Abstract
Intimal hyperplasia (IH) remains the major cause of intermediate and long-term failure of vascular grafts and endovascular interventions. Arteries are subjected to a significant longitudinal stress in addition to the shear stress and tensile stress from the blood flow. The aim of this study was to determine the effect of axial stretch on cell proliferation and IH in arteries. Porcine carotid arteries, intact or endothelial cell (EC) denudated, were maintained ex vivo at different stretch ratios (1.3, 1.5, and 1.8) and flow rates (16 or 160 mL/min) while remaining at physiologic pressure for 7 days. The viability of the arteries was verified with norepinephrine, carbachol, and sodium nitroprusside stimulations, and the cell proliferation was detected using bromodeoxyuridine labeling and immunostaining. Our results showed that the axial stretch ratio did not significantly affect intimal thickness and cell proliferation in normal arteries. However, axial stretch increased the neointimal thickness in EC denudated arteries cultured under low flow conditions. The cell proliferation increased significantly in the intima and inner half of the media of the EC denudated arteries under normal or elevated axial stretch in comparison to intact arteries at the same stretch ratio. These results demonstrated that axial stretch with EC denudation and low flow increases neointimal formation and cell proliferation in the arteries.
Keywords: Longitudinal stretch, Axial strain, Endothelial cell denudation, Neointima, Cell proliferation, Ex vivo, Blood vessel, Porcine
INTRODUCTION
Intimal hyperplasia (IH) remains the major cause of intermediate and long-term failure of vascular grafts and endovascular interventions.3,26,42 IH is characterized by migration and proliferation of vascular smooth muscle cells (VSMC) with deposition of extracellular components in the intima.31,38 It decreases lumen size and, together with thrombosis and atherosclerosis, eventually leads to total failure of vascular grafts and endovascular interventions.3,26,42 For example, the recurrence rate of in-stent restenosis after conventional percutaneous transluminal coronary angioplasty has been reported to be 10–60%.27,36 The cumulative primary patency rates for iliac endovascular procedures are 76%, 59%, and 49% at 1, 3, and 5 years, respectively. 27 Therefore, it is important to understand the causes and development of IH.
The main factors that provoke IH include lumen injury and mechanical stresses in arteries. Denudation of the endothelial layer occurs during endovascular procedures and leads to intimal thickening due to deposition of platelets, monocytes, extracellular matrix, and proliferation and migration of VSMC into the intimal layer.1,10,12,48 Increased circumferential stretch caused by high blood pressure or stent insertion promotes VSMC proliferation and IH.9,11 Intermittent and chronic reduction of blood flow also induces development of IH.25,28,33,37 Ward et al. showed that low flow after angioplasty significantly increased VSMC proliferation and IH in comparison to normal and high flow.46
Arteries in vivo are also under significant axial tension that changes due to aging, vascular grafting, or reconstructive vascular surgery.16,20,34,35,40 Coronary arteries are subjected to cyclic stretch that varies during a cardiac cycle.8 Bending and stretching our legs change the axial tension in the femoral arteries.4,5,24 The changing tension may affect the femoral arteries injured from endovascular procedures or implanted stents. The axial tension may also increase locally in stented arteries.13 It has been shown that elevated axial stretch stimulates cell proliferation,7,18,23 which is a key event in the development of IH. However, the direct effect of axial stretch on the development of IH is unclear. The effect of elevated and diminished axial stretch on the development of IH, especially at the presence of endothelial cell (EC) injury needs to be investigated in order to better understand the development of IH.
The aim of this study was to determine the effect of axial stretch on cell proliferation and intimal thickening in normal arteries cultured under physiological flow conditions, and in EC denudated arteries under low flow conditions. We used an ex vivo perfusion organ culture model to achieve good control of the mechanical environment.6,7,17–19,39
MATERIALS AND METHODS
Artery Specimens
Porcine common carotid arteries were harvested from farm pigs (250–300 lb) at a local slaughterhouse and transported to our laboratory in ice-cold PBS (Dulbecco’s phosphate buffered saline, Sigma) supplemented with 1% antibiotic–antimycotic solution (Sigma). Inside a biological safety cabinet (Forma Scientific), all arteries were washed with sterile PBS and loose connective tissue was removed. Arteries were inflated briefly with air to detect any leaks. Segments of 3–4 cm in length were prepared for organ culture as described below.
Artery Organ Culture
The ex vivo perfusion organ culture system was described previously in detail.17,18 Briefly, the system consisted of a flow loop with a vessel chamber, a media reservoir, a peristaltic roller pump, and a pressure meter. Artery segments were mounted to diameter matched stainless steel cannulae in the vessel chambers which were filled with bath medium. Media perfusion to the arteries were driven by a peristaltic roller pump at a controlled flow rate and pulse frequency. The perfusion and bath media consisted of cell culture medium DMEM (Sigma, D1152), supplemented with sodium bicarbonate (3.7 g/L, Sigma), l-glutamine (2 mM, Sigma), calf serum (CS, 10%, Sigma), HEPES buffer solution (25 mM, Gibco), and antibiotic– antimycotic solution (1%, Sigma). Dextran (50 g/L; Sigma) was added to the perfusion medium to adjust its viscosity close to that of human blood (4 cP). Bromodeoxyuridine (BrdU, 5 µg/mL, Sigma) was added to the perfusion medium at the first day of the experiment to label the nuclei of newly proliferated cells. The flow loops were then placed in water-jacketed incubators at 37 °C and aerated with 5% CO2. To avoid possible arterial trauma or shock from pressure and flow, the pump speed was gradually increased to the designed flow rate. The flow rate was set to 160 mL/min in EC intact arteries to generate a normal arterial shear stress of 1.5 Pa.17,18 The flow rate was set to 16 mL/min in EC denudated arteries to generate a low shear stress of 0.2 Pa. Meanwhile, the mean pressure and pulse amplitude were gradually increased to reach a pulsatile pressure oscillating between 80 and 120 mmHg by adjusting the resistance clamp and the length of the T-end tube.17,18 The pressure was monitored through an A–D board connected to a computer and recorded using Data Translation Scope software (Data Translation).
Axial Stretch
To determine the effect of axial stretch on intimal thickening, arteries were cultured at three different stretch ratios (relaxed, control, and stretched). The axial stretch ratios were achieved by adjusting the position of the cannulae.18 The stretched arteries were cultured at an elevated axial stretch ratio of λ = 1.8 while the relaxed arteries were cultured at a reduced stretch ratio of λ = 1.3. The control arteries were cultured at a normal stretch ratio of λ = 1.5, which is the in vivo stretch ratio previously reported for the porcine common carotid arteries.17
Endothelium Denudation
To assess the effect of axial stretch in the case of EC denudation and low flow, which are known to cause IH, the endothelium was denudated mechanically in a subset of arteries using a custom made cotton tip. Lumen of the EC denudated arteries and the normal fresh arteries were stained with 0.5% Evans blue dye for 30 min to verify the level of endothelium denudation. The arteries were also stained with Hoechst 33258 (1 mg/L, Molecular Probes) in a humid incubator for 30 min at 37 °C for fluorescent microscopy to further verify the level of EC denudation.
