Abstract
Autoaggressive, myelin-reactive T cells are involved in multiple sclerosis and its prototype experimental autoimmune encephalomyelitis (EAE) in mice. A peripheral negative feedback mechanism involving regulatory CD4+ and CD8+T cells (Treg) operates to suppress disease-mediating T cell responses. We have recently characterized a novel population of Qa-1a-restricted, TCR-peptide-reactive CD8αα+TCRαβ+ Treg that induce apoptotic depletion of the encephalitogenic Vβ8.2 cells in vivo and provide protection from EAE. Here we have used mice deficient in perforin, Fas/FasL and IFN-γ molecules to investigate their role in Treg-mediated regulation of EAE. Data show that Fas/FasL interactions are not involved, but regulation mediated by Treg is dependent on the presence of IFN-γ and the Perforin pathway. These data provide a molecular mechanism of Treg-mediated killing of the pathogenic T cells and have important implications in the design of immune interventions for demyelinating disease.
Keywords: regulatory T cells, Fas/FasL, perforin, EAE, TCR vaccination
Introduction
Experimental autoimmune encephalomyelitis (EAE) in rodents is a prototype for multiple sclerosis (MS) in humans; both are autoimmune diseases resulting from aggressive immune responses that destroy the myelin sheath surrounding axonal processes within the central nervous system. T cells have been demonstrated to be mediators of EAE (1,2), and are detected in the inflammatory infiltrate lesions in the brain and spinal cord of MS patients (3,4). On the one hand, functional cytotoxic CD8+ T cells reactive to MBP have been shown to induce EAE (1, 2, 5), on the other, CD8+ T cells with regulatory functions can suppress EAE disease processes (6–10). The Fas/FasL pathway has been implicated in both the regulation of disease (11) and pathogenesis associated with CD8+ T cells during EAE (12–14). Thus, dual roles for both CD8+ T cells and Fas/FasL pathway in EAE have been reported. Here we investigate if CD8+ Treg utilize the Fas pathway in their mechanism of action.
The role of regulatory T cells is one of the most promising areas of autoimmune disease research and has become an important new target for therapeutic intervention. Both regulatory CD4+ and CD8+ T cells have been shown to be protective against MS and EAE (8, 9, 15–18). In the EAE model we have delineated a negative feedback regulatory pathway that involves CD4+ and CD8αα+TCRαβ+ Treg operating in unison to down-regulate the disease-mediating Vβ8.2 CD4+ T cell population (8–10, 15, 19).
EAE is characterized by inflammation and demyelination of the central nervous system (CNS), and is mediated by dysregulated myelin-reactive T cells that can be induced in a variety of mouse strains by active immunization with specific myelin antigens or passive transfer of activated myelin-reactive T cells. In the H-2u mice EAE is mediated by a population of predominantly TCRVβ8.2+ CD4+ T cells recognizing an immunodominant determinant from the N-terminal of myelin basic protein (MBP) (20–22). In H-2u mice EAE is generally monophasic, and most animals recover spontaneously. These mice are then resistant to re-induction of disease. Previous studies have shown that CD8+ T cells perform an essential role in protecting H-2u mice from re-induction of disease (23–25). Here we have examined the mechanism of recovery from MBPAc1–9-induced EAE and how it is facilitated by the coordinated action of regulatory CD4+ and CD8αα+TCRαβ+ Treg cells that target the disease mediating Vβ8.2+ T cells (10, 15, 19). Regulatory CD4+ cells predominantly express the Vβ14 TCR and recognize a framework 3 region determinant (B5 peptide, aa 76–101) from the Vβ8.2 chain. These CD4+ Treg function to provide help in the recruitment and/or activation of CD8αα+TCRαβ+ Treg, which ultimately kill the disease-mediating Vβ8.2+ T cell population by inducing apoptotic cell death (8, 9, 19). We have recently cloned and characterized these regulatory CD8+ T cells (8, 9). They have a distinctive phenotype in that they express the CD8αα homodimer along with the TCRαβ chain, and they are predominantly Vβ6TCR+. CD8αα+TCRαβ+ Treg recognize a determinant (p42–50, aa 42–50) from the conserved CDR1/2 region of the Vβ8.2 chain in the context of a non-classical MHC class Ib molecule, Qa-1a (8, 9).
While it has been demonstrated that CD8αα+TCRαβ+ Treg target and kill activated Vβ8.2+ CD4+ T cells expressing p42–50 (8), the mechanism of killing has yet to be delineated. Several possible mechanisms of cell death exist. These include the induction of pathways intrinsic to the cell, such as the pro-apoptotic molecule BIM (26), and extrinsic pathways mediated by the actions of other cells, such as perforin and those of the tumor necrosis factor family including Fas/FasL, TNF-alpha and TRAIL.
In lpr and gld mice, which have autosomal recessive mutations and lack functional Fas and FasL, respectively, there is a significant amelioration of EAE induced by active immunization with MBP (12–14). This suggests that Fas interactions might play a role in the augmentation of EAE. However, there are experiments suggesting that Fas may have a dual role in the course of EAE. While FasL deficient donor cells fail to transfer disease, a deficiency in host FasL prolongs the clinical symptoms (11). This suggests that the Fas pathway may be important in the regulation of EAE. Furthermore, human studies have shown elevated expression of FAS and its ligands in MS lesions (27, 28). In respect to a role in the regulation of MS, functional Fas was demonstrated to be expressed on MBP-reactive T cells isolated from MS patients (29, 30). This suggests that autoaggressive CD4+ T cells may be targeted for down-regulation through Fas.
In addition to Fas/FasL, the perforin pathway has been implicated to play a role in the mechanism of regulation of EAE. Perforin deficient C57/BL6 mice were shown to display a more severe course of chronic relapsing EAE (31). Furthermore, studies recently reported that CD8+ Tregs that mediate protection against EAE produce perforin (32) and fail to control disease in perforin-deficient mice (33).
