Abstract
Glial cell line-derived neurotrophic factor (GDNF) is a potent factor for the ventral mesencephalic dopamine neurons. However, studies on the Gdnf gene deleted (Gdnf −/−) mouse have been limited to fetal tissue since these mice die prematurely. To evaluate long-term effects of Gdnf gene deletion, this study involves co-grafts of ventral mesencephalon (VM) and lateral ganglionic eminence (LGE) derived from different Gdnf genotypes. The VM/LGE co-grafts were evaluated at 3, 6, and 12 months for tyrosine hydroxylase (TH) -positive cell survival and nerve fiber formation in the LGE co-transplant, visualized by dopamine- and cyclic AMP-regulated phosphoprotein relative molecular mass 32,000 (DARPP-32) -immunoreactivity. Cell counts revealed no difference in TH-positive neurons between Gdnf genotypes at 3 months postgrafting. At 6 months, a significant reduction in cell number was observed in the Gdnf−/− grafts. In fact, in the majority of the Gdnf −/− VM/LGE transplant had degenerated. At 12 months, a reduction in cell number was seen in both Gdnf−/− and Gdnf+/− compared to wildtype transplants. In the Gdnf −/− grafts, TH-negative inclusion-like structures were present in the cytoplasm of the TH-positive neurons at 3 months. These structures were also found in the Gdnf+/− transplants at 12 months, but not in Gdnf +/+ controls at any time point. In Gdnf +/+ grafts, TH-positive nerve fiber innervation of the striatal co-grafts was dense and patchy and overlapped with clusters of DARPP-32-positive neurons. This overlap did mismatch in the Gdnf+/− grafts, while the TH-positive innervation was sparse in the Gdnf−/− transplants and the DARPP-32-positive neurons were widespread distributed. In conclusion, GDNF is essential for long-term maintenance of both the VM TH-positive neurons and for the striatal tissue, and appears crucial for generation of a proper organization of the striatum.
Keywords: GDNF, transplant, substantia nigra, striatum, DARPP-32, Gdnf knockout
INTRODUCTION
The development of the dopamine neurons in the nigrostriatal system has been well characterized: generation occurs during the early development and after generation they migrate into a ventral-lateral direction upon leaving the proliferative zone (Olson and Seiger, 1972, Lauder and Bloom, 1974, Vitalis et al., 2005, Gates et al., 2006). After migration, the nigral neurons extend their axons to enter the striatal anlage, the lateral ganglionic eminence (LGE). The dopamine nerve fiber innervation is developed along the lateral border of the striatum, and then in dopamine-dense islands in the striatal anlage (Olson et al., 1972, Voorn et al., 1988). These dopamine-dense patches is overlapping with specific areas that correspond to the developing striatal striosomes (Graybiel, 1984, van der Kooy and Fishell, 1987). The areas between these striosomes are developed into the matrix compartment of the striatum, although it takes place at a later stage than the development of the striosomes (Fishell and van der Kooy, 1987).
Many studies have been devoted to find attractants that promote the dopamine nerve fiber growth, especially in the context of promoting regeneration from dopamine neurons in Parkinson's disease. Among numerous neurotrophic factors that have been evaluated for their effects on the ventral mesencephalic (VM) dopamine neurons, glial cell line-derived neurotrophic factor (GDNF) has gained great attention. GDNF was purified and cloned in 1993 (Lin et al., 1993), and results have demonstrated to improve graft survival as well as exert neuroprotective and neurorestorative properties on the midbrain dopamine neurons (Strömberg et al., 1993, Beck et al., 1995, Tomac et al., 1995a, Rosenblad et al., 1996, Sinclair et al., 1996, Granholm et al., 1997, Yurek, 1998). Indeed, GDNF infused into the brains of parkinsonian patients significantly reversed the symptoms with improved activities of daily living, increase in [18F]-dopamine uptake, and sprouting of dopamine nerve fibers in the putamen (Gill et al., 2003, Love et al., 2005, Slevin et al., 2005). Thus, GDNF appears as a potential candidate for treatment in Parkinson's disease in terms of rescuing the reminiscent dopamine neurons and inducing dopamine nerve fiber sprouting, although the role of GDNF during normal adulthood remains unknown.
Studies concerning the presence or absence of GDNF have been performed using the Gdnf knockout (Gdnf−/−) mice. However, these mice lack kidneys, ureters, and the enteric system, and therefore die shortly after birth (Moore et al., 1996, Pichel et al., 1996, Sánchez et al., 1996). Consequently, it has not been possible to study GDNF-dependency on the dopamine system during adulthood. However, studies on the Gdnf heterozygous (Gdnf+/−) mice, which have reduced striatal GDNF levels, demonstrate accelerated loss of mesencephalic dopamine neurons compared to normal aging (Airavaara et al., 2004, Boger et al., 2006). To further explore the importance of GDNF on the nigrostriatal system, transplanting Gdnf−/− tissue enable studies on GDNF-dependency from development to adulthood. In the present study, fetal VM and LGE was co-grafted into the lateral ventricles of wildtype mice to create a nigrostriatal microcircuit that was developed and maintained in the absence or presence of GDNF. The co-grafts were morphologically evaluated at 3, 6, and 12 months postgrafting.
EXPERIMENTAL PROCEDURES
Animals
Gdnf−/− mice on a C57Bl/6J background were used for this study (Pichel et al., 1996). Gdnf +/− mice were mated to obtain Gdnf +/+, Gdnf +/−, and Gdnf−/− fetuses. Graft recipients were Gdnf +/+ female mice around 3–4 months old. Animals were housed in a temperature controlled and 12h light/dark cycle environment with free access to food and water. The animal experiments had been approved by the local ethics committee, which is in the accordance with NIH guidelines.
Intracranial transplantation
For collecting tissue for transplantation, pregnant Gdnf +/− mice at embryonic day (E) 14 were deeply anesthetized with 4% isofluran (Baxter Medical AB, Kista, Sweden) using Univentor 400 anaesthesia machine (AgnThos, Stockholm, Sweden). Following dislocation of the neck, the fetuses were obtained from the abdominal cavity and placed in a sterile Petri dish. The tip of the tails were collected from all fetuses and kept on ice during the operation procedure. The VM and the LGE were dissected from the fetuses and kept in Dulbecco's modified Eagle's medium (DMEM; Gibco, Grand Island, USA) aseptically. Meanwhile, adult Gdnf +/+ mice were anesthetised with isofluran and placed in a stereotaxic frame. A hole was drilled in the cranium at 0.8 mm in a mediolateral direction, at the bregma level. The VM and LGE tissues were inserted into the lateral ventricle with a push-pull cannula that was lowered 3.5 mm below the dura mater. Every VM/LGE co-transplant originated from the same fetus, such that co-grafts from Gdnf+/+ (n=32), Gdnf+/− (n=40), Gdnf−/−(n=18) were included in this study. Graft survival time was 3 (Gdnf+/+ n=16, Gdnf+/− n=7, Gdnf−/−n=8), 6 (Gdnf+/+ n=11, Gdnf+/− n=17, Gdnf−/−n=6), and 12 (Gdnf+/+ n=5, Gdnf+/− n=16, Gdnf−/−n=4) months.
Tissue preparation
For evaluating tissue, mice with grafts were deeply anesthetized with pentobarbital (60 mg/ml, i.p.), and sacrificed by intracardial perfusion. Ca2+-free Tyrode solution was used to rinse the blood system followed by fixation of the tissue with 100 ml 4% paraformaldehyde in 0.1 M phosphate buffer (pH=7.4). The brains were postfixed in 4% paraformaldehyde for 1–2 h after dissection and then transferred to 10% sucrose in 0.1 M phosphate buffer with 0.01% sodium azide. The sucrose solution was changes for several times to rinse paraformaldehyde from the tissue.
