Abstract
During Ca2+ transport by sarcoplasmic reticulum Ca2+-ATPase, the conformation change of ADP-sensitive phosphoenzyme (E1PCa2) to ADP-insensitive phosphoenzyme (E2PCa2) is followed by rapid Ca2+ release into the lumen. Here, we find that in the absence of K+, Ca2+ release occurs considerably faster than E1PCa2 to E2PCa2 conformation change. Therefore, the lumenal Ca2+ release pathway is open to some extent in the K+-free E1PCa2 structure. The Ca2+ affinity of this E1P is as high as that of the unphosphorylated ATPase (E1), indicating the Ca2+ binding sites are not disrupted. Thus, bound K+ stabilizes the E1PCa2 structure with occluded Ca2+, keeping the Ca2+ pathway to the lumen closed. We found previously (Yamasaki, K., Wang, G., Daiho, T., Danko, S., and Suzuki, H. (2008) J. Biol. Chem. 283, 29144–29155) that the K+ bound in E2P reduces the Ca2+ affinity essential for achieving the high physiological Ca2+ gradient and to fully open the lumenal Ca2+ gate for rapid Ca2+ release (E2PCa2 → E2P + 2Ca2+). These findings show that bound K+ is critical for stabilizing both E1PCa2 and E2P structures, thereby contributing to the structural changes that efficiently couple phosphoenzyme processing and Ca2+ handling.
Keywords: Bioenergetics, Calcium ATPase, Calcium Transport, Kinetics, Sarcoplasmic Reticulum, Phosphorylated Intermediate, Structure And Function
Introduction
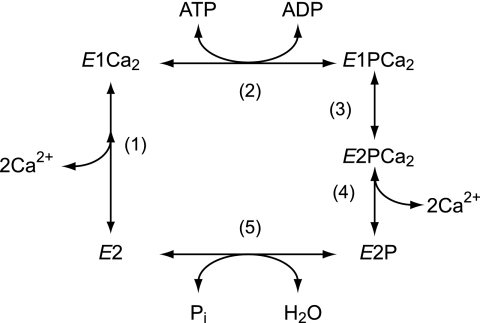
Sarcoplasmic reticulum (SR)2 Ca2+-ATPase (SERCA1a) catalyzes Ca2+ transport coupled with ATP hydrolysis against an ∼10,000-fold concentration gradient (1–9). The ATPase is first activated by the binding of two cytoplasmic Ca2+ ions at the transport sites with a submicromolar high affinity (E2 to E1Ca2, see step 1 in Fig. 1) and then autophosphorylated at Asp351 by ATP to form a phosphoenzyme intermediate (EP) (step 2). This EP is “ADP-sensitive” (E1P) because it is rapidly dephosphorylated by ADP in the reverse reaction. Upon E1P formation, the bound Ca2+ ions are occluded in the transport sites (E1PCa2). Subsequently, E1PCa2 undergoes its isomeric transition to an ADP-insensitive form (E2P), i.e. loss of ADP sensitivity, which results in a large reduction of Ca2+ affinity and opening of the lumenal release gate, i.e. Ca2+ deocclusion and release (steps 3–4). Ca2+ release in step 4 is very rapid, so that an E2PCa2 intermediate state does not accumulate and in fact had never been found until we recently established its existence (10–13) and successfully trapped it for the first time (14). Finally, E2P is hydrolyzed back to the inactive E2 form (step 5).
FIGURE 1.
Reaction scheme of Ca2+-ATPase.
In E1PCa2 → E2P + 2Ca2+, the A domain rotates parallel to the membrane plane and the P domain inclines to the A domain, thereby associating with each other to produce a compactly organized and inclined headpiece (15–27). This tight structure is stabilized by critical interaction networks between the A and P domains at three regions (10–14) (see Fig. 9 for details). The rotation and inclination of the domains result in motions and rearrangements of the transmembrane helices thereby disrupting the Ca2+ sites and opening the lumenal gate. In the P domain, there is a specific K+ binding site (28); K+ binding here is crucial for rapid hydrolysis of E2P (28–30). Recently, we further found (13) that the K+ in E2P is critical for reducing the lumenal Ca2+ affinity that is required to achieve the high physiological Ca2+ gradient and for rapid Ca2+ release (E2PCa2 → E2P + 2Ca2+). Thus, bound K+ contributes to stabilization of the compactly organized and inclined E2P structure with its disrupted Ca2+ sites and fully opened lumenal gate, probably by cross-linking the P domain with the A domain/M3-linker (13).
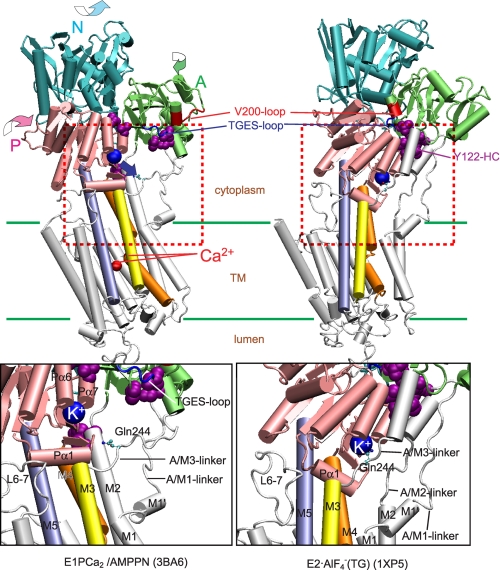
FIGURE 9.
Structural change during E1PCa2 → E2P + 2Ca2+ and movement of the K+ binding site. The structural change is modeled on the crystal structures with bound K+, E1PCa2·AMPPN, and E2·AlF4− (Protein Data Bank codes 3BA6 (22) and 1XP5 (20), respectively). The two structures are aligned with the static M8–10 helices. The approximate position of the transmembrane region (TM) is shown by green lines. The area indicated by red dashed lines in the whole molecule is enlarged in the lower panel. The motions of each of N, P, and A domains during E1PCa2·AMPPN → E2·AlF4− are indicated by curved arrows. Note that the K+ site with bound K+ on the P domain moves down to the Gln244 region on the A/M3-linker (blue arrow), thus likely cross-linking the P domain with the A/M3-linker. There are three critical interaction networks to realize and stabilize the compactly organized E2P structure. They are Tyr122 HC forming a hydrophobic interaction cluster (violet Van der Waals spheres), the Val200 loop (red loop), and TGES184 (blue loop) (10–13). Crystal structures of E2·BeF3− (21, 22), which are analogs of the E2P ground state (25), are not used here because they were formed without K+ (although the above noted changes are also seen with the E2·BeF3− crystals).
Despite these findings on the Ca2+ release process and E2P, a possible role for K+ in E1PCa2 has not been explored. The K+ site is situated at the bottom of the P-domain near the cytoplasmic ends of the transmembrane helices. Therefore, the lack of K+ binding might have a serious effect on the stability of the helices and Ca2+ handling in E1PCa2. The E2-E1Ca2 transition is markedly retarded, and its equilibrium is affected by the absence of K+ (31–33).
In this study, we explore a possible role of K+ in E1PCa2 especially in regard to Ca2+ occlusion. Results reveal that K+-free E1PCa2 has an open Ca2+ pathway to the lumen. Thus, the Ca2+ binding sites face the lumen, and Ca2+ can be released. The absence of K+ does not reduce the high Ca2+ affinity (Ca2+ site coordination probably unchanged), and yet the cytoplasmic gate is closed, and the lumenal gate is open. These changes probably do not involve large motions of the cytoplasmic domains and transmembrane helices. Therefore, bound K+ likely stabilizes the Ca2+ occluded structure of E1PCa2 by simply keeping the lumenal Ca2+ pathway closed. The structural role of K+ in E1PCa2 is discussed in detail using crystal structures of Ca2+-ATPase with bound K+.
