Abstract
Background
Several malignancies are known to exhibit a “field-effect” whereby regions beyond tumor boundaries harbor histological or molecular changes that are associated with cancer. We sought to determine if histologically benign prostate epithelium collected from men with prostate cancer exhibits features indicative of pre-malignancy or field effect.
Methods
Prostate needle biopsies from 15 men with high grade(Gleason 8–10) prostate cancer and 15 age- and BMI-matched controls were identified from a biospecimen repository. Benign epithelia from each patient were isolated by laser capture microdissection. RNA was isolated, amplified, and used for microarray hybridization. Quantitative PCR(qPCR) was used to determine the expression of specific genes of interest. Alterations in protein expression were analyzed through immunohistochemistry.
Results
Overall patterns of gene expression in microdissected benign-associated benign epithelium (BABE) and cancer-associated benign epithelium (CABE) were similar. Two genes previously associated with prostate cancer, PSMA and SSTR1, were significantly upregulated in the CABE group(FDR <1%). Expression of other prostate cancer-associated genes, including ERG, HOXC4, HOXC5 and MME, were also increased in CABE by qRT-PCR, although other genes commonly altered in prostate cancer were not different between the BABE and CABE samples. The expression of MME and PSMA proteins on IHC coincided with their mRNA alterations.
Conclusion
Gene expression profiles between benign epithelia of patients with and without prostate cancer are very similar. However, these tissues exhibit differences in the expression levels of several genes previously associated with prostate cancer development or progression. These differences may comprise a field effect and represent early events in carcinogenesis.
Keywords: Prostate cancer, gene regulation, carcinogenesis
INTRODUCTION
Prostate cancer is a multifocal disease, with 67–96% of radical prostatectomy specimens containing more than one focus of disease (1–7). Among several explanations, this multifocality suggests that factors which underlie the development of prostate cancer may affect the entire prostate or at least separate foci within the same prostate. Molecular changes occurring as a result of carcinogenic stimuli may be detected throughout the prostate, similar to other solid tumors, in what has been termed a “field effect” or “field cancerization” (8, 9). Histologic studies have demonstrated changes in the nucleus of benign epithelia adjacent to cancer which are not detectable by light microscopy (10). Additionally, several studies have provided evidence that histologically benign tissue adjacent to prostate cancer harbors molecular changes in both normal adjacent epithelium and stroma (11–16), however these studies have key design issues that limit their interpretation.
The molecular characterization of normal prostate epithelium involves several important methodologic issues with substantial implications for data interpretation. First, many studies consider the histologically normal tissue either immediately adjacent to prostate cancer or within the cancerous prostate gland as the reference “normal” tissue, and this practice is questionable in light of potential field effect changes. Second, the tissue adjacent to cancer may contain changes that are intimately associated with carcinogenesis and lend insight into mechanisms of cancer initiation. Lastly, the ideal reference “normal” prostate tissue would originate from age-matched men with no evidence of prostate cancer, which, given the high prevalence of prostate cancer, would require, at minimum, a prostate biopsy to rule out occult cancer. Previous studies have been hampered by this lack of appropriate reference control tissues.
The objective of this study was to compare the transciptomes from benign prostatic epithelia of men with high grade prostate cancer to benign prostatic epithelia of age- and BMI-matched men who have undergone multiple negative prostate biopsies without evidence of prostate cancer. Differences in gene profiles may identify important pathways that are involved in the earliest stages of prostate cancer development. These results may provide targets for cancer prevention or biomarkers for diagnostics in early detection and improve our understanding of the early events in prostate carcinogenesis.
MATERIALS AND METHODS
Study Design and Tissue Acquisition
Between May 2003 and July 2006, 367 patients undergoing prostate needle biopsy for suspicion of prostate cancer (elevated serum prostate-specific antigen [PSA] and/or abnormal digital rectal examination) consented to acquisition of four extra prostate biopsies for research studies, in addition to a 12-core biopsy regimen for standard pathologic examination. Patients who underwent previous hormonal therapy, chemotherapy, radiation therapy, treatment with 5-alpha reductase inhibitors, or testosterone replacement were excluded. Each research biopsy specimen was immediately embedded in polyethylene glycol freezing media (Tissue-Tek OCT Compound, Sakura Finetek, Torrance, CA), placed in isopentane that was precooled in liquid nitrogen, and stored at −80°C. All protocols for tissue acquisition and processing were approved by the local Institutional Review Board. As part of this protocol, each patient underwent written informed consent for research tissue acquisition prior to prostate needle biopsy. Among the 367 patients, 135 (37%) were diagnosed with prostate cancer; of these patients 21 (16%) were diagnosed with high-grade (Gleason 8–10) prostate cancer on biopsy. 189 patients (51%) had no evidence of cancer on prostate biopsy; 56 of these patients (30%) had a second or third set of biopsies that also showed no evidence of carcinoma. The remaining 43 patients (12%) had atypical small acinar proliferation (ASAP) or high grade prostatic intraepithelial neoplasia (PIN) on prostate biopsy.
Target sample size for this study was set to 20 samples per group, which would yield a power of 80% to detect 2-fold differences in expression of genes with a standard deviation of gene expression of 0.75 at a false discovery rate set to 5%. In a previous study on the effect of tissue ischemia on prostatic epithelial gene expression (17), only 10% of genes had a standard deviation ≥ 0.75; therefore power for the majority of genes would be higher than 80%. The achieved sample size was only 15 per group, because several of the samples from men with high-grade cancer had little or no normal epithelia in the prostate biopsy specimen available for analysis. With this reduced sample size, the power to detect a 2-fold difference in expression of genes with standard deviations of 0.75, 0.50 and 0.25 was 65%, 81% and >99%, respectively. Our study cohort was composed of 30 total patients: 1.) Control group: Benign prostate associated benign epithelium (BABE) patients, n=15. Fifteen patients were chosen from the 56 patients with multiple negative prostate biopsies. Men with multiple negative prostate biopsies were used to diminish the likelihood of false negative results from a single biopsy session. 2.) Cases: Cancer associated benign epithelium (CABE) patients, n=15. Fifteen patients were chosen from the 21 patients with high grade (Gleason sum 8–10) prostate cancer. Eight of these 15 men with high grade cancer on biopsy subsequently underwent retropubic radical prostatectomy; seven had high volume, bilateral, high-grade disease and one patient was down-graded to Gleason 4+3 on final pathology. To prevent potential bias from prostate cancer risk factors that may also affect prostate gene expression, patients were stratified based on age (5-year groups) and BMI (< 30 or ≥ 30) and matched between two patient groups (CABE vs BABE).
Tissue Processing and Laser Capture Microdissection
Eight micrometer sections of the frozen biopsy tissue from the 15 cases and 15 controls were placed on slides and stained with hematoxylin and eosin. We used needle biopsy cores for these studies, as we have previously shown that surgical resection of the prostate gland can introduce significant alterations in the expression of a substantial number of genes (17). The consistency of this approach also eliminated methodological differences in comparing tissues collected from various forms of surgical removal, e.g. organ-donor prostate tissue vs. radical prostatectomy prostate tissue. A board-certified pathologist (BSK) reviewed the slides to ensure that all cancer, PIN, ASAP, inflammation and atrophy were excluded from the captured areas. Approximately two thousand cells from histologically benign epithelial glands were captured using laser capture microdissection (LCM). Representative pictures were saved before, during and after dissection for pathologic confirmation.