Contractility Assay
Arteries were challenged with norepinephrine (NE), carbachol (carbamylcholine chloride, CCh), and sodium nitroprusside (SNP, all from Sigma) at the end of the 7-day organ culture. The axial stretch ratio of both the relaxed and stretched arteries was adjusted to the in vivo stretch ratio before testing while the pressure and flow were kept at the same level as during the organ culture. The outer diameters of the arteries were captured by a CCD camera controlled by a computer. After the baseline data were recorded, NE was added to the perfusion medium to a final concentration of 1 µM. CCh and SNP (a final concentration of 1 µM) were added with 10 min time intervals depending on the vessel response. The diameters were measured later from the images using Image-Pro Plus software (Media Cybernetics). The contraction and relaxation of the arteries were calculated as the percentage change of diameter compared to its initial baseline diameter.
Histology
Arteries were fixed overnight with 10% buffered formalin at 100 mmHg and preserved in 70% alcohol until histology preparation. Later, the specimens were divided into three equal ring segments, processed for paraffin embedding and transverse sectioning. Consecutive 5 µm sections were processed for hematoxylin and eosin staining and anti-BrdU staining for light microscopy and fluorescent microscopy, respectively.
Intimal Thickness Measurement
The hematoxylin and eosin stained transverse sections were examined and imaged under an Olympus light microscope with a computer controlled digital camera. A total of 12 locations were captured and stored for each artery. The intimal thickness was measured using Image-Pro Plus software and averaged to represent the value for the artery.
Cell Proliferation Labeling and Anti-BrdU Immunostaining
The BrdU-positive nuclei were detected using a BrdU staining kit (Labeling and Detection Kit I, Roche Diagnostics) as described previously.18,19 All nuclei were counterstained with Hoechst 33258 (1 mg/L, Molecular Probes) in a humid incubator for 30 min at 37 °C. The sections were mounted with cover slips using Fluormount-G (Southern Biotechnology Associates) for fluorescence microscopy.
Cell Counting
Images of sections with anti-BrdU immunostaining were acquired using a digital camera with an Olympus fluorescent microscope through a 10× objective. A DAPI filter and a FITC filter were used to detect total nuclei and BrdU-positive nuclei, respectively. A total of 12 locations were acquired for each artery. For each location the BrdU-positive nuclei and the total nuclei were counted using the Image-Pro Plus software. The number of BrdU-positive nuclei were divided by the total number of nuclei and the quotient was defined as the BrdU index to quantify the cell proliferation at the location. The BrdU index of all 12 locations was then averaged to represent the value for the artery. To further quantify the distribution of proliferating cells across the artery wall, BrdU index were determined for the intima, inner media, and the outer media (the media was equally divided into two layers).19
SMC Immunostaining
To identify SMCs within the neointima, we used anti-smooth muscle alpha-actin staining using an automated staining method (BenchMark XT IHC/ISH, Ventana) modified from our previous immunostaining protocol.30 Briefly, the microsections were deparaffined, processed with blocking agent, and incubated with Monoclonal Mouse Anti-Human Smooth Muscle Actin (Clone 1A4, Dako) for 24 min at 37 °C. The Enhanced V-Red Detection kit (catalog # 760-031, Ventana Medical Systems) was used to detect the alpha smooth muscle actin. Briefly, the microsections were incubated with universal biotinylated secondary antibody for 8 min at 37 °C followed by incubation with an streptavidin–alkaline phosphatase conjugate for 12 min at 37 °C.
Statistical Analysis
All values of measurements were presented as the mean ± SD for each artery. Statistical significance between means was determined using the ANOVA test followed by Tukey’s multiple pairwise comparison test or a Student’s t-test. The significance level was set as a p value < 0.05.
RESULTS
Overall, a total of six groups of carotid arteries were cultured for 7 days under three different axial stretch ratios (relaxed, control, and stretched). These six groups include three groups of intact arteries cultured under normal arterial flow rate (wall shear stress of 1.5 Pa) at the three stretch ratios and three groups of EC denudated arteries cultured under low flow rate (wall shear stress of 0.2 Pa) at the three stretch ratios.
Contractile Responses
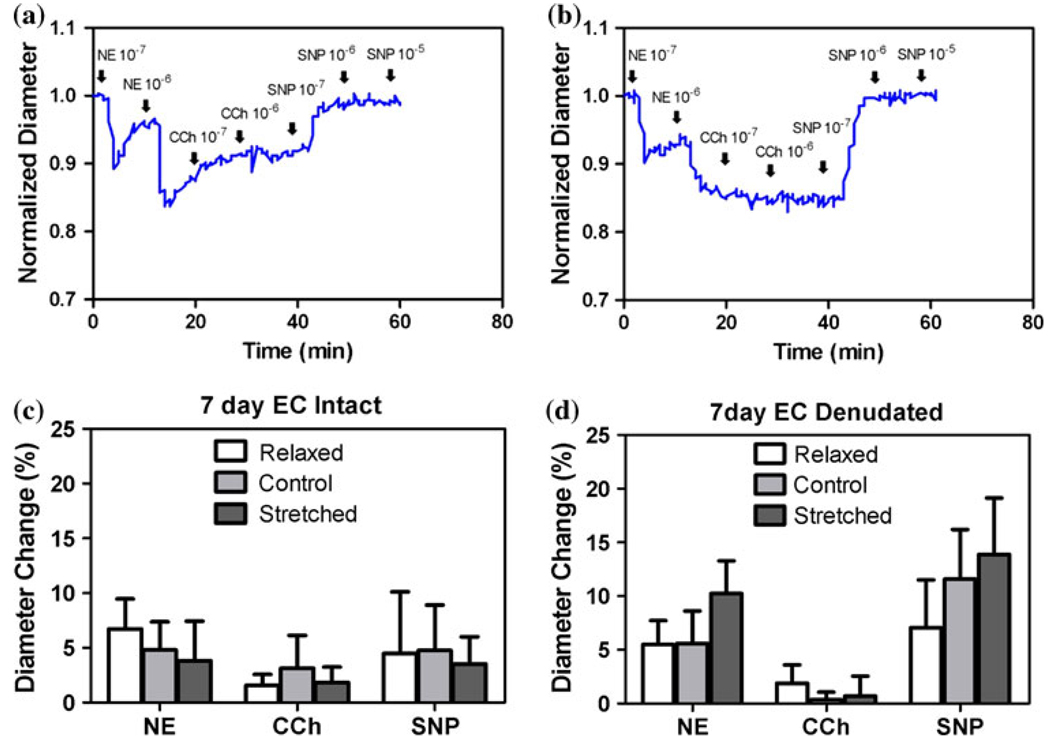
Arteries demonstrated strong responses to agonists after 7 days in organ culture (Fig. 1). The outer diameter of the arteries decreased as the arteries contracted in response to NE stimulation and then increased as the arteries relaxed in response to CCh and SNP. In both EC intact and denudated groups, difference in stretch ratio did not affect vasomotor responses to agonists. EC denudated arteries showed minimal response to CCh. In addition, all the EC denudated arteries contracted in response to NE and about 50% of the arteries tested showed spontaneous rhythmic contractions (SRC).