EXPERIMENTAL PROCEDURES
Preparation of SR Vesicles
SR vesicles were prepared from rabbit skeletal muscle as described (34). The phosphorylation site content in the vesicles determined according to Barrabin et al. (35) was 4.49 ± 0.22 nmol/mg vesicle protein (n = 5).
Determination of EP
SR vesicles were phosphorylated with [γ-32P]ATP as described in the legends to Figs. 2–6. In the experiments performed in Fig. 2, aliquots of the reaction mixture were spotted on the HAWP membrane filter (Millipore) and washed continuously with a chasing solution for the periods indicated. At the end of chase, the reaction was terminated by washing with 0.1 m HCl. To determine the amount of E2P in the phosphorylation mixture, the membrane was washed with an ADP solution for 1 s and then with 0.1 m HCl. The membrane was dried, and the radioactivity was measured by digital autoradiography. In Figs. 3 and 6, total EP was measured by quenching the phosphorylation reaction (in a test tube) with 5% (v/v) ice-cold trichloroacetic acid containing Pi, whereas for E2P determination, the reaction was chased with ADP for 1 s and quenched by addition of the trichloroacetic acid. The precipitated proteins were separated by 5% SDS-PAGE at pH 6.0 according to Weber and Osborn (36). The radioactivity associated with the separated Ca2+-ATPase was quantitated by digital autoradiography (37). Rapid kinetic measurements in Fig. 3 were performed with a handmade rapid mixing apparatus (38).
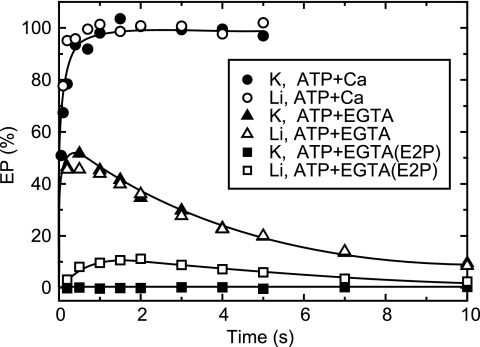
FIGURE 2.
Time courses of EP decay and Ca2+ release in the presence or absence of 0.1 m K+. A, the Ca2+-ATPase in SR vesicles (20 μg protein/ml) was phosphorylated for 10 s with 100 μm [γ-32P]ATP in 10 μm nonradioactive CaCl2 (closed circles and, in inset, triangles), or with 100 μm nonradioactive ATP in 10 μm 45CaCl2 (open circles), 0.1 m KCl, 3 μm A23187. Then, 50 μl of the reaction mixture was spotted on the membrane and washed for the time periods on the abscissa with a chasing solution containing 1 mm EGTA, 0.1 m KCl, 3 μm A23187. The amount of bound 45Ca2+ (open circles) and the total amount of EP (open triangles in inset) were determined. The amounts of E2P (closed triangles in inset) were determined by a subsequent washing by a solution containing 1 mm ADP, 1 mm EGTA, and 0.1 m KCl. The amount of E1P (closed circles) was calculated by subtracting the amount of E2P from total amount of EP. B, the Ca2+-ATPase in SR vesicles was phosphorylated in the presence of 0.1 m KCl as in A and spotted on the filter. Then, the filter was washed with the EGTA solution (and subsequently with the ADP solution for the E2P determination) containing 0.1 m LiCl instead of KCl, otherwise as in A. Solid lines in A and B show the least squares fit to a single exponential. The rates (s−1) for E1P decay and the Ca2+ release were 0.66 and 0.58 (A) and 0.21 and 0.81 (B), respectively.
FIGURE 3.
EP formation and decay in a single turnover. All of the solutions contained 0.1 m KCl (closed symbols) or LiCl (open symbols). SR vesicles (20 μg/ml) were incubated in 10 μm CaCl2, and EP formation was initiated by mixing with an equal volume of a solution containing 20 μm [γ-32P]ATP and 10 mm EGTA (triangles and squares) or 10 μm CaCl2 (circles). The total amount of EP was determined with the addition of trichloroacetic acid (circles and triangles). To determine the amount of E2P (squares), the phosphorylated sample was mixed with an equal volume of a solution containing 2 mm ADP and 5 mm EGTA, and then the reaction was terminated by trichloroacetic acid at 1 s after the ADP addition.
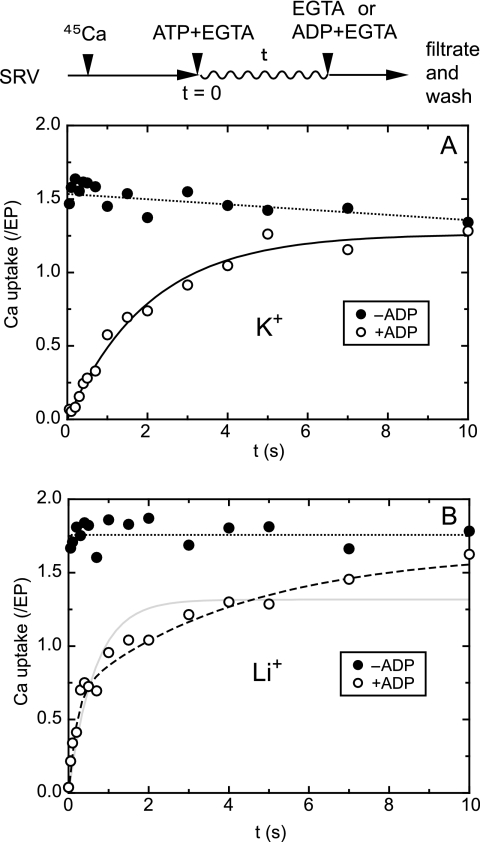
FIGURE 4.
45Ca2+ uptake in a single turnover of EP with and without K+. All of the solutions contained 0.1 m KCl (A) or LiCl (B). In the absence of the Ca2+ ionophore, SR vesicles (SRV; 20 μg/ml) were first incubated with 10 μm 45CaCl2 for ∼10 min, and then Ca2+ uptake in a single turnover of EP was initiated by mixing with an equal volume of a solution containing 20 μm ATP and 2 mm EGTA, as described in Fig. 3. After the indicated periods, the reaction was chased with an equal volume of a solution containing 2 mm EGTA without (closed circles) or with (open circles) 2 mm ADP. The mixture was immediately spotted on the membrane and washed for ∼10 s with 1 ml of a 2 mm EGTA solution. The amount of 45Ca2+ on the membrane, i.e. transported into the vesicles and/or remained bound to the ATPase and not released to cytoplasmic side, was normalized to the maximum total amount of EP formed immediately after the addition ATP and EGTA (Fig. 3). In A, the time course obtained with the ADP chase was best described by a single exponential Ca2+ uptake (solid line) with a rate constant of 0.49 s−1 and maximum Ca2+/EP value of 1.26. In B, it was best described by a double exponential (broken line) with a rate constant and maximum Ca2+/EP value of 5.1 s−1 and 0.66 for the fast phase and 0.24 s−1 and 0.98 for the slow phase (but it was not described by a single exponential increase shown by solid line with the rate constant of 1.54 s−1 and maximum value of 1.31). Note also that without the ADP addition, almost of all the bound Ca2+ ions are transported into the vesicles during the ∼10-s EGTA wash because the single turnover of EP is nearly completed in this period (see Fig. 3).