In this study we investigated whether the CD8αα+TCRαβ+ Treg use the Fas/FasL or perforin pathways to down-regulate the disease-mediating Vβ8.2+ CD4+ cells in EAE. By inducing active or passive EAE in B10.PL.lpr and B10.PL.gld mice, we have concluded that Fas is not involved in CD8αα+TCRαβ+ Treg-mediated regulation of EAE. However, using perforin deficient mice we have demonstrated the importance of this pathway in CD8αα+TCRαβ+ Treg mediated protection from EAE. Additionally, a Th-1-like milieu, in particular IFN-γ, is necessary for Treg-mediated regulation of disease. These finding have implications not only in the understanding of the mechanism of regulation, but also in the design of potential immunotherapies in MS.
Materials and Methods
Mice
B10.PL wildtype mice were purchased from The Jackson Laboratory (Bar Harbor, ME). B10.PL.lpr and B10.PL.gld mice were provided by Dr. John Russell (Washington University, St. Louis, MO). B10.PL.perforin −/− mice were generated by backcrossing C57BL/6-perforin−/− mice (Jackson Laboratory) onto B10.PL background for 8 generations. Mice were kept under pathogen-free conditions in our own colony at the Torrey Pines Institute for Molecular Studies (TPIMS, La Jolla, CA). Female mice, aged-matched at 8–16 weeks, were used in the described experiments. Treatment of animals was in compliance with federal and institutional guidelines, and approved by the TPIMS institute animal care and use committee.
Peptides
TCR peptides were synthesized by S. Horvath (California Institute of Technology, Pasadena, CA) using a solid phase technique on a peptide synthesizer (430A; Applied Biosystems) and purified on a reverse phase column by HPLC, as described earlier (34). TCR Vβ8.2 chain peptides are as follows (single-letter amino acid code): p42–50, aa 42–50: GLRLIHYSY; p69–77, aa 69–77: PSQENFSLI; B5, aa 76–101: LILELATPSQTSVYFCASGDAGGGYE and B1, aa 1–30: EAAVTQSPKNKVAVTGGKVTLSCNQTNNHNL. Myelin basic protein peptide: MBP Ac1–9 (AcASQKRPSQR), was purchased from Macromolecular Resources, Colorado State University.
Induction and clinical evaluation of disease
For active EAE induction, mice were immunized s.c with 100–150 µg of guinea pig MBP or Ac1–9 emulsified in CFA; 0.15 µg pertussis toxin (List Biological, Campbell, CA) was injected i.p. in 200 µl saline on day 0 and day 2. For passive induction of disease, B10.PL wildtype mice were immunized with 400 µg MBP. Ten days later lymphocytes were harvested and cultured in the presence of MBP and IL-12. Nine days later 1.85 ×106 cells/mouse were injected i.v into recipient mice. Mice were observed daily for the clinical appearance of EAE. Disease severity was scored on a 5-point scale (15): 1, Flaccid tail; 2, hind limb weakness; 3, hind limb paralysis; 4, whole body paralysis; 5, death.
Measurement of antigen-specific proliferative and cytokine responses
Draining lymph nodes of mice were removed 10 days after s.c. immunization with MBP, and single cell suspensions were prepared (8). Lymph node cells (8 × 105 cells per well) were cultured in 96-well microtiter plates with peptides at concentrations ranging from 0.0128 µg/ml to 40 µg/ml. IFN-γ cytokine secretion in the assay supernatant was determined using standard sandwich ELISA technique, as described earlier (8). All capturing and detecting antibody pairs were purchased from PharMingen (San Diego, CA). Proliferation was assayed by the addition of 1 µCi [3H]thymidine (International Chemical and Nuclear, Irvine, CA) per well for the last 18 hours of a 5-day culture. Incorporation of 3H-label was measured by liquid scintillation counting.
Statistical Analysis
Statistical differences between groups were evaluated using a Mann-Whitney test using GraphPad Prism 4.03 software. p < 0.05 was considered statistically significant.
Results
B10.PL.lpr mice are protected from active EAE following vaccination with a CD8αα+TCRαβ+ Treg-inducing peptide
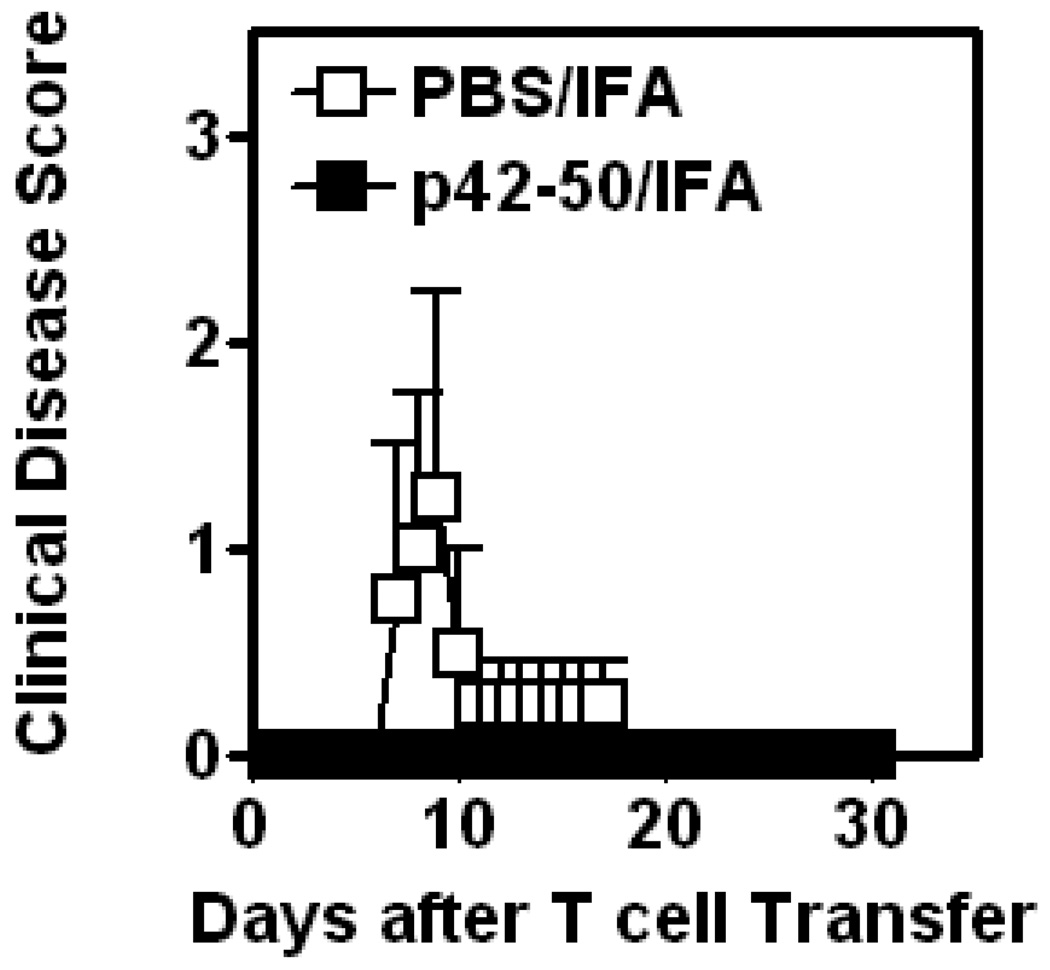
Wildtype B10.PL, lpr and gld mice were vaccinated i.p with either PBS/IFA as a control or the TCR-peptide p42–50 emulsified in IFA. 7 days later EAE was actively induced by MBP Ac1–9/CFA/PTx immunization. As shown in Figure 1 (left panel), the wildtype mice vaccinated with PBS/IFA developed disease, whereas wildtype mice that had received p42–50/IFA were completely protected from EAE. This is consistent with our earlier data that demonstrated CD8αα+TCRαβ+ Treg induction by TCR peptide p42–50 vaccination protected B10/PL mice against EAE (8). Interestingly TCR-peptide vaccination also significantly protected B10.PL.lpr mice from EAE (see Table 1 and Fig.1 – middle panel). This suggests that FAS had no significant role in the disease protection mediated by p42–50-reactive CD8αα+TCRαβ+ Treg. B10.Pl.gld mice, which lack functional FasL, proved resistant to the active induction of EAE (Fig.1 – right panel), in agreement with previous reports that the presence of FasL is necessary for the development of EAE (12–14).
