Abstract
BACKGROUND
Samarium-153 ethylenediamine tetramethylene phosphoric acid (153Sm-EDTMP) is a radiopharmaceutical that has been used to treat osteosarcoma. The authors conducted a phase 2 study to test safety and response of high-risk osteosarcoma to tandem doses of 153Sm-EDTMP and to determine correlation between radiation delivered by low and high administered activities.
METHODS
Patients with recurrent, refractory osteosarcoma detectable on standard 99mTc bone scan received a low dose of 153Sm-EDTMP (37.0–51.8 MBq/kg), followed upon count recovery by a second, higher dose (222 MBq/kg). Fourteen days later, patients were rescued with autologous hematopoietic stem cells. The authors assessed response to therapy, performed dosimetry to determine the relationship between administered activity and tumor absorbed dose, and investigated whether changes in 2-(fluorine-18) fluoro-2-deoxy-d-glucose (18F-FDG) tumor uptake upon hematologic recovery reflected disease response.
RESULTS
Nine patients were given tandem doses of 153Sm-EDTMP; 2 received only the initial dose because of disease progression. Six patients experienced radiographic disease stabilization, but this was not considered a response, so the study was terminated early. There was a linear relationship between administered activity and tumor absorbed dose, but there was no correlation between change in 18F-FDG positron emission tomography tumor uptake and tumor absorbed dose or time to progression. The median time to progression for the entire group was 79 days.
CONCLUSIONS
Tandem doses of 153Sm-EDTMP were safe for this cohort of heavily pretreated patients with very high-risk disease. The strong correlation between absorbed dose and administered activity within each evaluable patient provides a methodology to individually tailor tandem doses of this agent.
Keywords: radiopharmaceuticals, targeted radiotherapy, osteosarcoma, bone neoplasm
Cure of osteosarcoma requires definitive control of all radiographically evident tumor as well as treatment of micrometastatic disease. Osteosarcoma is a relatively radioresistant tumor; administration of <60 grays (Gy) is associated with only transient tumor control, and viable tumor has been found in amputation specimens even after treatment with 80 Gy or more.1–3 This has limited the applicability of radiotherapy, because such doses are associated with significant normal tissue injury. Surgery is therefore the primary modality for local control.
Targeted radiation therapy has been pursued as an alternative to external beam radiotherapy, and 1 of the most promising agents being developed for this purpose is samarium-153 ethylenediamine tetramethylene phosphoric acid (153Sm-EDTMP). The radioisotope 153Sm emits an electron (beta particle) with an average energy of 224 keV (approximate range of 0.5 mm), which is appropriate for targeted cytotoxicity. Its photon emissions include a 103-keV photon appropriate for scintigraphic imaging, thereby allowing both confirmation of agent localization and quantification of the absorbed dose delivered to target lesions. 153Sm has a physical half-life of 46.3 hours, allowing therapy to be delivered safely in the outpatient setting.
There have been several published studies of 153Sm-EDTMP for the treatment of high-risk osteosarcoma. A phase 1 study of high-dose 153Sm-EDTMP found that activities of up to 11.1 GBq/kg (30 mCi/kg) could be administered with autologous stem cell support, with myelosuppression as the only significant toxicity.4 The addition of gemcitabine as a radiation sensitizer did not significantly improve response.5 We recently completed a dose finding study of 153Sm-EDTMP, designed to identify a quantity of activity of this agent that could be administered to heavily pretreated osteosarcoma patients and allow hematopoietic recovery in 6 weeks without hematopoietic stem cell support.6 Patients were treated with 37 to 51.8 MBq/kg (1.0–1.4 mCi/kg), and transient disease stabilization was achieved in 5 (38%) of 13 patients. We subsequently designed this phase 2 study to 1) test the safety and response of high-risk osteosarcoma to high-dose 153Sm-EDTMP and 2) determine the correlation between administered activity and tumor absorbed dose in patients treated with tandem doses of radiopharmaceutical, allowing future use of a low tracer dose to predict an individualized, higher dose that would reliably result in a tumor absorbed dose in the tumoricidal range.
Our goal was to develop a methodology for designing a safe, effective treatment regimen integrating radiation therapy into the treatment of high-risk osteosarcoma, potentially overcoming the treatment resistance that characterizes this often incurable disease.
MATERIALS AND METHODS
Eligibility Requirements
Eligible patients were between the ages of 13 and 50 years and had high-risk osteosarcoma, defined as a tumor that was recurrent, refractory to conventional therapy, or metastatic, with either >4 pulmonary lesions, bone metastases, or both. Patients were required to have measurable disease detectable on a standard 99mTc bone scan. Adequate renal and hematologic function was required (including an absolute neutrophil count [ANC] ≥ 500/mm3 and a platelet count ≥50,000/ mm3). Subjects must have recovered fully from the effects of prior chemotherapy, but no specific treatment-free interval was required. Informed consent was obtained according to institutional policies. Patients were excluded if they had a Karnofsky performance status <50%, were pregnant or breast feeding, or had prior radiotherapy to all areas of active disease. The study was listed on clinicaltrials.gov (NCT00 245011) and approved by the institutional review board of Johns Hopkins University. All patients provided informed consent.
Treatment Plan
Before administration of 153Sm-EDTMP, collection of autologous hematopoietic stem cells was required. Either bone marrow or peripheral blood stem cells were considered acceptable, and the stem cell product was not dictated by the study. The initial treatment of 153Sm-EDTMP was part of a concurrent dose finding study6; the range of activities was 37 to 51.8 MBq/kg (1.0–1.4 mCi/ kg). Those patients ineligible for the dose finding study, or who declined to participate, were treated with 44.4 MBq/kg (1.2 mCi/kg). The drug was administered in the Radiation Oncology outpatient clinic. After imaging, patients were followed until hematologic recovery, defined as ANC ≥500/mm3 and platelet count ≥50,000/mm3. Patients were evaluated for disease response upon hematologic recovery from the first dose of 153Sm-EDTMP.