For evaluating fetal brains, the whole head was immersion fixed in 4% paraformaldehyde over night. The tissue was collected from E19 fetuses of Gdnf+/+, Gdnf+/−, and Gdnf−/−mutant mice (n=3 for each genotype), and the tails from each fetus were collected to be genotyped. After fixation, the tissue was rinsed in sucrose solution and then processed as described for transplanted brain tissue.
Genotyping
DNA was extracted from tail tissue of fetal donors and analyzed by using polymerase chain reaction (PCR) to determine the Gdnf genotype. To extract DNA from the cells, each tail tissue sample was homogenized with 200µl lysis-buffer (25mM NaOH and 0.2 mM EDTA; pH=12) and placed in a thermomixer (Thermomixer Compact, Eppendorf) for 1h at 95°C. Thereafter, samples were cooled to 4°C and 200 µl of a neutralizing buffer (40 mM Tris-HCl; pH=5) was added. DNA samples were stored at −20°C. When preparing for the PCR reaction, each reaction tube was loaded with a total volume of 20 µl containing 13 µl dH2O, 0.4 µl TAQ polymerase 5U (Fermentas), 2 µl 10X buffer (Fermentas), 2 µl MgCl2 from 25 mM (Fermentas), 0.3 µl sense primer and 0.3 µl antisense primer (Promega), 0.5 µl dNTP 10 mM (Fermentas), and finally 1.5 µl of DNA sample or dH2O for negative controls. Two sets of primers were used to identify the presence of Gdnf +/+ gene and Gdnf −/−gene, respectively. For detection of the Gdnf +/+ genes, primer sense – 5´-CCA GAG AAT TCC AGA GGG AAA GGT C-3´ and antisense– 5´-CAG ATA CAT CCA CAC CGT TTA GCG G-3´ was added. To detect Gdnf−/−gene, primer sense – 5´- CGG AGC CGG TTG GCG CTA CCG G-3´ and antisense – 5´-ACG ACT CGG ACC GCC ATC GGT G-3´ were utilized. PCR was performed in a thermal cycler (PTC-200, MJ Research, Inc.) programmed to amplify the DNA during 40 cycles totally. The program was initiated with a denaturation step at 92°C for 4 minutes and thereafter followed by 40 cycles where each cycle consisted of 1 minute denaturation at 92°C, 1 minute annealing at 56°C and 2 minute elongation at 72°C. PCR products were stored at −20° C until analyzed on a 2% agarose gel (Fermentas) by gel electrophoresis. A 100 basepair (bp) DNA ladder (Fermentas) was applied as a reference. DNA fragments consisting of 344 bp was detected for the Gdnf +/+ allele and fragments of 255 bp indicated Gdnf −/−. For Gdnf +/−, one DNA product of each reaction and size was received.
Immunohistochemistry
The brains were frozen with gaseous CO2 and cut into 14 µm thick sections using a cryostat. The tissue sections were thawed onto slides coated with gelatine/chrome alune. The sections were then washed in phosphate buffered saline (PBS; 0.1 M, pH=7.4) for 15 min and subsequently mounted with 90% glycerol in PBS until processed for antibody incubations. Sections were incubated with primary antibodies, raised in mouse or rabbit against the indirect dopamine marker tyrosine hydroxylase (TH; mouse-anti-TH, 1:1500; Immunostar, Inc; Hudson, WI, USA; rabbit-anti-TH, 1:300; Pel-Freeze; Rogers, AR, USA) and the striatal cell marker dopamine and cyclic AMP-regulated phosphoprotein relative molecular mass 32,000 (DARPP-32; raised in rabbit, 1:600; Cell Signaling Technology; Danvers, MA, USA). To distinguish between subtypes of dopamine neurons antibodies against G-protein Activated Inwardly Rectifying Potassium Channel 2 (GIRK2; raised in rabbit, 1:25; Millipore, Solna, Sweden), aldehyde dehydrogenase 1A1 (ALDH1; raised in rabbit; 1:100, Abcam, Cambridge, UK), and calbindin (raised in mouse; 1:100; Sigma, Saint Louis, Missouri, USA) were used in the combination with antibodies against TH. Incubation in antibodies raised against Iba1 in rabbit (1:1000; Wako Pure Chemical Industries, Ltd, Osaka Japan) and glucose transporter 1 (Glut1; raised in rabbit; 1:250; Abcam, Cambridge, UK) were utilized to visualize microglia and blood capillaries, respectively. In addition, antibodies against α-synuclein (raised in rabbit; 1:50; Cell Signaling Technology, Danvers, MA) were combined with TH-immunohistochemistry. The sections were incubated with primary antibodies for 48 h at 4°C. Before addition of the secondary antibodies, sections were incubated for 15 minutes with 5% goat serum in PBS to block unspecific binding. Incubation with the secondary antibodies were performed at room temperature for 1h, and the secondary antibodies used were Alexa Fluor® 594 (Invitrogen, Molecular probes; Eugene, Oregon, USA) goat anti-mouse IgG or goat-anti-rabbit IgG, and Alexa Fluor® 488 goat anti-rabbit IgG or goat-anti-mouse IgG in the dilution of 1:500. The following combinations were performed: TH/DARPP-32, TH/GIRK2, TH/ALDH1, TH/calbindin, and TH/α-synuclein. All antibodies were diluted in PBS containing 0.3% Triton X-100 and each incubation step was followed by rinsing with PBS. After rinsing, the sections were mounted with 90% glycerol diluted in PBS.
Evaluation and statistics
All transplants and E19 fetal brains were evaluated using fluorescence microscopy. TH-positive cells with entire cell nuclei were counted in every fourth section throughout the transplants, giving an estimation of the dopamine cell survival. Since the TH-positive neurons were estimated to be approximately 20 µm in diameter and the thickness of the sections was 14 µm, every TH-positive neuron was only counted once. Thus, the estimated cell number cannot be interpreted as absolute cell number since the volume of the transplants is too small for application of stereology. The GIRK-, ALDH1- and calbindin-positive neurons that also expressed TH-immunoreactivity, were counted to estimate the proportions of A9 and A10 TH-positive neurons in the transplants. One-factor analysis of variance (ANOVA) was performed to detect variances in mean values of cell survival between different GDNF genotypes. Additional post hoc (Bonferroni) tests were performed to determine pair wise differences. To detect differences between groups over time, two-factor ANOVA was performed. The significance level was set at p ≤ 0.05. The results are expressed as mean value ± SEM.