Captured individual specimens were lysed, and RNA was isolated using the Arcturus PicoPure RNA Isolation Kit (Molecular Devices, Sunnyvale, CA) according to manufacturer’s protocol. Samples were treated with Rnase-free Dnase (Qiagen, Valencia, CA) to minimize DNA contamination. This RNA was converted to a cDNA library and amplified and amino-allyl labeled using the TransPlex WTA Kit (Sigma-Aldrich Corp, St. Louis, MO) and its purity was confirmed by spectrophotometric analysis.
To provide a reference standard RNA for use on cDNA microarrays, we isolated total RNA from LNCaP, DU145, PC3, and CWR22 cell lines (American Type Culture Collection, Manassas, VA) growing at log phase in dye-free RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS; Invitrogen, Carlsbad, CA). RNA was purified using Trizol (Invitrogen) following the manufacturer’s protocol. This RNA was amplified using the TransPlex WTA Kit following procedures used for the study.
Microarray Hybridization and Analysis
Two micrograms of amplified amino-allyl cDNA from each was labeled with Cy3 fluorescent dye, and the reference standard was labeled with Cy5 fluorescent dye, both as described previously (18). Samples were then hybridized to Agilent 44K whole human genome expression oligonucleotide microarray slides (Agilent Technologies, Inc., Santa Clara, CA) following the manufacturer’s suggested protocols. Fluorescence array images were collected for both Cy3 and Cy5 using the Agilent DNA microarray fluorescent scanner G2565BA, and Agilent Feature Extraction software was used to grid, extract image intensities, and normalize data. Spots of poor quality, as determined by the software, were removed from further analysis. The microarray data for this experiment has been deposited in the Gene Expression Omnibus (GEO) database, available at www.ncbi.nlm.nih.gov/geo, under accession number GSEXXXXX.
To compare the overall expression patterns of all radical prostatectomy cancer samples to their patient-matched normal samples, the filtered log-ratio measurements were analyzed by using the Significance Analysis of Microarrays (SAM) procedure (www.stat.stanford.edu/∼tibs/SAM) (19). In this analysis, an unpaired two-sample t-test was used to determine which genes were significantly differentially expressed between cancer-associated benign epithelium and benign-associated benign epithelium, and initial determination of significantly altered genes was determined using false discovery rate of 5% (q-value <0.05). Prior to analysis, probes which had average signal below 300 or greater than 30% missing values were excluded from further analysis. Hierarchical clustering of the data revealed subtle clustering on date of microarray scan, potentially a result of covariates which could not be completely accounted for in the analysis. This effect reflected in the cluster analysis potentially diminished the number of significant genes between the CABE and BABE groups found in this study.
Pathway Analysis
Further evaluation of the microarray data was performed using Gene Set Enrichment Analysis (GSEA) (20) and Ingenuity Pathway Analysis. GSEA was used to compare expression differences between BABE and CABE in the prostate cancer molecular signature identified in a meta-analysis of microarray data on prostate cancer gene expression(21). Two separate gene sets based on this meta-analysis were created, one with 40 genes identified as increased in prostate cancer relative to benign tissue, and another with 39 genes decreased in prostate cancer (Supplemental Table 2). Genes differentially expressed between CABE and BABE with p-value <0.05 were ranked based on their relative expression in CABE vs. BABE. GSEA then determined if there was significant relative enrichment in either CABE or BABE in the expression of the genes listed in each gene set. Significance was determined at FDR of 0.25 as recommended by GSEA User Guide (http://www.broadinstitute.org/gsea/doc/GSEAUserGuideFrame.html?Interpreting_GSEA).
Ingenuity Pathway Analysis (Ingenuity Systems, www.ingenuity.com) was utilized to determine if any biologic functions or pathways were altered between the CABE and BABE groups. A dataset containing the genes altered at p<0.05 level between the CABE and BABE groups was uploaded into the application. Each identifier was mapped to its corresponding object in Ingenuity’s Knowledge Base. The functional analysis identified the biologic functions that were most significant in the dataset. The network analysis identified networks containing greatest number of affected molecules from the dataset. Right-tailed Fisher’s exact test was used to calculate the p-values for both the functional analysis and the identification of pathways, with all those referenced in the text and in the Supplemental Data having p<0.05.
Quantitative Reverse-Transcription PCR (qRT-PCR)
We used qRT-PCR to validate microarray results for selected genes. Primers specific for the genes of interest were designed using the web-based primer design service Primer3 provided by the Whitehead Institute for Biomedical Research (http://fokker.wi.mit.edu/cgi-bin/primer3/primer3_www.cgi). Primer sequences are listed in Supplementary Data Table 1. We determined acceptable performance characteristics of the PCR primers using normal human prostate cDNA, Biolase Taq polymerase (Bioline Inc., Randolph, MA), and the GeneAmp PCR system 9700 (Applied Biosystems, Foster City, CA) as previously described.(17). The WTA amplified cDNA was used as a template for the reaction, and each patient sample was individually analyzed for each gene of interest. Relative quantification of gene expression by quantitative PCR (40 cycles of 60°C annealing, 72°C extension, and 95°C melting) was performed on a 7700 Sequence Detector using SYBR Green Master mix and gene-specific primers following the manufacturer’s recommendations. Gene expression differences between the BABE and CABE groups were determined by student’s t-test using Graphpad Prism version 5.00 (Graphpad Software, San Diego, CA).
TMPRSS2:ERG fusion PCR
The WTA amplified cDNA was used as a template for PCR to examine for evidence of TMPRSS2 and ERG gene fusion using primers to the first exon of TMPRSS2 (T1) and the fourth exon of ERG (E4) (Supplementary Data). Total RNA from VCap cells was converted to cDNA using Superscript II (Invitrogen) according to manufacturer’s protocol. WTA amplified cDNA was also generated from VCap cells, and both total VCap cDNA and WTA amplified VCap cDNA were used as positive controls. Biolase Taq enzyme (Bioline, Randolph,MA) was used for the reaction according to manufacturer’s specifications with 10 pM of each primer. Samples were denatured at 94°C for 3 min, followed by 35 cycles of replication (94°C for 45 seconds, 57°C for 45 seconds and 72°C for 1.5 minutes) and final extension at 72°C for 10 minutes. Samples were analyzed by 1.5% agarose gel electrophoresis.
Immunohistochemistry
Five micron sections of prostate biopsy tissue from a random subset of cases and controls were used for immunohistochemical (IHC) analysis of PSMA and CD10 expression. Four cases and four controls were used for IHC. Target Retrieval Solution pH 9 EDTA (DAKO, Denmark) was used for antigen retrieval, first for 20 minutes in a vegetable steamer, followed by a 20 minute cool down at room temperature. Endogenous peroxidase activity was blocked using a 3% hydrogen peroxide solution for 8 minutes. Sections were then blocked for 10 minutes in 15% swine serum, and primary antibodies were applied for 1 hour at room temperature. Both PSMA (DAKO) and CD10 (Zymed, San Francisco, CA) were used at a final dilution of 1:200. This antibody dilution is within the linear range of the antibody concentration curve. Sections were incubated for 30 minutes with Envision secondary Reagent (DAKO) and developed with 2 applications of DAB horse radish peroxidase substrate and chromagen for 4 minutes each. Slides were counterstained with hematoxylin (DAKO), dehydrated, and coverslipped.