Figure 1. Vaccination with p42–50 protects B10.PL.lpr mice from active EAE.
Groups (4 mice in each) of wildtype B10.PL., B10.PL.lpr and B10.PL.gld mice were vaccinated i.p with 10 µg p42–50/IFA or PBS/IFA. 7 days later EAE was actively induced following immunization with MBPAc1–9/CFA/PTx. Mice were monitored daily for signs of clinical disease, and mean disease score (+/− SEM) of each group is shown. Data are representative of 3 independent experiments.
Table 1. Vaccination with p42–50 protects B10.PL.gld and B10.LPR mice from passively or actively induced EAE, respectively.
Groups (7–8 mice in each) of B10.PL.gld mice (gld) were vaccinated i.p with 10 µg p42–50/IFA or PBS/IFA. 7 days later, mice received 1.85×106 MBP-reactive CD4+ T cells i.v. Groups (8 mice in each) of B10.PL.lpr mice (lpr) were vaccinated i.p with 10 µg p42–50/IFA or PBS/IFA. 7 days later EAE was actively induced following immunization with MBPAc1–9/CFA/PTx. Mice were monitored daily for signs of clinical disease, and maximum disease score for each mouse and mean day of onset for each group is shown.
| Mice | Treatment | Incidence (maximum score for each mouse) |
Mean day of onset |
|---|---|---|---|
| Passive EAE (gld) | |||
| PBS/IFA | 7/7 (3,2,2,2,1,1,1) | 6.5 | |
| p42–50 | 1/8 (1,0,0,0,0,0,0,0) | 15 | |
| Active EAE (lpr) | |||
| PBS/IFA | 7/8 (4,3,3,3,2,2,1,0) | 11 | |
| p42–50 | 2/8 (1,1,0,0,0,0,0,0) | 11.5 |
TCR-peptide p42–50 vaccination protects B10.PL.gld mice from passive EAE
Due to the failure of B10.PL.gld mice to develop active EAE following MBP Ac1–9/CFA/PTx immunization, disease was induced passively. One week after vaccination with p42–50 or vehicle alone mice were injected with MBP-reactive disease-mediating T cells derived from wildtype B10.PL mice. Studying passive disease in this system allowed us to determine the effect of the lack of FasL on the host CD8αα+TCRαβ+ Treg alone, since MBP-reactive disease-mediating cells originated from wildtype mice and expressed functional Fas and FasL. PBS/IFA vaccinated B10.PL.gld mice developed clinical disease on transfer of disease-mediating cells (Figure 2 and Table 1). In contrast, p42–50/IFA vaccinated B10.PL.gld mice were almost completely protected from disease. This suggests that the lack of FasL on TCR-peptide-induced CD8αα+TCRαβ+ Treg does not affect their ability to mediate protection against EAE.
Figure 2. Vaccination with p42–50 protects B10.PL.gld mice from passive EAE.
Groups (4 mice in each) of B10.PL.gld mice were vaccinated i.p with 10 µg p42–50/IFA or PBS/IFA. 7 days later, mice received 1.85×106 MBP-reactive CD4+ T cells i.v. Mice were monitored daily for signs of clinical EAE disease. Mean score (+/− SEM) of each group is shown. Data are representative of two independent experiments.
TCR-peptide p42–50 vaccination fails to protect B10.PL.peforin−/− mice from active EAE
CD8αα+TCRαβ+ Treg induction following TCR-peptide p42–50 vaccination efficiently protected against EAE in FAS/FASL –deficient mice. Thus, we hypothesized that another pathway must be responsible for the Treg-mediated killing of MBP-reactive disease-mediating Vβ8.2+ CD4+ T cells. Along with FAS, the peforin pathway operates as a major mechanism in CD8+ T cell-mediated cytotoxicity (35). We investigated whether TCR-peptide p42–50 vaccination could protect perforin−/− mice from MBP-induced disease. Table 2 shows that TCR-peptide p42–50 vaccination significantly protects wild type B10.PL mice from EAE with 4 out of 11 mice developing only mild clinical symptoms. While the disease incidence and severity in perforin −/− mice vaccinated with TCR-peptide p42–50 was similar to that of the PBS-vaccinated control mice. These results indicate that TCR-peptide p42–50 vaccination in the absence of perforin fails to induce a protective Treg response. Thus, the perforin pathway appears to be an essential mechanism utilized by the CD8αα+TCRαβ+ Treg population for down-regulation and protection against EAE.