RESULTS
Survival of TH-positive neurons in the VM portion of co-grafts
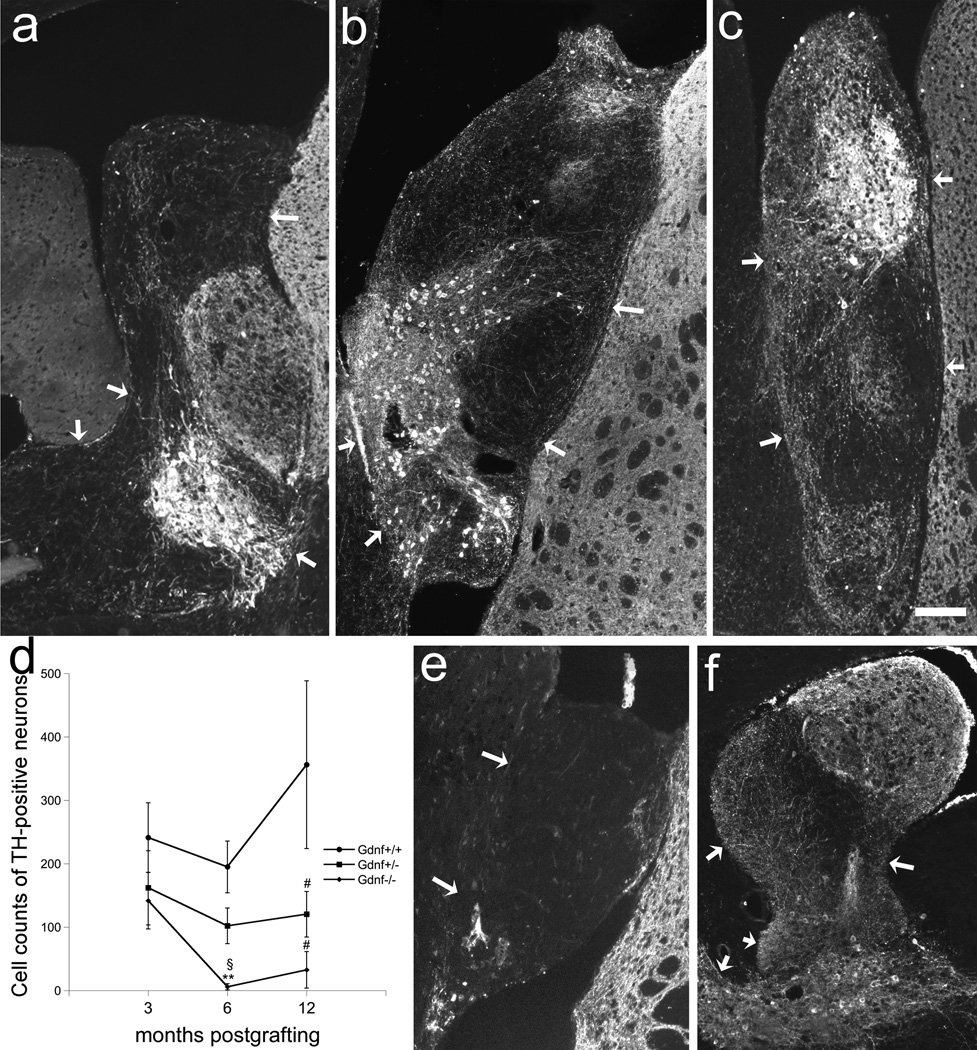
The present study was performed to establish long-term effects of Gdnf gene deletion on the nigrostriatal microcircuit. For this purpose, VM and LGE from Gdnf +/+, Gdnf +/−, and Gdnf−/−fetuses were co-grafted into the lateral ventricles of adult wildtype mice, and evaluated at 3, 6, and 12 months postgrafting. The VM portion of the co-grafts was identified by the presence of TH-positive neurons (Fig. 1). Cell counts of TH-positive neurons revealed significant differences between the Gdnf genotypes (F2,88=9.680; p=0.000; two-factor ANOVA). At 6 months, a significant reduction in TH-immunoreactive cell number was seen in the Gdnf −/−grafts when comparing with Gdnf +/+ tissue (F2,31=5.602; p=0.008, one-factor ANOVA). Likewise at 12 months postgrafting, a reduction in TH-positive neurons were noted in Gdnf −/−(F2,22=4.626; p=0.034; one-factor ANOVA) as well as in Gdnf +/− (F2,22=4.626; p=0.045; one-factor ANOVA) -derived transplants compared to wildtype controls (Fig. 1d). However, at the early time point of 3 months, cell survival did not differ between Gdnf genotypes (Fig. 1).
Figure 1.
Fetal VM-LGE co-transplants at 3 months from Gdnf +/+ (a), Gdnf+/− (b), and Gdnf −/−(c) genotypes, and at 6 months from Gdnf −/−(e) and 12 months from Gdnf +/+ (f) genotypes implanted to the lateral ventricle and visualized with TH-immunohistochemistry. In grafts with survival time of 3 months (a–c), the number of TH-positive neurons did not differ between Gdnf genotypes (d; n=16 for Gdnf +/+, n=7 for Gdnf+/−, n=8 for Gdnf −/−). At 6 months postgrafting, the survival of TH-positive neurons was significantly reduced compared to cell counts in Gdnf +/+ transplants (p<0.01; n= 11 for Gdnf +/+, n=17 for Gdnf +/−, n=6 for Gdnf −/−), and at 12 months a reduction in cell counts was also noted in the the Gdnf +/− compared to controls (p<0.05; n=5 for Gdnf +/+, n=16 for Gdnf +/−, n=4 for Gdnf −/−). At 6 months most of the tissue had degenerated in transplants derived from Gdnf −/− tissue (e), while the control transplant had a healthy appearance with dense patches of TH-positive nerve fibers in the striatal co-transplants at 12 months (f). Arrows mark graft-host interface. ** p<0.01 compared to Gdnf +/+ at 6 months, § p<0.05 compared to Gdnf−/−at 3 months, # p<0.05 compared to Gdnf +/+ at 12 months. Scale bar: a–c, f = 400 µm, e = 100 µm.
Whilst the TH-positive neuronal survival remained constant over time in the Gdnf +/+ and Gdnf +/− grafts, a significant reduction was detected in transplants derived from Gdnf −/−tissue (F2,15=4.399; p=0.041; Fig. 1d). In transplants derived from Gdnf −/−tissue, surviving TH-positive neurons were only found in two cases (n=6) of the Gdnf −/−at the 6-month time point, while all grafts derived from Gdnf +/+ had surviving transplants at all time points (Fig. 1d–f). Moreover, in Gdnf −/−transplants, the whole nigrostriatal co-graft had degenerated, or graft volume was drastically diminished, in addition to the disappearance of the TH-positive neurons (Fig. 1e). The lateral ventricle had been enlarged by graft emplacement in all cases, and when graft had disappeared, the enlarged ventricles were remained.