The scoring was performed on a 4-tiered scale. Both staining intensity and the percentage of positive glands were included in the overall score for each case. The study pathologist (BSK) was blinded to cancer status while performing the scoring. The scores from 2 slides from the same block that were stained on separate days were averaged. In addition, if multiple blocks existed from the same patient, the average score was calculated across the blocks. Mean IHC scores between the groups were then compared using student’s t-test.
RESULTS
Patient Demographics and Clinical Characteristics
The characteristics of the two populations used in this study are given in Table 1. The two groups were similar in age, BMI and family history of prostate cancer. Serum PSA levels were significantly higher in cancer, and the two groups also differed in terms of race, with four African Americans and one Pacific Islander in the CABE group, while all BABE patients were Caucasian. One control patient was removed from the study as he developed prostate cancer during the period of data analysis.
Table 1.
Patient demographics
| Control | Cancer | p-value | ||
|---|---|---|---|---|
| Age (yrs) | 64.8 | 67 | 0.36 | |
| BMI | 28 | 26.8 | 0.37 | |
| Mean PSA (ng/ml) | 10.6 | 18.4 | 0.04 | |
| Family History | 0 | 3 | 0.11 | |
| Race | White | 15 | 10 | 0.05 |
| African American | 0 | 4 | ||
| Pacific Islander | 0 | 1 | ||
Gene Expression Profiles from Benign Prostate Epithelium Differ Depending on Cancer Status
BABE and CABE samples had similar overall gene expression profiles. Table 2 gives the distribution of differences in genes expression between CABE and BABE specimens for 17,933 cDNAs. Expression differences in the table are categorized symmetrically above and below zero in units of log2, which are also shown as relative expression on a linear scale. In approximately 98% of transcripts, the relative expression of normal epithelium between CABE and BABE specimens was between 0.67 and 1.49. However, there were several transcripts with greater expression differences.
Table 2.
Distribution of Differences in Expression of 17,933 Transcripts Comparing Cancer-Associated Normal Epithelium(CABE) to Benign-Associated Normal Epithelium
| Absolute Difference (CABE/Benign log2) |
Relative Difference (CABE/Benign ratio) |
Total | |
|---|---|---|---|
| No. | % | ||
| > + 1.58 | > 3.0 | 1 | 0.01 |
| + (1.32–1.57) | 2.50 – 2.99 | 3 | 0.02 |
| + (1.00–1.31) | 2.00 – 2.49 | 11 | 0.06 |
| + (0.58–0.99) | 1.50 – 1.99 | 60 | 0.33 |
| + (0.00v0.57) | 1.00 – 1.49 | 8529 | 47.56 |
| − (0.01–0.57) | 0.67–0.99 | 9266 | 51.67 |
| − (0.58–0.99) | 0.50–0.66 | 52 | 0.29 |
| − (1.00–1.31) | 0.40–0.49 | 7 | 0.04 |
| − (1.32–1.57) | 0.34–0.39 | 1 | 0.01 |
| < −1.58 | <− 0.33 | 3 | 0.02 |
| Total | 17933 | 100 | |
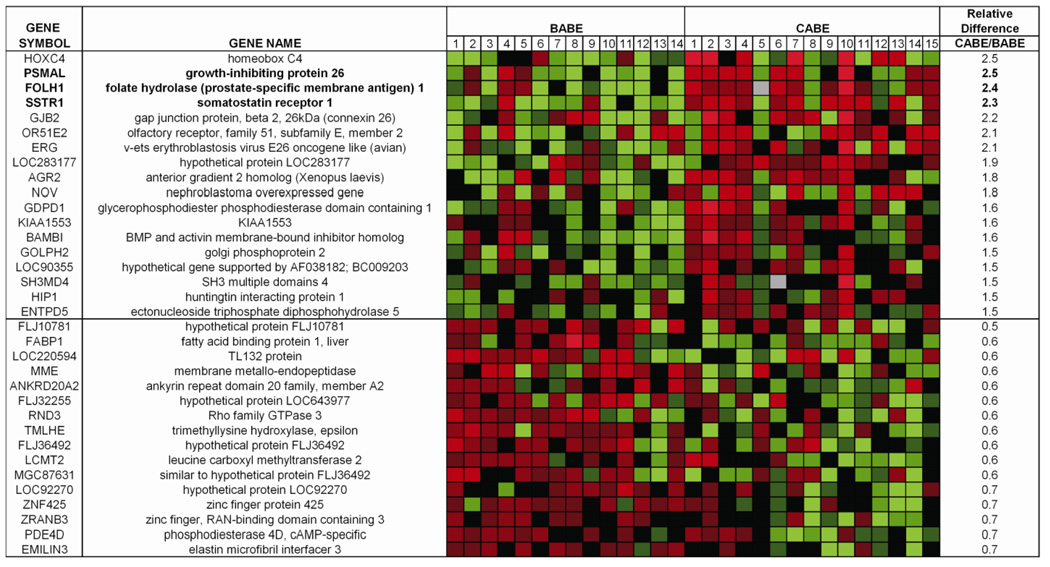
Of the 17,933 array probes with expression above background levels and with at least a 2-fold difference from the mean in at least 2 samples, 3 genes (FOLH1, PSMAL and SSTR1) were significantly different between the two groups after correcting for multiple comparisons using the Significance Analysis of Microarrays (SAM) algorithm (q<0.01). Although no other genes were significantly different by q value, many genes differed by t-test (p≤ 0.05) and had differences > 1.5 fold between the two groups (Figure 1). These included a number of genes previously implicated in prostate carcinogenesis, including ERG, CD 10/ MME and Hox C4 (22–24), as have FOLH1 and SSTR1 (25–31). The array data were not significantly different when only Caucasian subjects were included in the analysis (Supplemental Figure 3). For reference, we have included the races of the individual CABE patients in the supplemental data (Supplemental Table 4).
Figure 1. Differential gene expression of BABE versus CABE patients.
Eighteen unique genes were up-regulated ≥1.5 fold with p < 0.05 in a paired two sample t-test. Sixteen unique genes were down-regulated ≥1.5 fold with p < 0.05 in a paired two sample t-test. Genes with FDR <1% between the two groups are shown in bold text. Relative Difference refers to expression in CABE patients relative to BABE patients.
Pathway Analysis
Although there were few statistical differences in expression by microarray analysis, specific pathway analysis may provide insight into potential global gene expression differences. Gene set enrichment analysis (GSEA) analysis was performed specifically examining the subset of genes found to be over- and under- expressed in prostate cancer in a recent meta-analysis (21). This analysis found significant enrichment of the prostate cancer over-expressed genes in the CABE group, while it found significant enrichment of the prostate cancer under-expressed genes in the BABE group (Supplementary Data Table 2 and 3). Further analysis of this gene set using Ingenuity Pathways analysis found enrichment of cancer-associated genes and pathways in the group of differentially expressed genes with p-value <0.05 (Supplementary Data Figure 1). (21) Taken together, these analyses suggested that there exists a gene expression fingerprint of cancer within the normal epithelium adjacent to prostate cancer.