The second treatment with 153Sm-EDTMP was 222 MBq/kg (6 mCi/kg) and was also administered in the Radiation Oncology outpatient clinic. Patients were given 500 mg calcium carbonate orally before infusion to decrease the risk of transient hypocalcemia. An electrocardiogram (EKG) and serum calcium level were checked before treatment, and intravenous fluids were administered at twice maintenance. Patients remained on a cardiac monitor, isolated behind protective shielding, until radioactivity fell to safe levels as determined by personnel from Medical Physics. An EKG was repeated before discharge from clinic.
Fourteen days after administration of the higher dose 153Sm-EDTMP, autologous hematopoietic stem cells were infused in the Pediatric Oncology outpatient clinic. Upon hematologic recovery and resolution of treatment-related toxicities, radiographic response was determined. Patients were offered surgical resection of amenable lesions, and resected lesions were evaluated for histologic evidence of response.
Response Evaluation
Upon hematologic recovery from each treatment, disease response was evaluated radiographically, by either computed tomography (CT) or magnetic resonance imaging (MRI). Radiographic response criteria were as follows: complete response = complete resolution of radiographic evidence of disease; partial response = >50% reduction in the sum of the products of perpendicular tumor diameters; minor response = a 25% to 50% reduction in the sum of the products of the tumor diameter without the emergence of new lesions, and no individual lesion progressing >25% in a single dimension; stable disease = no progressive disease; progressive disease = emergence of new lesions or progression of any lesion by >25% in a single dimension. Clinical response was defined as a minor radiographic response or better.
Toxicities were graded according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events (version 3.0).
Radiation Dosimetry
153Sm-EDTMP whole body planar scans were performed 4, 24, and 48 to 72 hours after each infusion using a GE Infinia Hawkeye gamma camera (GE Healthcare, Waukesha, Wis) and an acquisition window centered on the 103-keV photopeak (±10%). Anterior/posterior whole body sweeps were performed at a detector speed of 8 to 10 cm/min with no contouring. Standard Medical Internal Radiation Dose Committee anteroposterior formalism7 was used to determine the activity in the lesions, while substituting in the build-up factor8 as a method for scatter correction. In addition, the measured lesion activities were adjusted to account for detector count rate saturation (Hobbs et al, unpublished data) and were then converted to absorbed dose in the OLINDA software package9 using the mass of the lesions, determined radiographically from CT or MRI.
2-(Fluorine-18) Fluoro-2-Deoxy-d-Glucose Positron Emission Tomography/CT Scans and Image Analysis
2-(Fluorine-18) fluoro-2-deoxy-d-glucose (18F-FDG) positron emission tomography (PET)/CT scans were performed using standard clinical protocols. Patients fasted for a minimum of 4 hours and had blood glucose levels <200 mg/dL before intravenous injection of a weight-adjusted dose of 18F-FDG (8.14 MBq/kg). Oral, but not intravenous, contrast material was administered for the CT portion of the study. After an approximately 60-minute radiotracer uptake phase, a combined PET/CT scan (Discovery LS or ST; GEHealthcare) was acquired.
The single-pixel maximum standardized uptake value adjusted for lean body mass (SUVmax) was determined for each lesion based on a 3-dimensional region of interest. These values were normalized to liver and mediastinal blood pool average activity adjusted for lean body mass (tumor SUVmax/reference SUVavg). The ratio of the normalized target lesion FDG uptake at the post-therapy scans to the baseline scans was calculated.
Statistical Analysis
This study was designed to determine the proportion of clinical tumor responses and the toxicities associated with high-dose 153Sm-EDTMP in patients with high-risk osteosarcoma using a 2-stage phase 2 clinical trial design. To estimate the probability of a clinical response to high-dose 153Sm-EDTMP, the proportion of patients with a clinical response was estimated, and a 95% confidence interval was estimated under the assumption that the underlying distribution is binomial. An optimum 2-stage design was intended to detect a 50% clinical response rate versus an estimated response rate of 25% among patients treated with chemotherapy alone, with a 10% type I and a 10% type II error. In the first stage, 10 evaluable patients were to be treated. If 2 or fewer of the 10 patients had a clinical response, the trial was to be stopped. Otherwise, an additional 17 patients would have been treated in the second stage. The treatment would have been recommended for further study if >9 of a total 27 patients had a clinical response after the second stage. This rule has a 90.3% chance of recommending the treatment for further study if the true response rate is 50%, and a 9.9% chance of inappropriately recommending the treatment for further study if the true response rate is only 25%. Time to tumor progression was estimated using the Kaplan-Meier method.
The other primary objective of this study was to examine whether measuring the tumor absorbed radiation dose after a low administered activity can be used to predict a higher administered activity that would reliably deliver a tumoricidal radiation dose before stem cell rescue. The relationship between tumor absorbed dose after low and high administered activities was modeled by simple linear regression. Tumor mass and SUV ratio were logarithm transformed to produce approximately normally distributed data, and Pearson correlation coefficients were calculated to assess the relationship between tumor absorbed dose and FDG-PET response and between the SUV ratio and time to progression.
