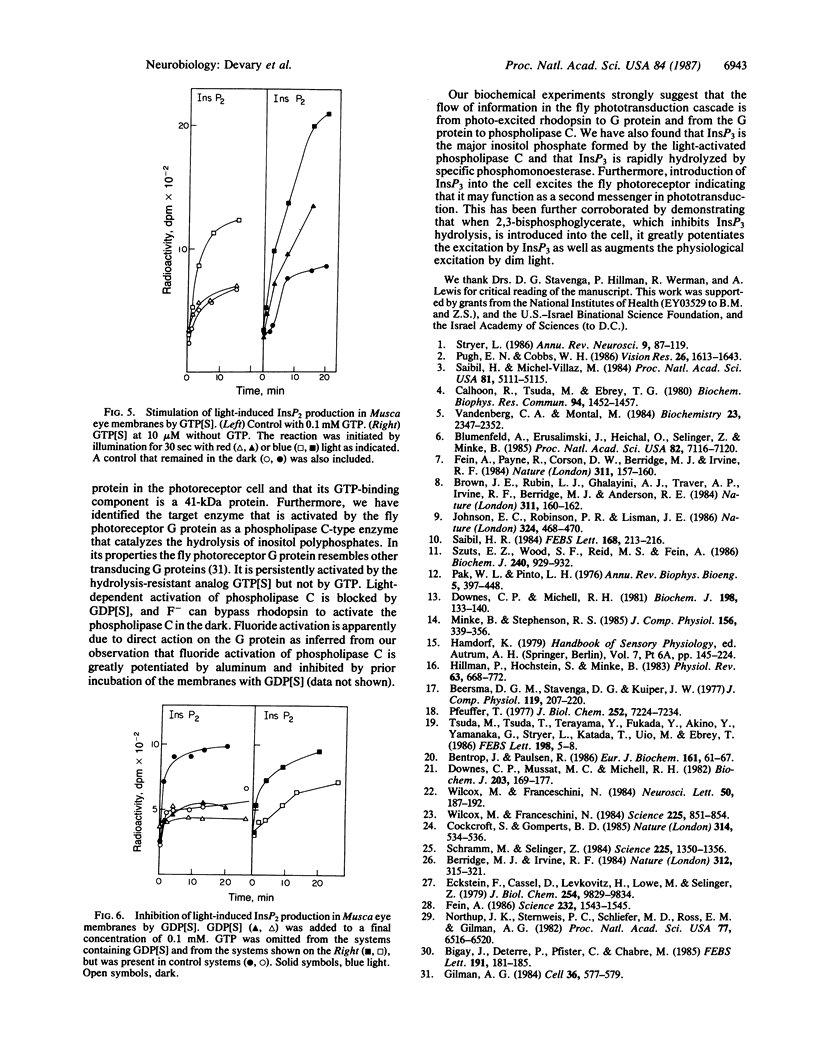
Abstract
Fly photoreceptor membranes were used to test the effect on defined biochemical reactions of light and of compounds causing photoreceptor excitation. Complementary electrophysiological studies examined whether putative second messengers excite the fly photoreceptor cells. This analysis revealed the following sequence of events: photoexcited rhodopsin activates a G protein by facilitating GTP binding. The G protein then activates a phospholipase C that generates inositol trisphosphate, which in turn acts as an internal messenger to bring about depolarization of the photoreceptor cell. Binding assays of GTP analogs and measurements of GTPase activity showed that there are 1.6 million copies of G protein per photoreceptor cell. The GTP binding component is a 41-kDa protein, and the light-activated GTPase is dependent on photoconversion of rhodopsin to metarhodopsin. Analysis of phospholipase C activity revealed that this enzyme is under stringent control of the G protein, that the major product formed is inositol trisphosphate, and that this product is rapidly hydrolyzed by a specific phosphomonoesterase. Introduction of inositol trisphosphate to the intact photoreceptor cell mimics the effect of light, and bisphosphoglycerate, which inhibits inositol trisphosphate hydrolysis, enhances the effects of inositol trisphosphate and of dim light. The interaction of photoexcited rhodopsin with a G protein is thus similar in both vertebrate and invertebrate photoreceptors. These G proteins, however, activate different photoreceptor enzymes: phospholipase C in invertebrates and cGMP phosphodiesterase in vertebrates.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bentrop J., Paulsen R. Light-modulated ADP-ribosylation, protein phosphorylation and protein binding in isolated fly photoreceptor membranes. Eur J Biochem. 1986 Nov 17;161(1):61–67. doi: 10.1111/j.1432-1033.1986.tb10124.x. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Bigay J., Deterre P., Pfister C., Chabre M. Fluoroaluminates activate transducin-GDP by mimicking the gamma-phosphate of GTP in its binding site. FEBS Lett. 1985 Oct 28;191(2):181–185. doi: 10.1016/0014-5793(85)80004-1. [DOI] [PubMed] [Google Scholar]
- Blumenfeld A., Erusalimsky J., Heichal O., Selinger Z., Minke B. Light-activated guanosinetriphosphatase in Musca eye membranes resembles the prolonged depolarizing afterpotential in photoreceptor cells. Proc Natl Acad Sci U S A. 1985 Oct;82(20):7116–7120. doi: 10.1073/pnas.82.20.7116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. E., Rubin L. J., Ghalayini A. J., Tarver A. P., Irvine R. F., Berridge M. J., Anderson R. E. myo-Inositol polyphosphate may be a messenger for visual excitation in Limulus photoreceptors. Nature. 1984 Sep 13;311(5982):160–163. doi: 10.1038/311160a0. [DOI] [PubMed] [Google Scholar]
- Calhoon R., Tsuda M., Ebrey T. G. A light-activated GTPase from octopus photoreceptors. Biochem Biophys Res Commun. 1980 Jun 30;94(4):1452–1457. doi: 10.1016/0006-291x(80)90582-3. [DOI] [PubMed] [Google Scholar]
- Cockcroft S., Gomperts B. D. Role of guanine nucleotide binding protein in the activation of polyphosphoinositide phosphodiesterase. Nature. 1985 Apr 11;314(6011):534–536. doi: 10.1038/314534a0. [DOI] [PubMed] [Google Scholar]
- Downes C. P., Michell R. H. The polyphosphoinositide phosphodiesterase of erythrocyte membranes. Biochem J. 1981 Jul 15;198(1):133–140. doi: 10.1042/bj1980133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downes C. P., Mussat M. C., Michell R. H. The inositol trisphosphate phosphomonoesterase of the human erythrocyte membrane. Biochem J. 1982 Apr 1;203(1):169–177. doi: 10.1042/bj2030169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckstein F., Cassel D., Levkovitz H., Lowe M., Selinger Z. Guanosine 5'-O-(2-thiodiphosphate). An inhibitor of adenylate cyclase stimulation by guanine nucleotides and fluoride ions. J Biol Chem. 1979 Oct 10;254(19):9829–9834. [PubMed] [Google Scholar]
