Abstract
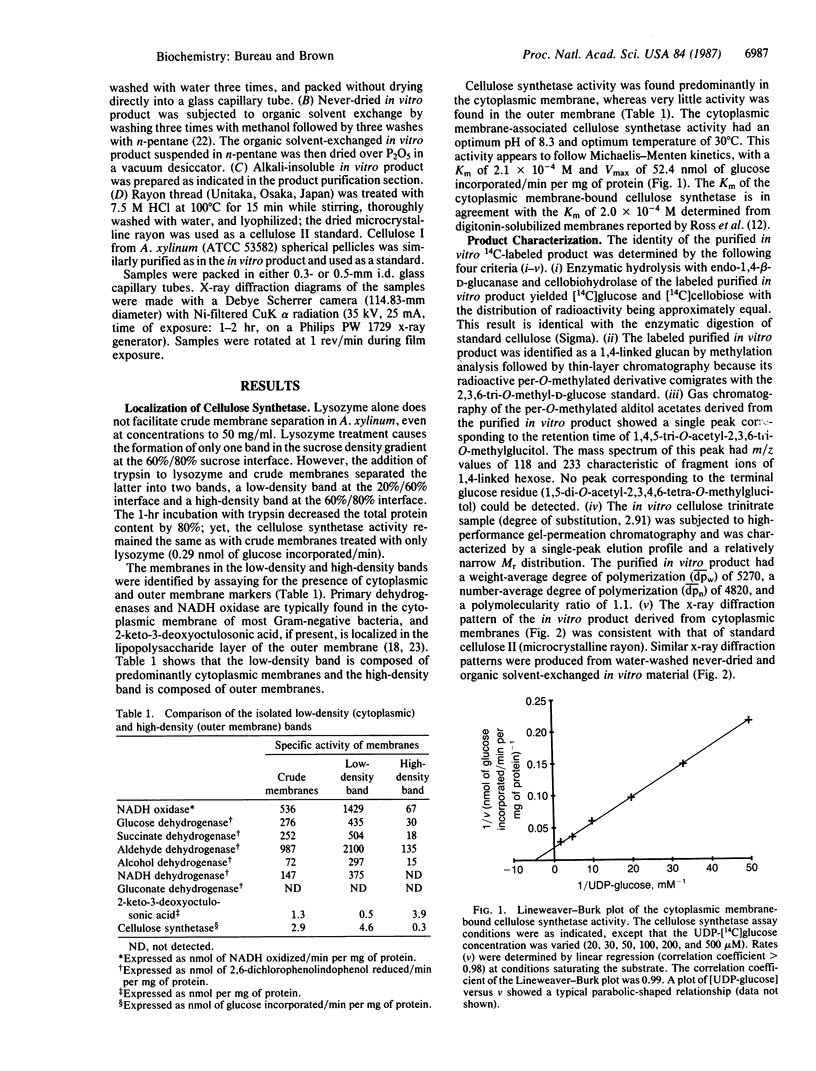
The cytoplasmic and outer membranes of Acetobacter xylinum (ATCC 53582) were isolated by discontinuous sucrose density ultracentrifugation. Both lysozyme (EC 3.2.1.17) and trypsin (EC 3.4.21.4) were required for efficient crude membrane separation. Primary dehydrogenases and NADH oxidase were used as cytoplasmic membrane markers, and 2-keto-3-deoxyoctulosonic acid was used to identify the outer membranes. Cellulose synthetase (UDP-glucose:1,4-β-D-glucan 4-β-D-glucosyltransferase; EC 2.4.1.12) activity was assayed as the conversion of radioactivity from UDP-[14C]glucose into an alkali-insoluble β-1,4-D-[14C]glucan. This activity was predominantly found in the cytoplasmic membrane. The cellulose nature of the product was demonstrated by (i) enzymatic hydrolysis followed by TLC, (ii) methylation analysis followed by TLC, and (iii) GC/MS. Further, the weight-average and number-average degree of polymerization of the in vitro product, determined by high-performance gel permeation chromatography, were 4820 and 5270, respectively. In addition, x-ray diffraction analysis indicated that the in vitro product is cellulose II, which is in contrast to the in vivo product—namely, cellulose I.