Confirmation of Cancer-Associated Genes that are Differentially Expressed in Benign Epithelium
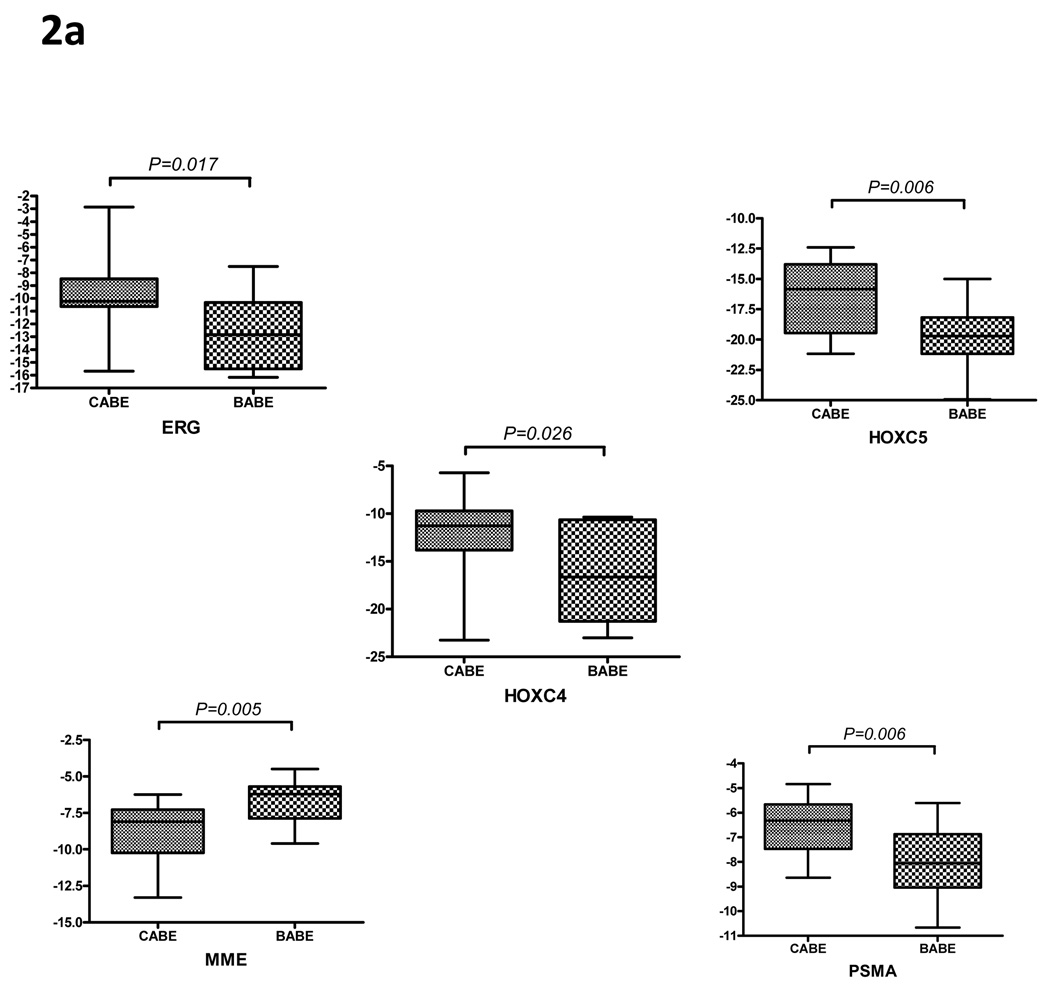
Microarray-based analysis of gene expression in BABE and CABE samples shown in Figure 1 suggest that molecular pathways associated with invasive prostate cancer were also altered in benign epithelium from prostates harboring high-grade malignant cells. (Figure 1) Using qRT-PCR, we confirmed the significantly increased expression of ERG (7-fold), HOXC4 (18-fold), HOXC5 (8-fold) and FOLH1 (3-fold) in benign epithelium acquired from prostate glands known to contain prostate adenocarcinoma (CABE) compared with benign epithelium from prostates in which no cancer was detected on multiple biopsies (BABE). We also determined that CD10/MME, whose expression is decreased in HGPIN and prostate cancer (24), was also decreased 4-fold in CABE relative to BABE samples by qRT-PCR. (Figure 2A, p<0.05 for all genes)
Figure 2. QPCR of prostate cancer genes in BABE versus CABE patients.
Quantitative reverse transcription polymerase chain reaction was used to examine the expression of (A) prostate cancer genes which have altered expression in CABE versus BABE patients based on the microarray data and (B) prostate cancer associated genes not altered between the two groups on microarray. Cycle threshold, denoted on the y-axis, is detected cycle number relative to housekeeping gene RPL13A(dCT). The data is expressed in the graphs as −1dCT to visually reflect the differences in expression between the two groups, thus in this format lower mean level corresponds to decreased gene expression.
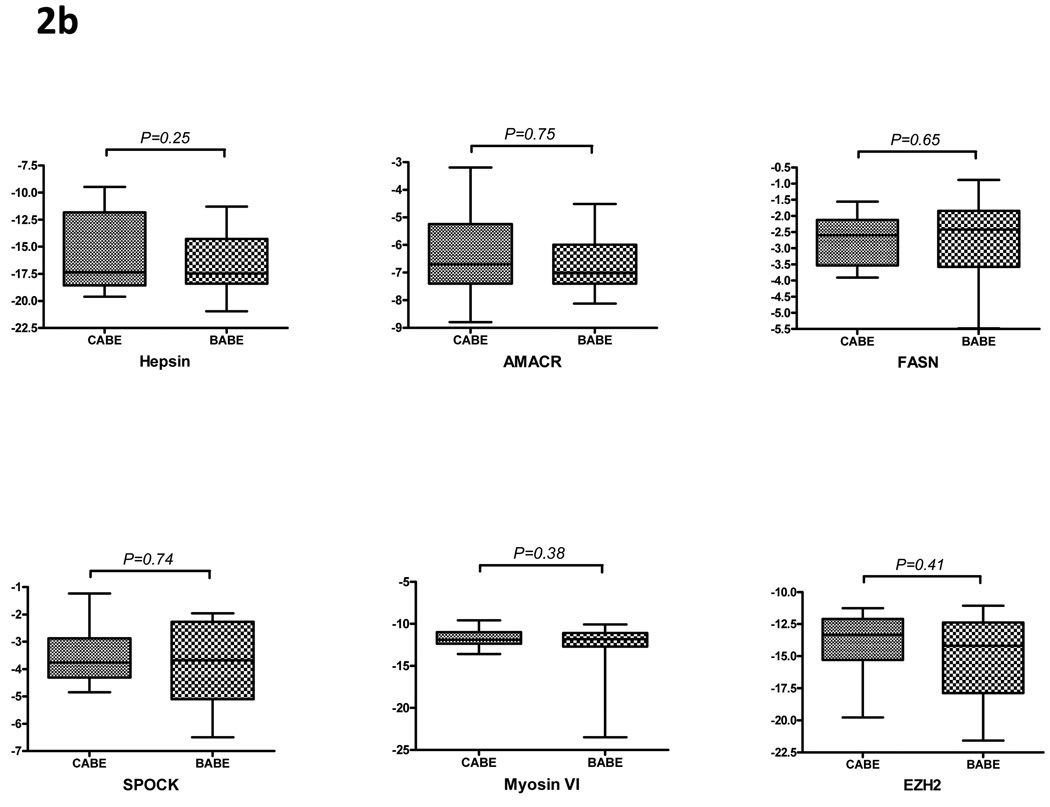
As several of these gene alterations correspond to those previously reported in cancer, one possible confounder of these findings would be contamination of laser-captured CABE with surrounding neoplastic epithelia or debris. Thus we evaluated several genes whose expression has been strongly increased in prostate cancer, including alpha-methylacyl CoA racemase (AMACR), hepsin (HPN), fatty acid synthase (FASN), myosin VI (MYO 6), SPOCK, TPD52 and EZH2. Quantitative measurements of these transcripts by qRT-PCR determined that none of these genes were significantly different in BABE versus CABE (Figure 2B), suggesting that adjacent cancer contamination was unlikely.