RESULTS
Patient Characteristics
Eleven subjects with high-grade osteoblastic osteosarcoma were enrolled in this study (Table 1), because 1 patient was removed from study before we were able to assess for response. The median age was 18 years (range, 14–30 years). Three had primary refractory disease, including 1 with a secondary osteosarcoma arising in the radiation field of a medulloblastoma that had been diagnosed and treated 6 years previously. The other 8 patients had recurrent disease: 1 patient in first relapse, 5 in second relapse (2 refractory to salvage therapy), 1 in third relapse, and 1 in fourth relapse. All of the patients were heavily pretreated with chemotherapy. Every patient had been previously treated with cisplatin, doxorubicin, and high-dose methotrexate. Ten of the patients were previously treated with ifosfamide, either as a single agent (1) or in combination with etoposide (9). Three had been treated with gemcitabine, 2 in combination with docetaxel and 1 as a single agent. Aerosolized granulocyte-macrophage colony-stimulating factor and local external beam radiotherapy had been administered to 3 patients. The patient with a history of medulloblastoma had received a full course of treatment for that tumor before the diagnosis of osteosarcoma.
Table 1.
Patient Characteristics
| Patient No. |
Sex | Age, y, mo |
Disease Status | Disease Sites | Previous Treatment | Other Medical Problems |
|---|---|---|---|---|---|---|
| 1 | F | 18, 7 | Recurrence 2 | L2 vertebral body, left lung | Cisplatin/doxorubicin/MTX | |
| 2 | M | 17, 6 | Primary refractory | Lungs, inguinal lymph nodes | Cisplatin/doxorubicin/MTX/VP-16/ifosfamide | |
| 3 | M | 17, 3 | Refractory recurrence 2 | Sacrum | Cisplatin/doxorubicin/MTX/VP-16/ifosfamide; inhaled GM-CSF; gemcitabine/Taxotere; local XRT, topotecan; samarium-153/gemcitabine | Cardiomyopathy |
| 4 | F | 30, 9 | Recurrence 4 | Chest wall | Cisplatin/doxorubicin/MTX; gemcitabine/Taxotere; ifosfamide | |
| 5 | M | 20, 2 | Refractory recurrence 2 | Lungs, rib, shoulder | Cisplatin/doxorubicin/MTX/VP-16/ifosfamide; XRT; inhaled GM-CSF | |
| 6 | M | 15, 5 | Primary refractory | Ribs, spine, right knee | Cisplatin/doxorubicin/MTX/VP-16/ifosfamide | |
| 7 | M | 15, 5 | Primary refractory (secondary to XRT for medulloblastoma) | Temporal bone | Cisplatin/doxorubicin/VP-16/ifosfamide (history of XRT and chemotherapy for medulloblastoma) | |
| 8 | F | 14, 3 | Recurrence 3 | Left lung, skull, patella, pelvis | Cisplatin/doxorubicin/MTX/VP-16/ifosfamide | |
| 9 | F | 19, 3 | Recurrence 2 | Skull, ribs, right femur, abdomen | Cisplatin/doxorubicin/MTX/VP-16/ifosfamide; XRT; inhaled GM-CSF | |
| 10 | M | 21, 2 | Recurrence 2 | Right upper lobe of lung, L4 and S1 vertebral bodies, right ilium, retroperitoneal soft tissue | Cisplatin/doxorubicin/MTX/VP-16/ifosfamide; gemcitabine | Asthma |
| 11 | F | 25, 6 | Recurrence 1 | Lungs, inguinal nodes | Cisplatin/doxorubicin/MTX/VP-16/ifosfamide |
F indicates female; MTX, methotrexate; M, male; GM-CSF, granulocyte-macrophage colony-stimulating factor; XRT, external radiation therapy.
All patients had disease that was detectable on standard 99mTc bone scan. Eight of the patients had bone lesions. Involved bones included vertebral bodies and sacrum in 4 patients and skull in 3. In addition, 6 of the patients had pulmonary metastases, and 2 had lymph node involvement. One of the patients had an anthracycline-related cardiomyopathy, although with medical therapy he had an adequate ejection fraction. There were no other significant comorbidities.
Toxicities
No immediate, infusion-related toxicities were noted during infusion of either low- or high-dose 153Sm-EDTMP. In particular, despite the risk of infusion-related hypocalcemia, no subject reported immediate numbness or tingling of the extremities, and there was no nausea or vomiting. Three subjects reported delayed numbness and tingling within 72 hours of infusion of the high dose, but only 1 of these was found to have mild hypocalcemia (grade 1), which was readily corrected. Most of the subjects experienced an increase in bone pain 12 to 48 hours after infusion of low-dose 153Sm-EDTMP (flair reaction) with subsequent improvement. Interestingly, this was not noted after the high-dose infusion.
All subjects had mild-to-moderate, self-limited pancytopenia after the low dose of 153Sm-EDTMP, as described previously.6 Three subjects required transfusion of red blood cells and/or platelets after low-dose 153Sm-EDTMP, and after high-dose 153Sm-EDTMP (but before peripheral blood stem cell [PBSC] or marrow reinfusion), 5 subjects required red blood cell and/or platelet transfusions. PBSC or marrow support was provided according to protocol on Day 14 after high-dose therapy. After stem cell infusion, all 8 evaluable subjects had recovery of neutropenia with ANC ≥500 within 2 to 4 weeks. All subjects were also lymphopenic. One subject had grade 2 lymphopenia, 5 subjects had grade 3 lymphopenia, and 3 subjects experienced grade 4 lymphopenia. The median day of lymphocyte nadir after administration of the high-dose treatment was Day 6 (range, 0–27).
Infectious complications occurred, but were manageable. After infusion of the lower 153Sm-EDTMP dose, 1 subject developed a fever with bacteremia that responded promptly to antibiotic treatment. After high-dose 153Sm-EDTMP, 4 of 9 subjects developed fever during their period of neutropenia. Two of these subjects had a documented infection (pneumonia in a patient with a chest wall mass and a urinary tract infection in a patient with a pelvic tumor), both of whom responded to antibiotic treatment.