FIGURE 1.
Top panels: Typical temporal diameter changes in response to norepinephrine (NE), carbachol (CCh), and sodium nitroprusside (SNP) in normal (a) and EC denudated (b) arteries. Bottom panels: The averaged percent diameter changes of normal (c) and EC denudated (d) arteries. Values are mean ± SD with n = 4 for each group.
Endothelium Denudation
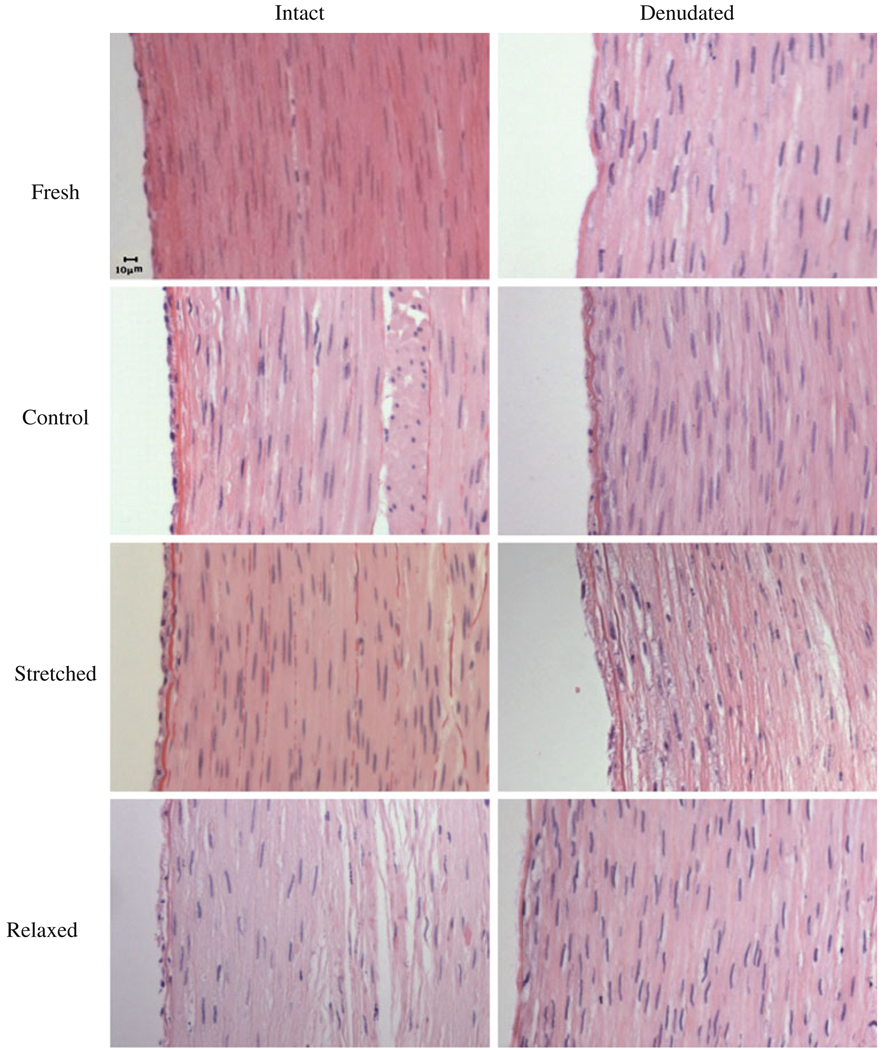
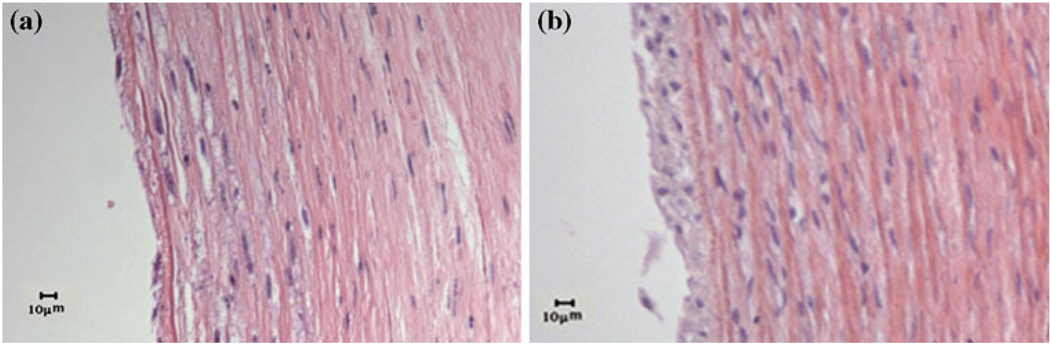
Both light and fluorescent microscopic examination confirmed that the EC were removed from the arterial wall after denudation while normal arteries demonstrated an intact single layer of EC (Fig. 2, top panels). After 7 days of organ culture, EC intact arteries still maintained intact EC layer while EC denudated arteries developed a thin layer of neointima (Fig. 2, middle and bottom panels).
FIGURE 2.
Micrographs of H&E stained cross sections of fresh arteries and arteries cultured for 7 days. First row: fresh arteries, second row: arteries cultured under normal axial stretch, third row: arteries cultured under elevated axial stretch, and fourth row: arteries cultured under relaxed axial stretch. Left column: EC intact arteries, right column: EC denudated arteries.
