 19:1027-1034 [DOI] [PubMed] [Google Scholar]
- Fedarko NS, Vetter UK, Weinstein S, Robey PG. (1992). Age-related changes in hyaluronan, proteoglycan, collagen, and osteonectin synthesis by human bone cells. J Cell Physiol 151:215-227 [DOI] [PubMed] [Google Scholar]
- Ferguson C, Alpern E, Miclau T, Helms JA. (1999). Does adult fracture repair recapitulate embryonic skeletal formation? Mech Dev 87:57-66 [DOI] [PubMed] [Google Scholar]
- Fuchs RK, Allen MR, Ruppel ME, Diab T, Phipps RJ, Miller LM, et al. (2008). In situ examination of the time-course for secondary mineralization of Haversian bone using synchrotron Fourier transform infrared microspectroscopy. Matrix Biol 27:34-41 [DOI] [PubMed] [Google Scholar]
- Goldman HM, McFarlin SC, Cooper DM, Thomas CD, Clement JG. (2009). Ontogenetic patterning of cortical bone microstructure and geometry at the human mid-shaft femur. Anat Rec (Hoboken) 292:48-64 [DOI] [PubMed] [Google Scholar]
- Gourion-Arsiquaud S, Burket JC, Havill LM, DiCarlo E, Doty SB, Mendelsohn R, et al. (2009a). Spatial variation in osteonal bone properties relative to tissue and animal age. J Bone Miner Res 24:1271-1281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourion-Arsiquaud S, Faibish D, Myers E, Spevak L, Compston J, Hodsman A, et al. (2009b). Use of FTIR spectroscopic imaging to identify parameters associated with fragility fracture. J Bone Miner Res 24:1565-1571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourion-Arsiquaud S, Allen MR, Burr DB, Vashishth D, Tang SY, Boskey AL. (2010). Bisphosphonate treatment modifies canine bone mineral and matrix properties and their heterogeneity. Bone 46:666-672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynpas M. (1993a). Age and disease-related changes in the mineral of bone. Calcif Tissue Int 53(Suppl 1):57-64 [DOI] [PubMed] [Google Scholar]
- Grynpas MD, Hancock RG, Greenwood C, Turnquist J, Kessler MJ. (1993b). The effects of diet, age, and sex on the mineral content of primate bones. Calcif Tissue Int 52:399-405 [DOI] [PubMed] [Google Scholar]
- Grynpas MD, Tupy JH, Sodek J. (1994). The distribution of soluble, mineral-bound, and matrix-bound proteins in osteoporotic and normal bones. Bone 15:505-513 [DOI] [PubMed] [Google Scholar]
- Grzesik WJ, Frazier CR, Shapiro JR, Sponseller PD, Robey PG, Fedarko NS. (2002). Age-related changes in human bone proteoglycan structure. Impact of osteogenesis imperfecta. J Biol Chem 277:43638-43647 [DOI] [PubMed] [Google Scholar]
- Hanschin RG, Stern WB. (1995). X-ray diffraction studies on the lattice perfection of human bone apatite (Crista iliaca). Bone 16(4 Suppl):355S-363S [PubMed] [Google Scholar]
- Havill LM. (2004). Osteon remodeling dynamics in Macaca mulatta: normal variation with regard to age, sex, and skeletal maturity. Calcif Tissue Int 74:95-102 [DOI] [PubMed] [Google Scholar]
- Henriksen K, Leeming DJ, Byrjalsen I, Nielsen RH, Sorensen MG, Dziegiel MH, et al. (2007). Osteoclasts prefer aged bone. Osteoporos Int 18:751-759 [DOI] [PubMed] [Google Scholar]
- Hirano T, Turner CH, Forwood MR, Johnston CC, Burr DB. (2000). Does suppression of bone turnover impair mechanical properties by allowing microdamage accumulation? Bone 27:13-20 [DOI] [PubMed] [Google Scholar]
- Huttner EA, Machado DC, de Oliveira RB, Antunes AG, Hebling E. (2009). Effects of human aging on periodontal tissues. Spec Care Dentist 29:149-155 [DOI] [PubMed] [Google Scholar]