Dosimetry
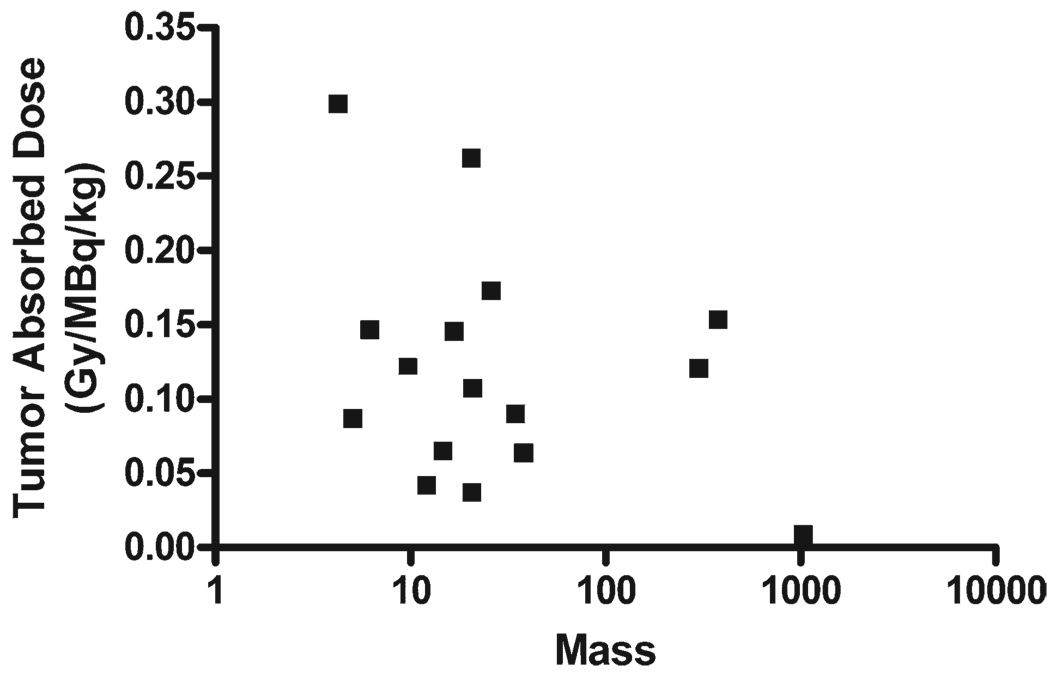
Visualization of the 103-keV photon emission allowed quantification of the tumor absorbed dose. Evaluable dosimetry data were available for 16 individual tumors from 7 patients. After administration of 222 MBq/kg, the absorbed dose varied from 1.8 to 66.2 Gy (median, 25.2). Target lesions varied from 4.3 to 1047 g (median, 20.8 g). There was no apparent correlation between absorbed dose and tumor mass (Fig. 1).
Figure 1.
Tumor absorbed dose versus mass is shown. Tumor absorbed dose was determined after infusion of high-activity samarium-153 ethylenediamine tetramethylene phosphoric acid and compared with tumor mass on a lesion-by-lesion basis for 16 individual tumors from 7 subjects. No correlation was seen (R2 = 0.11, P = .208). Gy indicates grays.
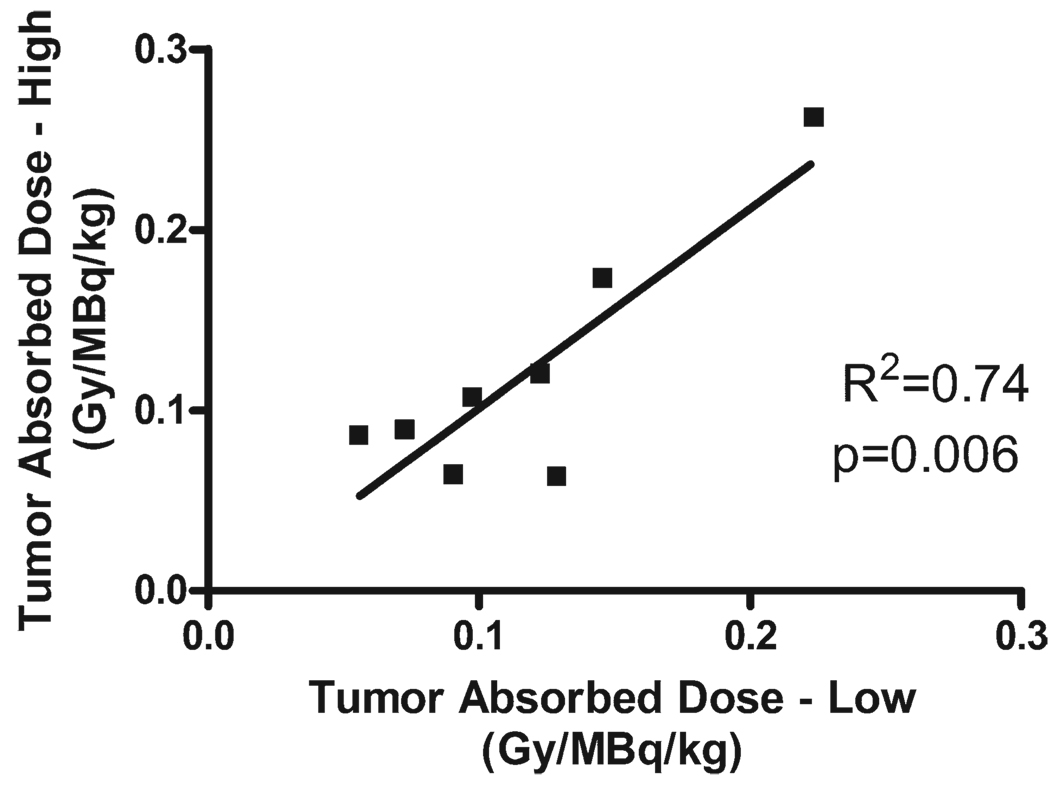
We observed variability of tumor absorbed dose of >2 orders of magnitude from patient to patient (0.008–0.3 Gy/MBq/kg), as has been reported in previous studies of high-dose 153Sm-EDTMP.4 Because we administered 2 different activities to each patient approximately 6 weeks apart, we examined the relationship between administered activity and dose absorbed by each individual tumor after standard and high doses. Adequate data were available for 8 individual tumors. We found that the absorbed dose after the low-activity treatment strongly predicted absorbed dose after the high-activity treatment (Fig. 2), with a coefficient of correlation of R2 = 0.74 (P = .006). The slope of the line relating absorbed dose after high activity as a function of absorbed dose after low activity is 1.106, with an intercept of −0.0094. The discrepancy between the number of tumors analyzed for dosimetry (16, Fig. 1) and the number of tumors evaluable for correlation between administered activity and absorbed dose (8, Fig. 2) is because of the appearance of new lesions in some patients between their low-dose and high-dose treatments.