- Fein A. Blockade of visual excitation and adaptation in Limulus photoreceptor by GDP-beta-S. Science. 1986 Jun 20;232(4757):1543–1545. doi: 10.1126/science.3487116. [DOI] [PubMed] [Google Scholar]
- Fein A., Payne R., Corson D. W., Berridge M. J., Irvine R. F. Photoreceptor excitation and adaptation by inositol 1,4,5-trisphosphate. Nature. 1984 Sep 13;311(5982):157–160. doi: 10.1038/311157a0. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. G proteins and dual control of adenylate cyclase. Cell. 1984 Mar;36(3):577–579. doi: 10.1016/0092-8674(84)90336-2. [DOI] [PubMed] [Google Scholar]
- Hillman P., Hochstein S., Minke B. Transduction in invertebrate photoreceptors: role of pigment bistability. Physiol Rev. 1983 Apr;63(2):668–772. doi: 10.1152/physrev.1983.63.2.668. [DOI] [PubMed] [Google Scholar]
- Johnson E. C., Robinson P. R., Lisman J. E. Cyclic GMP is involved in the excitation of invertebrate photoreceptors. Nature. 1986 Dec 4;324(6096):468–470. doi: 10.1038/324468a0. [DOI] [PubMed] [Google Scholar]
- Northup J. K., Sternweis P. C., Smigel M. D., Schleifer L. S., Ross E. M., Gilman A. G. Purification of the regulatory component of adenylate cyclase. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6516–6520. doi: 10.1073/pnas.77.11.6516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pak W. L., Pinto L. H. Genetic approach to the study of the nervous system. Annu Rev Biophys Bioeng. 1976;5:397–448. doi: 10.1146/annurev.bb.05.060176.002145. [DOI] [PubMed] [Google Scholar]
- Pfeuffer T. GTP-binding proteins in membranes and the control of adenylate cyclase activity. J Biol Chem. 1977 Oct 25;252(20):7224–7234. [PubMed] [Google Scholar]
- Pugh E. N., Jr, Cobbs W. H. Visual transduction in vertebrate rods and cones: a tale of two transmitters, calcium and cyclic GMP. Vision Res. 1986;26(10):1613–1643. doi: 10.1016/0042-6989(86)90051-9. [DOI] [PubMed] [Google Scholar]
- Saibil H. R. A light-stimulated increase of cyclic GMP in squid photoreceptors. FEBS Lett. 1984 Mar 26;168(2):213–216. doi: 10.1016/0014-5793(84)80248-3. [DOI] [PubMed] [Google Scholar]
- Saibil H. R., Michel-Villaz M. Squid rhodopsin and GTP-binding protein crossreact with vertebrate photoreceptor enzymes. Proc Natl Acad Sci U S A. 1984 Aug;81(16):5111–5115. doi: 10.1073/pnas.81.16.5111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schramm M., Selinger Z. Message transmission: receptor controlled adenylate cyclase system. Science. 1984 Sep 21;225(4668):1350–1356. doi: 10.1126/science.6147897. [DOI] [PubMed] [Google Scholar]
- Stryer L. Cyclic GMP cascade of vision. Annu Rev Neurosci. 1986;9:87–119. doi: 10.1146/annurev.ne.09.030186.000511. [DOI] [PubMed] [Google Scholar]
- Szuts E. Z., Wood S. F., Reid M. S., Fein A. Light stimulates the rapid formation of inositol trisphosphate in squid retinas. Biochem J. 1986 Dec 15;240(3):929–932. doi: 10.1042/bj2400929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg C. A., Montal M. Light-regulated biochemical events in invertebrate photoreceptors. 2. Light-regulated phosphorylation of rhodopsin and phosphoinositides in squid photoreceptor membranes. Biochemistry. 1984 May 22;23(11):2347–2352. doi: 10.1021/bi00306a004. [DOI] [PubMed] [Google Scholar]
- Wilcox M., Franceschini N. Illumination induces dye incorporation in photoreceptor cells. Science. 1984 Aug 24;225(4664):851–854. doi: 10.1126/science.6206565. [DOI] [PubMed] [Google Scholar]
- Wilcox M., Franceschini N. Stimulated drug uptake in a photoreceptor cell. Neurosci Lett. 1984 Sep 7;50(1-3):187–192. doi: 10.1016/0304-3940(84)90484-1. [DOI] [PubMed] [Google Scholar]