Abstract
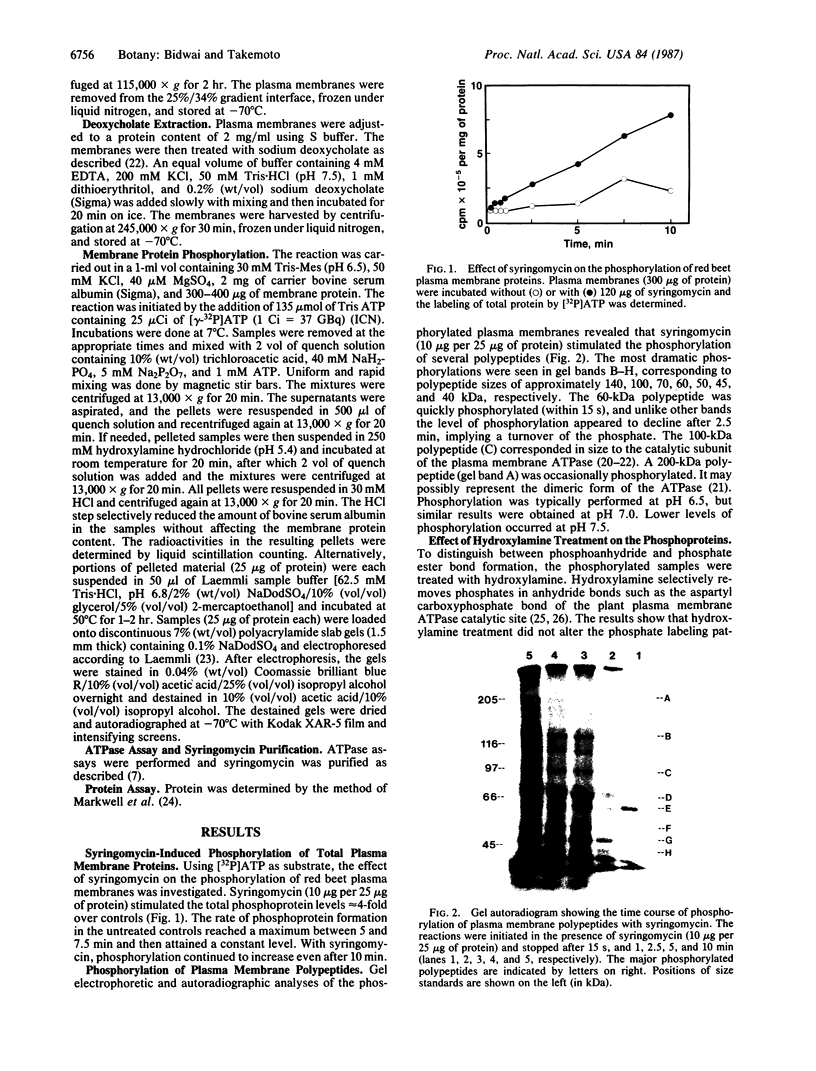
Syringomycin, a peptide toxin and a virulence factor produced by the bacterial phytopathogen Pseudomonas syringae pv. syringae, stimulated the phosphorylation of several plasma membrane polypeptides of red beet storage tissue. Among these was a 100-kDa polypeptide, which corresponds in size to the proton pump ATPase. The phosphorylations were insensitive to hydroxylamine, indicating that the polypeptide phosphorylated intermediates involved phosphate ester bonds characteristic of protein kinase-mediated phosphorylation. Phosphorylation of the 100-kDa polypeptide and of most of the other polypeptides was reduced or eliminated by extraction of the membranes with 0.1% (wt/vol) sodium deoxycholate, a treatment that also eliminated the ability of the toxin to stimulate ATPase activity. Phosphorylation of the 100-kDa polypeptide was highest with 10-20 μg of syringomycin; the same amounts gave the highest degree of ATPase activity stimulation. Phosphorylation of some of the polypeptides was eliminated or decreased by the Ca2+ chelator EGTA. Addition of excess Ca2+ restored the phosphorylation of most of these polypeptides. We conclude that syringomycin acts by stimulating an endogenous membrane protein kinase activity, which results in the phosphorylation of several membrane polypeptides. One of the phosphorylated polypeptides corresponds in size to the proton pump ATPase.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bidwai A. P., Zhang L., Bachmann R. C., Takemoto J. Y. Mechanism of Action of Pseudomonas syringae Phytotoxin, Syringomycin : Stimulation of Red Beet Plasma Membrane ATPase Activity. Plant Physiol. 1987 Jan;83(1):39–43. doi: 10.1104/pp.83.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briskin D. P., Leonard R. T. Phosphorylation of the adenosine triphosphatase in a deoxycholate-treated plasma membrane fraction from corn roots. Plant Physiol. 1982 Nov;70(5):1459–1464. doi: 10.1104/pp.70.5.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briskin D. P., Poole R. J. Characterization of the solubilized plasma membrane ATPase of red beet. Plant Physiol. 1984 Sep;76(1):26–30. doi: 10.1104/pp.76.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briskin D. P., Poole R. J. Evidence for a beta-Aspartyl Phosphate Residue in the Phosphorylated Intermediate of the Red Beet Plasma Membrane ATPase. Plant Physiol. 