Effect of Axial Stretch on Intimal Thickness
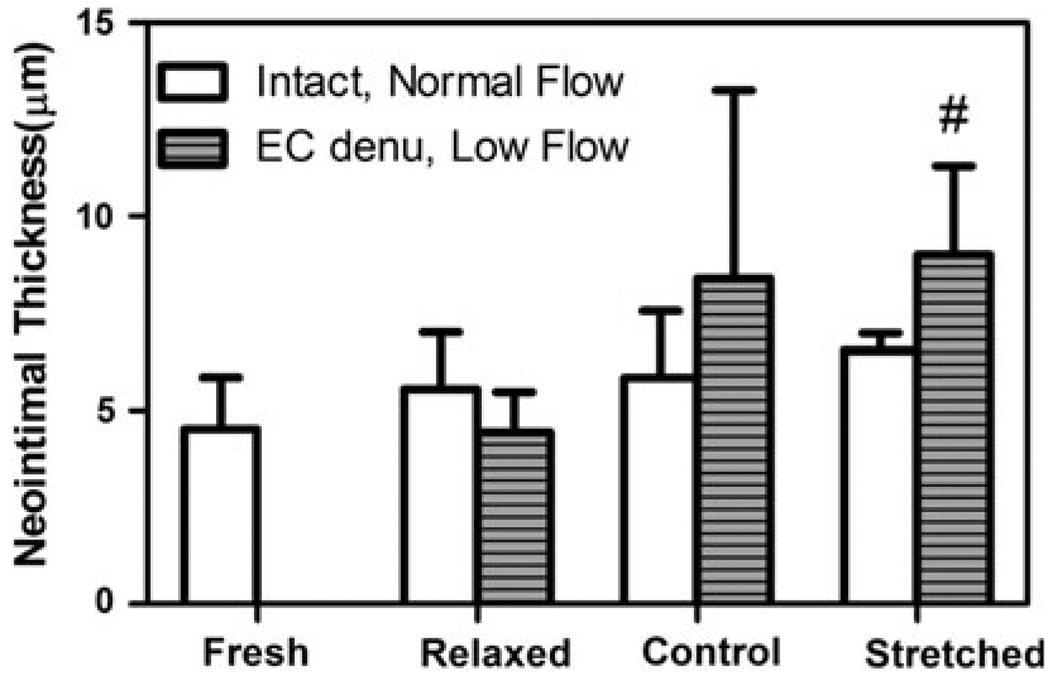
In EC intact groups, the relaxed (λ = 1.3), control (λ = 1.5), and stretched (λ = 1.8) arteries developed little neointima. In the EC denudated groups, neointima developed depending on the axial stretch ratio. The intimal thickness increased with increasing axial stretch in the denudated groups (with a correlation coefficient r = 0.57, p = 0.03). Intimal thickness in the stretched denudated arteries was significantly higher than in the relaxed denudated arteries (p < 0.05), though it was not significantly different from the control stretched arteries (Fig. 3). A two-way ANOVA was used to determine the effects of axial stretch and EC denudation on the development of IH. It showed that the axial stretch ratio had a clear effect on the intimal thickness (p < 0.05). However, there was no significant difference between the EC intact and EC denudated arteries (power β = 0.23). Additionally, there was no significant interaction between stretch ratio and EC denudation (β = 0.31).
FIGURE 3.
Comparison of intimal thickness in intact arteries cultured under normal flow for 7 days and EC denudated arteries cultured under low flow for 7 days. Arteries were cultured at different stretch ratios: relaxed (1.3), control (1.5), and stretched (1.8). Values are mean ± SD. n = 3, 5, and 4 for the relaxed, normal, and stretched groups of intact arteries; n = 6, 4, and 5 for the relaxed, normal, and stretched groups of EC denudated groups. #p < 0.05 vs. EC denudated, relaxed group.
Effect of Axial Stretch on Cell Proliferation
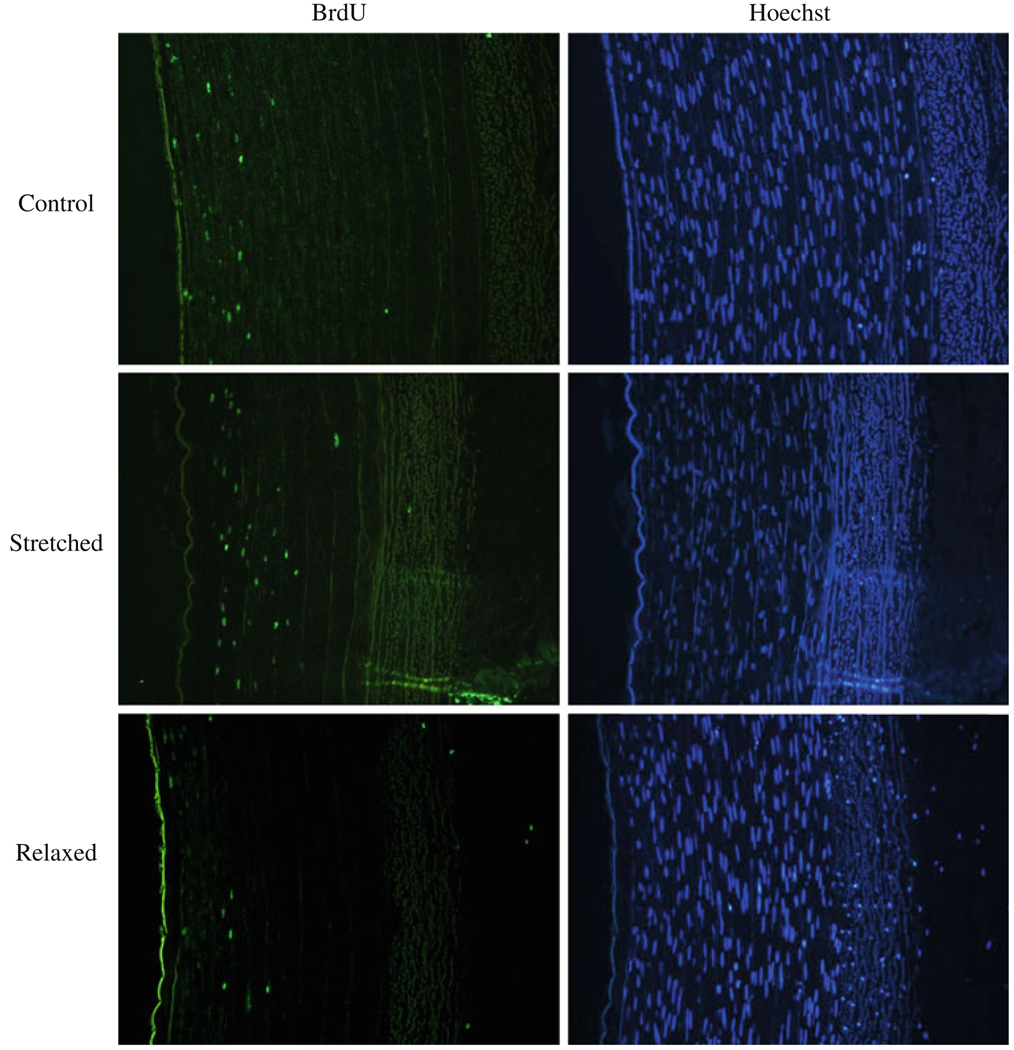
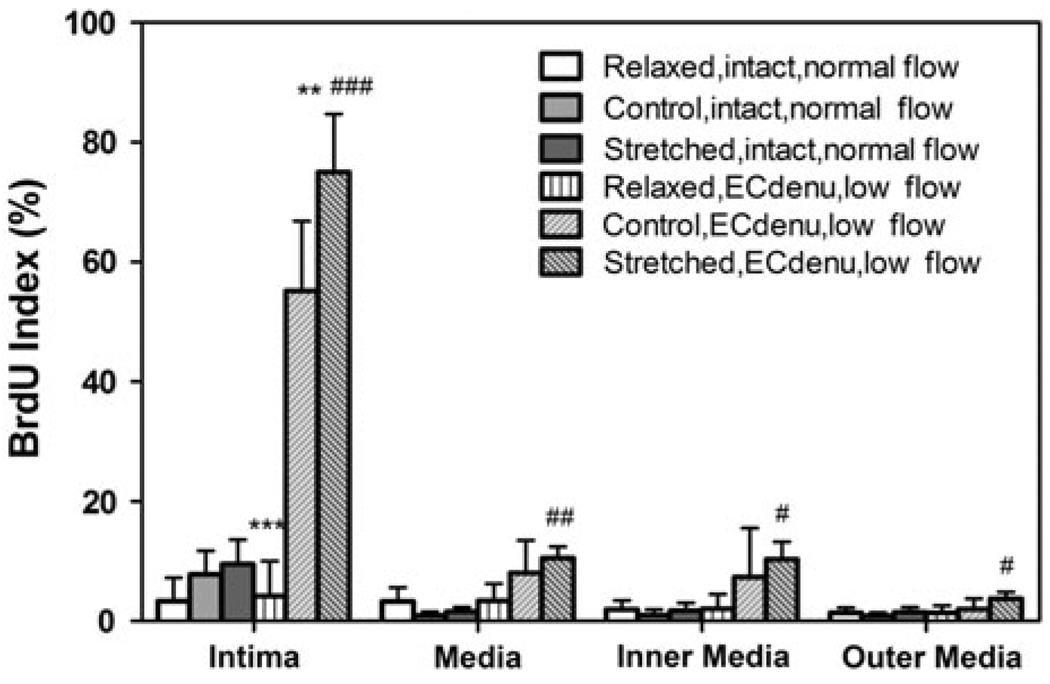