Table 2. Vaccination with p42–50 fails to protect Perforin −/− mice from active EAE.
Groups (7–11 mice in each) of wildtype B10.PL and perforin −/− mice were vaccinated i.p with 10 µg p42–50/IFA or PBS/IFA. 7 days later EAE was actively induced following immunization with MBPAc1–9/CFA/PTx. Mice were monitored daily for signs of clinical disease, and maximum disease score for each mouse and mean day of onset for each group is shown.
| Mice | Treatment | Incidence (maximum score for each mouse) |
Mean day of onset |
|---|---|---|---|
| Perforin +/+ | PBS | 9/9 (5,4,3,3,2,2,2,1,1) | 11.3 |
| p42–50 | 4/11 (3,1,1,1,0,0,0,0,0,0,0) | 8.9 | |
| Perforin −/− | PBS | 7/7 (5,5,5,4,4,4,3) | 9.1 |
| p42–50 | 11/11 (5,5,5,5,4,4,4,4,3,3,1) | 10.3 |
MBP-reactive encephalitogenic CD4+ T cell response is suppressed in mice vaccinated with p42– 50
We aimed to determine whether p42–50-mediated protection from EAE is associated with the suppression of MBP-reactive CD4+ T cells. Wildtype B10.PL mice were vaccinated i.p with p42–50/IFA or PBS/IFA 8 days before the s.c immunization with MBP Ac1–20/CFA/PTx. On day 10 draining lymph nodes (DLNs) were harvested, and proliferative recall responses to MBP Ac1–20 (Fig.3. upper panels) or IFN-γ cytokine secretion (Fig.3. lower panels) from mice vaccinated with p42–50/IFA versus PBS/IFA were examined. Vaccination with p42–50 was associated with suppression of proliferation and IFN-γ secretion in response to MBP Ac1–20. This suggests that p42–50-mediated suppression results in significant inhibition of both proliferation and effector function of the encephalitogenic MBP-reactive CD4+ T cells.
Figure 3. The anti-MBP T cell response is suppressed in mice vaccinated with p42–50.
Groups (4–5 mice in each) of mice were vaccinated i.p with 10 µg p42–50. 8 days later mice were injected with 100 µg MBPAc1–20/CFA/PTx. 10 days later DLN were harvested, and proliferation (upper panel) and IFN-γ secretion (lower panel) in response to an in vitro recall at an optimal concentration of 10 µg/ml MBPAc1–20 was analyzed. Data are representative of two independent experiments.
TCR-peptide-mediated protection from EAE requires a Th1-environment
We and others have previously demonstrated that the suppressive potential of regulatory T cells is significantly influenced by the cytokine milieu in which they are primed (6, 36–39). Optimal Treg priming occurs in a Th-1 cytokine environment, and under Th2-conditions Treg are inefficient in regulating EAE. First, we confirmed in the wildtype B10.PL mice that CD4+ Treg-stimulating TCR-peptide B5 vaccination under Th2-priming conditions does not protect mice from EAE (Fig.4 upper panel). The mean EAE disease scores depicted in Figure 4a confirmed that under Th2 conditions, mice vaccinated with TCR-peptide B5 develop exacerbated EAE when compared to a TCR-peptide B1 control. On the other hand, B5 peptide vaccination under Th1 conditions protects against EAE.
Figure 4. Successful TCR-peptide vaccination requires a Th1 environment.
Groups (6–8 mice in each) of mice were nasally instilled with 10 µg B5 either without cytokines or with IL-4 (10 pg/mouse) or IL-12 (0.3 µg/mouse) to deviate the response towards a Th2 or Th1 direction, respectively (top panel). Active EAE was induced five days later with MBP/CFA/PTx. Mean disease score of each group is shown. IFN-γ is required for p42–50 to protect against EAE (lower panel). Groups (6–8 mice in each) of B10.PL IFN-γ knockout mice were vaccinated with PBS/IFA or p42–50/IFA as indicated. 7 days later mice were immunized with 200 µg MBPAc1–9/CFA/PTx for the active induction of EAE. Mean clinical disease score is shown.
Next, we investigated whether p42–50 vaccination was similarly influenced by the cytokine milieu. We used IFN-γ knockout mice to examine whether the absence of this cytokine influenced the functional outcome of p42–50-mediated regulation of EAE. B10.PL IFNγ −/− mice were vaccinated with 20 µg p42–50/IFA or PBS/IFA. 7 days later mice were immunized with Ac1–9/CFA/PTx to induce EAE. Figure 4 (lower panel) shows vaccination of IFN-γ deficient mice does not protect against EAE, whereas p42–50 vaccination does prevent disease in wildtype B10.PL (Fig.1 left panel).
To further investigate the role of the Th1/Th2 cytokine milieu in the activation of CD8αα+TCRαβ+ Treg and protection against EAE, wildtype B10.PL or B10.PL.lpr mice were vaccinated with p42–50 in a Th2-like environment. This was achieved by vaccinating mice with p42–50 in the presence of IL-4. Figure 5 shows that p42–50/IFA vaccination of wildtype B10.PL or Fas deficient B10.PL.lpr mice in the presence of IL-4 does not protect animals from disease, but rather exacerbates EAE in comparison to PBS/IFA. This indicates that the Fas pathway does not play a significant role in CD8αα+TCRαβ+ Treg-mediated positive or negative modulation of EAE. In summary, the above data demonstrate that the induction of CD8αα+TCRαβ+ Treg is dependent on Th1 cytokines, but its mechanism of suppression is independent of the Fas/FasL pathway.
Figure 5. Vaccination with p42–50 in a Th2-like environment potentiates EAE in B10.PL.wt and B10.PL.lpr mice.
Groups (6–8 mice in each) of mice were vaccinated with 10 µg p42–50 in the presence of IL-4 (10 pg/mouse) to create a Th2 environment. Seven days later EAE was actively induced by MBPAc1–9/CFA/PTx immunization. Mean disease score of each group is shown.