Immunohistochemistry
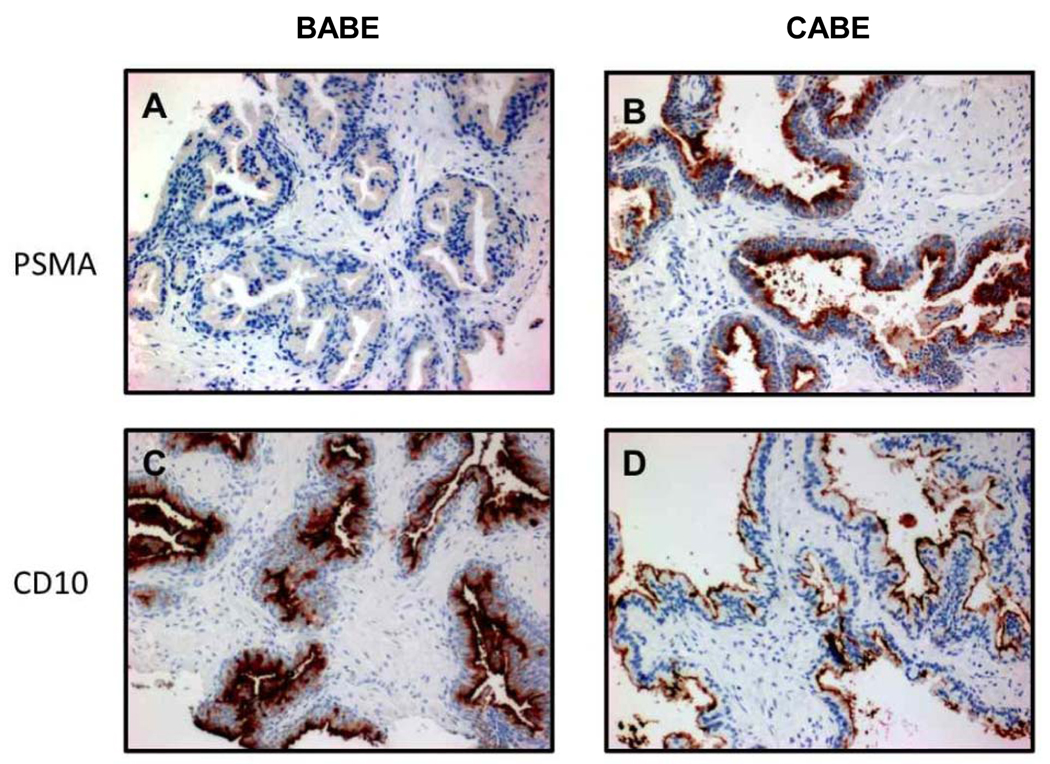
There was a significant difference in protein expression of PSMA and CD10 in BABE compared to CABE (Figure 3 and Table 3). The mean IHC staining intensity score for PSMA expression in BABE was 0.88 ± 0.69, while it was 1.98 ± 1.04 in CABE, p = 0.003. Conversely, the mean IHC staining intensity score for CD10 was 2.88 ± 0.35 in CABE and 1.43 ± 1.12 in BABE, p = 0.003. PSMA and CD10 proteins were localized at the luminal aspect of the prostate epithelium, consistent with previous reports (27, 32) and confirms the specific reactivity of the antibody clones. These IHC results confirm the transcript gene expression data, which demonstrate an increased PSMA and decreased CD10 expression in CABE compared to BABE.
Figure 3. PSMA and CD10 expression in BABE and CABE.
Representative immunohistochemical staining of formalin-fixed, paraffin embedded tissue with PSMA and CD10 antibodies. Panels A and C are from the same biopsy from BABE, and panels B and D are from the same biopsy of CABE. Tissues in panels A and B were stained with the PSMA antibody, and panels C and D were stained with CD 10.
Table 3.
PSMA and CD10 immunohistochemistry scores
| Mean IHC Score ± standard deviation | |||
|---|---|---|---|
| BABE (n=4) | CABE (n=4) | p-value | |
| PSMA | 0.88 ± 0.69 | 1.98 ± 1.04 | 0.003 |
| CD10 | 2.88 ± 0.35 | 1.43 ± 1.12 | 0.003 |
TMPRSS2:ERG Fusion analysis
Recent studies have demonstrated that a fusion between the TMPRSS2 gene and the ERG gene is a frequent event in prostate cancer, and that this recombination event may account for the increased expression of ERG in prostate cancer. This fusion is thought to occur early in prostate carcinogenesis, as it is found in precancerous lesions such as prostatic intraepithelial neoplasia (PIN). We investigated if the TMPRSS2:ERG recombination event was responsible for the elevated levels of ERG in the CABE group through PCR analysis. We found evidence of the gene fusion in one of the samples, which also was the sample with highest ERG expression on microarray and QPCR analysis. (Supplemental Data, Figure 2) This specific sample was re-reviewed by two pathologists (BSK and LDT), and was found to contain no histologic evidence of cancer or PIN.
DISCUSSION
In this study, examining gene expression in histologically normal epithelium from those with and without biopsy-proven prostate cancer, we found that overall expression patterns were similar, but distinct differences in specific genes were apparent between the two groups. We also demonstrated that these significant transcript differences were associated with biologically plausible pathways of prostate cancer carcinogenesis, suggestive of the existence of a field effect.
Our study differs from prior studies utilizing microarray expression data to compare normal tissue from prostatectomy specimens with normal prostate tissue obtained from organ donors (12, 33) or prostate needle biopsy tissue from healthy controls with low risk of prostate cancer (34). These other studies each found >1000 gene differences between their respective equivalent CABE and BABE groups, and there are several potential reasons for our disparate findings. First, we used prostate biopsy specimens that were processed in exactly the same method, namely immediately freezing the specimen within seconds of retrieval by needle biopsy. We have previously found that surgical manipulation and tissue ischemia have an effect on gene expression profiles in prostate tissue (17), and the other studies utilized surgical samples and/or organ donor tissues with variable handling and processing. Second, we utilized LCM in order to examine changes that are found in a specific histologic cell type, as opposed to utilizing homogenized whole prostate tissue with variable stromal-to-epithelial ratios as in the other studies. For example, differences in stromal gene expression or small, unrecognized foci of prostate cancer may have contributed to the large differences in gene expression seen in prior studies. Importantly, prostate gene expression may be affected by age, race and BMI, all of which impact prostate cancer risk. In all prior studies, age was significantly different between cancer and control groups, and race and BMI were not provided. Imbalance in these factors, most notably age, may account for some of the gene expression differences in the previous studies, and we stratified for these factors a priori in our study design. On the other hand, the elevated PSA in our control group may have minimized the expression differences in our study, as we did not determine the source of elevated PSA (,benign prostatic hyperplasia (BPH), inflammation or occult cancer)., Despite this, as stated previously, the risk of occult cancer in our control patients given their prior negative biopsies is low.