FIGURE 5.
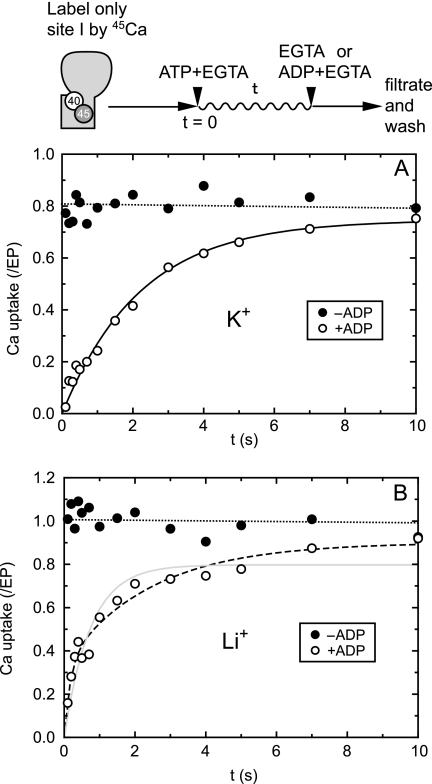
Uptake of site I-bound 45Ca2+ in a single turnover of EP with and without K+. SR vesicles were incubated with 10 μm 45Ca2+ as in Fig. 4 and diluted by an equal volume of a solution containing 2 mm nonradioactive CaCl2 and 0.1 m KCl (A) or LiCl (B), and further incubated for 10 s. By this incubation, site I of the two Ca2+ sites (I, II) is labeled with 45Ca2+ due to Ca2+ exchange with site II (see supplemental Fig. S1) (42–44). Then, 45Ca2+ uptake assay in a single turnover was performed as in Fig. 4. In A, the time course obtained with the ADP-chase was best described by a single exponential Ca2+ uptake (solid line) with a rate constant of 0.45 s−1 and maximum Ca2+/EP value of 0.75. In B, it was best described by a double exponential increase (broken line) with a rate constant and maximum value of 6.0 s−1 and 0.34 for the fast phase and 0.41 s−1 and 0.56 for the slow phase (but not described by a single exponential increase shown as solid line with the rate constant of 1.36 s−1 and the maximum value of 0.80).
FIGURE 6.
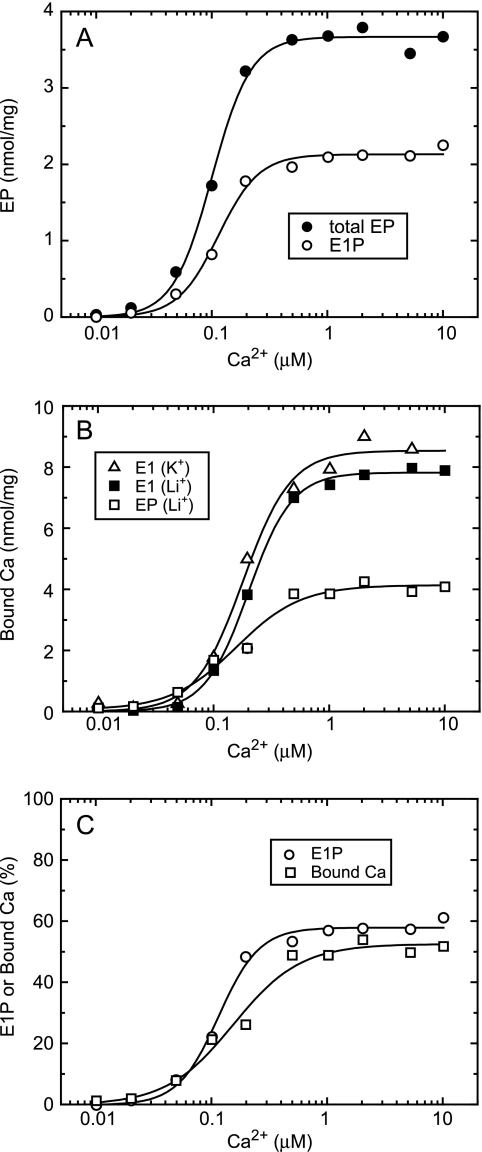
Ca2+ dependence of E1P accumulation and Ca2+ binding in steady state. A, SR vesicles (200 μg/ml) were phosphorylated at 4 °C for 30 s with 100 μm [γ-32P]ATP in 3 μm A23187, 0.1 m LiCl, and 20 μm CaCl2 with various concentrations of EGTA to give the indicated free Ca2+ concentrations. The total amount of EP (closed circles) and amount of E1P (open circles) were determined as described in Fig. 3. Solid lines show the least squares fit to the Hill equation. The maximum, K0.5, and Hill coefficient for the total amount of EP were 3.67 nmol/mg, 0.10 μm, and 2.6, respectively, and those for E1P were 2.12 nmol/mg, 0.11 μm, and 2.5, respectively. B, SR vesicles were phosphorylated with 100 μm ATP (open squares) or incubated without ATP (closed squares and open triangles) in 20 μm 45CaCl2 with various concentrations of EGTA and 0.1 m LiCl (squares) or KCl (triangles), otherwise as described in A. Then, 50 μl of the reaction mixture was spotted on the membrane, and the amount of 45Ca2+ specifically bound to the ATPase was determined. Solid lines show the least squares fit to the Hill equation. The maximum, K0.5, and Hill coefficient were 8.54 nmol/mg, 0.18 μm, and 2.1 (open triangles), 7.82 nmol/mg, 0.20 μm, and 2.3 (closed squares), and 4.14 nmol/mg, 0.15 μm, and 1.5 (open squares). C, the amount of E1P (open circles) in A and that of bound 45Ca2+ under the phosphorylating condition (open squares) in B in the absence of K+ are replotted after normalization to the maximum total amount of EP and to the maximum 45Ca2+ binding under the nonphosphorylating condition (E1) in the absence of K+, respectively, and shown as % values. Solid lines show the least squares fit to the Hill equation, and the maximum values were 58% for E1P and 52% for bound 45Ca2+, respectively.
Determination of Bound Ca2+
In the experiments performed in Figs. 2 and 6, SR vesicles were incubated with 45CaCl2 as per the figure legends, and an aliquot of reaction mixture was spotted on the HAWP membrane filter (Millipore). Then, the membrane was perfused with a chasing solution for indicated time periods using a rapid filtration apparatus RFS-4 (Bio-Logic, Claix, France). To estimate nonspecific 45Ca2+ binding, the same experiments were done in the presence of 1 μm thapsigargin. Specific 45Ca2+ binding was obtained after subtracting this nonspecific binding.
Ca2+ Uptake into SR Vesicles in a Single Turnover of EP
In the experiments performed in Figs. 4 and 5, SR vesicles were incubated with 45Ca2+, and a single turnover of EP was initiated by adding ATP and excess EGTA using the handmade rapid mixing apparatus. After chasing the reaction, the mixture was spotted on the membrane filter and washed for ∼10 s by an EGTA solution, as described in the figure legends. The background level of 45Ca2+ was determined without ATP and subtracted. This background level was <3% of the maximum Ca2+ uptake level.
Miscellaneous
All of the reactions were performed at 4 °C in 7 mm MgCl2 and 50 mm MOPS/Tris (pH 7.3). Protein concentrations were determined by the method of Lowry et al. (39) with bovine serum albumin as a standard. Free Ca2+ concentrations were calculated by the Calcon program. Data were analyzed by nonlinear regression using the program Origin (Microcal Software, Inc., Northampton, MA). Three-dimensional models of the enzyme were produced by the program VMD (40).