Figure 2.
Tumor absorbed dose correlation between low and high administered activities is shown. Tumor absorbed dose of radiation was determined after administration of low- and high-activity samarium-153 ethylenediamine tetramethylene phosphoric acid. There was a linear correlation (slope of the linear regression line is 1.106, and the y intercept is −0.0094), with R2 = 0.74 and P = .006. Gy indicates grays.
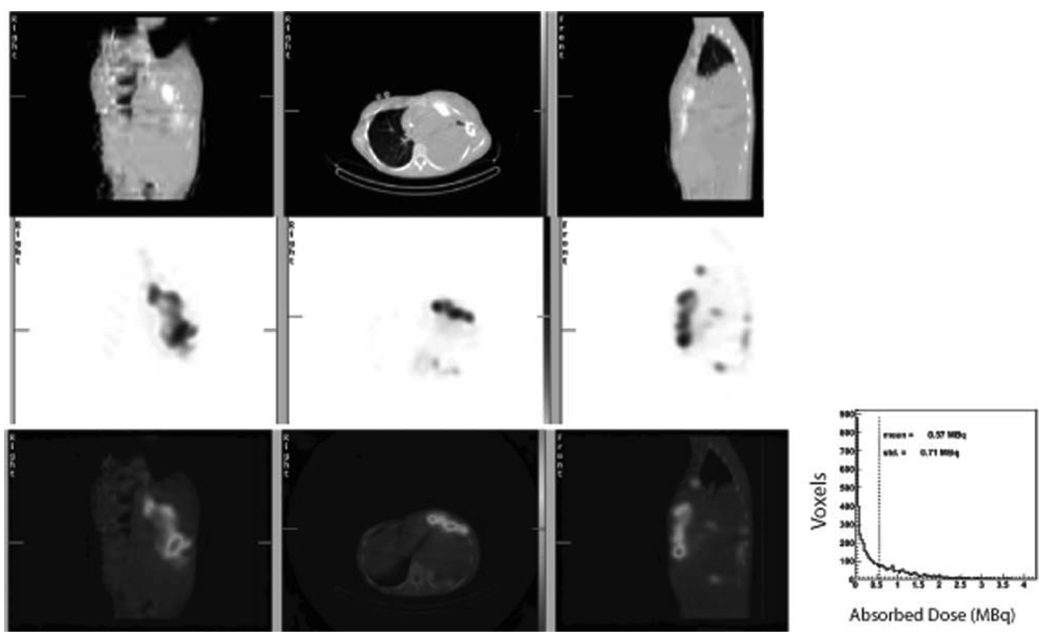
In addition to whole body planar scans, some patients underwent single-photon emission computed tomography (SPECT) imaging with accompanying CT scan 4 hours after administration of high-dose 153Sm-EDTMP to allow a more detailed analysis of the distribution of radioactivity within the larger tumors. Activity distribution was quite heterogeneous (Fig. 3).
Figure 3.
Heterogeneity of activity distribution is shown. The fused single-photon emission computed tomography (SPECT)/computed tomography (CT) from Patient 4 (Table 1) shows nonuniform distribution of activity in the large chest wall mass. The top row shows a coronal (left), axial (center), and sagittal (right) CT image. The center row shows corresponding SPECT images, and the bottom row shows the fused images. The extent of the heterogeneity in the distribution of activity is quantified in the histogram (inset). The histogram shows the amount of uptake at 4 hours postinfusion for each voxel in the large anterior tumor. The mean voxel activity (voxel size = 4.42 × 4.42 × 4.42 mm3) at 4 hours was 0.57 MBq, with a standard deviation of 0.71 MBq. This degree of heterogeneity is typical of most tumors quantified with SPECT imaging.