Although the genes found in this study require further investigation and validation, these results have clear clinical implications and applications. The existence of these alterations in gene expression prior to histologic change suggests that they may be the earliest events on the pathway to overt prostatic malignancy. As such, they provide insight into mechanisms of prostate cancer development and serve as potential targets for early intervention or prevention of prostate cancer. They may also serve as molecular markers for men who are at higher risk for prostate cancer diagnosis (e.g. family history) or occult prostate cancer, and may allow for patient stratification in terms of repeat biopsy or follow up. Lastly, the existence of a field effect has implications for focal therapy for prostate cancer (e.g., high intensity focused ultrasound, cryotherapy), as treatment of the index tumor alone will potentially fail to extirpate pre-neoplastic epithelia.
Several genes that were found to be differentially expressed between CABE and BABE have a noteworthy biology, including ERG, a gene which has been extensively investigated since the recent discovery of genetic translocations between TMPRSS2 and members of the ETS gene family, resulting in ERG overexpression in prostate cancer (22, 35–39). It is interesting that ERG expression was upregulated in benign epithelial cells from patients with prostate cancer in the absence of the common TMPRSS2 translocation, suggesting other mechanisms are at work to increase ERG expression in these cells. Our findings suggest that ERG overexpression occurs diffusely in prostatic epithelia prone to carcinogenesis and may be one of the earliest changes on the path to overt cancer. Another interesting finding is the increase in PSMA expression in the CABE specimens. PSMA functions as a folate hydrolase, potentially increasing folate availability (40). Experimental evidence has demonstrated that folate may increase the proliferative ability prostate cancer cells through PSMA (41), and recently oral folic acid supplementation in the setting of a randomized colorectal cancer clinical trial was associated with an increased risk of prostate cancer diagnosis (42). It is conceivable that overexpression of PSMA by “normal” epithelial cells may provide an early growth advantage through increased folate availability and propensity toward malignancy. HOXC4 and HOXC5, members of the HOXC gene family, were also upregulated and are found to be increased in malignant prostate cell lines, primary prostate tumors as well as lymph node metastasis (23). While the mechanism of tumorigenesis in response to alterations in HOX gene expression is unclear, HOXC4 overexpression has been linked to increased androgen receptor activity in vitro (23). Of interest regarding the Ingenuity analysis is that FOLH1 and HOXC4, two genes identified as increased in CABE samples, are increased in response to estradiol in prostate and breast cancer cell lines, respectively(Supplemental Figure 1). Selective estrogen receptor modulators (SERMs) have demonstrated benefit as potential chemoprotective agents for prostate cancer in phase 2 studies, and effects on these two genes may have a role in this function. Lastly, MME/ CD10 was found to be decreased in the CABE group, similar to its decreased expression in prostate cancer (24, 43, 44). Potential mechanisms by which MME contributes to tumorigenesis include interactions withheat shock proteins (45) and Akt activity (46), and loss of MME expression portends a higher risk of biochemical recurrence (47).
Potential limitations deserve mention. One limitation of this study is the lack of confirmation that our BABE control group did not indeed harbor occult prostate cancer despite multiple negative biopsies. While such a confirmatory study is not possible, studies suggest that the likelihood of a false negative biopsy decreases with multiple negative biopsies (48, 49), and the probability of finding a high grade or high volume prostate cancer is unlikely(49). Second, our findings certainly may be due to a field effect caused by the tumor microenvironment rather than due to changes that initiate tumor formation. To address this important issue, one would need to compare biopsies from men who would later develop cancer to biopsies from men who never developed cancer, an ongoing research pursuit. Furthermore, we are not certain that detected transcript differences yield corresponding protein level changes in all genes or, more importantly, altered function, although we were able to demonstrate altered protein levels for PSMA and MME by IHC, which corresponded with their changes at the mRNA transcript level. Due to the limited number of patients in our tissue bank with high grade prostate cancer and the fact that some of these patients did not have normal epithelia on the biopsy tissue, our sample size was another limitation. This may increase the potential for false negatives, and thus it is possible that more genes are differentially expressed between the BABE and CABE groups. As a result, the power to detect differences in gene expression ≤ 1.5 fold was modest; thus some biologically important differences gene expression may not have been detected. Another limitation is that we only characterized changes that are detectable in the normal epithelium associated with high grade prostate cancer; further studies are needed to determine if these findings can be generalized to all patients with prostate cancer, in particular those with lower Gleason grades. Lastly, gene expression signatures in epithelium may be influenced by or as a result of changes in the associated stroma, and we did not interrogate the associated prostatic stroma for molecular alterations as has been reported by others (50).
Several additional strengths of our study also merit review. Our controls were age- and BMI-matched in an attempt to minimize the effects of variables which have a known effect on prostate cancer risk, and thus possibly normal prostate epithelial gene expression. Although there was some racial disparity between our cases and controls, in a separate analysis, we confirmed that our gene expression findings are unchanged when comparing only Caucasians in CABE vs. BABE specimens (Supplemental Data, Figure 3). In addition, while the use of biopsies does not totally rule out occult cancer, it does eliminate the potential confounding effects of surgical manipulation and ischemia on gene expression (17). We utilized laser captured microdissected tissue in order to minimize the cancer and stromal contamination, as well as the potential effects of inflammation and atrophy on our prostatic epithelial gene profiles. Lastly, through qPCR we were also able to demonstrate that the observed alterations in gene expression were not likely to be the result of cancer contamination.
In conclusion, we have demonstrated that there are gene expression differences between histologically normal prostatic epithelium in men with prostate cancer when compared to normal prostatic epithelium from men without prostate cancer. Several genes that we identified have been associated with prostate cancer in prior studies, although the exact mechanisms of cancer induction by these genes remain to be determined. One of these genes is ERG, whose elevated expression in these normal epithelia from men with prostate cancer does not appear to be due to the TMPRSS2:ERG fusion described in a majority of prostate cancers. Further studies are needed to determine if these expression changes precede the appearance of frank malignancy.
TRANSLATIONAL RELEVANCE
Analyses of prostatectomy specimens have determined that prostate cancer is multifocal in the majority of cases. Many human cancers which are multifocal or recurrent have such characteristics due to “field cancerization” or “field effect”, in which the surrounding histologically normal tissue have molecular changes which increase their propensity for carcinogenesis. In this study, comparison of gene expression profiles in benign epithelia from men with prostate cancer to those of men without prostate cancer reveal differences in several genes associated with prostate cancer. These findings have implications on the mechanism of prostate tumorigenesis, as these genes are likely to have an early role in this process. These may prove useful as molecular markers of occult prostate cancer and/or proclivity for future cancer development. Lastly, this field effect presents a potential shortcoming in the use of focal therapies for prostate cancer, such as cryotherapy and high-intensity focused ultrasound (HIFU).
Supplementary Material
Acknowledgments
Acknowledgment of research support: This work was supported by grant support including DK65083 (DWL), the Pacific Northwest Prostate Cancer SPORE CA97186 (DWL and PSN), and the Fred Hutchinson Cancer Research Center P30 CA015704.