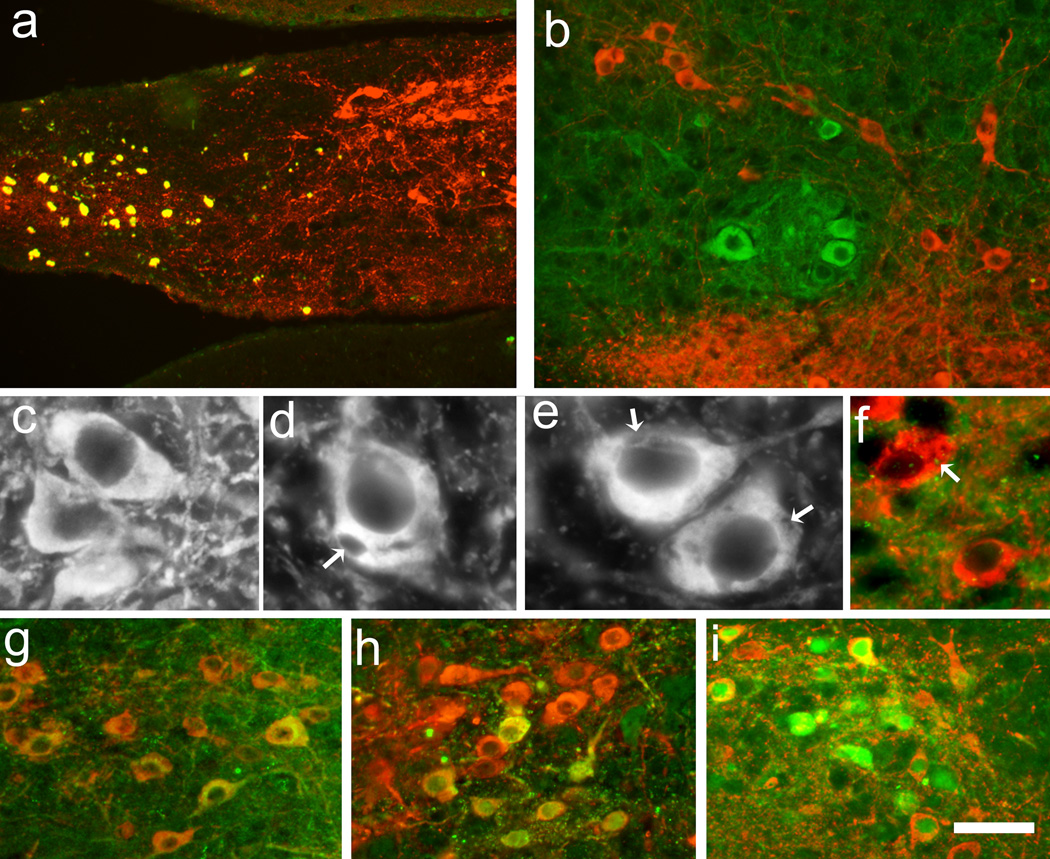
In some of the Gdnf −/−transplants at 3 months, degenerating areas was found as determined by highly autofluorescent debris, although viable TH-positive neurons were present (Fig. 2a). Furthermore, in several transplants TH-negative inclusion-like spots were found in the cytoplasm of several TH-positive neurons (Fig. 2e). However, double immunoreactivity using antibodies against TH and α-synuclein revealed that α-synuclein-positive dots were present within the cytoplasm of these TH-positive neurons, and in rare cases the α-synuclein-positive dots overlapped with the inclusion-like structures (Fig. 2f). These TH-negative inclusion-like structures were not found in the transplants derived from Gdnf +/+ tissue at any time point evaluated, while they were present in grafts from Gdnf+/− at 12 months (Fig. 2c–d).
Figure 2.
TH-immunohistochemistry from VM portion of VM-LGE co-transplants at 3 (a, b, e–i) and 12 (c, d) months after grafting demonstrated that grafts derived from Gdnf −/−tissue had autofluorescent debris in parts of the transplant (a); debris were positive in all filters although the section had been incubated with only TH and its secondary antibodies i.e. debris become yellow when screening for several filters. The TH-positive neurons appeared healthy in Gdnf +/+ transplants at 12 months (c), while neurons in VM derived from Gdnf +/− tissue had TH-negative inclusion-like structures (d; see arrows). Similar structures were often obvious in Gdnf−/−transplants already at 3 months (e), and double-labeling with α-synuclein demonstrated dots in the cytoplasm of neurons exhibiting TH-negative structures (f). Investigating the presence of TH/GIRK2- (g), TH/ALDH1- (h), and TH/calbindin- (i) immunoreactivity demonstrated no difference in number of neurons between genotypes at 3 months postgrafting, although many neurons expressing GIRK2 were negative to TH-immunoreactivity (b). TH-immunoreactivity = red or white; GIRK2 (b, g), α-synuclein (f), ALDH1 (h), and calbindin (i) = green. Scale bar: a = 90 µm, b = 45 µm, c, d, e = 12.5 µm, f = 25 µm, and g, h, i = 50 µm.
Evaluation of the number of neurons that were TH/GIRK2 double labeled demonstrated no significant differences between genotypes at 3 months postgrafting, where approximately 10% of the TH-positive neurons expressed GIRK2. Similar results were achieved when studying TH combined with ALDH1 (Fig. 2g, h). The number of TH-positive neurons double labeled with calbindin was calculated to around 20% of the total number of TH-positive neurons (Fig. 2i) with no difference between genotypes. Thus, there were few TH-positive neurons that displayed co-labeling with TH and either GIRK2, ALDH1 or calbindin, however, many TH-negative neurons were positive to all makers, especially GIRK2 (Fig. 2b).
TH-positive nerve fiber innervation of LGE tissue
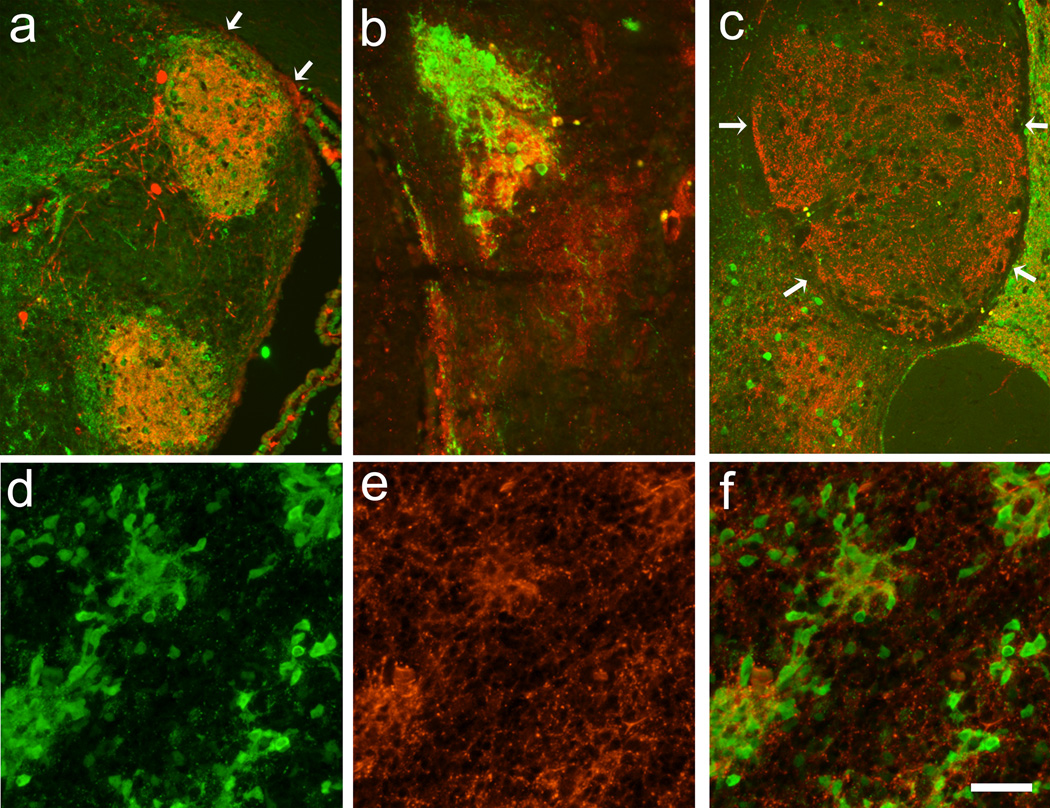
The striatal portion of co-grafts was identified by the absence of TH-positive neurons and the presence of DARPP-32-positive cells. In the striatal portion of the co-grafts derived from Gdnf +/+ tissue, the TH-positive innervation formed a dense, patchy nerve fiber network, leaving areas around the patches TH-negative (Fig. 3a). The presence of these TH-dense areas overlapped with dense clusters of DARPP-32-positive neurons. This innervation pattern was seen throughout all time points in the Gdnf +/+-derived transplants.