Discussion
We previously reported that suppression of EAE by TCR-peptide-reactive CD8αα+TCRαβ+ Treg was dependent on the induction of apoptotic cell death in the MBP-reactive disease-mediating Vβ8.2+ CD4+ T cell population (8, 9, 19). Here we have investigated the mechanism of killing by CD8αα+TCRαβ+ Treg and found that this immune-regulatory mechanism is independent of the Fas pathway. Additionally, successful regulation is dependent upon Treg priming in the presence of a Th1-like milieu, and in particularly IFN-γ.
It has now been amply demonstrated that CD8+ T cells have an immunoregulatory role in EAE and MS (7–10, 16, 23–25, 40, 41). In addition to TCR-peptide immunization (8–10, 15), other immunotherapies have demonstrated the ability to induce CD8+ Treg in humans and in animal models of MS (6, 16, 40, 42). T cell vaccination with attenuated autoreactive CD4+ T cells has been shown to induce CD8+ T cell responses to protect against EAE and MS pathology (6, 16). Furthermore, Karandikar and colleagues detected the upregulation of CD8+ T cell responses in MS patients to levels comparable to healthy controls after glatiramer acetate (copaxone) therapy (40). Additionally, copaxone-induced CD8+ Treg showed cytotoxic function and killed activated CD4+ T cells (42).
Fas (CD95) and its natural ligand, FasL (CD95L), are type I and type II transmembrane proteins and members of the TNF/nerve growth receptor and TNF families, respectively. FasL is up-regulated when the TCR is engaged, and on ligation with Fas apoptosis is induced in the Fas-bearing cell (43, 44). CD8+ T cells, CD4+CD25+, and δγ T cells with regulatory function have been shown to use the Fas-killing pathway (45, 46). It is clear from our data (Figs. 1 and 2, and Table 1) that vaccination with CD8+ T cell-reactive peptide p42–50 prevents both active and passive induction of EAE in B10.PL.lpr and B10.PL.gld mice. These findings clearly indicate that CD8αα+TCRαβ+ Treg function was not hindered in the absence of Fas/FasL.
Another mechanism of suppression mediated by regulatory CD8+ T cells could involve the secretion of the TNF-family member cytokine TRAIL (47). EAE has previously been shown to be exacerbated after the blockade of TRAIL (48). However, cytokine secretion analysis and gene array analysis of our CD8αα+TCRαβ+ Treg clones (8, 9) did not reveal TRAIL production by these cells. Thus it is unlikely that TRAIL operates in the suppression mediated by TCR-peptide-reactive CD8αα+TCRαβ+ Treg.
We finally considered the role of the perforin pathway in the mechanism of killing mediated by CD8αα+TCRαβ+ Treg. The mechanism of perforin-induced death involves the direct action of perforin and granzymes on the target cell. Perforin creates a pore in the target cell wall, which allows granzyme to enter and subsequently kill the cell (35). Familial lymphohistocytosis occurs in humans with perforin gene defects, and is characterized by accumulation of activated T cells (49). Interestingly, perforin deficient mice have been shown to be compromised in their ability to down-regulate the expansion of activated T cells (50). Furthermore, perforin knockout C57BL/6 mice display a more severe form of antigen-induced EAE in comparison to wildtype and lpr and gld mutants (31). It was recently demonstrated that perforin contributed to the mechanism of CD8+ Treg-mediated suppression of disease-causing CD4+ T cells in an EAE model (32). York et al. demonstrated that adoptive transfer of MOG35–55-reactive CD8+ T cells could protect against EAE. The CD8+ Treg produced perforin and killed MOG-loaded CD4+ T cells. However, experiments still need to be performed in this model to confirm a role for perforin in CD8+ Treg-mediated protection from EAE. Lu et al. have recently demonstrated non-classical MHC class I-restricted CD8+ T cells isolated from MOG-immunized wildtype or FasL deficient mice successfully suppressed EAE-mediating CD4+ T cells both in vivo and in vitro, while CD8+ T cells from perforin knockout mice failed to demonstrate any suppressive activity (33). Consistent with this, our data (Table 2) suggest that p42–50-induced Qa-1a-restricted CD8αα+TCRαβ+ Treg do not regulate EAE in perforin−/− H-2u mice. Thus, perforin, but not Fas/FasL pathway, is utilized by non-classical MHC class 1a-restricted CD8αα+ Tregs.
Interestingly, in addition to CD8+ Treg, several recent studies have demonstrated a role for the perforin pathway in CD4+FoxP3+ Treg mediated immune regulation (51, 52). Boissonnas et al., using two-photon microscopy elegantly demonstrated that FoxP3 Tregs mediated DC death in a perforin dependent manner in tumor draining lymph nodes (52). Thus, in addition to the secretion of immunosuppressive cytokines, such as TGF-β and IL-10, the production of perforin may also represent an important pathway in the cell extrinsic regulatory mechanisms utilized by Tregs.
In accordance with previous findings regarding the CD4+ Treg-inducing TCR peptide vaccination protocol (36–39), we found the efficacy of TCRVβ8.2-peptide p42–50 vaccination to be vulnerable to the cytokine conditions that exist when the peptide is administered. Only under Th1 conditions did p42–50 vaccination confer protection from EAE. When mice were vaccinated with p42–50 and the immune response was deviated to Th2, regulation was lost and p42–50-immunized mice contracted more severe disease than the PBS/IFA control group (Figure 4 and 5). This is in accordance with the spontaneous CD8αα+TCRαβ+ Treg priming that occurs in the Th1 inflammatory environment associated with active EAE (53). The source of Th1 cytokines could be the disease-mediating CD4+ T cell population or “helper” CD4+ Treg (1, 15, 53, 54). Our recent studies indicate that IFN-γ secretion by CD4+ Treg is essential for their helper function in the efficient induction of CD8αα+TCRαβ+ Treg (Pedersen et al., in preparation).