RESULTS
Time Courses of EP Decay and Ca2+ Release
The Ca2+-ATPase in SR vesicles was phosphorylated with MgATP in the presence of 0.1 m K+, 10 μm Ca2+, and Ca2+-ionophore A23187 (Fig. 2, A and B). The reaction reaches steady state within a few seconds, and almost all of the Ca2+-ATPase is in the ADP-sensitive form of EP (E1P) because of the rate-limiting E1P to E2P transition followed by rapid E2P hydrolysis in the presence of K+ (29, 30).
When the reaction was chased with excess EGTA in 0.1 m K+, the amount of EP decreases in a single exponential time course, and the EP during the decay is almost all ADP sensitive (Fig. 2A). The bound Ca2+ decreases concomitantly with E1P decay, i.e. E1PCa2 to E2P transition. The result agrees with the established mechanism that the two Ca2+ ions are occluded in E1PCa2, and Ca2+ release into the lumen occurs very rapidly after the rate-limiting E1PCa2 to E2PCa2 transition, E1PCa2 → E2PCa2 → E2P + 2Ca2+ (11–14). Thus, the EP transition and Ca2+ release are tightly coupled in the presence of K+.
Surprisingly, when E1PCa2 formed as above in 0.1 m K+, and A23187 was chased with excess EGTA in the absence of K+, the Ca2+ release is considerably (∼3×) faster than the E1P decay via its transition to E2P (Fig. 2B). The result shows that in the absence of K+, there is an E1P species without bound Ca2+ and that the Ca2+ ions are released from E1PCa2. We found essentially the same results in the presence of choline chloride in place of LiCl without K+ (data not shown).
45Ca2+ Uptake in Single Turnover of E1PCa2
We then examined whether this rapid Ca2+ release from E1PCa2 in the absence of K+ upon the EGTA chase occurs to the lumenal side or cytoplasmic side of the membrane. For this purpose, we performed a 45Ca2+ uptake assay in a single turnover of E1PCa2 in the absence of ionophore, i.e. with sealed SR vesicles. In Fig. 3, for the single turnover of E1PCa2, the Ca2+-ATPase in the vesicles in 10 μm Ca2+ was phosphorylated by a simultaneous addition of [γ-32P]ATP and excess EGTA in either the presence or the absence of 0.1 m K+. Approximately half of the ATPase is phosphorylated rapidly to form E1PCa2 both in the presence and absence of K+, and then EP decays slowly, in contrast to the full phosphorylation achieved without the removal of Ca2+. In sealed vesicles (without A23187), EP decays at the same rate in the presence or absence of K+. In the presence of K+, nearly all EP is E1P (ADP-sensitive), whereas in the absence of K+, E2P increases slowly to ∼20% at ∼2 s of the maximum amount of EP formed immediately after the ATP addition.
Then, in Fig. 4 (closed circles), the 45Ca2+ uptake assay during a single turnover of E1PCa2 was performed by membrane filtration with an EGTA chase, i.e. with extensive EGTA washing of the filter for ∼10 s under otherwise the same conditions as in the single turnover of E1PCa2 in Fig. 3. During the ∼10 s of EGTA washing, nearly all EP was dephosphorylated (Fig. 3) as we intended; therefore, all of the bound 45Ca2+ in EP was released even at the first time point (0.1 s after the start when nearly all EP is E1PCa2) either to the cytoplasmic side or lumenal side. If released to the cytoplasmic side, the 45Ca2+ will be lost from the filter by the EGTA wash, and levels will be reduced significantly from the ideal stoichiometry of two Ca2+ ions transported in a single turnover of E1PCa2. However, the results (closed circles) clearly show a maximum uptake of ∼1.7 Ca2+ per EP in 0.1 m K+ and an even higher uptake of 1.8∼1.9 without K+, very close to the ideal stoichiometry. Therefore, during a single turnover, the bound 45Ca2+ ions in E1PCa2 formed in the absence of K+ are not released to the cytoplasmic side but to the lumen. It is concluded that in E1PCa2 without K+, the cytoplasmic gate is closed, but a Ca2+ pathway to the lumen exists. Thus, the Ca2+ binding sites face the lumen.
ADP Chase during Single Turnover of 45Ca2+ Uptake
In Fig. 4 (open circles), we assessed at each time point during the single turnover of E1PCa2 the amount of 45Ca2+ remaining on the filter with the vesicles. For this purpose, we chased the reaction with ADP and excess EGTA at each time point, i.e. dephosphorylating to E1Ca2 very rapidly in the reverse reaction and removing 45Ca2+ released to the cytoplasmic side. Both in the presence and absence of K+, at 0.1 s (first time point) immediately after the ATP/EGTA addition, nearly maximum EP is already formed (all E1PCa2, Fig. 3), and all of the bound 45Ca2+ is removed by the ADP chase. Then, in the presence of 0.1 m K+ (A), the ADP-insensitive fraction and the amount of 45Ca2+ released into the lumen increased exponentially due to the forward E1PCa2 decay via its transition to E2P with Ca2+ release as expected from the established transport mechanism. In fact, the time course agreed with that of EP decay via the rate-limiting E1PCa2 to E2P transition (Fig. 3, closed triangles).
On the other hand, in the absence of K+ (B), 45Ca2+, and the ADP-insensitive fraction increase very rapidly (within the initial ∼0.5 s) and suddenly slow, showing a clear biphasic time course. The second slow phase occurs at nearly the same rate as the EP decay via the E1P to E2P transition (Fig. 3, open triangles) and the single exponential 45Ca2+ uptake in the presence of K+ (A), i.e. the normal transport process E1PCa2 → E2PCa2 → E2P + 2Ca2+. The initial rapid phase occurs at a significantly faster rate and to a higher extent than in E2P formation (Fig. 3) and therefore cannot be accounted for simply by formation of E2P. Actually, the initial phase is even faster than the Ca2+ release from K+-free E1PCa2 revealed upon excess EGTA addition (without ADP) in A23187 in Fig. 2. The results suggest that, in K+-free E1PCa2, different types of Ca2+ sites are produced in the initial rapid phase; the Ca2+ ions are not released to the cytoplasmic side even upon ADP-induced reverse dephosphorylation.
Behavior of 45Ca2+ at Site I in E1PCa2
We examined whether the above observed biphasic kinetics revealed by the ADP chase is related to the heterogeneity of the Ca2+ sites I and II in E1Ca2. In E1Ca2, Ca2+ bound at site II is rapidly exchanged with the cytoplasmic Ca2+, and the Ca2+ bound at the deeper site I can be released to the cytoplasm only when site II is vacant (15, 41–44). Therefore, we first labeled site I with 45Ca2+ by exchanging the site II-bound 45Ca2+ with nonradioactive Ca2+ (supplemental Fig. S1). In Fig. 5B, we clearly observed a biphasic 45Ca2+ increase in the ADP-insensitive fraction in the absence of K+ as in Fig. 4B. The only difference is that, as expected, the total amount of 45Ca2+ uptake (0.8–1.0 Ca2+ per EP) is half of that in Fig. 4 in which both sites I and II are labeled by 45Ca2+. The results show that the heterogeneity of the two Ca2+ sites I and II in E1Ca2 is not related to the biphasic 45Ca2+ increase revealed by the ADP chase in Fig. 4B. Furthermore, we observed a nonsequential release of two Ca2+ ions from E1PCa2 to the lumenal side upon removal of free Ca2+ in the presence of A23187 without ADP, which therefore is not related to the biphasic 45Ca2+ increase in Fig. 4B (supplemental Fig. S2) (58, 59).