Key to Abbreviations
- BABE
benign-associated benign epithelium
- CABE
cancer-associated benign epithelium
- MME/CD10
membrane metallo-endopeptidase/ cluster of differentiation 10
- PSMA/FOLH1
prostate-specific membrane antigen/ folate hydrolase 1
- HOXC4
homeobox C4
- HOXC5
homeobox C5
- SSTR
somatostatin receptor
- ERG
v-ets avian erythroblastosis virus E26 oncogene homolog
- TMPRSS2
transmembrane protease, serine 2
- HPN
hepsin
- FASN
fatty acid synthase
- AMACR
alpha-methylacyl-CoA racemase
- SPOCK
sparc/osteonectin, CWCV, and kazal-like domains proteoglycan
- EZH2
enhancer of zeste, Drosophila, homolog 2
Footnotes
Authors’ disclosures: none
References
- 1.Wise AM, Stamey TA, McNeal JE, Clayton JL. Morphologic and clinical significance of multifocal prostate cancers in radical prostatectomy specimens. Urology. 2002;60:264–269. doi: 10.1016/s0090-4295(02)01728-4. [DOI] [PubMed] [Google Scholar]
- 2.Eichelberger LE, Cheng L. Does pT2b prostate carcinoma exist? Critical appraisal of the 2002 TNM classification of prostate carcinoma. Cancer. 2004;100:2573–2576. doi: 10.1002/cncr.20305. [DOI] [PubMed] [Google Scholar]
- 3.Douglas TH, McLeod DG, Mostofi FK, et al. Prostate-specific antigen-detected prostate cancer (stage T1c): an analysis of whole-mount prostatectomy specimens. Prostate. 1997;32:59–64. doi: 10.1002/(sici)1097-0045(19970615)32:1<59::aid-pros8>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 4.Cheng L, Jones TD, Pan CX, Barbarin A, Eble JN, Koch MO. Anatomic distribution and pathologic characterization of small-volume prostate cancer (<0.5 ml) in whole-mount prostatectomy specimens. Mod Pathol. 2005;18:1022–1026. doi: 10.1038/modpathol.3800431. [DOI] [PubMed] [Google Scholar]
- 5.Byar DP, Mostofi FK. Carcinoma of the prostate: prognostic evaluation of certain pathologic features in 208 radical prostatectomies. Examined by the step-section technique. Cancer. 1972;30:5–13. doi: 10.1002/1097-0142(197207)30:1<5::aid-cncr2820300103>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 6.Boccon-Gibod LM, Dumonceau O, Toublanc M, Ravery V, Boccon-Gibod LA. Micro-focal prostate cancer: a comparison of biopsy and radical prostatectomy specimen features. Eur Urol. 2005;48:895–899. doi: 10.1016/j.eururo.2005.04.033. [DOI] [PubMed] [Google Scholar]
- 7.Arora R, Koch MO, Eble JN, Ulbright TM, Li L, Cheng L. Heterogeneity of Gleason grade in multifocal adenocarcinoma of the prostate. Cancer. 2004;100:2362–2366. doi: 10.1002/cncr.20243. [DOI] [PubMed] [Google Scholar]
- 8.Nonn L, Ananthanarayanan V, Gann PH. Evidence for field cancerization of the prostate. Prostate. 2009 doi: 10.1002/pros.20983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Braakhuis BJ, Tabor MP, Kummer JA, Leemans CR, Brakenhoff RH. A genetic explanation of Slaughter's concept of field cancerization: evidence and clinical implications. Cancer Res. 2003;63:1727–1730. [PubMed] [Google Scholar]
- 10.Mairinger T, Mikuz G, Gschwendtner A. Nuclear chromatin texture analysis of nonmalignant tissue can detect adjacent prostatic adenocarcinoma. Prostate. 1999;41:12–19. doi: 10.1002/(sici)1097-0045(19990915)41:1<12::aid-pros3>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 11.Tolonen TT, Tommola S, Jokinen S, Parviainen T, Martikainen PM. Bax and Bcl-2 are focally overexpressed in the normal epithelium of cancerous prostates. Scand J Urol Nephrol. 2007;41:85–90. doi: 10.1080/00365590601181257. [DOI] [PubMed] [Google Scholar]
- 12.Yu YP, Landsittel D, Jing L, et al. Gene expression alterations in prostate cancer predicting tumor aggression and preceding development of malignancy. J Clin Oncol. 2004;22:2790–2799. doi: 10.1200/JCO.2004.05.158. [DOI] [PubMed] [Google Scholar]
- 13.Uetsuki H, Tsunemori H, Taoka R, Haba R, Ishikawa M, Kakehi Y. Expression of a novel biomarker, EPCA, in adenocarcinomas and precancerous lesions in the prostate. J Urol. 2005;174:514–518. doi: 10.1097/01.ju.0000165154.41159.b1. [DOI] [PubMed] [Google Scholar]
- 14.Hanson JA, Gillespie JW, Grover A, et al. Gene promoter methylation in prostate tumor-associated stromal cells. J Natl Cancer Inst. 2006;98:255–261. doi: 10.1093/jnci/djj051. [DOI] [PubMed] [Google Scholar]
- 15.Mehrotra J, Varde S, Wang H, et al. Quantitative, spatial resolution of the epigenetic field effect in prostate cancer. Prostate. 2008;68:152–160. doi: 10.1002/pros.20675. [DOI] [PubMed] [Google Scholar]
- 16.Ananthanarayanan V, Deaton RJ, Yang XJ, Pins MR, Gann PH. Alpha-methylacyl-CoA racemase (AMACR) expression in normal prostatic glands and high-grade prostatic intraepithelial neoplasia (HGPIN): association with diagnosis of prostate cancer. Prostate. 2005;63:341–346. doi: 10.1002/pros.20196. [DOI] [PubMed] [Google Scholar]
- 17.Lin DW, Coleman IM, Hawley S, et al. Influence of surgical manipulation on prostate gene expression: implications for molecular correlates of treatment effects and disease prognosis. J Clin Oncol. 2006;24:3763–3770. doi: 10.1200/JCO.2005.05.1458. [DOI] [PubMed] [Google Scholar]
- 18.True L, Coleman I, Hawley S, et al. A molecular correlate to the Gleason grading system for prostate adenocarcinoma. Proc Natl Acad Sci U S A. 2006;103:10991–10996. doi: 10.1073/pnas.0603678103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci U S A. 2001;98:5116–5121. doi: 10.1073/pnas.091062498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Subramanian A, Tamayo P, Mootha VK, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rhodes DR, Barrette TR, Rubin MA, Ghosh D, Chinnaiyan AM. Meta-analysis of microarrays: interstudy validation of gene expression profiles reveals pathway dysregulation in prostate cancer. Cancer Res. 2002;62:4427–4433. [PubMed] [Google Scholar]
- 22.Tomlins SA, Rhodes DR, Perner S, et al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science. 2005;310:644–648. doi: 10.1126/science.1117679. [DOI] [PubMed] [Google Scholar]
- 23.Miller GJ, Miller HL, van Bokhoven A, et al. Aberrant HOXC expression accompanies the malignant phenotype in human prostate. Cancer Res. 2003;63:5879–5888. [PubMed] [Google Scholar]