BrdU staining clearly demonstrated new cell proliferation in all cultured arteries (Fig. 4). In EC intact arteries, there was no overall difference in cell proliferation among the control, relaxed, and stretched groups although the stretched arteries had slightly higher BrdU index in the intima than the relaxed arteries did (Fig. 5). In the EC denudated groups, however, the axial stretch demonstrated a clear effect on the cell proliferation (Figs. 4 and 5). The effect of the axial stretch ratio on cell proliferation was more pronounced than on the intimal thickness in the EC denudated groups. Cell proliferation in the stretched arteries was significantly higher than the relaxed and control arteries in intima (p < 0.001 and p < 0.01, respectively). Cell proliferation in the control arteries was also significantly higher than the relaxed arteries (p < 0.001). The increase in cell proliferation in intima demonstrated a strong correlation with the level of the axial stretch ratio (R2 = 0.94). When the control and stretched EC denudated groups were compared to the corresponding intact groups, the BrdU indices in the intima and inner media layers of the EC denudated arteries were significantly higher (p < 0.001). On the other hand, the relaxed arteries showed no statistical difference in cell proliferation between the EC intact and the EC denudated groups. Immunostaining confirmed the SMCs in the neointima (Fig. 6).
FIGURE 4.
Micrographs (10×) of BrdU stained arterial cross sections of EC denudated arteries cultured for 7 days. First row: arteries cultured under normal axial stretch, second row: arteries cultured under elevated axial stretch, and third row: arteries cultured under relaxed axial stretch. Left column: BrdU-positive nuclei, right column: Hoechst counterstaining.
FIGURE 5.
Comparison of BrdU index in intact arteries and EC denudated arteries cultured under normal or low flow for 7 days. Values are mean ± SD. n = 3, 8, 8 for the relaxed, normal, and stretched groups of intact arteries; n = 4 for all EC denudated groups. ** p < 0.01, *** p < 0.001 vs. control EC denu group; # p < 0.05, ## p < 0.01, ### p < 0.001 vs. relaxed EC denu group.
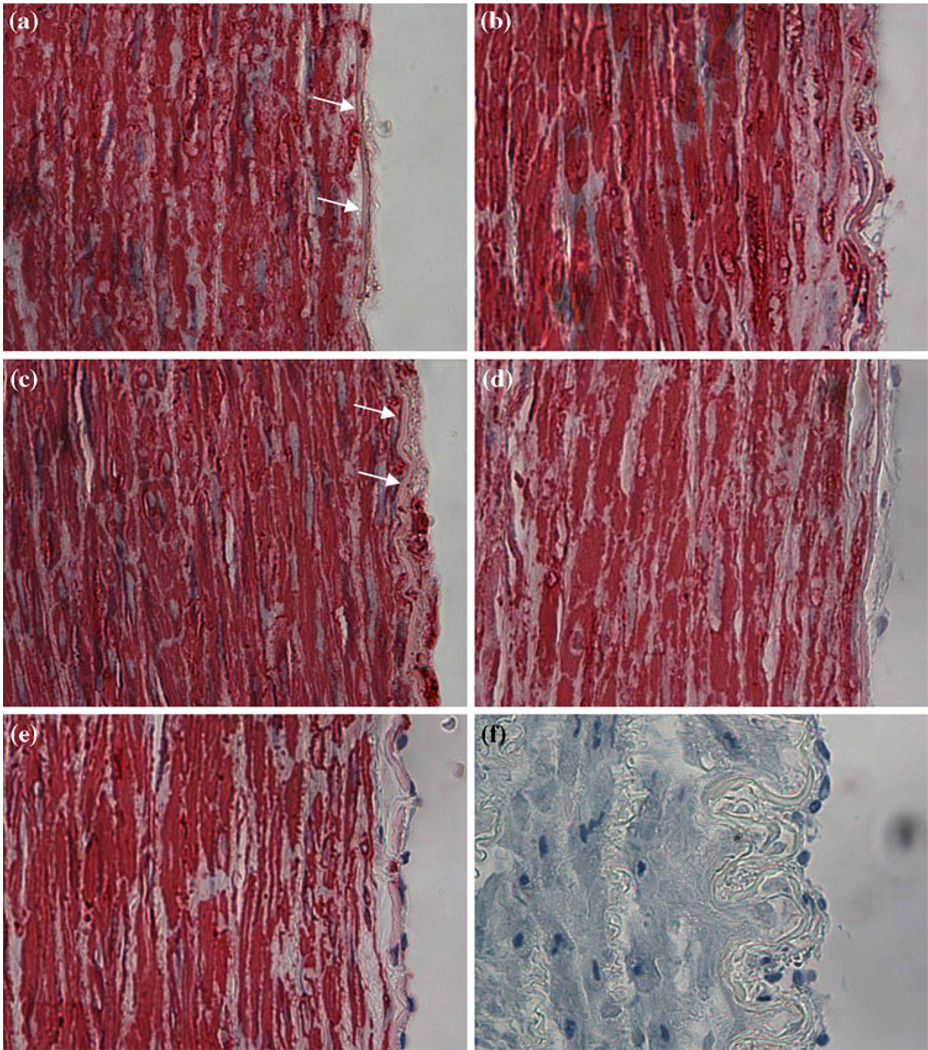
FIGURE 6.