1983 Aug;72(4):1133–1135. doi: 10.1104/pp.72.4.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briskin D. P., Poole R. J. Plasma membrane ATPase of red beet forms a phosphorylated intermediate. Plant Physiol. 1983 Mar;71(3):507–512. doi: 10.1104/pp.71.3.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briskin D. P., Thornley W. R., Roti-Roti J. L. Target molecular size of the red beet plasma membrane ATPase. Plant Physiol. 1985 Jul;78(3):642–644. doi: 10.1104/pp.78.3.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss J. E., Stull J. T. Measurement of chemical phosphate in proteins. Methods Enzymol. 1983;99:7–14. doi: 10.1016/0076-6879(83)99035-3. [DOI] [PubMed] [Google Scholar]
- Caroni P., Carafoli E. Regulation of Ca2+-pumping ATPase of heart sarcolemma by a phosphorylation-dephosphorylation Process. J Biol Chem. 1981 Sep 25;256(18):9371–9373. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- McDonough J. P., Mahler H. P. Covalent phosphorylation of the Mg2+-dependent ATPase of yeast plasma membranes. J Biol Chem. 1982 Dec 25;257(24):14579–14581. [PubMed] [Google Scholar]
- Paliyath G., Poovaiah B. W. Calmodulin inhibitor in senescing apples and its physiological and pharmacological significance. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2065–2069. doi: 10.1073/pnas.81.7.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tognoli L., Colombo R. Protein phosphorylation in intact cultured sycamore (Acer pseudoplatanus) cells and its response to fusicoccin. Biochem J. 1986 Apr 1;235(1):45–48. doi: 10.1042/bj2350045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veluthambi K., Poovaiah B. W. Calcium- and calmodulin-regulated phosphorylation of soluble and membrane proteins from corn coleoptiles. Plant Physiol. 1984 Oct;76(2):359–365. doi: 10.1104/pp.76.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veluthambi K., Poovaiah B. W. Calcium-promoted protein phosphorylation in plants. Science. 1984 Jan 13;223(4632):167–169. doi: 10.1126/science.223.4632.167. [DOI] [PubMed] [Google Scholar]
- Zhang L., Takemoto J. Y. Mechanism of action of Pseudomonas syringae phytotoxin, syringomycin. Interaction with the plasma membrane of wild-type and respiratory-deficient strains of Saccharomyces cerevisiae. Biochim Biophys Acta. 1986 Sep 25;861(1):201–204. doi: 10.1016/0005-2736(86)90581-x. [DOI] [PubMed] [Google Scholar]