Figure 3.
LGE portions of VM-LGE co-transplants at 3 months postgrafting (a–c) in grafts derived from Gdnf +/+ (a), Gdnf +/− (b), and Gdnf −/−(c) tissues demonstrating TH (red) and DARPP-32 (green) –immunoreactivities. The distribution of TH-positive nerve fibers was patchy and dense and overlapped with areas dense with DARPP-32-positive neurons in Gdnf +/+ transplants (a), while these DARPP-32-positive areas mismatched somewhat with TH in grafts from Gdnf +/− (b). In transplants from Gdnf −/−, TH-positive nerve fibers never formed a dense patchy distribtion, and the expression of DARPP-32 was poor (c). In E19 Gdnf −/−fetal striatum, TH-positive nerve fibers did form a patchy distribution, which overlapped well with the presence of DARPP-32-positive neurons (d–f). Arrows delineate graft-host interface. Scale bar: a, c = 200 µm, b, d, e, f = 100 µm.
In Gdnf +/−-derived striatal grafts, the innervation pattern was slightly different compared to what was found in Gdnf +/+ transplants: in addition to the dense TH-positive areas, similar to those found in Gdnf +/+ -derived striatum, a less dense innervation was found between the TH-dense patches in the striatal transplants. Interestingly, the dense TH-positive areas did not always completely overlap with the presence of dense DARPP-32-positive cell clusters (Fig. 3b). The organization of the striatum in the Gdnf +/− did not differ between time points.
The organization of striatal portion of co-grafts derived from Gdnf −/−tissue differed from the other genotypes in that they did not express dense clusters of DARPP-32-positive neurons. The presence of DARPP-32-positive neurons was less frequent than in the other genotypes and the neurons were distributed in a widespread manner. The TH-positive nerve fiber innervation of the striatal portion was not displayed in dense patches, but sparse and widespread (Fig. 3c). This organization of the Gdnf −/−striatal transplants was true for all time points when viable grafts were found. Thus, at the longer time points not only the VM portion of the grafts had degenerated but also the striatal tissue. In a few cases, reminiscent tissue was found, often both TH- and DARPP-32-negative (Fig. 1e). The presence of surviving transplants at earlier time points was indicated by enlargement of the ventricle that once had been transplanted.
The presence of microglia in the transplant
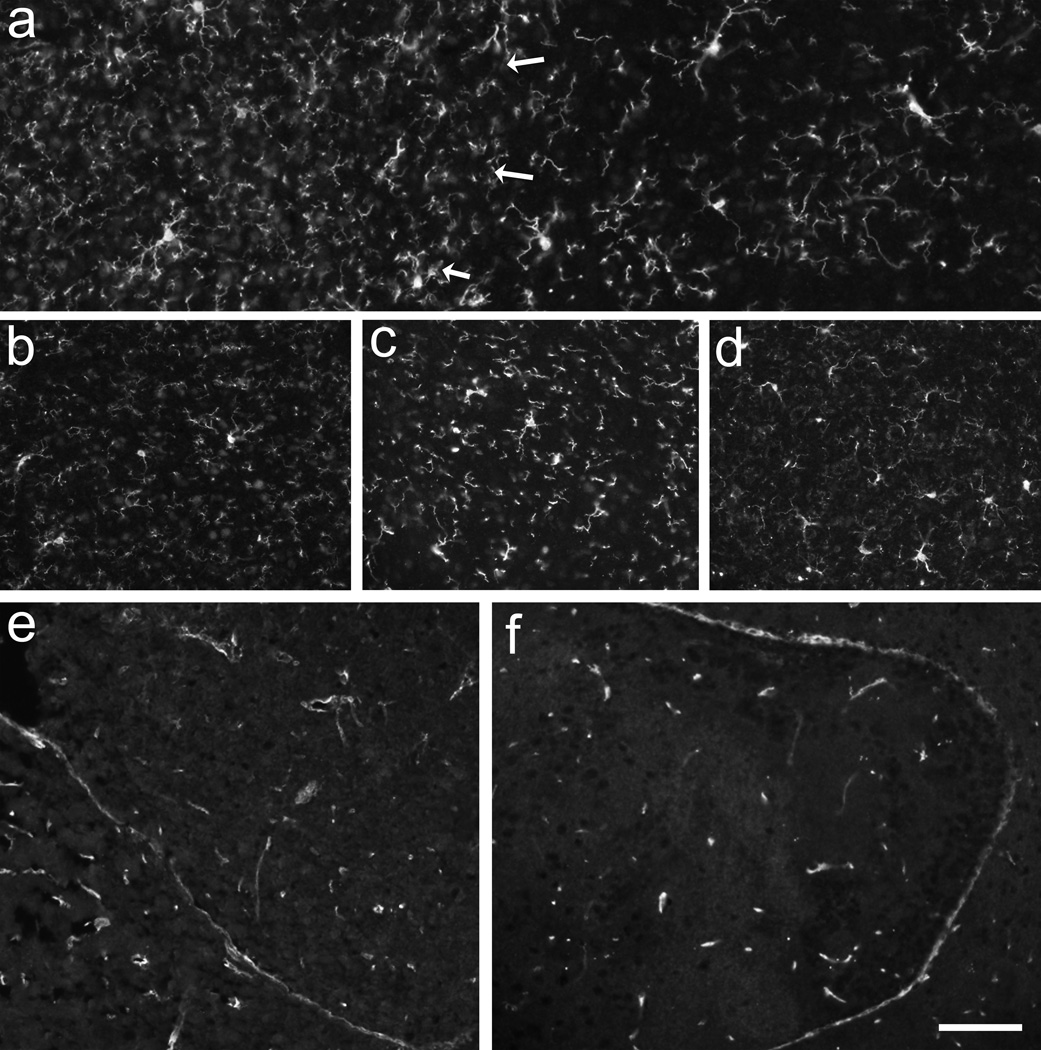
Iba1-immunoreactivity revealed no difference in density of the transplants over time or between Gdnf genotypes. Interestingly, the density of Iba1-immunoreactivity was slightly higher in the striatal portion of co-grafts compared to the VM (Fig. 4a), a finding that was true for all Gdnf genotypes. The striatal density was similar to that seen in the host striatum. In transplants that expressed autofluorescent debris, i.e. signs for degeneration, the density of Iba1-immunoreactive microglia was similar to other transplants.
Figure 4.
The pan-microglia marker Iba1-immunoreactivity (a–d) demonstrated that the density of microglia was slightly higher in the striatal portion of co-grafts (a; left to arrows) compared to the VM (a; right). There was no increase in microglia over time as found at 12 months postgrafting in Gdnf +/+ (a) compared to Gdnf +/− (b) transplants, although the Gdnf +/− transplants had a significant loss of TH-positive neurons at this time point. Comparing the VM portion of co-grafts, no difference between Iba1-positive microglia was demonstrated between Gdnf +/+ (c) and Gdnf −/−(d) at 3 months postgrafting. Using Glut1-immunohistochemistry to study blood vessel support in the transplants demonstrated that both the Gdnf+/+ (e) and Gdnf−/−(f) transplants had good and evenly distributed blood vessel support. Scale bar: a–d =50 µm, e, f = 100 µm.
Blood capillaries in the transplants
The presence of blood capillaries in the transplants was evaluated using Glut1-immunoreactivity. The results revealed that the density and morphology of the blood vessels were similar in all transplants (Fig. 4e, f). The density of Glut1-positive capillaries in the transplants did not differ from that found in the host brains.