Micrographs (40×) of smooth muscle α-actin stained arterial cross sections of EC denudated arteries and EC intact arteries. EC denudated arteries were cultured under relaxed (a), normal (b), and stretched (c) axial stretch ratios. Arrows on panels (a) and (b) indicate the internal elastic lamina; intimal hyperplasia is significantly more developed in panel (c) when compared to panel (a). A representative image of an intact artery cultured under relaxed stretch ratio is shown (d). (e) is a fresh artery and (f) is a negative control stain. Negative stain is seen in the intima of EC intacted arteries (d and e).
DISCUSSION
We studied the effect of axial stretch on intimal thickening and cell proliferation in porcine arteries in organ culture. Since the effects of low flow and EC injury have been well documented,1,10,12,48 our goal was not to determine the effects of each of these two factors. Instead, the aim of this study was to determine the effect of axial stretch in conjunction with these two factors—a worst case scenario. While normal arterial flow is often restored after endovascular procedures injured arteries may be subjected to low flow and elevated axial stretch.13,46 Therefore, the current results are useful in evaluating the combined effect of EC denudation, low flow, and axial stretch ratio on neointima formation.
Our results showed that axial stretch did not affect intimal thickness in normal arteries. However, with EC denudation and low flow conditions, the axial stretch ratio significantly affected intimal thickness and cell proliferation. In these denudated arteries, cell proliferation was significantly lower in the relaxed arteries than in the control and the stretched arteries. Intimal thickness and medial cell proliferation both demonstrated a strong correlation with the axial stretch ratio. Additionally, compared to the normal intact arteries, the EC denudated arteries had significantly higher cell proliferation when they were cultured under control or stretched stretch ratios.
In stretched arteries with EC denudation, the cell proliferation was significantly higher in the intima than in the media; within the media, cell proliferation was significantly higher in the inner layer than in the outer layer (see Figs. 4 and 5). This may be due to the proliferation stimulated by EC injury. Our results showed similar cell proliferation between the outer and inner media of the normal, intact arteries after 7 days in organ culture, suggesting that the axial stretch did not lead to the increase in cell proliferation at the inner layers. This conclusion agrees with our previous results of arteries cultured under hypertensive pressure or elevated axial stretch for 7 days7,19,29 but differ from the results of arteries cultured under elevated axial stretch for 5 days.18 The difference may due to the different culture time. The rate of cell proliferation most likely peaked at 5 days and returned to baseline levels at 7 days.2,45 In contrast, EC denudation did not affect cell proliferation in the relaxed group with a reduced axial stretch ratio. It is possible that arterial response to the reduced axial stretch takes longer time as suggested by a previous study.22
Our results showed that while the cell proliferation was very significantly increased, intimal thickening in EC denudated arteries was less significant after seven day organ culture. For EC denudated arteries, the intimal thickness was significantly higher in the stretched group than the relaxed group but not significantly higher than the control group. The insignificant difference between the stretched group and the normal stretched group could be due to the small sample size which resulted in a statistical power of β = 0.64. More likely, it could be due to either the short culture period or an insufficient level of axial stretch. This speculation is supported by two observations, first, while a few of the sample sizes were small, a significant difference in cell proliferation was detected with a reasonable statistical power (β = 0.92). Second, previous studies have shown that intimal thickening takes 7–14 days to develop47 and our culture period falls at the minimum of this time frame. Indeed, we see a much greater intimal thickness in these arteries after 14 days in organ culture (Fig. 7). These results agree with results by Guerin et al. which demonstrate that smooth muscle cell proliferation was maximal in the artery wall between 7 and 14 days after stent insertion in a human mammary artery.12 Anti-smooth muscle alpha-actin staining demonstrated that SMCs migrated in the neointima which is consistent with both our previous observation of the locally injured porcine carotid arteries30 and the study by Guerin et al. which found that SMCs migrated into the lumen of the artery after stent placement.12
FIGURE 7.
Micrographs of H&E stained cross sections of arteries after 14 days in organ culture (a: EC intact, b: EC denudated).
The vasomotor response after 7-day organ culture was strong, with no statistical differences between the stretched and control arteries. These results agree with previous reports7,17 that arteries are viable after 5–7 day organ culture on both control and stretched arteries. Vasomotor response induced by CCh was smaller in EC denudated arteries than in the intact arteries. This difference is due to the fact that CCh acts through the NO-pathway that is EC dependent.18,41,43 In our contractility testing, some arteries showed SRC. The SRC is another indicator that arteries were viable and functioning after 7 days of organ culture. Previous studies have shown that SRC was presented in isolated radial arteries (21% of the arteries) with or without EC44 and the presence of SRC potentiates the nor-epinephrine and KCI-induced contractions.32
The pulse frequency was 1 Hz in the low flow arteries and 3 Hz in the normal flow group. Previous studies have shown that a variation of pulsatile frequency at 1–3 Hz had much less significant effect on EC gene expression than the level of shear stress in the range of 0–1.5 Pa.21 Therefore, we assumed that the effect of pulse frequency in our experiment is minimal although further study is needed to clarify the effect of pulse frequency on IH development.
Vascular surgery and interventions are often associated with EC injury and alteration of axial stretch to the arteries. Our results indicated that moderate axial stretch may stimulate smooth muscle cell proliferation but not lead to intimal thickening,18 however, the changes in axial stretch combined with EC injury could have a significant effect on the cell proliferation and intimal thickness in the arteries. These results may be useful in treatment and prevention of intimal thickness and restenosis. Techniques to elongate arteries should be carefully applied to avoid EC injury.7,18 Arteries and vascular grafts should be maintained at physiological axial tension and avoid over stretch from vascular intervention to attenuate cell proliferation and intimal thickening. On the other hand, low stretch ratio should be avoided to maintain normal vascular function and prevent vessel tortuosity.14,15,22
ACKNOWLEDGMENTS
This work was supported by NIH grant GM008194-25S10080. It was also partially supported by NSF grant 0602834, Texas Higher Educational Coordinating Board grant 003659-0014-2006, and NSF of China Grant 10928206. The authors thank the Granzins at New Braunfels, TX and Wiatrek at Poth, TX for generously providing the arteries for this work and thank Dr. John Zhang’s lab, Dr. William W. Mogan, and Mr. Kurtis Johnson for their help in this work.