Disease Response
Clinical response
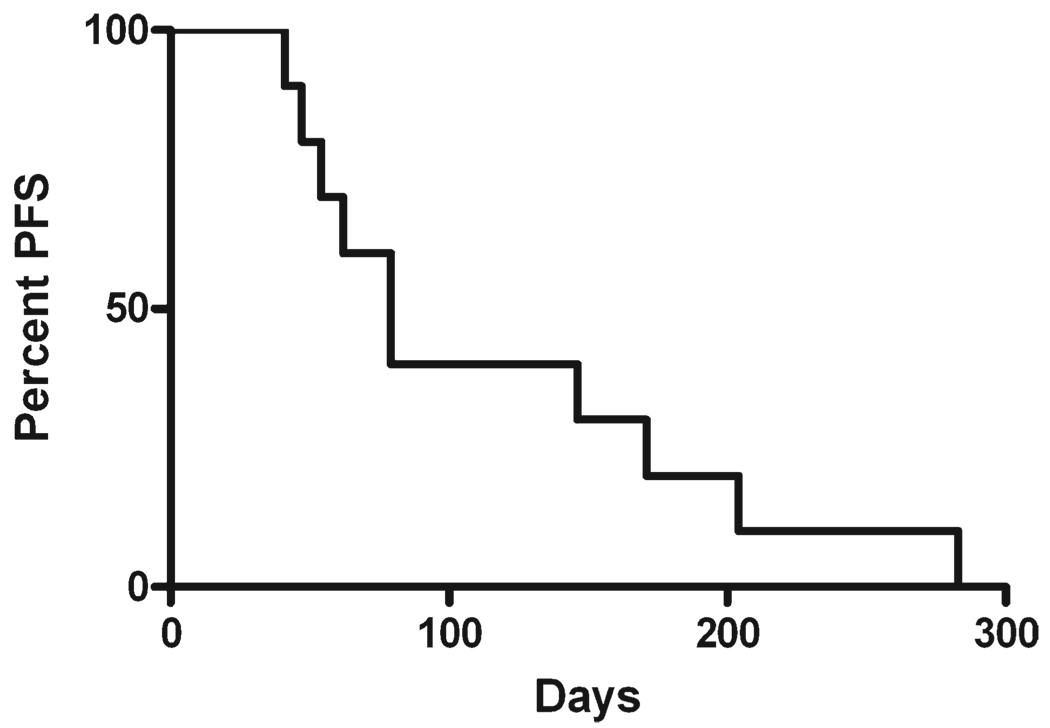
Patients were administered tandem doses of 153Sm-EDTMP as treatment for their high-risk osteosarcoma. Disease status was evaluated after recovery from the first, lower dose, and again after recovery from the higher dose. Six of the patients experienced disease stabilization at some point during the protocol. Two patients had stable disease after the low-dose treatment but progressed after high dose, 2 patients progressed after the low dose but had disease stabilization after the high dose, and 2 had stable disease throughout the study. Five patients had no evidence of disease stabilization at any point. The median time to progression was 79 days for the entire cohort (Fig. 4), and 142 days for the 4 patients with disease stabilization after the higher dose. Two patients achieved prolonged survival (990 and 1432 days) despite eventual disease progression, received subsequent treatments, but eventually died of their disease. Despite these results, the study was terminated early for lack of efficacy, because patients with stable disease did not meet the protocol definition of clinical response.
Figure 4.
Time to disease progression is shown. A Kaplan-Meier survival curve indicates the number of days from treatment with low-activity samarium-153 ethylenediamine tetramethylene phosphoric acid until the first evaluation that revealed radiographic evidence of progressive disease. PFS indicates progression-free survival.
PET response
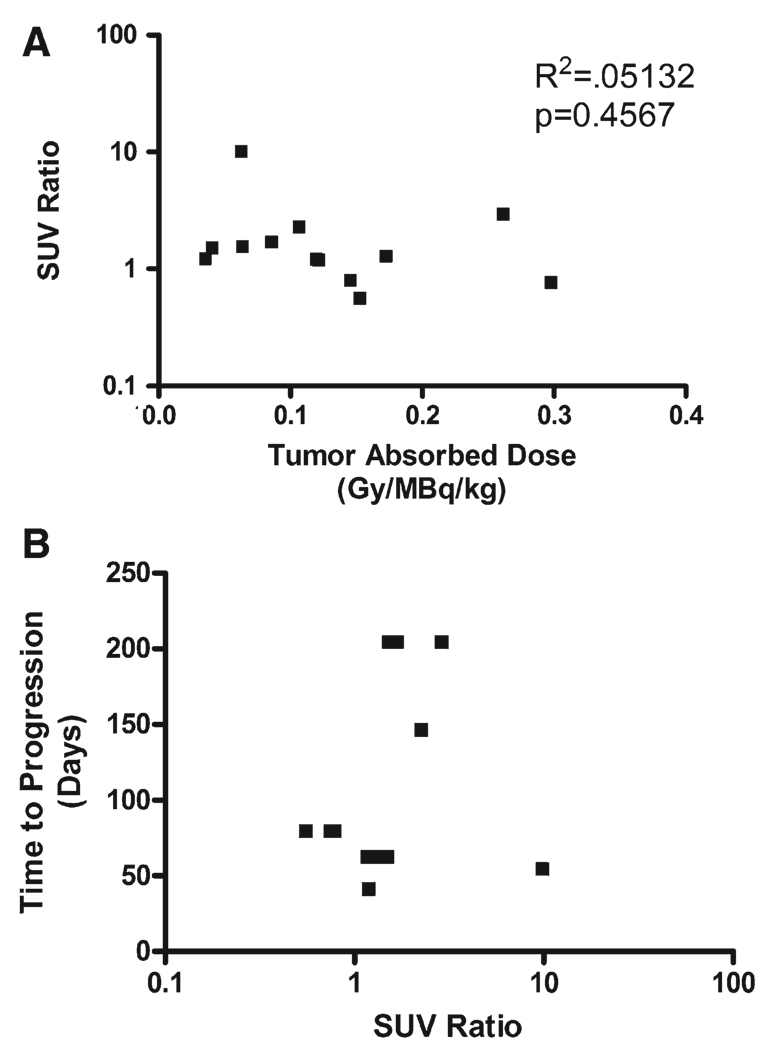
Patients had a scan before and after 153Sm-EDTMP therapy. Post-therapy PET/CT was performed at a median of 34 days (range, 27–68 days). A total of 18 lesions in 6 of the subjects were evaluated by PET. Nine of these lesions were radiographically stable from baseline scan through end of treatment, and the other 9 lesions showed radiographic progression during that time. For the 9 stable lesions, the change in SUVmax ranged from −44% to +307% (median,+53%), and for the 9 progressive lesions, the change ranged from −14% to +890% (median, +50%). Our hypothesis was that the higher tumor absorbed dose would be associated with a reduction in SUVmax, but we did not find any significant relationship (Fig. 5A). We also evaluated whether a reduction in SUVmax is associated with longer time to progression, but found no significant correlation (Fig. 5B). Three of the lesions that showed radiographic progression were noted to have significant central necrosis on CT scan, 2 of which had no significant change in SUV, and 1 of which had an 890% increase in SUVmax despite the necrotic appearance on scan.