TH- and DARPP-32-immunoreactivity in E19 fetuses
In the striatum of E19 fetuses, the distribution of TH and DARPP-32 was similar in all Gdnf genotypes. TH-immunoreactive nerve fibers were found along the lateral rim of corpus callosum and in TH-dense islands. These areas overlapped with areas demonstrating high density of DARPP-32- immunoreactivity (Fig. 3d–f).
DISCUSSION
GDNF is known to be an efficient factor to promote survival and growth from VM dopamine neurons. In this study, the presence and absence of GDNF was evaluated for the survival and maintenance of the nigrostriatal system. The results revealed that both the VM TH-positive neurons and the striatum need GDNF for long-term survival. The short-term survival of the TH-positive neurons in VM-LGE co-transplants was not dependent on GDNF supply; hence transplants that were developed in the absence of GDNF displayed similar numbers of surviving neurons as those producing GDNF. On the contrary, cell survival for time periods longer than 3 months required GDNF. Furthermore, the organization of the striatal co-graft was changed in the absence of GDNF such that the dopamine nerve fibers did not manage to form the patchy innervation and the DARPP-32-positive neurons were not organized into dense clusters as found in the Gdnf+/+ and Gdnf+/− transplants.
The reduced dopamine neuron survival is in accordance with a previous study where grafted VM dopamine neurons from Gdnf −/−tissue degenerated, unless the grafted tissue had been incubated in GDNF prior to transplantation (Granholm et al., 2000). In the present study, absence of GDNF severely affected the number of TH-positive neurons during long-term time points, however, not at the 3-month time point. This observation is further supported by the fact that no morphological change in the dopamine system is found in newborn Gdnf null mutated fetuses (Pichel et al., 1996). Loss of TH-positive neurons was also found in the Gdnf+/−-derived transplants, however, this effect was delayed and observed first at 12 months postgrafting. In Gdnf +/− adult mice, the VM dopamine system has a faster onset of degeneration of dopamine neurons compared to normal aging in the wildtype mouse, which also is supported by age-related changes in behavior (Boger et al., 2006). Furthermore, in the conditional Gdnf null-mutated mouse the dopamine neurons die upon GDNF ablation during adulthood (Pascual et al., 2008). The mechanism for this GDNF-dependency during adulthood but not during development is yet not understood. One possible explanation is based on the location for the GDNF expression: GDNF mRNA and protein are expressed in the striatum, while its receptors are expressed by nigral neurons (Trupp et al., 1997). GDNF is then thought to act via uptake into the striatal dopamine nerve terminals to be retrogradely transported to the cell body to exert its action (Tomac et al., 1995b). Thus, with reduced GDNF levels in the striatum, as in the Gdnf +/− mice, the dopamine neurons appear more vulnerable (Boger et al., 2007, Boger et al., 2008). Even though no toxic challenge was performed in the present study, the lack of GDNF was sufficient to loose the TH-positive neurons. Alternatively, other neurotrophic factors belonging to the same family, such as neurturin, artemin, and persephin, might be produced in sufficient levels to act through the GDNF receptors and thereby compensate the early but not the late loss of GDNF. Indeed, these levels are downregulated in the adult compared to developing striata (Schaar et al., 1993, Strömberg et al., 1993, Ikeda et al., 1999).
The survival of TH-positive neurons at 3 months after grafting and the significant loss of neurons at the longer time points in Gdnf −/−transplants could not be correlated to a specific subpopulation of A9 or A10 VM dopamine neurons, but rather seemed as a non-specific loss of TH-positive neurons. Survival effects of GDNF on subpopulation/s of VM dopamine neurons are contradictory, such that enhanced survival has been contributed to TH/calbindin-positive as well as TH-positive/calbindin-negative neurons (Johansson et al., 1995, Meyer et al., 1999). Calbindin is known to be expressed mainly in the A10 dopamine neurons while GIRK2 and ALDH1 are mainly markers for the A9 dopamine neurons (Gerfen et al., 1985, McCaffery and Drager, 1994, Schein et al., 1998, Inanobe et al., 1999, Chung et al., 2005). However, it was recently demonstrated that the dose of GDNF determines which subpopulation of dopamine neurons that is affected, such that one single dose promotes the nigral A9 neurons and a low, continuously given dose promotes the A10 neurons (Borgal et al., 2007). Thus, both the A9 and A10 dopamine neurons appear to be affected by GDNF. The lack of GDNF in the present study affected all subtypes of VM dopamine neurons such that none of them survived. Similar findings were observed in conditional Gdnf knockout mice, where both A9 and A10 dopamine neurons degenerated to the same degree (Pascual et al., 2008). Therefore, it is likely that GDNF is essential for both the A9 and A10 neurons.
TH-negative inclusion-like structures were found in transplants derived from Gdnf −/−and Gdnf+/− tissues at 3 and 12 months, respectively, while these structures were not found in the Gdnf +/+ grafts at any time point. Attempts were made to characterize these structures with immunohistochemistry for α-synuclein as aggregated α-synuclein is a major component of the Lewy bodies (Spillantini et al., 1997). However, the presence of α-synuclein-immunoreactivity in the cytoplasm could generally not be localized to the TH-negative areas, indicating that the inclusion-like structures found in the transplants failed to be correlated with Lewy bodies, even though they were frequently found in transplants with great loss of TH-positive neurons.
The lack of GDNF affected the striatal portion of the co-grafts. In fact, the clusters of DARPP-32-positive neurons were present in the striatum of Gdnf +/+ but not in the Gdnf −/−transplants. Interestingly, no difference was found between Gdnf genotypes concerning the DARPP-32/TH-positive distribution in the striatum of E19 fetuses. This suggests that in the absence of GDNF, the striatum is normally developed during fetal stages, while it becomes malformed during postnatal development or adulthood. The presence of DARPP-32-positive neuronal clusters coincided with dense islands of TH-immunoreactivity in the Gdnf +/+ transplants. Because the TH-positive nerve fiber innervation of the striatal co-graft was present at 3 months without being distributed in patches in the Gdnf−/−-derived striatum, it is therefore likely that the widespread distribution of the DARPP-32-positive neurons in the Gdnf −/−-derived transplants was not caused by a selective degeneration prior to evaluation but rather a consequence of the lack of GDNF during postnatal development. Thus, if the DARPP-32-positive neurons had been distributed in patches prior to the time for evaluation, the TH-positive nerve fibers had then reorganized or never followed the presence of DARPP-32. Instead, the widespread TH-positive innervation pattern was similar to what is found when VM TH-positive neurons innervate normal, adult, dopamine-denervated striatal tissue, i.e. no patchy innervation is then produced (Strömberg et al., 1997). Therefore, it seems that the loss of organization of the DARPP-32/TH-positive patchy distribution in the striatal transplants results as a consequence of the absence of GDNF postnatally, especially since GDNF is expressed in a patchy distribution during development both in striatal grafts of normal tissue and in in situ development (Lopez-Martin et al., 1999).
The differences in number of surviving TH-positive neurons, nerve fiber innervation, and organization of the DARPP-32-immunoreactive neurons in transplants of different Gdnf genotypes might be a consequence of immunological reaction. Furthermore, the loss of tissue in the Gdnf −/−-derived transplants might be a consequence of insufficient blood supply. Therefore the presence of the pan-microglia marker Iba1 and Glut1 were evaluated. However, there was neither differences between genotypes in the presence of microglia nor were there any differences in the density of blood capillaries, suggesting that the loss of tissue in the Gdnf −/− -derived transplants was not caused by immune reaction or lack of support from blood vessels but a consequence of the lack of GDNF.