- Ikeda T, Nagai Y, Yamaguchi A, Yokose S, Yoshiki S. (1995). Age-related reduction in bone matrix protein mRNA expression in rat bone tissues: application of histomorphometry to in situ hybridization. Bone 16:17-23 [DOI] [PubMed] [Google Scholar]
- Jayo MJ, Jerome CP, Lees CJ, Rankin SE, Weaver DS. (1994). Bone mass in female cynomolgus macaques: a cross-sectional and longitudinal study by age. Calcif Tissue Int 54:231-236 [DOI] [PubMed] [Google Scholar]
- Jepsen KJ. (2009). Systems analysis of bone. Wiley Interdiscip Rev Syst Biol Med 1:73-88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Mishima H, Sakai S, Liu YK, Ohyabu Y, Uemura T. (2008). Gene expression analysis of major lineage-defining factors in human bone marrow cells: effect of aging, gender, and age-related disorders. J Orthop Res 26:910-917 [DOI] [PubMed] [Google Scholar]
- Jilka RL, Weinstein RS, Takahashi K, Parfitt AM, Manolagas SC. (1996). Linkage of decreased bone mass with impaired osteoblastogenesis in a murine model of accelerated senescence. J Clin Invest 97:1732-1740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jilka RL, Weinstein RS, Parfitt AM, Manolagas SC. (2007). Quantifying osteoblast and osteocyte apoptosis: challenges and rewards. J Bone Miner Res 22:1492-1501 [DOI] [PubMed] [Google Scholar]
- Jonasson G, Jonasson L, Kiliaridis S. (2006). Changes in the radiographic characteristics of the mandibular alveolar process in dentate women with varying bone mineral density: a 5-year prospective study. Bone 38:714-721 [DOI] [PubMed] [Google Scholar]
- Jowsey J. (1966). Studies of Haversian systems in man and some animals. J Anat 100(Pt 4):857-864 [PMC free article] [PubMed] [Google Scholar]
- Kanis JA. (2002). Diagnosis of osteoporosis and assessment of fracture risk. Lancet 359:1929-1936 [DOI] [PubMed] [Google Scholar]
- Kashima TG, Nishiyama T, Shimazu K, Shimazaki M, Kii I, Grigoriadis AE, et al. (2009). Periostin, a novel marker of intramembranous ossification, is expressed in fibrous dysplasia and in c-Fos–overexpressing bone lesions. Hum Pathol 40:226-237 [DOI] [PubMed] [Google Scholar]
- Kaste SC, Kasow KA, Horwitz EM. (2007). Quantitative bone mineral density assessment in malignant infantile osteopetrosis. Pediatr Blood Cancer 48:181-185 [DOI] [PubMed] [Google Scholar]
- Kavukcuoglu NB, Denhardt DT, Guzelsu N, Mann AB. (2007). Osteopontin deficiency and aging on nanomechanics of mouse bone. J Biomed Mater Res A 83:136-144 [DOI] [PubMed] [Google Scholar]
- Kawashima Y, Fritton JC, Yakar S, Epstein S, Schaffler MB, Jepsen KJ, et al. (2009). Type 2 diabetic mice demonstrate slender long bones with increased fragility secondary to increased osteoclastogenesis. Bone 44:648-655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy BK, Kaeberlein M. (2009). Hot topics in aging research: protein translation, 2009. Aging Cell 8:617-623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krall EA, Garcia RI, Dawson-Hughes B. (1996). Increased risk of tooth loss is related to bone loss at the whole body, hip and spine. Calcif Tissue Int 59:433-437 [DOI] [PubMed] [Google Scholar]
- Kribbs PJ, Chestnut CH, 3rd, Ott SM, Kilcoyne RF. (1989). Relationships between mandibular and skeletal bone in an osteoporotic population. J Prosthet Dent 62:703-707 [DOI] [PubMed] [Google Scholar]
- Kuhn LT, Grynpas MD, Rey CC, Wu Y, Ackerman JL, Glimcher MJ. (2008). A comparison of the physical and chemical differences between cancellous and cortical bovine bone mineral at two ages. Calcif Tissue Int 83:146-154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuro-o M. (2009). Klotho and aging. Biochim Biophys Acta 1790:1049-1058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, et al. (1997). Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 390:45-51 [DOI] [PubMed] [Google Scholar]
