Abstract
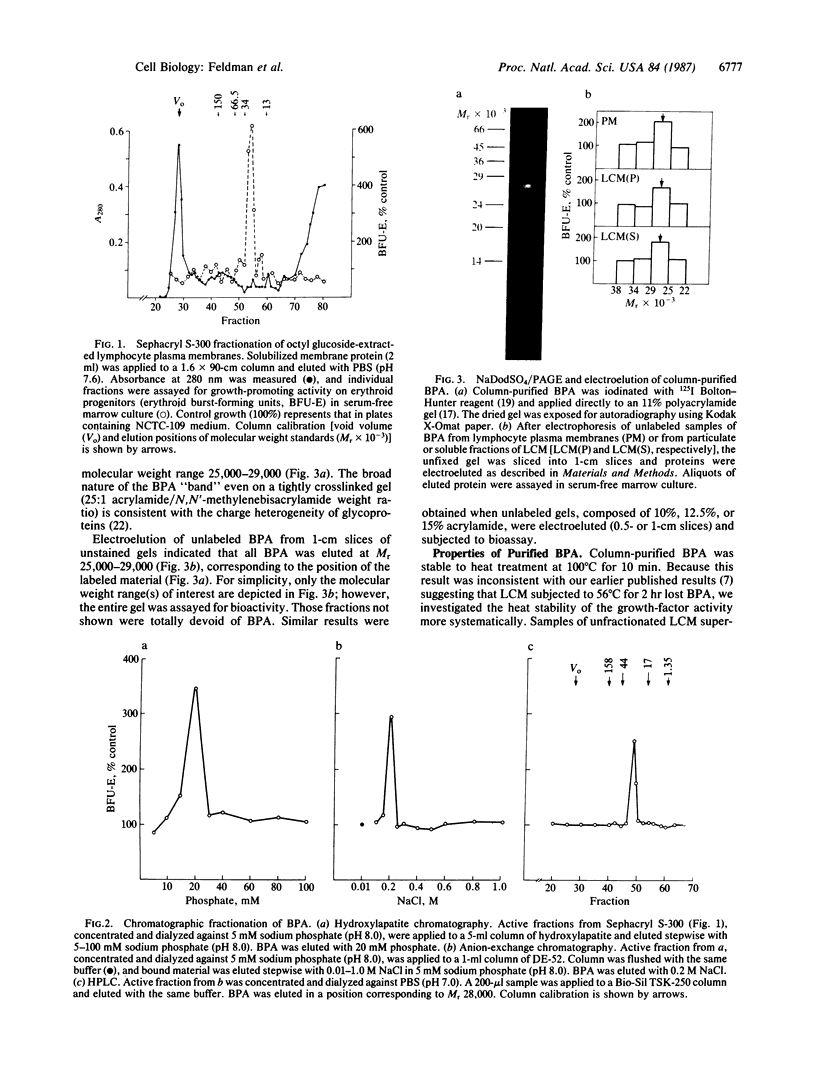
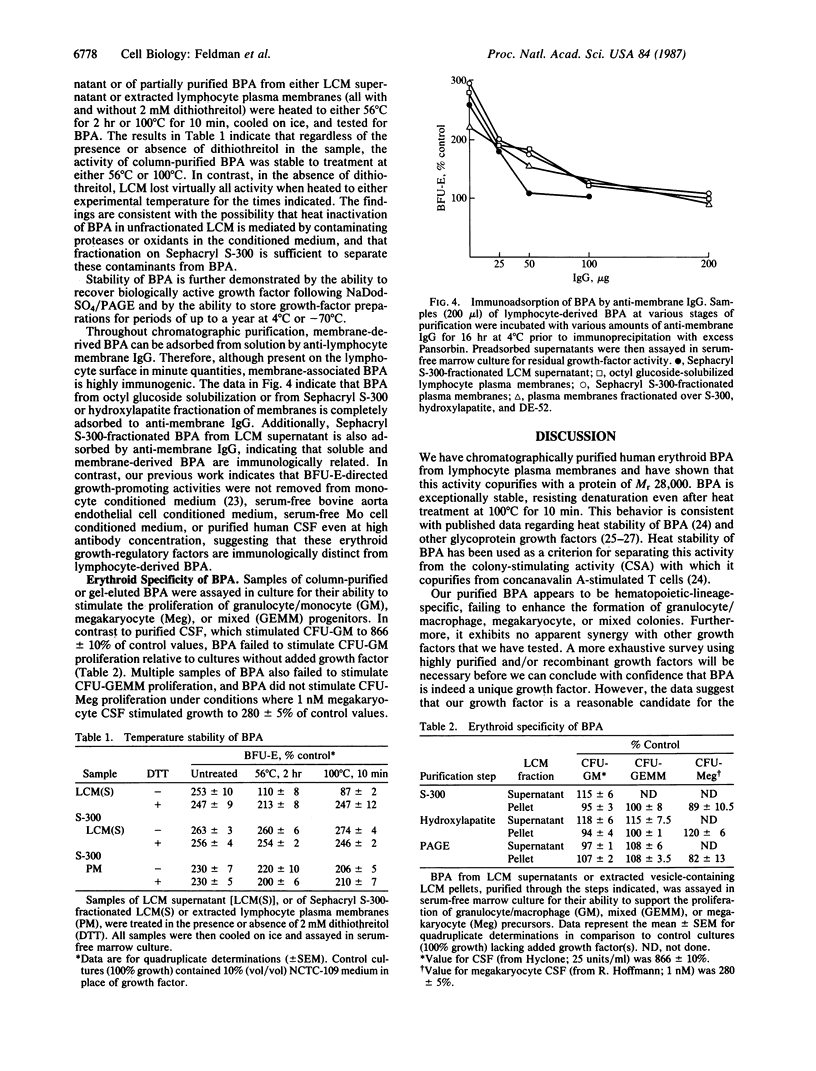
We have purified erythroid burst-promoting activity (BPA) from human lymphocyte plasma membranes by detergent extraction followed by gel-filtration, ion-exchange, and hydroxylapatite chromatography. BPA is a heat-stable integral membrane glycoprotein of Mr 28,000 by gel filtration whose activity is eluted from NaDodSO4/polyacrylamide gels as a broad band at Mr 25,000-29,000. The growth stimulator appears to be erythroid-specific, stimulating proliferation of the human erythroid burst-forming unit (BFU-E) by up to 600% of control values when tested in serum-free bone marrow culture. In contrast, it is devoid of granulocyte/macrophage colony-stimulating factor activity and has a negligible effect on the formation of human megakaryocyte and mixed hematopoietic colonies. Polyclonal anti-lymphocyte membrane IgG, which neutralizes BPA expression in culture, completely absorbs BPA from all lymphocyte-derived sources [solubilized lymphocyte plasma membranes, membrane-containing vesicles shed into lymphocyte conditioned medium (LCM) and soluble vesicle-free LCM supernatants], suggesting that soluble and membrane-derived lymphocyte BPA are antigenically related. This membrane glycoprotein may be an important mediator of proximal cellular interactions that are known to promote erythropoiesis in vitro.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aye M. T. Erythroid colony formation in cultures of human marrow: effect of leukocyte conditioned medium. J Cell Physiol. 1977 Apr;91(1):69–77. doi: 10.1002/jcp.1040910108. [DOI] [PubMed] [Google Scholar]
- Bolton A. E., Hunter W. M. The labelling of proteins to high specific radioactivities by conjugation to a 125I-containing acylating agent. Biochem J. 1973 Jul;133(3):529–539. doi: 10.1042/bj1330529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dainiak N., Cohen C. M. Regulation of human erythroid proliferation in vitro by leukocyte surface components. Ann N Y Acad Sci. 1985;459:129–142. doi: 10.1111/j.1749-6632.1985.tb20821.x. [DOI] [PubMed] [Google Scholar]
- Dainiak N., Cohen C. M. Surface membrane vesicles from mononuclear cells stimulate erythroid stem cells to proliferate in culture. Blood. 1982 Sep;60(3):583–594. [PubMed] [Google Scholar]