CONCLUSIONS
Taken together, VM TH-positive neurons are developed without GDNF but require GDNF for long-term maintenance, while the striatum needs GDNF both during development and in the adulthood. In the absence of GDNF, the striatum cannot arrange into striatal patchy organization with areas dense in TH- and DARPP-32- immunoreactivity that is found during normal development. These data suggest that GDNF is crucial for maintenance of both the VM and the striatum, which strongly supports the importance of the clinical trials of GDNF infusions into patients suffering from Parkinson's disease (Gill et al., 2003, Love et al., 2005, Slevin et al., 2005).
ACKNOWLEDGEMENTS
This study was supported by the Swedish Research Council grant #09917, the Umeå University Medical Faculty Foundations, and National Institutes of Aging grant #AG023630.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Airavaara M, Planken A, Gäddnäs H, Piepponen TP, Saarma M, Ahtee L. Increased extracellular dopamine concentrations and FosB/DFosB expression in striatal brain areas of heterozygous GDNF knockout mice. Eur J Neurosci. 2004;20:2336–2344. doi: 10.1111/j.1460-9568.2004.03700.x. [DOI] [PubMed] [Google Scholar]
- Beck KD, Valverde J, Alexi T, Poulsen K, Moffat B, Vandlen RA, Rosenthal A, Hefti F. Mesencephalic dopaminergic neurons protected by GDNF from axotomy-induced degeneration in the adult brain. Nature. 1995;373:339–341. doi: 10.1038/373339a0. [DOI] [PubMed] [Google Scholar]
- Boger HA, Middaugh LD, Huang P, Zaman V, Smith AC, Hoffer BJ, Tomac AC, Granholm AC. A partial GDNF depletion leads to earlier age-related deterioration of motor function and tyrosine hydroxylase expression in the substantia nigra. Exp Neurol. 2006;202:336–347. doi: 10.1016/j.expneurol.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Boger HA, Middaugh LD, Patrick KS, Ramamoorthy S, Denehy ED, Zhu H, Pacchioni AM, Granholm AC, McGinty JF. Long-term consequences of methamphetamine exposure in young adults are exacerbated in glial cell line-derived neurotrophic factor heterozygous mice. J Neurosci. 2007;27:8816–8825. doi: 10.1523/JNEUROSCI.1067-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boger HA, Middaugh LD, Zaman V, Hoffer B, Granholm AC. Differential effects of the dopamine neurotoxin MPTP in animals with a partial deletion of the GDNF receptor, GFR alpha1, gene. Brain Res. 2008;1241:18–28. doi: 10.1016/j.brainres.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgal L, Hong M, Sadi D, Mendez I. Differential effects of glial cell line-derived neurotrophic factor on A9 and A10 dopamine neuron survival in vitro. Neuroscience. 2007;147:712–719. doi: 10.1016/j.neuroscience.2007.03.057. [DOI] [PubMed] [Google Scholar]
- Chung S, Hedlund E, Hwang M, Kim DW, Shin B-S, Hwang D-Y, Kang UJ, Isacson O, Kim K-S. The homeodomain transcription factor Pitx3 facilitates differentiation of mouse embryonic stem cells into AHD2-expressing dopaminergic neurons. Mol Cell Neurosci. 2005;28:241–252. doi: 10.1016/j.mcn.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Fishell G, van der Kooy D. Pattern formation in the striatum: developmental changes in the distribution of striatonigral neurons. J Neurosci. 1987;7:1969–1978. doi: 10.1523/JNEUROSCI.07-07-01969.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gates MA, Torres EM, White A, Fricker-Gates RA, Dunnett SB. Re-examining the ontogeny of substantia nigra dopamine neurons. Eur J Neurosci. 2006;23:1384–1390. doi: 10.1111/j.1460-9568.2006.04637.x. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Baimbridge KG, Miller JJ. The neostriatal mosaic: Compartmental distribution of calcium-binding protein and parvalbumin in the basal ganglia of the rat and monkey. Proc Natl Acad Sci USA. 1985;82:8780–8784. doi: 10.1073/pnas.82.24.8780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill SS, Patel NK, Hotton GR, O'Sullivan K, McCarter R, Bunnage M, Brooks DJ, Svendsen CN, Heywood P. Direct brain infusion of glial cell line-derived neurotrophic factor in Parkinson disease. Nat Med. 2003;9:589–595. doi: 10.1038/nm850. [DOI] [PubMed] [Google Scholar]
- Granholm A-C, Mott JL, Bowenkamp K, Eken S, Henry S, Hoffer BJ, Lapchak PA, Palmer MR, van Horne C, Gerhardt GA. Glial cell line-derived neurotrophic factor improves survival of ventral mesencephalic grafts to the 6-hydroxydopamine lesioned striatum. Exp Brain Res. 1997;116:29–38. doi: 10.1007/pl00005741. [DOI] [PubMed] [Google Scholar]
- Granholm A-C, Reyland M, Albeck D, Sanders L, Gerhardt g, Hoerning G, Shen L, Westphal H, Hoffer B. Glial cell line-derived neurotrophic factor is essential for postnatal survival of midbrain dopamine neurons. J Neurosci. 2000;20:3182–3190. doi: 10.1523/JNEUROSCI.20-09-03182.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graybiel AM. Correspondence between the dopamine islands and striosomes of the mammalian striatum. Neurosci. 1984;13:1157–1187. doi: 10.1016/0306-4522(84)90293-8. [DOI] [PubMed] [Google Scholar]
- Ikeda T, Xia XY, Xia YX, Ikenoue T, Choi BH. Expression of glial cell line-derived neurotrophic factor in the brain and cerebrospinal fluid of the developing rat. Int J Dev Neurosci. 1999;17:681–691. doi: 10.1016/s0736-5748(99)00057-x. [DOI] [PubMed] [Google Scholar]
- Inanobe A, Yoshimoto Y, Horio Y, Morishige K, Hibino H, Matsumoto S, Tokunaga Y, Maeda T, Hata Y, Takai Y, Kurachi Y. Characterization of G-protein-gated K+ channels composed of Kir3.2 subunits in dopaminergic neurons of the substantia nigra. J Neurosci. 1999;19:1006–1017. doi: 10.1523/JNEUROSCI.19-03-01006.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson M, Friedemann M, Hoffer B, Strömberg I. Effects of glial cell line-derived neurotrophic factor on developing and mature ventral mesencephalic grafts in oculo. Exp Neurol. 1995;134:25–34. doi: 10.1006/exnr.1995.1033. [DOI] [PubMed] [Google Scholar]
- Lauder J, Bloom F. Ontogeny of monoamine neurons in the locus coeruleus, raphe nuclei and substantia nigra of the rat. J Comp Neurol. 1974;155:469–482. doi: 10.1002/cne.901550407. [DOI] [PubMed] [Google Scholar]
- Lin L-FH, Doherty DH, Lile JD, Bektesh S, Collins F. GDNF: A glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science. 1993;260:1130–1132. doi: 10.1126/science.8493557. [DOI] [PubMed] [Google Scholar]