Significantly, CD8αα+TCRαβ+ Treg preferentially target the Th1-like cells and not Th2 cells (6, 37, 53). Killing of a major population of autoaggressive Th1-like cells results in the deviation of the overall immune response to the self-antigen in a non-pathogenic Th2 direction (37, 39). CD8αα+TCRαβ+ Treg may target Th1 cells due to an increased susceptibility of these cells to apoptosis, the involvement of IFN-γ, or enhanced display of TCR-peptide/Qa-1 complexes from efficient immunoproteasome dependent processing. Enhancement of costimulatory molecules on the target Th1 cells may also facilitate recognition by the CD8αα+TCRαβ+ Treg. This is supported by a recent report that detected up-regulated CD80 and CD86 co-stimulatory molecules on the cell surface of Th1 cells upon activation, and blockade of the B7 molecules attenuated the efficacy of T cell vaccination using these cells in an EAE model (55).
Delineating the killing mechanism involved in the CD8αα+TCRαβ+ T cell-mediated regulation of EAE will provide essential information on a pathway that can be targeted to down-regulate aberrant T cell responses. It will also assist in the design of novel therapies to treat diseases characterized by pathogenic T cell responses to self-antigen, such as MS, Rheumatoid Arthritis and Type 1 Diabetes.
Acknowledgments
This work was supported by the National Institutes of Health grant RO1AI052227, MSNRI and DNRG to V Kumar.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Steinman L. Multiple sclerosis: a coordinated immunological attack against myelin in the central nervous system. Cell. 1996;85:299. doi: 10.1016/s0092-8674(00)81107-1. [DOI] [PubMed] [Google Scholar]
- 2.Martin R. HLA class I: friend and foe of multiple sclerosis. Nat Med. 2008;14:1150. doi: 10.1038/nm1108-1150. [DOI] [PubMed] [Google Scholar]
- 3.Traugott U, Reinherz EL, Raine CS. Multiple sclerosis: distribution of T cell subsets within active chronic lesions. Science. 1983;219:308. doi: 10.1126/science.6217550. [DOI] [PubMed] [Google Scholar]
- 4.Hauser SL, Bhan AK, Gilles F, Kemp M, Kerr C, Weiner HL. Immunohistochemical analysis of the cellular infiltrate in multiple sclerosis lesions. Ann. Neurol. 1986;19:578. doi: 10.1002/ana.410190610. [DOI] [PubMed] [Google Scholar]
- 5.Huseby ES, Liggitt D, Brabb T, Schnabel B, Ohlen C, Goverman J. A pathogenic role for myelin-specific CD8(+) T cells in a model for multiple sclerosis. J Exp. Med. 2001;194:669. doi: 10.1084/jem.194.5.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiang H, Braunstein NS, Yu B, Winchester R, Chess L. CD8+ T cells control the TH phenotype of MBP-reactive CD4+ T cells in EAE mice. Proc Natl Acad Sci U S A. 2001;98:6301. doi: 10.1073/pnas.101123098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hu D, Ikizawa K, Lu L, Sanchirico ME, Shinohara ML, Cantor H. Analysis of regulatory CD8 T cells in Qa-1-deficient mice. Nat Immunol. 2004;5:516. doi: 10.1038/ni1063. [DOI] [PubMed] [Google Scholar]
- 8.Tang X, Maricic I, Purohit N, Bakamjian B, Reed-Loisel LM, Beeston T, Jensen P, Kumar V. Regulation of immunity by a novel population of Qa-1-restricted CD8alphaalpha+TCRalphabeta+ T cells. J Immunol. 2006;177:7645. doi: 10.4049/jimmunol.177.11.7645. [DOI] [PubMed] [Google Scholar]
- 9.Tang X, Maricic I, Kumar V. Anti-TCR antibody treatment activates a novel population of nonintestinal CD8 alpha alpha+ TCR alpha beta+ regulatory T cells and prevents experimental autoimmune encephalomyelitis. J Immunol. 2007;178:6043. doi: 10.4049/jimmunol.178.10.6043. [DOI] [PubMed] [Google Scholar]
- 10.Smith TR, Kumar V. Revival of CD8(+) Treg-mediated suppression. Trends Immunol. 2008;29:337. doi: 10.1016/j.it.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 11.Sabelko-Downes KA, Cross AH, Russell JH. Dual role for Fas ligand in the initiation of and recovery from experimental allergic encephalomyelitis. J Exp. Med. 1999;189:1195. doi: 10.1084/jem.189.8.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sabelko KA, Kelly KA, Nahm MH, Cross AH, Russell JH. Fas and Fas ligand enhance the pathogenesis of experimental allergic encephalomyelitis, but are not essential for immune privilege in the central nervous system. J Immunol. 1997;159:3096. [PubMed] [Google Scholar]
- 13.Sabelko-Downes KA, Gimenez MT, Suvannavejh GC, Miller SD, Russell JH. Genetic control of pathogenic mechanisms in autoimmune demyelinating disease. J Neuroimmunol. 2000;110:168. doi: 10.1016/s0165-5728(00)00350-7. [DOI] [PubMed] [Google Scholar]
- 14.Waldner HR, Sobel A, Howard E, Kuchroo VK. Fas- and FasL-deficient mice are resistant to induction of autoimmune encephalomyelitis. J Immunol. 1997;159:3100. [PubMed] [Google Scholar]
- 15.Kumar V, Sercarz EE. The involvement of T cell receptor peptide-specific regulatory CD4+ T cells in recovery from antigen-induced autoimmune disease. J Exp. Med. 1993;178:909. doi: 10.1084/jem.178.3.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang J, Medaer R, Stinissen P, Hafler D, Raus J. MHC-restricted depletion of human myelin basic protein-reactive T cells by T cell vaccination. Science. 1993;261:1451. doi: 10.1126/science.7690157. [DOI] [PubMed] [Google Scholar]