- LaMothe JM, Hepple RT, Zernicke RF. (2003). Selected contribution: bone adaptation with aging and long-term caloric restriction in Fischer 344 x Brown-Norway F1-hybrid rats. J Appl Physiol 95:1739-1745 [DOI] [PubMed] [Google Scholar]
- Lee CC, Fletcher MD, Tarantal AF. (2005). Effect of age on the frequency, cell cycle, and lineage maturation of rhesus monkey (Macaca mulatta) CD34+ and hematopoietic progenitor cells. Pediatr Res 58:315-322 [DOI] [PubMed] [Google Scholar]
- Lee HW, Blasco MA, Gottlieb GJ, Horner JW, Greider CW, DePinho RA. (1998). Essential role of mouse telomerase in highly proliferative organs. Nature 392:569-574 [DOI] [PubMed] [Google Scholar]
- Leeming DJ, Henriksen K, Byrjalsen I, Qvist P, Madsen SH, Garnero P, et al. (2009). Is bone quality associated with collagen age? Osteoporos Int 20:1461-1470 [DOI] [PubMed] [Google Scholar]
- LeGeros RZ. (2002). Properties of osteoconductive biomaterials: calcium phosphates. Clin Orthop Relat Res 395:81-98 [DOI] [PubMed] [Google Scholar]
- Lian JB, Stein GS. (2008). Osteoblast biology. In: Osteoporosis. 3rd ed. Marcus R, Feldman D, Nelson D, Rosen CJ, editors. New York, NY: Academic Press, Chapter 6, pp. 93-128 [Google Scholar]
- Liang S, Hosur KB, Domon H, Hajishengallis G. (2010). Periodontal inflammation and bone loss in aged mice. J Periodontal Res (Epub ahead of print, March 9, 2010) (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling Y, Rios HF, Myers ER, Lu Y, Feng JQ, Boskey AL. (2005). DMP1 depletion decreases bone mineralization in vivo: an FTIR imaging analysis. J Bone Miner Res 20:2169-2177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loong CK, Rey C, Kuhn LT, Combes C, Wu Y, Chen S, et al. (2000). Evidence of hydroxyl-ion deficiency in bone apatites: an inelastic neutron-scattering study. Bone 26:599-602 [DOI] [PubMed] [Google Scholar]
- Lu C, Hansen E, Sapozhnikova A, Hu D, Miclau T, Marcucio RS. (2008). Effect of age on vascularization during fracture repair. J Orthop Res 26:1384-1389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manolagas SC. (2010). From estrogen-centric to aging and oxidative stress: a revised perspective of the pathogenesis of osteoporosis. Endocr Rev, 31:266-300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manolagas SC, Parfitt AM. (2010). What old means to bone. Trends Endocrinol Metab 21:369-374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin GM. (2005). Genetic modulation of senescent phenotypes in Homo sapiens. Cell 120:523-532 [DOI] [PubMed] [Google Scholar]
- Martin GM, Sprague CA, Epstein CJ. (1970). Replicative life-span of cultivated human cells. Effects of donor’s age, tissue, and genotype. Lab Invest 23:86-92 [PubMed] [Google Scholar]
- Masuda H, Chikuda H, Suga T, Kawaguchi H, Kuro-o M. (2005). Regulation of multiple ageing-like phenotypes by inducible klotho gene expression in klotho mutant mice. Mech Ageing Dev 126:1274-1283 [DOI] [PubMed] [Google Scholar]
- Matsuo K. (2009). Cross-talk among bone cells. Curr Opin Nephrol Hypertens 18:292-297 [DOI] [PubMed] [Google Scholar]
- McCalden RW, McGeough JA, Barker MB, Court-Brown CM. (1993). Age-related changes in the tensile properties of cortical bone. The relative importance of changes in porosity, mineralization, and microstructure. J Bone Joint Surg Am 75:1193-1205 [DOI] [PubMed] [Google Scholar]