Figure 5.
Comparison between positron emission tomography (PET) response, tumor absorbed dose, and clinical response is shown. (A) The change in PET activity in response to the high administered activity of samarium-153 ethylenediamine tetramethylene phosphoric acid (153Sm-EDTMP) was compared with tumor absorbed dose. For ease of analysis, we determined an standardized uptake value (SUV) ratio, defined as the ratio between the SUVmax post-therapy and the SUVmax pretreatment, both normalized to liver activity. This data transformation allows a logarithmic plot to be evaluated. No correlation between tumor absorbed dose and change in SUV ratio was seen (R2 = 0.051, P = .457). (B) The number of days from treatment with high-activity 153Sm-EDTMP until the first evaluation that revealed radiographic evidence of progressive disease was compared with the response of each tumor as determined by 2-(fluorine-18) fluoro-2-deoxy-d-glucose PET. No correlation is seen between time to tumor progression and SUV ratio (R2 = 0.0031, P = 0.857).
DISCUSSION
We report here the results of our clinical trial investigating the safety and effectiveness of tandem doses of 153Sm-EDTMP for the treatment of patients with high-risk osteosarcoma. It was feasible to deliver this therapy to a cohort of heavily pretreated patients with a minimum of toxicity, mostly limited to myelosuppression. Of the 9 subjects treated as intended, 4 experienced disease stabilization, including 2 patients whose disease had progressed after the initial low dose. Our results suggest that even as a single agent, 153Sm-EDTMP may have some activity for high-risk osteosarcoma, causing radiographic disease stabilization.
Two of our subjects had lesions visible on 99mTc bone scan that were all extraosseous (Patients 2 and 11 from Table 1). Patient 2 had progression-free survival of 283 days. This patient had persistent disease (pulmonary nodules on CT scan, positive bone scan in pelvic lymph nodes, and a positive FDG-PET scan) after being treated with methotrexate, cisplatin, doxorubicin, ifosfamide, and etoposide as a subject on COG study AOST0121. After being treated with tandem doses of 153Sm-EDTMP (which resulted in disease stabilization), staged bilateral thoracotomies were performed to remove his pulmonary nodules, and the aggregate necrosis of these 41 nodules was >95%. The combination of significant tumor necrosis and prolonged progression-free survival suggests a significant response to this treatment. Although Patient 11 had progression-free survival of only 79 days, both patients had disease stabilization, suggesting that 153Sm-EDTMP may have activity in tumors that do not involve bone.
Anatomic imaging may underestimate response, as calcified osteosarcoma may not shrink despite cytotoxicity. We had hoped that functional imaging with FDG-PET might provide evidence of cellular response, because a growing literature suggests the utility of FDG-PET scanning in assessing the response of sarcomas to treatment.10–12 We observed no correlation between change in SUVmax and response to treatment with 153Sm-EDTMP. This could be because of the relatively small number of patients available for analysis, but could also reflect a tumor inflammatory reaction to this particular treatment, because some tumors had clearly necrotic centers after treatment (suggesting response), but nevertheless had marked increases in SUVmax. We do not believe PET scanning will be useful for evaluating response to treatment with this agent, although it may be quite useful with other agents. Until methodology can be found that accurately evaluates for response in osteosarcoma, histology remains the gold standard.
One controversial question regarding the use of 153Sm-EDTMP has been whether extraosseous lesions respond to this treatment. One of our subjects with disease limited to lung parenchyma and lymph nodes had a prolonged period of progression-free survival, and by histology an aggregate of >95% necrosis of his pulmonary nodules, and another patient with pulmonary parenchymal disease also had disease stabilization, strongly supporting the idea that this agent is cytotoxic to extraosseous disease.
Although we found evidence of activity (disease stabilization and tumor necrosis), eventually all patients progressed. One way to improve the efficacy of 153Sm-EDTMP would be to increase the administered activity, because there were no significant nonhematologic toxicities, and Anderson et al administered up to 1110 MBq/kg, also with insignificant nonhematologic toxicity.4 A major goal of this study was to determine whether we could tailor dosing, using a low dose to select the subsequent higher dose. This approach clearly requires correlation between tumor absorbed dose after 2 different administered activities within the same patient. Previous studies, from our group and from others, have demonstrated significant variability in tumor absorbed dose from 1 patient to the next.4–6 In contrast, we found a tight linear correlation between absorbed dose after the low administered activity and absorbed dose after the high administered activity, compared tumor by tumor, so variability previously reported probably reflects interpatient variability. It therefore should be feasible to design a study that delivers tandem doses of 153Sm-EDTMP to patients, with the second administered activity determined based on the absorbed dose measured after the first treatment. This approach would allow the administration of an individually targeted tumoricidal absorbed dose of radiopharmaceutical. In such a study, the time between treatments would be minimized to prevent disease progression after the lower administered activity.