These results show that there are two different types of E1PCa2, i.e. the normal Ca2+-occluded E1PCa2 and another E1PCa2 species that possesses lumen-facing Ca2+ binding sites (opened lumenal pathway) and a closed cytoplasmic gate. The results further indicate that in the absence of K+, the E1PCa2 species with the lumen-facing Ca2+ binding sites is rapidly produced from normal E1PCa2, and this process is revealed by the ADP chase as the initial rapid phase in Fig. 4B (see more in “Discussion” and a schematic model in Fig. 7).
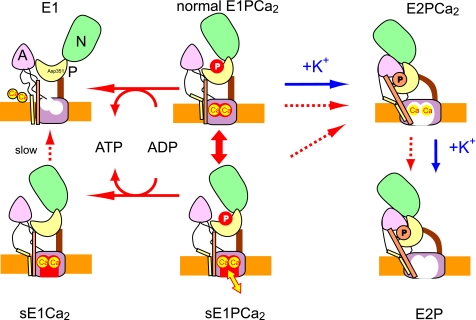
FIGURE 7.
Schematic model for roles of K+ in EP processing and Ca2+ handling in Ca2+ transport. sE1PCa2 is an E1PCa2 species formed without K+ possessing a closed cytoplasmic gate and lumen-facing Ca2+ binding sites (an opened lumenal pathway) with high Ca2+ affinity (Fig. 6). sE1PCa2 is in rapid equilibrium with the normal E1PCa2. Here, s denotes silent because this species is apparently absent in the presence of K+ and also because the bound Ca2+ ions are not released to the cytoplasmic side even upon ADP-induced reverse dephosphorylation (to sE1Ca2) in contrast to the normal E1PCa2 reverse dephosphorylation. Actual active Ca2+ transport is achieved by a large reduction of the Ca2+ affinity during the normal sequence E1PCa2 → E2PCa2 → E2P + 2Ca2+ (blue arrows). The schematic is based on crystal structural models for the ADP-sensitive and -insensitive EP states and E1Ca2, with the positions of the cytoplasmic N, P, and A domains, and membrane (orange layer) being approximate. The Ca2+ sites in the transmembrane domain are depicted as occluded (closed cytoplasmic and lumenal gates) in normal E1PCa2, as lumen-facing and high Ca2+ affinity with the closed cytoplasmic gate in sE1PCa2 and sE1Ca2, and as lumenally opened with reduced Ca2+ affinity in E2P and E2PCa2 (immediately before the Ca2+ release).
Affinity of E1P for Lumenal Ca2+ in Absence of K+
In Fig. 6, we assessed the Ca2+ affinity of the transport sites exposed to the lumen in K+-free E1PCa2 by determining the Ca2+ binding to E1P in steady state in the presence of A23187. In Fig. 6A, the total amount of EP increased with increasing Ca2+ concentration and reached its maximum level at ∼0.5 μm Ca2+ due to high affinity Ca2+ binding at the transport sites (E2 to E1Ca2 transition). The total amount of EP at saturating Ca2+ was half of the maximum Ca2+ binding in E1Ca2 (B); therefore, all Ca2+-ATPases are phosphorylated at saturating Ca2+. As replotted in Fig. 6C, ∼60% of the maximum total amount of EP was E1P in steady state at saturating Ca2+ under these conditions.
In Fig. 6B, the amount of bound Ca2+ in steady state in the presence of A23187 was determined without washing the filter so as not to alter the equilibrium. As replotted in Fig. 6C with % values relative to the maximum Ca2+ binding in E1Ca2, the bound Ca2+ under the phosphorylating condition without K+ increases concomitantly with an increase in E1P, and their relative values are nearly the same. Note that if the affinity of the lumen-facing Ca2+ sites of E1P without K+ is significantly lower than that of the high Ca2+ affinity in E1 for the phosphorylation, the Ca2+ binding curve would be shifted significantly to higher Ca2+ concentrations, and the relative value of the bound Ca2+ would become significantly smaller than that of E1P in the 0.1–10 μm range. However, this is obviously not the case. We conclude that the affinity of the lumen-facing Ca2+ sites of K+-free E1P is as high as the cytoplasmic Ca2+ affinity in E1.
DISCUSSION
Ca2+ Release from E1PCa2 in Absence of K+
Our studies show that in the absence of K+, Ca2+ is released from E1PCa2 to the lumenal side. This Ca2+ release obviously precedes the conversion of the ADP-sensitive EP (E1P) to ADP-insensitive one (E2P); thus, there is a K+-free E1P species without bound Ca2+ (Fig. 2B). Evidently, a Ca2+ pathway from the transport sites to the lumen is open at least to some extent in this species. K+, probably bound to its specific site in the ATPase (28), therefore plays a critical role in E1PCa2 to stabilize the transport sites in an occluded state. Notable also is our finding that the Ca2+ affinity of the sites facing the lumen in K+-free E1PCa2 is as high as the cytoplasmic Ca2+ affinity in the unphosphorylated E1 state (Fig. 6). Thus, the Ca2+ binding sites are not disrupted in this K+-free E1PCa2 structure, suggesting that the opening of the lumenal Ca2+ pathway does not involve large structural changes such as those that occur during the EP conformation change. The observation also means that such a Ca2+-ATPase species cannot be involved in producing a Ca2+ gradient across the membrane and therefore is unlikely to contribute significantly to active Ca2+ transport. This is because, without a reduction in Ca2+ affinity, lumenal Ca2+ would rebind at low concentrations and inhibit the pump.
Our kinetic analysis of the lumenal Ca2+-induced reverse conversion E2P + 2Ca2+ ↔ E2PCa2 ↔ E1PCa2 in wild type Ca2+-ATPase (13) has revealed that the K+ in E2P is critical for lowering the lumenal Ca2+ affinity and for fully opening the lumenal gate, thereby accomplishing the high physiological Ca2+ gradient and rapid Ca2+ release E2PCa2 → E2P + 2Ca2+. K+ stabilizes the E2P structure with disrupted Ca2+ sites and a fully open lumenal gate. In the absence of K+, the lumenal Ca2+ affinity of E2P is ∼2000 times lower than in E1P (K0.5 values, 0.4 mm (13) and 0.15 μm (Fig. 6), respectively). Therefore, the large structural change associated with the EP conformation change is obviously required, even in the absence of K+, for disrupting the Ca2+ sites. K+ binding in E2PCa2/E2P further reduces the Ca2+ affinity to a level (K0.5 value, 1.5 mm (13)) appropriate for producing the high physiological Ca2+ gradient across the membrane.
Thus, bound K+ stabilizes both the Ca2+ occluded structure of E1PCa2 and the Ca2+-released structure of E2P. Thereby, K+ critically contributes to the successive structural changes and ensures strict and efficient coupling for EP processing and Ca2+ handling in E1PCa2 → E2PCa2 → E2P + 2Ca2+, key events for Ca2+ transport. Also notable is the fact that the K+ bound in the P domain is crucial for producing a catalytic site structure in E2P appropriate for its accelerated hydrolysis (28–30).