- Mellibovsky L, Bustamante M, Lluch P, Nogues X, Grinberg D, Balcells S, et al. (2007). Bone mass of a 113-year-old man. J Gerontol A Biol Sci Med Sci 62:794-795 [DOI] [PubMed] [Google Scholar]
- Melton LJ., 3rd (1996). Epidemiology of hip fractures: implications of the exponential increase with age. Bone 18(3 Suppl):121S-125S [DOI] [PubMed] [Google Scholar]
- Meneghini C, Dalconi MC, Nuzzo S, Mobilio S, Wenk RH. (2003). Rietveld refinement on x-ray diffraction patterns of bioapatite in human fetal bones. Biophys J 84:2021-2029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer RA, Jr, Tsahakis PJ, Martin DF, Banks DM, Harrow ME, Kiebzak GM. (2001). Age and ovariectomy impair both the normalization of mechanical properties and the accretion of mineral by the fracture callus in rats. J Orthop Res 19:428-435 [DOI] [PubMed] [Google Scholar]
- Miller LM, Little W, Schirmer A, Sheik F, Busa B, Judex S. (2007). Accretion of bone quantity and quality in the developing mouse skeleton. J Bone Miner Res 22:1037-1045 [DOI] [PubMed] [Google Scholar]
- Mohajery M, Brooks SL. (1992). Oral radiographs in the detection of early signs of osteoporosis. Oral Surg Oral Med Oral Pathol 73:112-117 [DOI] [PubMed] [Google Scholar]
- Mohammad AR, Bauer RL, Yeh CK. (1997). Spinal bone density and tooth loss in a cohort of postmenopausal women. Int J Prosthodont 10:381-385 [PubMed] [Google Scholar]
- Mohsin S, O’Brien FJ, Lee TC. (2006). Microcracks in compact bone: a three-dimensional view. J Anat 209:119-124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriya Y, Ito K, Murai S. (1998). Effects of experimental osteoporosis on alveolar bone loss in rats. J Oral Sci 40:171-175 [DOI] [PubMed] [Google Scholar]
- Muller M. (2009). Cellular senescence: molecular mechanisms, in vivo significance, and redox considerations. Antioxid Redox Signal 11:59-98 [DOI] [PubMed] [Google Scholar]
- Nagaraja S, Lin AS, Guldberg RE. (2007). Age-related changes in trabecular bone microdamage initiation. Bone 40:973-980 [DOI] [PubMed] [Google Scholar]
- Naik AA, Xie C, Zuscik MJ, Kingsley P, Schwarz EM, Awad H, et al. (2009). Reduced COX-2 expression in aged mice is associated with impaired fracture healing. J Bone Miner Res 24:251-264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi R, Shimizu M, Mori M, Akiyama H, Okudaira S, Otsuki B, et al. (2006). Secreted frizzled-related protein 4 is a negative regulator of peak BMD in SAMP6 mice. J Bone Miner Res 21:1713-1721 [DOI] [PubMed] [Google Scholar]
- Negri AL. (2005). The klotho gene: a gene predominantly expressed in the kidney is a fundamental regulator of aging and calcium/phosphorus metabolism. J Nephrol 18:654-658 [PubMed] [Google Scholar]
- Nieves JW, Bilezikian JP, Lane JM, Einhorn TA, Wang Y, Steinbuch M, et al. (2009). Fragility fractures of the hip and femur: incidence and patient characteristics. Osteoporos Int 29:399-408 [DOI] [PubMed] [Google Scholar]
- Novak A, Dedhar S. (1999). Signaling through beta-catenin and Lef/Tcf. Cell Mol Life Sci 56:523-537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyman JS, Roy A, Acuna RL, Gayle HJ, Reyes MJ, Tyler JH, et al. (2006). Age-related effect on the concentration of collagen crosslinks in human osteonal and interstitial bone tissue. Bone 39:1210-1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyman JS, Roy A, Tyler JH, Acuna RL, Gayle HJ, Wang X. (2007). Age-related factors affecting the postyield energy dissipation of human cortical bone. J Orthop Res 25:646-655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien FJ, Brennan O, Kennedy OD, Lee TC. (2005). Microcracks in cortical bone: how do they affect bone biology? Curr Osteoporos Rep 3:39-45 [DOI] [PubMed] [Google Scholar]