An alternative approach would be to combine highdose 153Sm-EDTMP with another agent, such as a vascular disrupting agent (such as combretastatin) or a traditional cytotoxic agent that might act as a radiosensitizer or may just act synergistically with targeted radiation. Another factor to be considered in increasing the effectiveness of this treatment is the heterogeneity of activity distribution, especially in large tumors. This heterogeneity, along with the short path length of the cytotoxic β particle, means that large areas of tumor are inadequately treated by 153Sm-EDTMP alone. A vascular targeting agent might allow for more homogeneous distribution of activity, improving tumor response. Such a combinatorial approach should be feasible given the lack of significant nonhematologic toxicity seen in patients treated with 153Sm-EDTMP as a single agent.
Another investigational bone-seeking radiopharmaceutical, 223Ra, is currently in clinical trials for prostate cancer bone metastases.13 This agent is an alpha emitter with a relatively short half-life and short path length.14 It remains to be seen whether beta-emitters, such as 153Sm or alpha-emitters, such as 223Ra, will provide a better combination of toxicity and efficacy.
In conclusion, we found that it is feasible and safe to deliver tandem doses of 153Sm-EDTMP to heavily pretreated patients with recurrent or refractory osteosarcoma. Disease stabilization was provided to 44% of patients with only minimal toxicity. Furthermore, we documented tumor response in extraosseous tumor, a finding that increases the population of patients who may benefit from this treatment. The strong correlation between absorbed dose after low and high administered activities within each evaluable patient provides a methodology to individually tailor radiopharmaceutical dosing in patients treated by our tandem approach.
Acknowledgments
Supported by EUSA Pharma (USA) Inc. (formerly Cytogen Corp.).
Footnotes
CONFLICT OF INTEREST DISCLOSURES
REFERENCES
- 1.Francis KC, Phillips R, Nickson JJ, Woodard HQ, Higinbotham NL, Coley BL. Massive preoperative irradiation in the treatment of osteogenic sarcoma in children: a preliminary report. Am J Roentgenol Radium Ther Nucl Med. 1954;72:813–818. [PubMed] [Google Scholar]
- 2.Jenkin RD, Allt WE, Fitzpatrick PJ. Osteosarcoma. An assessment of management with particular reference to primary irradiation and selective delayed amputation. Cancer. 1972;30:393–400. doi: 10.1002/1097-0142(197208)30:2<393::aid-cncr2820300215>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 3.Lee ES, Mackenzie DH. Osteosarcoma. A study of the value of preoperative megavoltage radiotherapy. Br J Surg. 1964;51:252–274. doi: 10.1002/bjs.1800510405. [DOI] [PubMed] [Google Scholar]
- 4.Anderson PM, Wiseman GA, Dispenzieri A, et al. High-dose samarium-153 ethylene diamine tetramethylene phosphonate: low toxicity of skeletal irradiation in patients with osteosarcoma and bone metastases. J Clin Oncol. 2002;20:189–196. doi: 10.1200/JCO.2002.20.1.189. [DOI] [PubMed] [Google Scholar]
- 5.Anderson PM, Wiseman GA, Erlandson L, et al. Gemcitabine radiosensitization after high-dose samarium for osteoblastic osteosarcoma. Clin Cancer Res. 2005;11(19 pt 1):6895–6900. doi: 10.1158/1078-0432.CCR-05-0628. [DOI] [PubMed] [Google Scholar]
- 6.Loeb DM, Garrett-Mayer E, Hobbs RF, et al. Dose-finding study of 153Sm-EDTMP in patients with poor-prognosis osteosarcoma. Cancer. 2009;115:2514–2522. doi: 10.1002/cncr.24286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siegel JA, Thomas SR, Stubbs JB, et al. MIRD pamphlet No. 16: Techniques for quantitative radiopharmaceutical biodistribution data acquisition and analysis for use in human radiation dose estimates. J Nucl Med. 1999;40:37S–61S. [PubMed] [Google Scholar]
- 8.Siegel JA, Wu RK, Maurer AH. The buildup factor: effect of scatter on absolute volume determination. J Nucl Med. 1985;26:390–394. [PubMed] [Google Scholar]
- 9.Stabin MG, Sparks RB, Crowe E. OLINDA/EXM: the second-generation personal computer software for internal dose assessment in nuclear medicine. J Nucl Med. 2005;46:1023–1027. [PubMed] [Google Scholar]
- 10.Benz MR, Czernin J, Allen-Auerbach MS, et al. FDG-PET/CT imaging predicts histopathologic treatment responses after the initial cycle of neoadjuvant chemotherapy in high-grade soft-tissue sarcomas. Clin Cancer Res. 2009;15:2856–2863. doi: 10.1158/1078-0432.CCR-08-2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hawkins DS, Conrad EU, III, Butrynski JE, Schuetze SM, Eary JF. [F-18]-fluorodeoxy-D-glucose-positron emission tomography response is associated with outcome for extremity osteosarcoma in children and young adults. Cancer. 2009;115:3519–3525. doi: 10.1002/cncr.24421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iagaru A, Masamed R, Chawla SP, Menendez LR, Fedenko A, Conti PS. F-18 FDG PET and PET/CT evaluation of response to chemotherapy in bone and soft tissue sarcomas. Clin Nucl Med. 2008;33:8–13. doi: 10.1097/RLU.0b013e31815c4fd4. [DOI] [PubMed] [Google Scholar]
- 13.Nilsson S, Franzen L, Parker C, et al. Bone-targeted radium-223 in symptomatic, hormone-refractory prostate cancer: a randomised, multicentre, placebo-controlled phase II study. Lancet Oncol. 2007;8:587–594. doi: 10.1016/S1470-2045(07)70147-X. [DOI] [PubMed] [Google Scholar]
- 14.Henriksen G, Fisher DR, Roeske JC, Bruland OS, Larsen RH. Targeting of osseous sites with alpha-emitting 223Ra: comparison with the beta-emitter 89Sr in mice. J Nucl Med. 2003;44:252–259. [PubMed] [Google Scholar]