Biphasic Ca2+ Release in ADP Chase of Single Turnover of E1PCa2 without K+
In Fig. 7, we provide a schematic model to show the roles of K+ in the Ca2+ transport and to account for the biphasic Ca2+ release from K+-free E1PCa2 following an ADP chase during a single turnover (Fig. 4B, open circles). The fast initial phase may be accounted for by the rapid formation of sE1PCa2, with lumen-facing, high affinity Ca2+ binding sites, in rapid equilibrium with normal E1PCa2. The bound 45Ca2+ ions cannot be released to the cytoplasmic side even upon ADP-induced reverse dephosphorylation (sE1Ca2) but only to the lumenal side (yellow arrow). Because sE1P has high affinity, Ca2+ rebinding occurs at low lumenal concentrations3 and inhibits flux through this pathway. The slow second phase (Fig. 4B) most probably reflects the E1PCa2 to E2P transition as in the single exponential Ca2+ uptake in 0.1 m K+ (Fig. 4A and Fig. 7, blue arrows). The formation of sE1PCa2 in rapid equilibrium with occluded E1PCa2 necessarily lowers the steady state level of the latter species and hence Ca2+ transport through the normal route. Thus, although progression to sE1PCa2 is relatively fast, this pathway cannot contribute to gradient formation and ultimately slows normal transport. It is concluded that K+ ensures the normal structural process for Ca2+ transport (blue arrows) by stabilizing the Ca2+-occluded structure of E1PCa2 and disallowing opening of a lumenal Ca2+ pathway (this study), and by stabilizing the E2P structure with disrupted Ca2+ sites (greatly reduced affinity) and a fully opened lumenal gate (13).
Structural Role of Bound K+ in E1PCa2
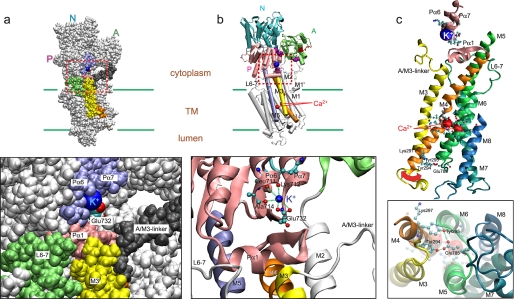
The crystal structures provide a likely structural role of bound K+ in E1PCa2. In structures analogous to K+-bound E1PCa2 (E1PCa2·AMPPN (22) and E1Ca2·AlF4− ·ADP as well as E1Ca2·AMPPCP (17)), K+ is specifically bound at the bottom part of the P domain and coordinated by the backbone carbonyl oxygens of Leu711, Lys712, and Ala714 on Pα6 (sixth P-domain α-helix) (near the catalytic Mg2+ site Asp703/Asp707 on Pα5 of this region) and by the Glu732 side chain oxygen on Pα7 (Fig. 8). The importance of Glu732 in the K+-induced acceleration of E2P hydrolysis was shown through mutations (28).
FIGURE 8.
Structure of E1PCa2 with bound K+. The crystal structure E1PCa2·AMPPN (Protein Data Bank code 3BA6) (22) is shown. Panel a, a space-filling model with K+ (blue), M3 (yellow), M4 (orange), L6–7 (lime), Pα1 (pink), Pα6/Pα7 (light purple), Glu732 (red and cyan), and A/M3-linker (dark gray). b, a schematic model with the view from the same direction as in a. K+ and Ca2+ are blue and red Van der Waals spheres, respectively. M3, M4, and M5 are yellow, orange, and light purple, respectively. The lower panels in a and b are the enlarged views of the areas surrounded by red broken line. In b, the coordination of K+ is shown by broken green lines. c, the helices for Ca2+ binding (M4, M5, M6, and M8) and K+ binding (Pα6/Pα7) and adjacent components (M3, M7, Pα1, and A/M3-linker) are depicted. A large red arrow suggests a possible motion of M3/M4L at the lumenal end to open the Ca2+ pathway. The residues involved in coordination of K+ and Ca2+ are depicted in ball and stick representation. Residues possibly forming interactions at the lumenal end (Tyr294, Tyr295, Lys297, and Glu785) are also depicted. The lower panel in c shows the view from the lumenal side.
The K+ ion and these ligands are distant from and not in direct contact with the transport sites from which Ca2+ release occurs. On the other hand, adjacent to the K+ binding site on Pα6/Pα7 is Pα1, which is directly linked with the cytoplasmic end of M4 within the P domain. Pα6, Pα7, and Pα1 constitute the bottom part of one-half of the P domain and move together as a body during the transport cycle (7, 18). Furthermore, Pα1 forms a hydrogen-bonding network with L6–7 (a cytoplasmic short loop linking M6 and M7) and top parts of M3/M5. This interaction network is critical for proper arrangement of the transmembrane helices (48–50). In fact, disruption of this network by mutations causes a marked retardation of the E2-E1 transition (48, 49).
Because the bound K+ is deeply embedded and ligated within this part of the P domain (Fig. 8a), its absence would allow more flexibility of the structural components, such as segmental fluctuations or wobbling, which in turn would impinge on the cytoplasmic regions of the transmembrane helices and probably destabilize the interaction network Pα1/L6–7/M3/M5. The absence of K+ in fact markedly retards the E2 to E1 transition (31, 32), and, as noted above, disruption of the Pα1/M3/M5/L6–7 interaction network markedly retards the E1-E2 transition and also the E1P to E2P conformation change (48–50). Opening of the lumenal pathway and Ca2+ release from E1PCa2 may be caused by such structural perturbations in the absence of bound K+.
As shown in the view from the lumen of the helices M4/M5/M6/M8 ligating Ca2+ in Fig. 8c, the space surrounded by these helices seems to be the only possible Ca2+ exit pathway. M3 is in close contact at the lumenal end with the lumenal part of M4 (M4L), and they are connected by a short lumenal loop (L3–4). During the EP conformation change and subsequent Ca2+ release (E1PCa2 → E2P + 2Ca2+), M3 and M4L incline together and move outward, thereby opening the putative Ca2+ release pathway (lumenal gate) (19). The M3/M4L motion is produced by the large rotation and inclination of the A and P domains and by the consequent significant motions and rearrangements of the helices M1∼6, in which M1/M2 as a rigid body pushes M4L to open the Ca2+ release gate (Fig. 9) (19). The large motions concomitantly disrupt the Ca2+ binding sites and reduce the Ca2+ affinity (19). In K+-free E1PCa2 (ADP sensitive), these domain motions have not yet taken place, and the Ca2+ sites are not disrupted and maintain a high affinity. Here, these motions are likely much less prominent and opening of the release pathway is simply the result of fluctuations and wobbling of the relevant helices, in particular M3/M4L.
The unique Ca2+ coordination and particular make up of the M3 and M4 helices lend themselves to creating a release pathway while maintaining a high affinity. The Ca2+ sites with properly positioned ligands are located at an unwound portion of the M4 helix creating intrinsic flexibility (Fig. 8). On the other hand, M3 is a continuous helix from the cytoplasmic to the lumenal end and is located at the periphery of the transmembrane domain and is not closely associated with other helices including M1/M2 (except for M4L at the lumenal end). Thus, in the crystal structures analogous to E1PCa2, M3 seems not to have much steric restriction against possible outward movement, a shift that would open the Ca2+ pathway. Therefore, if the cytoplasmic region of M3 is not fixed as occurs in the absence of bound K+, its lumenal part and the associated M4L may become more mobile. Wobbling here could allow the Ca2+ pathway to fluctuate between a closed and open state. The Ca2+ sites are not necessarily disrupted because of the flexibility of the unwound structure of M4 and because the large motions of the A–P domains do not occur. (These are the motions that disrupt the Ca2+ sites by inclining the cytoplasmic region of M4/M5.) Also, M3 is not involved directly in the Ca2+ ligation.