- Lopez-Martin E, Caruncho HJ, Rodriguez-Pallares J, Guerra MJ, Labandeira-Garcia JL. Striatal dopaminergic afferents concentrate in GDNF-positive patches during development and in developing intrastriatal striatal grafts. J Comp Neurol. 1999;406:199–206. doi: 10.1002/(sici)1096-9861(19990405)406:2<199::aid-cne5>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Love S, Plaha P, Patel NK, Hotton GR, Brooks DJ, Gill SS. Glial cell line-derived neurotrophic factor induces neuronal sprouting in human brain. Nat Med. 2005;11:703–704. doi: 10.1038/nm0705-703. [DOI] [PubMed] [Google Scholar]
- McCaffery P, Drager UC. High levels of a retinoic acid-generating dehydrogenase in the meso-telencephalic dopamine system. Proc Natl Acad Sci USA. 1994;91:7772–7776. doi: 10.1073/pnas.91.16.7772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer M, Zimmer J, Seiler RW, Widmer HR. GDNF increases the density of cells containing calbindin but not of cells containing calretinin in cultured rat and human fetal nigral tissue. Cell Transplant. 1999;8:25–36. doi: 10.1177/096368979900800112. [DOI] [PubMed] [Google Scholar]
- Moore MW, Klein RD, Fariñas I, Sauer H, Armanini M, Phillips H, Reichert LF, Ryan AM, Carever-Moore K, Rosenthal A. Renal and neuronal abnormalities in mice lacking GDNF. Nature. 1996;382:76–79. doi: 10.1038/382076a0. [DOI] [PubMed] [Google Scholar]
- Olson L, Seiger A. Early prenatal ontogeny of central monoamine neurons in the rat: fluorescence histochemical observations. Z Anat Entwicklungsgesch. 1972;137:301–316. doi: 10.1007/BF00519099. [DOI] [PubMed] [Google Scholar]
- Olson L, Seiger Å, Fuxe K. Heterogeneity of striatal and limbic dopamine innervation: Highly fluorescent islands in developing and adult rats. Brain Res. 1972;44:283–288. doi: 10.1016/0006-8993(72)90385-x. [DOI] [PubMed] [Google Scholar]
- Pascual A, Hidalgo-Figueroa M, Piruat JI, Pintado CO, Gomez-Diaz R, Lopez-Barneo J. Absolute requirement of GDNF for adult catecholaminergic neuron survival. Nat Neurosci. 2008;11:755–761. doi: 10.1038/nn.2136. [DOI] [PubMed] [Google Scholar]
- Pichel JG, Shen L, Sheng HZ, Granholm AC, Drago J, Grinberg A, Lee EJ, Huang SP, Saarma M, Hoffer BJ, Sariola H, Westphal H. Defects in enteric innervation and kidney development in mice lacking GDNF. Nature. 1996;382:73–76. doi: 10.1038/382073a0. [DOI] [PubMed] [Google Scholar]
- Rosenblad C, Martinez-Serrano A, Björklund A. Glial cell line-derived neurotrophic factor increases survival, growth, and function of intrastriatal fetal nigral dopaminergic grafts. Neurosci. 1996;75:979–985. doi: 10.1016/0306-4522(96)00343-0. [DOI] [PubMed] [Google Scholar]
- Sánchez MP, Silos-Santiago I, Frisén J, He B, Lira SA, Barbacid M. Renal agenesis and the absence of enteric neurons in mice lacking GDNF. Nature. 1996;382:70–73. doi: 10.1038/382070a0. [DOI] [PubMed] [Google Scholar]
- Schaar DG, Sieber BA, Dreyfus CF, Black IB. Regional and cell-specific expression of GDNF in rat brain. Exp Neurol. 1993;124:368–371. doi: 10.1006/exnr.1993.1207. [DOI] [PubMed] [Google Scholar]
- Schein J, Hunter D, Roffler-Tarlov S. Girk2 expression in the ventral midbrain, cerebellum, and olfactory bulb and its relationship to the murine mutation weaver. Dev Biol. 1998;204:432–450. doi: 10.1006/dbio.1998.9076. [DOI] [PubMed] [Google Scholar]
- Sinclair SR, Svendsen CN, Torrs EM, Martin D, Fawcett JW, Dunnett SB. GDNF enhances cell survival and fibre outgrowth in embryonic nigral grafts. NeuroRep. 1996;7:2547–2552. doi: 10.1097/00001756-199611040-00029. [DOI] [PubMed] [Google Scholar]
- Slevin JT, Gerhardt GA, Smith CD, Gash DM, Kryscio R, Young B. Improvement of bilateral motor functions in patients with Parkinson disease through the unilateral intraputaminal infusion of glial cell line-derived neurotrophic factor. J Neurosurg. 2005;102:216–222. doi: 10.3171/jns.2005.102.2.0216. [DOI] [PubMed] [Google Scholar]
- Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997;388:839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- Strömberg I, Björklund L, Johansson M, Tomac A, Collins F, Olson L, Hoffer B, Humpel C. Glial cell line-derived neurotrophic factor (GDNF) is expressed in the developing but not adult striatum and stimulates developing dopamine neurons in vivo. Exp Neurol. 1993;124:401–412. doi: 10.1006/exnr.1993.1214. [DOI] [PubMed] [Google Scholar]
- Strömberg I, Björklund L, Förander P. The age of striatum determines the pattern and extent of dopaminergic innervation: A nigrostriatal double graft study. Cell Transplant. 1997;6:287–296. doi: 10.1177/096368979700600311. [DOI] [PubMed] [Google Scholar]
- Tomac A, Lindqvist E, Lin L-F, Ögren SO, Young D, Hoffer BJ, Olson L. Protection and repair of the nigrostriatal dopaminergic system by GDNF in vivo. Nature. 1995a;373:335–339. doi: 10.1038/373335a0. [DOI] [PubMed] [Google Scholar]
- Tomac A, Widenfalk J, Lin LF, Kohno T, Ebendal T, Hoffer BJ, Olson L. Retrograde axonal transport of glial cell line-derived neurotrophic factor in the adult nigrostriatal system suggests a trophic role in the adult. Proc Natl Acad Sci U S A. 1995b;92:8274–8278. doi: 10.1073/pnas.92.18.8274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trupp M, Belluardo N, Funakoshi H, Ibanez CF. Complementary and overlapping expression of glial cell line-derived neurotrophic factor (GDNF), c-ret proto-oncogene, and GDNF receptor-alpha indicates multiple mechanisms of trophic actions in the adult rat CNS. J Neurosci. 1997;17:3554–3567. doi: 10.1523/JNEUROSCI.17-10-03554.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Kooy D, Fishell G. Neuronal birthdate underlies the development of striatal compartments. Brain Res. 1987;401:155–161. doi: 10.1016/0006-8993(87)91176-0. [DOI] [PubMed] [Google Scholar]
- Vitalis T, Cases O, Parnavelas JG. Development of the dopaminergic neurons in the rodent brainstem. Exp Neurol. 2005;191 Suppl 1:S104–S112. doi: 10.1016/j.expneurol.2004.05.044. [DOI] [PubMed] [Google Scholar]
- Voorn P, Kalsbeek A, Jorritsma-Byham B, Groenewegen H. The pre- and postnatal development of the dopaminergic cell groups in the ventral mesencephalon and the dopaminergic innervation of the striatum of the rat. Neurosci. 1988;25:857–887. doi: 10.1016/0306-4522(88)90041-3. [DOI] [PubMed] [Google Scholar]
- Yurek DM. Glial cell line-derived neurotrophic factor improves survival of dopaminergic neurons in transplants of fetal ventral mesencephalic tissue. Exp Neurol. 1998;153:195–202. doi: 10.1006/exnr.1998.6884. [DOI] [PubMed] [Google Scholar]