- 17.Viglietta V, Baecher-Allan C, Weiner HL, Hafler DA. Loss of functional suppression by CD4+CD25+ regulatory T cells in patients with multiple sclerosis. J Exp. Med. 2004;199:971. doi: 10.1084/jem.20031579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reddy J, Waldner H, Zhang X, Illes Z, Wucherpfennig KW, Sobel RA, Kuchroo VK. Cutting edge: CD4+CD25+ regulatory T cells contribute to gender differences in susceptibility to experimental autoimmune encephalomyelitis. J Immunol. 2005;175:5591. doi: 10.4049/jimmunol.175.9.5591. [DOI] [PubMed] [Google Scholar]
- 19.Madakamutil LT, Maricic I, Sercarz E, Kumar V. Regulatory T cells control autoimmunity in vivo by inducing apoptotic depletion of activated pathogenic lymphocytes. J Immunol. 2003;170:2985. doi: 10.4049/jimmunol.170.6.2985. [DOI] [PubMed] [Google Scholar]
- 20.Acha-Orbea H, Mitchell DJ, Timmermann L, Wraith DC, Tausch GS, Waldor MK, Zamvil SS, McDevitt HO, Steinman L. Limited heterogeneity of T cell receptors from lymphocytes mediating autoimmune encephalomyelitis allows specific immune intervention. Cell. 1988;54:263. doi: 10.1016/0092-8674(88)90558-2. [DOI] [PubMed] [Google Scholar]
- 21.Urban JL, Kumar V, Kono DH, Gomez C, Horvath SJ, Clayton J, Ando DG, Sercarz EE, Hood L. Restricted use of T cell receptor V genes in murine autoimmune encephalomyelitis raises possibilities for antibody therapy. Cell. 1988;54:577. doi: 10.1016/0092-8674(88)90079-7. [DOI] [PubMed] [Google Scholar]
- 22.Kumar V, Kono DH, Urban JL, Hood L. The T-cell receptor repertoire and autoimmune diseases. Annu. Rev. Immunol. 1989;7:657. doi: 10.1146/annurev.iy.07.040189.003301. [DOI] [PubMed] [Google Scholar]
- 23.Sun D, Ben-Nun A, Wekerle H. Regulatory circuits in autoimmunity: recruitment of counter-regulatory CD8+ T cells by encephalitogenic CD4+ T cell lines. Eur. J. Immunol. 1988;18:1993. doi: 10.1002/eji.1830181219. [DOI] [PubMed] [Google Scholar]
- 24.Jiang H, Zhang SI, Pernis B. Role of CD8+ T cells in murine experimental allergic encephalomyelitis. Science. 1992;256:1213. doi: 10.1126/science.256.5060.1213. [DOI] [PubMed] [Google Scholar]
- 25.Koh DR, Fung-Leung WP, Ho A, Gray D, cha-Orbea H, Mak TW. Less mortality but more relapses in experimental allergic encephalomyelitis in CD8−/− mice. Science. 1992;256:1210. doi: 10.1126/science.256.5060.1210. [DOI] [PubMed] [Google Scholar]
- 26.Bouillet P, Metcalf D, Huang DC, Tarlinton DM, Kay TW, Kontgen F, Adams JM, Strasser A. Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science. 1999;286:1735. doi: 10.1126/science.286.5445.1735. [DOI] [PubMed] [Google Scholar]
- 27.Dowling P, Shang G, Raval S, Menonna J, Cook S, Husar W. Involvement of the CD95 (APO-1/Fas) receptor/ligand system in multiple sclerosis brain. J Exp. Med. 1996;184:1513. doi: 10.1084/jem.184.4.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.D'Souza SD, Bonetti B, Balasingam V, Cashman NR, Barker PA, Troutt AB, Raine CS, Antel JP. Multiple sclerosis: Fas signaling in oligodendrocyte cell death. J Exp. Med. 1996;184:2361. doi: 10.1084/jem.184.6.2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pelfrey CM, Tranquill LR, Boehme SA, McFarland HF, Lenardo MJ. Two mechanisms of antigen-specific apoptosis of myelin basic protein (MBP)-specific T lymphocytes derived from multiple sclerosis patients and normal individuals. J Immunol. 1995;154:6191. [PubMed] [Google Scholar]
- 30.Ichikawa H, Ota K, Iwata M. Increased Fas antigen on T cells in multiple sclerosis. J Neuroimmunol. 1996;71:125. doi: 10.1016/s0165-5728(96)00149-x. [DOI] [PubMed] [Google Scholar]
- 31.Malipiero U, Frei K, Spanaus KS, Agresti C, Lassmann H, Hahne M, Tschopp J, Eugster HP, Fontana A. Myelin oligodendrocyte glycoprotein-induced autoimmune encephalomyelitis is chronic/relapsing in perforin knockout mice, but monophasic in Fasand Fas ligand-deficient lpr and gld mice. Eur. J Immunol. 1997;27:3151. doi: 10.1002/eji.1830271211. [DOI] [PubMed] [Google Scholar]
- 32.York NR, Mendoza JP, Ortega SB, Benagh A, Tyler AF, Firan M, Karandikar NJ. Immune regulatory CNS-reactive CD8+ T cells in experimental autoimmune encephalomyelitis. J. Autoimmun. 2010 doi: 10.1016/j.jaut.2010.01.003. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lu L, Kim HJ, Werneck MB, Cantor H. Regulation of CD8+ regulatory T cells: Interruption of the NKG2A-Qa-1 interaction allows robust suppressive activity and resolution of autoimmune disease. Proc Natl Acad Sci U S A. 2008;105:19420. doi: 10.1073/pnas.0810383105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clark-Lewis I, Aebersold R, Ziltener H, Schrader JW, Hood LE, Kent SB. Automated chemical synthesis of a protein growth factor for hemopoietic cells, interleukin-3. Science. 1986;231:134. doi: 10.1126/science.3079915. [DOI] [PubMed] [Google Scholar]
- 35.Kagi D, Vignaux F, Ledermann B, Burki K, Depraetere V, Nagata S, Hengartner H, Golstein P. Fas and perforin pathways as major mechanisms of T cell-mediated cytotoxicity. Science. 1994;265:528. doi: 10.1126/science.7518614. [DOI] [PubMed] [Google Scholar]
- 36.Kumar V, Sercarz E. Induction or protection from experimental autoimmune encephalomyelitis depends on the cytokine secretion profile of TCR peptide-specific regulatory CD4 T cells. J Immunol. 1998;161:6585. [PubMed] [Google Scholar]