Interestingly, at the lumenal end of M4L (Fig. 8c), there are bulky and hydrophobic residues (Tyr294/Tyr295/Lys297), which may form hydrogen bonds, e.g. Tyr294/Tyr295 with Glu785 on L5–6. Lys297 seems to seal the Ca2+ channel (51). Tyr295 is important for Ca2+ transport activity and stabilizing E2 relative to E1 (52). These residues may possibly function as the lumenal plug, and M3/M4L wobbling may destabilize their interactions helping to open the Ca2+ pathway in K+-free E1PCa2.
Importantly, in the crystal structures of analogues of E1PCa2, the cytoplasmic Ca2+ gate is closed by the Ca2+ ligand Glu309 because Leu65 on M1 locks the Glu309 side chain configuration by van der Waals contact (8, 9, 18, 53). Our observation shows that this cytoplasmic gate is closed in E1PCa2 even without bound K+, and therefore, the Glu309-gating with Leu65 has not been affected.
Movement of K+ Binding Site during E1PCa2 → E2P + 2Ca2+
K+-bound crystal structures E1PCa2·AMPPN and E2·AlF4− may be used as a model for the overall change in E1PCa2 → E2P + 2Ca2+ (Fig. 9). Hence, the P domain inclines to the A domain that also rotates and inclines (curved arrows), thus producing the A–P domain association in the most compactly organized and inclined headpiece structure, the Ca2+-released E2P. With this change, the cytoplasmic region of M4/M5 in the P domain inclines and disrupts the Ca2+ sites (19). M2 inclines with the A domain motion and consequently M1, which forms a rigid V-shaped body with M2, pushes against the lumenal part of M4, and opens the lumenal gate (19).
In these structural changes, the K+ site with bound K+ on the P domain moves down to the Gln244 region on the A/M3-linker (blue arrow) and brings in the Gln244 side chain (or neighboring residues) as an additional coordination ligand. Thus bound K+ likely cross-links the bottom part of the P domain and the A/M3-linker. This cross-link must contribute to the stabilization of the compactly organized and inclined E2P structure with disrupted Ca2+ sites and fully opened lumenal gate (13).
The A/M1′-linker of correct length has a critical function in inclining and compacting the E2P structure (14, 27). The structure is stabilized by three critical interaction networks; at the Tyr122 HC (hydrophobic interaction cluster involving the A and P domains and M2), at the Val200 loop (ionic and hydrogen bonding interactions with the P domain residues), and at the TGES184 loop (hydrogen bonding interactions with the P domain residue in the catalytic site) (10–14). The TGES184 loop of the rotated A domain protrudes into the catalytic site and blocks attack of ADP on the Asp351 phosphate (causing the loss of ADP sensitivity). The Tyr122 HC is produced upon A–P domain inclination induced by tension on the A/M1′-linker (14, 27) and is critical for reducing the Ca2+ affinity and opening the lumenal gate, i.e. to deocclude/release Ca2+, E2PCa2 → E2P (11–13). All of the interaction networks are essential for these changes and are also necessary for the formation of the catalytic site with hydrolytic activity (10–13). Importantly, the Val200 loop and Tyr122 HC are situated at the top and bottom of the A–P domain interface, respectively, and bound K+ is lower down and close to the membrane domain. Thus, these interaction networks including the K+ site are situated at positions most appropriate for stabilizing the compactly organized and inclined (thus strained) structure of E2P.
Ca2+ Release into Cytoplasm and Uncoupling
It was previously observed with SR Ca2+-ATPase (54–57) that Ca2+ in E1PCa2 can be released to cytoplasm upon direct hydrolysis to E1Ca2 (not via its transition to E2P) under specific conditions such as with a raised lumenal Ca2+ level. This causes ATP hydrolysis without Ca2+ transport resulting in uncoupling. de Meis and co-workers (54–56) further suggested that such uncoupled ATP hydrolysis functions as a heat-producing entity. This finding obviously differs from ours in that in K+-free E1PCa2, the Ca2+ release pathway into the lumen is open, and the phosphoenzyme is not directly hydrolyzed.
In summary, we have found that E1PCa2 without bound K+ has a perturbed structure with at least a partially open lumenal Ca2+ release pathway and still with the Ca2+ sites maintaining a high affinity. Thus, in the natural E1PCa2 structure, bound K+ stabilizes the Ca2+ in an occluded form by not allowing the pathway to open. Bound K+ also stabilizes E2P following disruption of the Ca2+ sites and full opening of the lumenal gate (13). Thus, bound K+ has a crucial role in EP processing and Ca2+ occlusion and release to the lumen in the sequence E1PCa2 → E2PCa2 → E2P + 2Ca2+.
Supplementary Material
Acknowledgments
We are grateful to Dr. Chikashi Toyoshima (University of Tokyo) for helpful discussions. We thank Dr. David B. McIntosh for reviewing and improving the manuscript.
This work was supported by a grant-in-aid for scientific research C (to K. Y.) and B (to H. S.) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1 and S2.
Note that the intravesicular volume of SR vesicles has been estimated to be in the range of 2–10 μl/mg protein (45, 46), and therefore, the release of Ca2+ bound in EP (∼8 nmol/mg protein) into the lumen in a single turnover might increase the lumenal Ca2+ to ∼0.8–4 mm. Although a fair amount of lumenal free Ca2+ may be removed by low affinity Ca2+ buffers such as calsequestrin (47), even a small rise in the lumenal Ca2+ level might result in rebinding of lumenal Ca2+ to sE1P because of its high affinity revealed in Fig. 6 (yellow arrow in Fig. 7).
- SR
- sarcoplasmic reticulum
- EP
- phosphoenzyme
- E1P
- ADP-sensitive phosphoenzyme
- E2P
- ADP-insensitive phosphoenzyme
- HC
- hydrophobic interaction cluster
- MOPS
- 3-(N-morpholino) propanesulfonic acid.