REFERENCES
- 1.Bayes-Genis A, Kantor B, Keelan PC, Altman JD, Lubbe DF, Kang JH, Schwartz RS. Restenosis and hyperplasia: animal models. Curr. Interv. Cardiol. Rep. 2000;2(4):303–308. [PubMed] [Google Scholar]
- 2.Boonen HC, Schiffers PM, Fazzi GE, Janssen GM, Daemen MJ, De Mey JG. DNA synthesis in isolated arteries. Kinetics and structural consequences. Am. J. Physiol. 1991;260(1 Pt 2):H210–H217. doi: 10.1152/ajpheart.1991.260.1.H210. [DOI] [PubMed] [Google Scholar]
- 3.Casterella PJ, Teirstein PS. Prevention of coronary restenosis. Cardiol. Rev. 1999;7(4):219–231. doi: 10.1097/00045415-199907000-00014. [DOI] [PubMed] [Google Scholar]
- 4.Cheng CP, Wilson NM, Hallett RL, Herfkens RJ, Taylor CA. In vivo MR angiographic quantification of axial and twisting deformations of the superficial femoral artery resulting from maximum hip and knee flexion. J. Vasc. Interv. Radiol. 2006;17(6):979–987. doi: 10.1097/01.RVI.0000220367.62137.e8. [DOI] [PubMed] [Google Scholar]
- 5.Choi G, Shin LK, Taylor CA, Cheng CP. In vivo deformation of the human abdominal aorta and common iliac arteries with hip and knee flexion: implications for the design of stent-grafts. J. Endovasc. Ther. 2009;16(5):531–538. doi: 10.1583/09-2806.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Conklin BS, Surowiec SM, Lin PH, Chen C. A simple physiologic pulsatile perfusion system for the study of intact vascular tissue. Med. Eng. Phys. 2000;22(6):441–449. doi: 10.1016/s1350-4533(00)00052-7. [DOI] [PubMed] [Google Scholar]
- 7.Davis NP, Han HC, Wayman B, Vito R. Sustained axial loading lengthens arteries in organ culture. Ann. Biomed. Eng. 2005;33(7):867–877. doi: 10.1007/s10439-005-3488-x. [DOI] [PubMed] [Google Scholar]
- 8.Ding Z, Friedman MH. Quantification of 3-D coronary arterial motion using clinical biplane cineangiograms. Int. J. Card. Imaging. 2000;16(5):331–346. doi: 10.1023/a:1026590417177. [DOI] [PubMed] [Google Scholar]
- 9.Drouilhet JC, III, Southern F, Williams DK, Brown AT, Eidt J, Moursi MM. Increased intimal hyperplasia after carotid endarterectomy in spontaneously hypertensive rats. Vasc. Surg. 2001;35(1):11–18. doi: 10.1177/153857440103500103. [DOI] [PubMed] [Google Scholar]
- 10.El Hamamsy I, Stevens LM, Vanhoutte PM, Perrault LP. Injury of the coronary endothelium at implantation increases endothelial dysfunction and intimal hyperplasia after heart transplantation. J. Heart Lung Transplant. 2005;24(3):251–258. doi: 10.1016/j.healun.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 11.Fridez P, Makino A, Miyazaki H, Meister JJ, Hayashi K, Stergiopulos N. Short-term biomechanical adaptation of the rat carotid to acute hypertension: contribution of smooth muscle. Ann. Biomed. Eng. 2001;29(1):26–34. doi: 10.1114/1.1342054. [DOI] [PubMed] [Google Scholar]
- 12.Guerin P, Rondeau F, Grimandi G, Heymann MF, Heymann D, Pillet P, Al Habash O, Loirand G, Pacaud P, Crochet D. Neointimal hyperplasia after stenting in a human mammary artery organ culture. J. Vasc. Res. 2004;41(1):46–53. doi: 10.1159/000076245. [DOI] [PubMed] [Google Scholar]
- 13.Gyongyosi M, Yang P, Khorsand A, Glogar D Austrian Wiktor Stent Study Group and European Paragon Stent Investigators. Longitudinal straightening effect of stents is an additional predictor for major adverse cardiac events. J. Am. Coll. Cardiol. 2000;35(6):1580–1589. doi: 10.1016/s0735-1097(00)00570-2. [DOI] [PubMed] [Google Scholar]
- 14.Han HC. A biomechanical model of artery buckling. J. Biomech. 2007;40(16):3672–3678. doi: 10.1016/j.jbiomech.2007.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Han HC. Blood vessel buckling within soft surrounding tissue generates tortuosity. J. Biomech. 2009;42(16):2797–2801. doi: 10.1016/j.jbiomech.2009.07.033. [DOI] [PubMed] [Google Scholar]
- 16.Han HC, Fung YC. Longitudinal strain of canine and porcine aortas. J. Biomech. 1995;28(5):637–641. doi: 10.1016/0021-9290(94)00091-h. [DOI] [PubMed] [Google Scholar]
- 17.Han HC, Ku DN. Contractile responses in arteries subjected to hypertensive pressure in seven-day organ culture. Ann. Biomed. Eng. 2001;29(6):467–475. doi: 10.1114/1.1376391. [DOI] [PubMed] [Google Scholar]
- 18.Han HC, Ku DN, Vito RP. Arterial wall adaptation under elevated longitudinal stretch in organ culture. Ann. Biomed. Eng. 2003;31(4):403–411. doi: 10.1114/1.1561291. [DOI] [PubMed] [Google Scholar]
- 19.Han HC, Marita S, Ku DN. Changes of opening angle in hypertensive and hypotensive arteries in 3-day organ culture. J. Biomech. 2006;39(13):2410–2418. doi: 10.1016/j.jbiomech.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 20.Han HC, Zhao L, Huang M, Hou LS, Huang YT, Kuang ZB. Postsurgical changes of the opening angle of canine autogenous vein graft. J. Biomech. Eng. 1998;120(2):211–216. doi: 10.1115/1.2798304. [DOI] [PubMed] [Google Scholar]
- 21.Himburg HA, Dowd SE, Friedman MH. Frequency-dependent response of the vascular endothelium to pulsatile shear stress. Am. J. Physiol. Heart Circ Physiol. 2007;293(1):H645–H653. doi: 10.1152/ajpheart.01087.2006. [DOI] [PubMed] [Google Scholar]
- 22.Jackson ZS, Dajnowiec D, Gotlieb AI, Langille BL. Partial off-loading of longitudinal tension induces arterial tortuosity. Arterioscler. Thromb. Vasc. Biol. 2005;25(5):957–962. doi: 10.1161/01.ATV.0000161277.46464.11. [DOI] [PubMed] [Google Scholar]
- 23.Jackson ZS, Gotlieb AI, Langille BL. Wall tissue remodeling regulates longitudinal tension in arteries. Circ. Res. 2002;90(8):918–925. doi: 10.1161/01.res.0000016481.87703.cc. [DOI] [PubMed] [Google Scholar]
- 24.Klein AJ, Chen SJ, Messenger JC, Hansgen AR, Plomondon ME, Carroll JD, Casserly IP. Quantitative assessment of the conformational change in the femoropopliteal artery with leg movement. Catheter Cardiovasc. Interv. 2009;74(5):787–798. doi: 10.1002/ccd.22124. [DOI] [PubMed] [Google Scholar]