Keywords: bacterial envelope; 1,4-β-D-glucan; enzyme localization; cellulose synthetase; cellulose II
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aloni Y., Cohen R., Benziman M., Delmer D. Solubilization of the UDP-glucose:1,4-beta-D-glucan 4-beta-D-glucosyltransferase (cellulose synthase) from Acetobacter xylinum. A comparison of regulatory properties with those of the membrane-bound form of the enzyme. J Biol Chem. 1983 Apr 10;258(7):4419–4423. [PubMed] [Google Scholar]
- Aloni Y., Delmer D. P., Benziman M. Achievement of high rates of in vitro synthesis of 1,4-beta-D-glucan: activation by cooperative interaction of the Acetobacter xylinum enzyme system with GTP, polyethylene glycol, and a protein factor. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6448–6452. doi: 10.1073/pnas.79.21.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun V. Covalent lipoprotein from the outer membrane of Escherichia coli. Biochim Biophys Acta. 1975 Oct 31;415(3):335–377. doi: 10.1016/0304-4157(75)90013-1. [DOI] [PubMed] [Google Scholar]
- Braun V., Rehn K. Chemical characterization, spatial distribution and function of a lipoprotein (murein-lipoprotein) of the E. coli cell wall. The specific effect of trypsin on the membrane structure. Eur J Biochem. 1969 Oct;10(3):426–438. doi: 10.1111/j.1432-1033.1969.tb00707.x. [DOI] [PubMed] [Google Scholar]
- Braun V., Wolff H. The murein-lipoprotein linkage in the cell wall of Escherichia coli. Eur J Biochem. 1970 Jun;14(2):387–391. doi: 10.1111/j.1432-1033.1970.tb00301.x. [DOI] [PubMed] [Google Scholar]
- Brown R. M., Jr, Willison J. H., Richardson C. L. Cellulose biosynthesis in Acetobacter xylinum: visualization of the site of synthesis and direct measurement of the in vivo process. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4565–4569. doi: 10.1073/pnas.73.12.4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colvin J. R., Leppard G. G. The biosynthesis of cellulose by Acetobacter xylinum and Acetobacter acetigenus. Can J Microbiol. 1977 Jun;23(6):701–709. doi: 10.1139/m77-105. [DOI] [PubMed] [Google Scholar]
- Cooper D., Manley R. S. Cellulose synthesis by Acetobacter xylinum. II. Investigation into the relation between cellulose synthesis and cell envelope components. Biochim Biophys Acta. 1975 Jan 13;381(1):97–108. doi: 10.1016/0304-4165(75)90192-0. [DOI] [PubMed] [Google Scholar]
- Filip C., Fletcher G., Wulff J. L., Earhart C. F. Solubilization of the cytoplasmic membrane of Escherichia coli by the ionic detergent sodium-lauryl sarcosinate. J Bacteriol. 1973 Sep;115(3):717–722. doi: 10.1128/jb.115.3.717-722.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLASER L. The synthesis of cellulose in cell-free extracts of Acetobacter xylinum. J Biol Chem. 1958 Jun;232(2):627–636. [PubMed] [Google Scholar]
- Haigler C. H., Brown R. M., Jr, Benziman M. Calcofluor white ST Alters the in vivo assembly of cellulose microfibrils. Science. 1980 Nov 21;210(4472):903–906. doi: 10.1126/science.7434003. [DOI] [PubMed] [Google Scholar]
- Harris P. J., Henry R. J., Blakeney A. B., Stone B. A. An improved procedure for the methylation analysis of oligosaccharides and polysaccharides. Carbohydr Res. 1984 Apr 2;127(1):59–73. doi: 10.1016/0008-6215(84)85106-x. [DOI] [PubMed] [Google Scholar]
- Hobot J. A., Carlemalm E., Villiger W., Kellenberger E. Periplasmic gel: new concept resulting from the reinvestigation of bacterial cell envelope ultrastructure by new methods. J Bacteriol. 1984 Oct;160(1):143–152. doi: 10.1128/jb.160.1.143-152.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F. C., Brown R. M., Jr, Cooper J. B., Delmer D. P. Synthesis of Fibrils in Vitro by a Solubilized Cellulose Synthase from Acetobacter xylinum. Science. 1985 Nov 15;230(4727):822–825. doi: 10.1126/science.230.4727.822. [DOI] [PubMed] [Google Scholar]
- Lugtenberg B., Van Alphen L. Molecular architecture and functioning of the outer membrane of Escherichia coli and other gram-negative bacteria. Biochim Biophys Acta. 1983 Mar 21;737(1):51–115. doi: 10.1016/0304-4157(83)90014-x. [DOI] [PubMed] [Google Scholar]
- Miura T., Mizushima S. Separation by density gradient centrifugation of two types of membranes from spheroplast membrane of Escherichia coli K12. Biochim Biophys Acta. 1968 Jan 3;150(1):159–161. doi: 10.1016/0005-2736(68)90020-5. [DOI] [PubMed] [Google Scholar]
- Osborn M. J., Gander J. E., Parisi E., Carson J. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Isolation and characterization of cytoplasmic and outer membrane. J Biol Chem. 1972 Jun 25;247(12):3962–3972. [PubMed] [Google Scholar]
- SCHRAMM M., HESTRIN S. Factors affecting production of cellulose at the air/liquid interface of a culture of Acetobacter xylinum. J Gen Microbiol. 1954 Aug;11(1):123–129. doi: 10.1099/00221287-11-1-123. [DOI] [PubMed] [Google Scholar]
- Schnaitman C. A. Solubilization of the cytoplasmic membrane of Escherichia coli by Triton X-100. J Bacteriol. 1971 Oct;108(1):545–552. doi: 10.1128/jb.108.1.545-552.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swissa M., Aloni Y., Weinhouse H., Benizman M. Intermediatry steps in Acetobacter xylinum cellulose synthesis: studies with whole cells and cell-free preparations of the wild type and a celluloseless mutant. J Bacteriol. 1980 Sep;143(3):1142–1150. doi: 10.1128/jb.143.3.1142-1150.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaar K. Visualization of pores (export sites) correlated with cellulose production in the envelope of the gram-negative bacterium Acetobacter xylinum. J Cell Biol. 1979 Mar;80(3):773–777. doi: 10.1083/jcb.80.3.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaman Z., Verwilghen R. L. Quantitation of proteins solubilized in sodium dodecyl sulfate-mercaptoethanol-Tris electrophoresis buffer. Anal Biochem. 1979 Nov 15;100(1):64–69. doi: 10.1016/0003-2697(79)90110-6. [DOI] [PubMed] [Google Scholar]