- Dainiak N., Feldman L., Cohen C. M. Neutralization of erythroid burst-promoting activity in vitro with antimembrane antibodies. Blood. 1985 Apr;65(4):877–885. [PubMed] [Google Scholar]
- Dainiak N., Kreczko S., Cohen A., Pannell R., Lawler J. Primary human marrow cultures for erythroid bursts in a serum-substituted system. Exp Hematol. 1985 Nov;13(10):1073–1079. [PubMed] [Google Scholar]
- Donahue R. E., Emerson S. G., Wang E. A., Wong G. G., Clark S. C., Nathan D. G. Demonstration of burst-promoting activity of recombinant human GM-CSF on circulating erythroid progenitors using an assay involving the delayed addition of erythropoietin. Blood. 1985 Dec;66(6):1479–1481. [PubMed] [Google Scholar]
- Feldman L., Cohen C. M., Dainiak N. In vitro release of physically separable factors from monocytes that exert opposing effects on erythropoiesis. Blood. 1986 May;67(5):1454–1459. [PubMed] [Google Scholar]
- Gauwerky C. E., Lusis A. J., Golde D. W. Human leukemia cell line K562 responds to erythroid-potentiating activity. Blood. 1982 Feb;59(2):300–305. [PubMed] [Google Scholar]
- Golde D. W., Bersch N., Quan S. G., Lusis A. J. Production of erythroid-potentiating activity by a human T-lymphoblast cell line. Proc Natl Acad Sci U S A. 1980 Jan;77(1):593–596. doi: 10.1073/pnas.77.1.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman J. W., Hall E. A., Miller K. L., Shinpock S. G. Interleukin 3 promotes erythroid burst formation in "serum-free" cultures without detectable erythropoietin. Proc Natl Acad Sci U S A. 1985 May;82(10):3291–3295. doi: 10.1073/pnas.82.10.3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman R., Yang H. H., Bruno E., Straneva J. E. Purification and partial characterization of a megakaryocyte colony-stimulating factor from human plasma. J Clin Invest. 1985 Apr;75(4):1174–1182. doi: 10.1172/JCI111813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jett M., Seed T. M., Jamieson G. A. Isolation and characterization of plasma membranes and intact nuclei from lymphoid cells. J Biol Chem. 1977 Mar 25;252(6):2134–2142. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Perper R. J., Zee T. W., Mickelson M. M. Purification of lymphocytes and platelets by gradient centrifugation. J Lab Clin Med. 1968 Nov;72(5):842–848. [PubMed] [Google Scholar]