- 25.Korshunov VA, Berk BC. Flow-induced vascular remodeling in the mouse: a model for carotid intima-media thickening. Arterioscler. Thromb. Vasc. Biol. 2003;23(12):2185–2191. doi: 10.1161/01.ATV.0000103120.06092.14. [DOI] [PubMed] [Google Scholar]
- 26.Koyama J, Owa M, Sakurai S, Shimada H, Hikita H, Higashikata T, Ikeda S. Relation between vascular morphologic changes during stent implantation and the magnitude of in-stent neointimal hyperplasia. Am. J. Cardiol. 2000;86(7):753–758. doi: 10.1016/s0002-9149(00)01075-4. [DOI] [PubMed] [Google Scholar]
- 27.Kudo T, Chandra FA, Ahn SS. Long-term outcomes and predictors of iliac angioplasty with selective stenting. J. Vasc. Surg. 2005;42(3):466–475. doi: 10.1016/j.jvs.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 28.Langille BL, Bendeck MP, Keeley FW. Adaptations of carotid arteries of young and mature rabbits to Intimal Thickness in Stretched Arteries reduced carotid blood flow. Am. J. Physiol. Heart Circ. Physiol. 1989;256(4):H931–H939. doi: 10.1152/ajpheart.1989.256.4.H931. [DOI] [PubMed] [Google Scholar]
- 29.Lee YU, Drury-Stewart D, Vito RP, Han HC. Morphologic adaptation of arterial endothelial cells to longitudinal stretch in organ culture. J. Biomech. 2008;41(15):3274–3277. doi: 10.1016/j.jbiomech.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee YU, Luo J, Sprague E, Han HC. Comparison of artery organ culture and co-culture models for studying endothelial cell migration and its effect on smooth muscle cell proliferation and migration. Ann. Biomed. Eng. 2010;38(3):801–812. doi: 10.1007/s10439-009-9877-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lehoux S, Castier Y, Tedgui A. Molecular mechanisms of the vascular responses to haemodynamic forces. J. Intern. Med. 2006;259(4):381–392. doi: 10.1111/j.1365-2796.2006.01624.x. [DOI] [PubMed] [Google Scholar]
- 32.Longmore J, Weston AH. The role of K+ channels in the modulation of vascular smooth muscle tone. In: Cook NS, editor. Potassium Channels: Structure, Classification, Function and Therapeutic Potential. New York: John Wiley & Sons/Ellis Horwood; 1990. pp. 259–272. [Google Scholar]
- 33.Loth F, Jones SA, Zarins CK, Giddens DP, Nassar RF, Glagov S, Bassiouny HS. Relative contribution of wall shear stress and injury in experimental intimal thickening at PTFE end-to-side arterial anastomoses. J. Biomech. Eng. 2002;124(1):44–51. doi: 10.1115/1.1428554. [DOI] [PubMed] [Google Scholar]
- 34.Lu Y, Huang Y, Zhao L, Li R, Kaijun S, Ma P, Chu X. Management of major arterial injuries of limbs: a study of 166 cases. Cardiovasc. Surg. 1993;1(5):486–488. [PubMed] [Google Scholar]
- 35.Meng X, Mavromatis K, Galis ZS. Mechanical stretching of human saphenous vein grafts induces expression and activation of matrix-degrading enzymes associated with vascular tissue injury and repair. Exp. Mol. Pathol. 1999;66(3):227–237. doi: 10.1006/exmp.1999.2260. [DOI] [PubMed] [Google Scholar]
- 36.Mitra AK, Agrawal DK. In stent restenosis: bane of the stent era. J. Clin. Pathol. 2006;59(3):232–239. doi: 10.1136/jcp.2005.025742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nanjo H, Sho E, Komatsu M, Sho M, Zarins CK, Masuda H. Intermittent short-duration exposure to low wall shear stress induces intimal thickening in arteries exposed to chronic high shear stress. Exp. Mol. Pathol. 2006;80(1):38–45. doi: 10.1016/j.yexmp.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 38.Newby AC, Zaltsman AB. Molecular mechanisms in intimal hyperplasia. J. Pathol. 2000;190(3):300–309. doi: 10.1002/(SICI)1096-9896(200002)190:3<300::AID-PATH596>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 39.Nichol JW, Petko M, Myung RJ, Gaynor JW, Gooch KJ. Hemodynamic conditions alter axial and circumferential remodeling of arteries engineered ex vivo. Ann. Biomed. Eng. 2005;33(6):725–732. doi: 10.1007/s10439-005-4494-8. [DOI] [PubMed] [Google Scholar]
- 40.Nichols WW, O'Rourke MF. 4th edn. London: Arnold; 1998. McDonald's Blood Flow in Arteries: Theoretical, Experimental, and Clinical Principles Chapter 4. [Google Scholar]
- 41.Pannangpetch P, Woodman OL. The effect of ischaemia on endothelium-dependent vasodilatation and adrenoceptor-mediated vasoconstriction in rat isolated hearts. Br. J. Pharmacol. 1996;117(6):1047–1052. doi: 10.1111/j.1476-5381.1996.tb16695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pasterkamp G, de Kleijn DPV, Borst C. Arterial remodeling in atherosclerosis, restenosis and after alteration of blood flow: potential mechanisms and clinical implications. Cardiovasc. Res. 2000;45(4):843–852. doi: 10.1016/s0008-6363(99)00377-6. [DOI] [PubMed] [Google Scholar]
- 43.Sato K, Ozaki H, Karaki H. Differential effects of carbachol on cytosolic calcium levels in vascular endothelium and smooth muscle. J. Pharmacol. Exp. Ther. 1990;255(1):114–119. [PubMed] [Google Scholar]
- 44.Stojnic N, Bukarica LG, Peric M, Bumbasirevic M, Lesic A, Lipkovski JM, Heinle H. Analysis of vasoreactivity of isolated human radial artery. J. Pharmacol. Sci. 2006;100(1):34–40. doi: 10.1254/jphs.fpe05004x. [DOI] [PubMed] [Google Scholar]
- 45.Sumpio BE, Banes AJ, Levin LG, Johnson G., Jr Mechanical stress stimulates aortic endothelial cells to proliferate. J. Vasc. Surg. 1987;6(3):252–256. [PubMed] [Google Scholar]
- 46.Ward MR, Tsao PS, Agrotis A, Dilley RJ, Jennings GL, Bobik A. Low blood flow after angioplasty augments mechanisms of restenosis: inward vessel remodeling, cell migration, and activity of genes regulating migration. Arterioscler. Thromb. Vasc. Biol. 2001;21(2):208–213. doi: 10.1161/01.atv.21.2.208. [DOI] [PubMed] [Google Scholar]
- 47.Weintraub WS. The pathophysiology and burden of restenosis. Am. J. Cardiol. 2001;100(5A):3K–9K. doi: 10.1016/j.amjcard.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 48.Willis AI, Pierre-Paul D, Sumpio BE, Gahtan V. Vascular smooth muscle cell migration: current research and clinical implications. Vasc. Endovasc. Surg. 2004;38(1):11–23. doi: 10.1177/153857440403800102. [DOI] [PubMed] [Google Scholar]