- 37.Kumar V, Maglione J, Thatte J, Pederson B, Sercarz E, Ward ES. Induction of a type 1 regulatory CD4 T cell response following V beta 8.2 DNA vaccination results in immune deviation and protection from experimental autoimmune encephalomyelitis. Int. Immunol. 2001;13:835. doi: 10.1093/intimm/13.6.835. [DOI] [PubMed] [Google Scholar]
- 38.Braciak TA, Pedersen B, Chin J, Hsiao C, Ward ES, Maricic I, Jahng A, Graham FL, Gauldie J, Sercarz EE, Kumar V. Protection against experimental autoimmune encephalomyelitis generated by a recombinant adenovirus vector expressing the V beta 8.2 TCR is disrupted by coadministration with vectors expressing either IL-4 or - 10. J Immunol. 2003;170:765. doi: 10.4049/jimmunol.170.2.765. [DOI] [PubMed] [Google Scholar]
- 39.Waisman A, Ruiz PJ, Hirschberg DL, Gelman A, Oksenberg JR, Brocke S, Mor F, Cohen IR, Steinman L. Suppressive vaccination with DNA encoding a variable region gene of the T-cell receptor prevents autoimmune encephalomyelitis and activates Th2 immunity. Nat. Med. 1996;2:899. doi: 10.1038/nm0896-899. [DOI] [PubMed] [Google Scholar]
- 40.Karandikar NJ, Crawford MP, Yan X, Ratts RB, Brenchley JM, Ambrozak DR, Lovett-Racke AE, Frohman EM, Stastny P, Douek DC, Koup RA, Racke MK. Glatiramer acetate (Copaxone) therapy induces CD8(+) T cell responses in patients. 2002 doi: 10.1172/JCI14380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Correale J, Villa A. Isolation and characterization of CD8+ regulatory T cells in multiple sclerosis. J Neuroimmunol. 2008 doi: 10.1016/j.jneuroim.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 42.Tennakoon DK, Mehta RS, Ortega SB, Bhoj V, Racke MK, Karandikar NJ. Therapeutic induction of regulatory, cytotoxic CD8+ T cells in multiple sclerosis. J Immunol. 2006;176:7119. doi: 10.4049/jimmunol.176.11.7119. [DOI] [PubMed] [Google Scholar]
- 43.Brunner T, Mogil RJ, LaFace D, Yoo NJ, Mahboubi A, Echeverri F, Martin SJ, Force WR, Lynch DH, Ware CF. Cell-autonomous Fas (CD95)/Fas-ligand interaction mediates activation-induced apoptosis in T-cell hybridomas. Nature. 1995;373:441. doi: 10.1038/373441a0. [DOI] [PubMed] [Google Scholar]
- 44.Ju ST, Panka DJ, Cui H, Ettinger R, El-Khatib M, Sherr DH, Stanger BZ, Marshak-Rothstein A. Fas(CD95)/FasL interactions required for programmed cell death after T-cell activation. Nature. 1995;373:444. doi: 10.1038/373444a0. [DOI] [PubMed] [Google Scholar]
- 45.Ponomarev ED, Dittel BN. Gamma delta T cells regulate the extent and duration of inflammation in the central nervous system by a Fas ligand-dependent mechanism. J Immunol. 2005;174:4678. doi: 10.4049/jimmunol.174.8.4678. [DOI] [PubMed] [Google Scholar]
- 46.Janssens W, Carlier V, Wu B, VanderElst L, Jacquemin MG, Saint-Remy JM. CD4+CD25+ T cells lyse antigen-presenting B cells by Fas-Fas ligand interaction in an epitope-specific manner. J Immunol. 2003;171:4604. doi: 10.4049/jimmunol.171.9.4604. [DOI] [PubMed] [Google Scholar]
- 47.Griffith TS, Kazama H, VanOosten RL, Earle JK, Jr, Herndon JM, Green DR, Ferguson TA. Apoptotic cells induce tolerance by generating helpless CD8+ T cells that produce TRAIL. J Immunol. 2007;178:2679. doi: 10.4049/jimmunol.178.5.2679. [DOI] [PubMed] [Google Scholar]
- 48.Hilliard B, Wilmen A, Seidel C, Liu TS, Goke R, Chen Y. Roles of TNF-related apoptosis-inducing ligand in experimental autoimmune encephalomyelitis. J Immunol. 2001;166:1314. doi: 10.4049/jimmunol.166.2.1314. [DOI] [PubMed] [Google Scholar]
- 49.Stepp SE, Dufourcq-Lagelouse R, Le DF, Bhawan S, Certain S, Mathew PA, Henter JI, Bennett M, Fischer A, de Saint BG, Kumar V. Perforin gene defects in familial hemophagocytic lymphohistiocytosis. Science. 1999;286:1957. doi: 10.1126/science.286.5446.1957. [DOI] [PubMed] [Google Scholar]
- 50.Badovinac VP, Tvinnereim AR, Harty JT. Regulation of antigen-specific CD8+ T cell homeostasis by perforin and interferon-gamma. Science. 2000;290:1354. doi: 10.1126/science.290.5495.1354. [DOI] [PubMed] [Google Scholar]
- 51.Grossman WJ, Verbsky JW, Barchet W, Colonna M, Atkinson JP, Ley TJ. Human T regulatory cells can use the perforin pathway to cause autologous target cell death. Immunity. 2004;21:589. doi: 10.1016/j.immuni.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 52.Boissonnas A, Scholar-Dahirel A, Simon-Biancal V, Pace L, Valet F, Kissenpfennig A, Sparwasser T, Malissen B, Fetler L, Amigorena S. Foxp3+ T cells induce perforin-dependent dendritic cell death in tumor-draining lymph nodes. Immunity. 2010;32:266. doi: 10.1016/j.immuni.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 53.Kumar V. Homeostatic control of immunity by TCR peptide-specific Tregs. J Clin Invest. 2004;114:1222. doi: 10.1172/JCI23166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bevan MJ. Helping the CD8(+) T-cell response. Nat Rev. Immunol. 2004;4:595. doi: 10.1038/nri1413. [DOI] [PubMed] [Google Scholar]
- 55.Yang Y, Ratts RB, Hussain RZ, Northrop SC, Ben LH, Lovett-Racke A, Racke MK. CD28:B7 interaction is necessary for the protective effect of T cell vaccination in EAE. Eur. J Immunol. 2007;37:2032. doi: 10.1002/eji.200636971. [DOI] [PubMed] [Google Scholar]