- O’Driscoll SW, Saris DB, Ito Y, Fitzimmons JS. (2001). The chondrogenic potential of periosteum decreases with age. J Orthop Res 19:95-103 [DOI] [PubMed] [Google Scholar]
- Paschalis EP, DiCarlo E, Betts F, Sherman P, Mendelsohn R, Boskey AL. (1996). FTIR microspectroscopic analysis of human osteonal bone. Calcif Tissue Int 59:480-487 [DOI] [PubMed] [Google Scholar]
- Paschalis EP, Betts F, DiCarlo E, Mendelsohn R, Boskey AL. (1997). FTIR microspectroscopic analysis of normal human cortical and trabecular bone. Calcif Tissue Int 61:480-486 [DOI] [PubMed] [Google Scholar]
- Pellegrino ED, Biltz RM. (1972). Mineralization in the chick embryo. I. Monohydrogen phosphate and carbonate relationships during maturation of the bone crystal complex. Calcif Tissue Res 10:128-135 [DOI] [PubMed] [Google Scholar]
- Pietschmann P, Skalicky M, Kneissel M, Rauner M, Hofbauer G, Stupphann D, et al. (2007). Bone structure and metabolism in a rodent model of male senile osteoporosis. Exp Gerontol 42:1099-1108 [DOI] [PubMed] [Google Scholar]
- Plantalech L, Guillaumont M, Vergnaud P, Leclercq M, Delmas PD. (1991). Impairment of gamma carboxylation of circulating osteocalcin (bone gla protein) in elderly women. J Bone Miner Res 6:1211-1216 [DOI] [PubMed] [Google Scholar]
- Płudowski P, Lebiedowski M, Olszaniecka M, Marowska J, Matusik H, Lorenc RS. (2006). Idiopathic juvenile osteoporosis—an analysis of the muscle-bone relationship. Osteoporos Int 17:1681-1690 [DOI] [PubMed] [Google Scholar]
- Provot S, Schipanni E, Wu J, Kronenberg H. (2008). Development of the skeleton. In: Osteoporosis. 3rd ed. Marcus R, Feldman D, Nelson D, Rosen CJ, editors. New York, NY: Academic Press, Chapter 10, pp. 241-270 [Google Scholar]
- Purnell JQ, Brandon DD, Isabelle LM, Loriaux DL, Samuels MH. (2004). Association of 24-hour cortisol production rates, cortisol-binding globulin, and plasma-free cortisol levels with body composition, leptin levels, and aging in adult men and women. J Clin Endocrinol Metab 89:281-287 [DOI] [PubMed] [Google Scholar]
- Ramanadham S, Yarasheski KE, Silva MJ, Wohltmann M, Novack DV, Christiansen B, et al. (2008). Age-related changes in bone morphology are accelerated in group VIA phospholipase A2 (iPLA2beta)-null mice. Am J Pathol 172:868-881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch F. (2005). Bone growth in length and width: the Yin and Yang of bone stability. J Musculoskelet Neuronal Interact 5:194-201 [PubMed] [Google Scholar]
- Rauch F, Travers R, Norman ME, Taylor A, Parfitt AM, Glorieux FH. (2002). The bone formation defect in idiopathic juvenile osteoporosis is surface-specific. Bone 31:85-89 [DOI] [PubMed] [Google Scholar]
- Rauner M, Sipos W, Pietschmann P. (2008). Age-dependent Wnt gene expression in bone and during the course of osteoblast differentiation. Age (Dordr) 30:273-282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey C, Renugopalakrishnan V, Collins B, Glimcher MJ. (1991a). Fourier transform infrared spectroscopic study of the carbonate ions in bone mineral during aging. Calcif Tissue Int 49:251-258 [DOI] [PubMed] [Google Scholar]