REFERENCES
- 1.Hasselbach W., Makinose M. (1961) Biochem. Z. 333, 518–528 [PubMed] [Google Scholar]
- 2.Ebashi S., Lipmann F. (1962) J. Cell Biol. 14, 389–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Inesi G., Sumbilla C., Kirtley M. E. (1990) Physiol. Rev. 70, 749–760 [DOI] [PubMed] [Google Scholar]
- 4.Møller J. V., Juul B., le Maire M. (1996) Biochim. Biophys. Acta 1286, 1–51 [DOI] [PubMed] [Google Scholar]
- 5.MacLennan D. H., Rice W. J., Green N. M. (1997) J. Biol. Chem. 272, 28815–28818 [DOI] [PubMed] [Google Scholar]
- 6.McIntosh D. B. (1998) Adv. Mol. Cell. Biol. 23A, 33–99 [Google Scholar]
- 7.Toyoshima C., Inesi G. (2004) Annu. Rev. Biochem. 73, 269–292 [DOI] [PubMed] [Google Scholar]
- 8.Toyoshima C. (2008) Arch. Biochem. Biophys. 476, 3–11 [DOI] [PubMed] [Google Scholar]
- 9.Toyoshima C. (2009) Biochim. Biophys. Acta 1793, 941–946 [DOI] [PubMed] [Google Scholar]
- 10.Kato S., Kamidochi M., Daiho T., Yamasaki K., Gouli W., Suzuki H. (2003) J. Biol. Chem. 278, 9624–9629 [DOI] [PubMed] [Google Scholar]
- 11.Yamasaki K., Daiho T., Danko S., Suzuki H. (2004) J. Biol. Chem. 279, 2202–2210 [DOI] [PubMed] [Google Scholar]
- 12.Wang G., Yamasaki K., Daiho T., Suzuki H. (2005) J. Biol. Chem. 280, 26508–26516 [DOI] [PubMed] [Google Scholar]
- 13.Yamasaki K., Wang G., Daiho T., Danko S., Suzuki H. (2008) J. Biol. Chem. 283, 29144–29155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Daiho T., Yamasaki K., Danko S., Suzuki H. (2007) J. Biol. Chem. 282, 34429–34447 [DOI] [PubMed] [Google Scholar]
- 15.Toyoshima C., Nakasako M., Nomura H., Ogawa H. (2000) Nature 405, 647–655 [DOI] [PubMed] [Google Scholar]
- 16.Toyoshima C., Nomura H. (2002) Nature 418, 605–611 [DOI] [PubMed] [Google Scholar]
- 17.Sørensen T. L., Møller J. V., Nissen P. (2004) Science 304, 1672–1675 [DOI] [PubMed] [Google Scholar]
- 18.Toyoshima C., Mizutani T. (2004) Nature 430, 529–535 [DOI] [PubMed] [Google Scholar]
- 19.Toyoshima C., Nomura H., Tsuda T. (2004) Nature 432, 361–368 [DOI] [PubMed] [Google Scholar]
- 20.Olesen C., Sørensen T. L.., Nielsen R. C., Møller J. V., Nissen P. (2004) Science 306, 2251–2255 [DOI] [PubMed] [Google Scholar]
- 21.Toyoshima C., Norimatsu Y., Iwasawa S., Tsuda T., Ogawa H. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 19831–19836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olesen C., Picard M., Winther A. M., Gyrup C., Morth J. P., Oxvig C., Møller J. V., Nissen P. (2007) Nature 450, 1036–1042 [DOI] [PubMed] [Google Scholar]
- 23.Danko S., Daiho T., Yamasaki K., Kamidochi M., Suzuki H., Toyoshima C. (2001) FEBS Lett. 489, 277–282 [DOI] [PubMed] [Google Scholar]
- 24.Danko S., Yamasaki K., Daiho T., Suzuki H., Toyoshima C. (2001) FEBS Lett. 505, 129–135 [DOI] [PubMed] [Google Scholar]
- 25.Danko S., Yamasaki K., Daiho T., Suzuki H. (2004) J. Biol. Chem. 279, 14991–14998 [DOI] [PubMed] [Google Scholar]
- 26.Danko S., Daiho T., Yamasaki K., Liu X., Suzuki H. (2009) J. Biol. Chem. 284, 22722–22735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Daiho T., Danko S., Yamasaki K., Suzuki H. (2010) J. Biol. Chem. 285, 24538–24547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sørensen T. L., Clausen J. D., Jensen A. M., Vilsen B., Møller J. V., Andersen J. P., Nissen P. (2004) J. Biol. Chem. 279, 46355–46358 [DOI] [PubMed] [Google Scholar]
- 29.Shigekawa M., Pearl L. J. (1976) J. Biol. Chem. 251, 6947–6952 [PubMed] [Google Scholar]
- 30.Shigekawa M., Dougherty J. P. (1978) J. Biol. Chem. 253, 1451–1457 [PubMed] [Google Scholar]
- 31.Medda P., Fassold E., Hasselbach W. (1987) Eur. J. Biochem. 165, 251–259 [DOI] [PubMed] [Google Scholar]
- 32.Lee A. G., Baker K., Khan Y. M., East J. M. (1995) Biochem. J. 305, 225–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Champeil P., Henao F., de Foresta B. (1997) Biochemistry 36, 12383–12393 [DOI] [PubMed] [Google Scholar]
- 34.Nakamura S., Suzuki H., Kanazawa T. (1994) J. Biol. Chem. 269, 16015–16019 [PubMed] [Google Scholar]
- 35.Barrabin H., Scofano H. M., Inesi G. (1984) Biochemistry 23, 1542–1548 [DOI] [PubMed] [Google Scholar]
- 36.Weber K., Osborn M. (1969) J. Biol. Chem. 244, 4406–4412 [PubMed] [Google Scholar]
- 37.Daiho T., Suzuki H., Yamasaki K., Saino T., Kanazawa T. (1999) FEBS Lett. 444, 54–58 [DOI] [PubMed] [Google Scholar]
- 38.Kanazawa T., Saito M., Tonomura Y. (1970) J. Biochem. 67, 693–711 [DOI] [PubMed] [Google Scholar]
- 39.Lowry O. H., Rosebrough N. J., Farr A. L., Randall R. J. (1951) J. Biol. Chem. 193, 265–275 [PubMed] [Google Scholar]
- 40.Humphrey W., Dalke A., Schulten K. (1996) J. Mol. Graph. 14, 33–38 [DOI] [PubMed] [Google Scholar]
- 41.Nakamura J. (1989) J. Biol. Chem. 264, 17029–17031 [PubMed] [Google Scholar]
- 42.Inesi G. (1987) J. Biol. Chem. 262, 16338–16342 [PubMed] [Google Scholar]
- 43.Petithory J. R., Jencks W. P. (1988) Biochemistry 27, 8626–8635 [DOI] [PubMed] [Google Scholar]
- 44.Orlowski S., Champeil P. (1991) Biochemistry 30, 352–361 [DOI] [PubMed] [Google Scholar]
- 45.Malan N. T., Sabbadini R., Scales D., Inesi G. (1975) FEBS Lett. 60, 122–125 [DOI] [PubMed] [Google Scholar]
- 46.Duggan P. F., Martonosi A. (1970) J. Gen. Physiol. 56, 147–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fleischer S., Inui M. (1989) Annu. Rev. Biophys. Biophys. Chem. 18, 333–364 [DOI] [PubMed] [Google Scholar]
- 48.Clausen J. D., Andersen J. P. (2004) J. Biol. Chem. 279, 54426–54437 [DOI] [PubMed] [Google Scholar]
- 49.Lenoir G., Picard M., Møller J. V., le Maire M., Champeil P., Falson P. (2004) J. Biol. Chem. 279, 32125–32133 [DOI] [PubMed] [Google Scholar]
- 50.Zhang Z., Lewis D., Sumbilla C., Inesi G., Toyoshima C. (2001) J. Biol. Chem. 276, 15232–15239 [DOI] [PubMed] [Google Scholar]
- 51.Chen L., Sumbilla C., Lewis D., Zhong L., Strock C., Kirtley M. E., Inesi G. (1996) J. Biol. Chem. 271, 10745–10752 [DOI] [PubMed] [Google Scholar]
- 52.Lee A. G. (2002) Biochim. Biophys. Acta 1565, 246–266 [DOI] [PubMed] [Google Scholar]
- 53.Einholm A. P., Vilsen B., Andersen J. P. (2004) J. Biol. Chem. 279, 15888–15896 [DOI] [PubMed] [Google Scholar]
- 54.de Meis L. (2001) J. Biol. Chem. 276, 25078–25087 [DOI] [PubMed] [Google Scholar]
- 55.Reis M., Farage M., de Souza A. C., de Meis L. (2001) J. Biol. Chem. 276, 42793–42800 [DOI] [PubMed] [Google Scholar]
- 56.Barata H., de Meis L. (2002) J. Biol. Chem. 277, 16868–16872 [DOI] [PubMed] [Google Scholar]
- 57.Yu X., Inesi G. (1995) J. Biol. Chem. 270, 4361–4367 [DOI] [PubMed] [Google Scholar]
- 58.Hanel A. M., Jencks W. P. (1991) Biochemistry 30, 11320–11330 [DOI] [PubMed] [Google Scholar]
- 59.Orlowski S., Champeil P. (1991) Biochemistry 30, 11331–11342 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.