- Porter P. N., Ogawa M. Characterization of human erythroid burst-promoting activity derived from bone marrow conditioned media. Blood. 1982 Jun;59(6):1207–1212. [PubMed] [Google Scholar]
- Porter P. N., Ogawa M., Leary A. G. Enhancement of the growth of human early erythroid progenitors by bone marrow conditioned media. Exp Hematol. 1980 Jan;8(1):83–88. [PubMed] [Google Scholar]
- Ross R., Vogel A. The platelet-derived growth factor. Cell. 1978 Jun;14(2):203–210. doi: 10.1016/0092-8674(78)90107-1. [DOI] [PubMed] [Google Scholar]
- Schrader J. W. The panspecific hemopoietin of activated T lymphocytes (interleukin-3). Annu Rev Immunol. 1986;4:205–230. doi: 10.1146/annurev.iy.04.040186.001225. [DOI] [PubMed] [Google Scholar]
- Sullivan R., Hesketh P. J., McCarroll L. A., Zuckerman K. S. Production of erythroid burst-promoting activity (BPA) and granulocyte-monocyte colony-stimulating activity (GM-CSA) by isolated human T-lymphocyte subpopulations. Exp Hematol. 1986 Aug;14(7):659–667. [PubMed] [Google Scholar]
- Udenfriend S., Stein S., Böhlen P., Dairman W., Leimgruber W., Weigele M. Fluorescamine: a reagent for assay of amino acids, peptides, proteins, and primary amines in the picomole range. Science. 1972 Nov 24;178(4063):871–872. doi: 10.1126/science.178.4063.871. [DOI] [PubMed] [Google Scholar]
- Westbrook C. A., Gasson J. C., Gerber S. E., Selsted M. E., Golde D. W. Purification and characterization of human T-lymphocyte-derived erythroid-potentiating activity. J Biol Chem. 1984 Aug 25;259(16):9992–9996. [PubMed] [Google Scholar]
- Wong G. G., Witek J. S., Temple P. A., Wilkens K. M., Leary A. C., Luxenberg D. P., Jones S. S., Brown E. L., Kay R. M., Orr E. C. Human GM-CSF: molecular cloning of the complementary DNA and purification of the natural and recombinant proteins. Science. 1985 May 17;228(4701):810–815. doi: 10.1126/science.3923623. [DOI] [PubMed] [Google Scholar]
- Wray W., Boulikas T., Wray V. P., Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981 Nov 15;118(1):197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]