- Rey C, Shimizu M, Collins B, Glimcher MJ. (1991b). Resolution-enhanced Fourier transform infrared spectroscopy study of the environment of phosphate ion in the early deposits of a solid phase of calcium phosphate in bone and enamel and their evolution with age: 2. Investigations in the nu3PO4 domain. Calcif Tissue Int 49:383-388 [DOI] [PubMed] [Google Scholar]
- Rey C, Hina A, Tofighi A, Glimcher MJ. (1995a). Maturational of poorly crystalline apatites – chemical and structural aspects in vivo and in vitro. Cells Materials 5:345-356 [Google Scholar]
- Rey C, Miquel JL, Facchini L, Legrand AP, Glimcher MJ. (1995b). Hydroxyl groups in bone mineral. Bone 16:583-586 [DOI] [PubMed] [Google Scholar]
- Reynolds RM, Dennison EM, Walker BR, Syddall HE, Wood PJ, Andrew R, et al. (2005). Cortisol secretion and rate of bone loss in a population-based cohort of elderly men and women. Calcif Tissue Int 77:134-138 [DOI] [PubMed] [Google Scholar]
- Richards JB, Kavvoura FK, Rivadeneira F, Styrkársdóttir U, Estrada K, Halldórsson BV, et al. (2009). Collaborative meta-analysis: associations of 150 candidate genes with osteoporosis and osteoporotic fracture. Ann Intern Med 151:528-537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie CS, Joshipura K, Hung HC, Douglass CW. (2002). Nutrition as a mediator in the relation between oral and systemic disease: associations between specific measures of adult oral health and nutrition outcomes. Crit Rev Oral Biol Med 13:291-300 [DOI] [PubMed] [Google Scholar]
- Rochefort GY, Pallu S, Benhamou CL. (2010). Osteocyte: the unrecognized side of bone tissue. Osteoporos Int (Epub ahead of print, March 4, 2010) (in press) [DOI] [PubMed] [Google Scholar]
- Sahin E, DePinho RA. (2010). Linking functional decline of telomeres, mitochondria and stem cells during ageing. Nature 464:520-528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito M, Marumo K. (2010). Collagen cross-links as a determinant of bone quality: a possible explanation for bone fragility in aging, osteoporosis, and diabetes mellitus. Osteoporos Int 21:195-214 [DOI] [PubMed] [Google Scholar]
- Salih DA, Brunet A. (2008). FoxO transcription factors in the maintenance of cellular homeostasis during aging. Curr Opin Cell Biol 20:126-136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanfilippo F, Bianchi AE. (2003). Osteoporosis: the effect on maxillary bone resorption and therapeutic possibilities by means of implant prostheses—a literature review and clinical considerations. Int J Periodontics Restorative Dent 23:447-457 [PubMed] [Google Scholar]
- Sarajlic N, Topic B, Brkic H, Alajbeg IZ. (2009). Aging quantification on alveolar bone loss. Coll Antropol 33:1165-1170 [PubMed] [Google Scholar]
- Schaffler MB, Choi K, Milgrom C. (1995). Aging and matrix microdamage accumulation in human compact bone. Bone 17:521-552 [DOI] [PubMed] [Google Scholar]
- Schneider S, Breit SM, Grampp S, Künzel WW, Liesegang A, Mayrhofer E, et al. (2004). Comparative assessment of bone mineral measurements obtained by use of dual-energy x-ray absorptiometry, peripheral quantitative computed tomography, and chemical-physical analyses in femurs of juvenile and adult dogs. Am J Vet Res 65:891-900 [DOI] [PubMed] [Google Scholar]
- Seeman E. (2003). The structural and biomechanical basis of the gain and loss of bone strength in women and men. Endocrinol Metab Clinic North Am 32:25-38 [DOI] [PubMed] [Google Scholar]
- Seeman E. (2009). Bone modeling and remodeling. Crit Rev Eukaryot Gene Expr 9:219-233 [DOI] [PubMed] [Google Scholar]