- 24.Dall'Era MA, True LD, Siegel AF, Porter MP, Sherertz TM, Liu AY. Differential expression of CD10 in prostate cancer and its clinical implication. BMC Urol. 2007;7:3. doi: 10.1186/1471-2490-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Troyer JK, Beckett ML, Wright GL., Jr Detection and characterization of the prostate-specific membrane antigen (PSMA) in tissue extracts and body fluids. Int J Cancer. 1995;62:552–558. doi: 10.1002/ijc.2910620511. [DOI] [PubMed] [Google Scholar]
- 26.Sweat SD, Pacelli A, Murphy GP, Bostwick DG. Prostate-specific membrane antigen expression is greatest in prostate adenocarcinoma and lymph node metastases. Urology. 1998;52:637–640. doi: 10.1016/s0090-4295(98)00278-7. [DOI] [PubMed] [Google Scholar]
- 27.Silver DA, Pellicer I, Fair WR, Heston WD, Cordon-Cardo C. Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin Cancer Res. 1997;3:81–85. [PubMed] [Google Scholar]
- 28.Kosari F, Munz JM, Savci-Heijink CD, et al. Identification of prognostic biomarkers for prostate cancer. Clin Cancer Res. 2008;14:1734–1743. doi: 10.1158/1078-0432.CCR-07-1494. [DOI] [PubMed] [Google Scholar]
- 29.Sinisi AA, Bellastella A, Prezioso D, et al. Different expression patterns of somatostatin receptor subtypes in cultured epithelial cells from human normal prostate and prostate cancer. J Clin Endocrinol Metab. 1997;82:2566–2569. doi: 10.1210/jcem.82.8.4142. [DOI] [PubMed] [Google Scholar]
- 30.Reubi JC, Waser B, Schaer JC, Markwalder R. Somatostatin receptors in human prostate and prostate cancer. J Clin Endocrinol Metab. 1995;80:2806–2814. doi: 10.1210/jcem.80.9.7673428. [DOI] [PubMed] [Google Scholar]
- 31.Halmos G, Schally AV, Sun B, Davis R, Bostwick DG, Plonowski A. High expression of somatostatin receptors and messenger ribonucleic acid for its receptor subtypes in organ-confined and locally advanced human prostate cancers. J Clin Endocrinol Metab. 2000;85:2564–2571. doi: 10.1210/jcem.85.7.6698. [DOI] [PubMed] [Google Scholar]
- 32.Freedland SJ, Seligson DB, Liu AY, et al. Loss of CD10 (neutral endopeptidase) is a frequent and early event in human prostate cancer. Prostate. 2003;55:71–80. doi: 10.1002/pros.10202. [DOI] [PubMed] [Google Scholar]
- 33.Chandran UR, Dhir R, Ma C, Michalopoulos G, Becich M, Gilbertson J. Differences in gene expression in prostate cancer, normal appearing prostate tissue adjacent to cancer and prostate tissue from cancer free organ donors. BMC Cancer. 2005;5:45. doi: 10.1186/1471-2407-5-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schlomm T, Hellwinkel OJ, Buness A, et al. Molecular cancer phenotype in normal prostate tissue. Eur Urol. 2009;55:885–890. doi: 10.1016/j.eururo.2008.04.105. [DOI] [PubMed] [Google Scholar]
- 35.Perner S, Demichelis F, Beroukhim R, et al. TMPRSS2:ERG fusion-associated deletions provide insight into the heterogeneity of prostate cancer. Cancer Res. 2006;66:8337–8341. doi: 10.1158/0008-5472.CAN-06-1482. [DOI] [PubMed] [Google Scholar]
- 36.Saramaki OR, Harjula AE, Martikainen PM, Vessella RL, Tammela TL, Visakorpi T. TMPRSS2:ERG Fusion Identifies a Subgroup of Prostate Cancers with a Favorable Prognosis. Clin Cancer Res. 2008;14:3395–3400. doi: 10.1158/1078-0432.CCR-07-2051. [DOI] [PubMed] [Google Scholar]
- 37.Mehra R, Tomlins SA, Yu J, et al. Characterization of TMPRSS2-ETS gene aberrations in androgen-independent metastatic prostate cancer. Cancer Res. 2008;68:3584–3590. doi: 10.1158/0008-5472.CAN-07-6154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Furusato B, Gao CL, Ravindranath L, et al. Mapping of TMPRSS2-ERG fusions in the context of multi-focal prostate cancer. Mod Pathol. 2008;21:67–75. doi: 10.1038/modpathol.3800981. [DOI] [PubMed] [Google Scholar]
- 39.Petrovics G, Liu A, Shaheduzzaman S, et al. Frequent overexpression of ETS-related gene-1 (ERG1) in prostate cancer transcriptome. Oncogene. 2005;24:3847–3852. doi: 10.1038/sj.onc.1208518. [DOI] [PubMed] [Google Scholar]
- 40.Pinto JT, Suffoletto BP, Berzin TM, et al. Prostate-specific membrane antigen: a novel folate hydrolase in human prostatic carcinoma cells. Clin Cancer Res. 1996;2:1445–1451. [PubMed] [Google Scholar]
- 41.Yao V, Bacich DJ. Prostate specific membrane antigen (PSMA) expression gives prostate cancer cells a growth advantage in a physiologically relevant folate environment in vitro. Prostate. 2006;66:867–875. doi: 10.1002/pros.20361. [DOI] [PubMed] [Google Scholar]
- 42.Cole BF, Baron JA, Sandler RS, et al. Folic acid for the prevention of colorectal adenomas: a randomized clinical trial. Jama. 2007;297:2351–2359. doi: 10.1001/jama.297.21.2351. [DOI] [PubMed] [Google Scholar]
- 43.Tawfic S, Niehans GA, Manivel JC. The pattern of CD10 expression in selected pathologic entities of the prostate gland. Hum Pathol. 2003;34:450–456. doi: 10.1016/s0046-8177(03)00077-7. [DOI] [PubMed] [Google Scholar]
- 44.Liu AY, Roudier MP, True LD. Heterogeneity in primary and metastatic prostate cancer as defined by cell surface CD profile. Am J Pathol. 2004;165:1543–1556. doi: 10.1016/S0002-9440(10)63412-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dall'Era MA, Oudes A, Martin DB, Liu AY. HSP27 and HSP70 interact with CD10 in C4-2 prostate cancer cells. Prostate. 2007;67:714–721. doi: 10.1002/pros.20558. [DOI] [PubMed] [Google Scholar]
- 46.Osman I, Dai J, Mikhail M, et al. Loss of neutral endopeptidase and activation of protein kinase B (Akt) is associated with prostate cancer progression. Cancer. 2006;107:2628–2636. doi: 10.1002/cncr.22312. [DOI] [PubMed] [Google Scholar]
- 47.Osman I, Yee H, Taneja SS, et al. Neutral endopeptidase protein expression and prognosis in localized prostate cancer. Clin Cancer Res. 2004;10:4096–4100. doi: 10.1158/1078-0432.CCR-04-0120. [DOI] [PubMed] [Google Scholar]
- 48.Keetch DW, Catalona WJ, Smith DS. Serial prostatic biopsies in men with persistently elevated serum prostate specific antigen values. J Urol. 1994;151:1571–1574. doi: 10.1016/s0022-5347(17)35304-1. [DOI] [PubMed] [Google Scholar]
- 49.Djavan B, Ravery V, Zlotta A, et al. Prospective evaluation of prostate cancer detected on biopsies 1, 2, 3 and 4: when should we stop? J Urol. 2001;166:1679–1683. [PubMed] [Google Scholar]
- 50.Dakhova O, Ozen M, Creighton CJ, et al. Global gene expression analysis of reactive stroma in prostate cancer. Clin Cancer Res. 2009;15:3979–3989. doi: 10.1158/1078-0432.CCR-08-1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.