- Somerville JM, Aspden RM, Armour KE, Armour KJ, Reid DM. (2004). Growth of C57BL/6 mice and the material and mechanical properties of cortical bone from the tibia. Calcif Tissue Int 74:469-475 [DOI] [PubMed] [Google Scholar]
- Southard KA, Southard TE, Schlechte JA, Meis PA. (2000). The relationship between the density of the alveolar process and that of post-cranial bone. J Dent Res 89:964-969 [DOI] [PubMed] [Google Scholar]
- Streckfus CF, Parsell DE, Streckfus JE, Pennington W, Johnson RB. (1999). Relationship between oral alveolar bone loss and aging among African-American and Caucasian individuals. Gerontology 45:110-114 [DOI] [PubMed] [Google Scholar]
- Suzuki H, Amizuka N, Oda K, Li M, Yoshie H, Ohshima H, et al. (2005). Histological evidence of the altered distribution of osteocytes and bone matrix synthesis in klotho-deficient mice. Arch Histol Cytol 68:371-381 [DOI] [PubMed] [Google Scholar]
- Szulc P, Seeman E. (2009). Thinking inside and outside the envelopes of bone: dedicated to PDD. Osteoporos Int 20:1281-1288 [DOI] [PubMed] [Google Scholar]
- Taguchi A, Suei Y, Ohtsuka M, Otani K, Tanimoto K, Hollender LG. (1999). Relationship between bone mineral density and tooth loss in elderly Japanese women. Dentomaxillofac Radiol 28:219-223 [DOI] [PubMed] [Google Scholar]
- Tanaka M, Ejiri S, Toyooka E, Kohno S, Ozawa H. (2002). Effects of ovariectomy on trabecular structures of rat alveolar bone. J Periodontal Res 37:161-165 [DOI] [PubMed] [Google Scholar]
- Tanaka M, Toyooka E, Kohno S, Ozawa H, Ejiri S. (2003). Long-term changes in trabecular structure of aged rat alveolar bone after ovariectomy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 95:495-502 [DOI] [PubMed] [Google Scholar]
- Tang SY, Zeenath U, Vashishth D. (2007). Effects of non-enzymatic glycation on cancellous bone fragility. Bone 40:1144-1151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarnowski CP, Ignelzi MA, Jr, Morris MD. (2002). Mineralization of developing mouse calvaria as revealed by Raman microspectroscopy. J Bone Miner Res 17:1118-1126 [DOI] [PubMed] [Google Scholar]
- Tommasini SM, Nasser P, Schaffler MB, Jepsen KJ. (2005). Relationship between bone morphology and bone quality in male tibias: implications for stress fracture risk. J Bone Miner Res 20:1372-1380 [DOI] [PubMed] [Google Scholar]
- Tommasini SM, Nasser P, Jepsen KJ. (2007). Sexual dimorphism affects tibia size and shape but not tissue-level mechanical properties. Bone 40:498-505 [DOI] [PubMed] [Google Scholar]
- Tong W, Glimcher MJ, Katz JL, Kuhn L, Eppell SJ. (2003). Size and shape of mineralites in young bovine bone measured by atomic force microscopy. Calcif Tissue Int 72:592-598 [DOI] [PubMed] [Google Scholar]
- Tosi LL, Boyan BD, Boskey AL. (2005). Does sex matter in musculoskeletal health? The influence of sex and gender on musculoskeletal health. J Bone Joint Surg Am 87:1631-1647 [DOI] [PubMed] [Google Scholar]
- Van Cauter E, Leproult R, Kupfer DJ. (1996). Effects of gender and age on the levels and circadian rhythmicity of plasma cortisol. J Clin Endocrinol Metab 81:2468-2473 [DOI] [PubMed] [Google Scholar]
- Vashishth D. (2007). Hierarchy of bone microdamage at multiple length scales. Int J Fatigue 29:1024-1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vashishth D, Gibson GJ, Khoury JI, Schaffler MB, Kimura J, Fyhrie DP. (2001). Influence of nonenzymatic glycation on biomechanical properties of cortical one. Bone 28:195-201 [DOI] [PubMed] [Google Scholar]
- Verborgt O, Gibson GJ, Schaffler MB. (2000). Loss of osteocyte integrity in association with microdamage and bone remodelling after fatigue in vivo. J Bone Miner Res 15:60-67 [DOI] [PubMed] [Google Scholar]
- Viguet-Carrin S, Follet H, Gineyts E, Roux JP, Munoz F, Chapurlat R, et al. (2010). Association between collagen cross-links and trabecular microarchitecture properties of human vertebral bone. Bone 46:342-347 [DOI] [PubMed] [Google Scholar]
- Von Wowern N, Stoltze K. (1978). Histoquantitation on small jaw specimens. Scand J Dent Res 86:193-199 [DOI] [PubMed] [Google Scholar]
- Wallace JM, Rajachar RM, Allen MR, Bloomfield SA, Robey PG, Young MF, et al. (2007). Exercise-induced changes in the cortical bone of growing mice are bone- and gender-specific. Bone 40:1120-1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Seeman E. (2008). Skeletal growth and peak bone strength. Best Pract Res Clin Endocrinol Metab 22:687-700 [DOI] [PubMed] [Google Scholar]
- Weiner S, Traub W. (1989). Crystal size and organization in bone. Connect Tissue Res 21:259-265 [DOI] [PubMed] [Google Scholar]
- Westerbeek ZW, Hepple RT, Zernicke RF. (2008). Effects of aging and caloric restriction on bone structure and mechanical properties. J Gerontol A Biol Sci Med Sci 63:1131-1136 [DOI] [PubMed] [Google Scholar]
- White SC, Rudolph DJ. (1999). Alterations of the trabecular pattern of the jaws in patients with osteoporosis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 88:628-635 [DOI] [PubMed] [Google Scholar]
- Williams BO, Insogna KL. (2009). Where Wnts went: the exploding field of Lrp5 and Lrp6 signaling in bone. J Bone Miner Res 24:171-178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo SL, Kuei SC, Amiel D, Gomez MA, Hayes WC, White FC, et al. (1981). The effect of prolonged physical training on the properties of long bone: a study of Wolff’s Law. J Bone Joint Surg Am 63:780-787 [PubMed] [Google Scholar]
- Wu M, Fannin J, Rice KM, Wang B, Blough ER. (2010). Effect of aging on cellular mechanotransduction. Ageing Res Rev (Epub ahead of print, November 20, 2009) (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Ackerman JL, Kim HM, Rey C, Barroug A, Glimcher MJ. (2002). Nuclear magnetic resonance spin-spin relaxation of the crystals of bone, dental enamel, and synthetic hydroxyapatites. J Bone Miner Res 17:472-480 [DOI] [PubMed] [Google Scholar]
- Yates LB, Karasik D, Beck TJ, Cupples LA, Kiel DP. (2007). Hip structural geometry in old and old-old age: similarities and differences between men and women. Bone 41:722-732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yerramshetty JS, Lind C, Akkus O. (2006). The compositional and physicochemical homogeneity of male femoral cortex increases after the sixth decade. Bone 39:1236-1243 [DOI] [PubMed] [Google Scholar]
- Zhou S, Greenberger JS, Epperly MW, Goff JP, Adler C, Leboff MS, et al. (2008). Age-related intrinsic changes in human bone-marrow-derived mesenchymal stem cells and their differentiation to osteoblasts. Aging Cell 7:335-343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W, Robey PG, Boskey AL. (2008). The regulatory role of matrix proteins in mineralization of bone. In: Osteoporosis. 3rd ed. Marcus R, Feldman D, Nelson D, Rosen CJ, editors. New York, NY: Academic Press, Chapter 9, pp. 191-240 [Google Scholar]
- Zuscik MJ, Hilton MJ, Zhang X, Chen D, O’Keefe RJ. (2008). Regulation of chondrogenesis and chondrocyte differentiation by stress. J Clin Invest 118:429-438 [DOI] [PMC free article] [PubMed] [Google Scholar]