Abstract
Hypoxia and the hypoxia-inducible factor (HIF) transcription factor regulate angiogenic-osteogenic coupling and osteoclast-mediated bone resorption. To determine how HIF might coordinate osteoclast and osteoblast function, we studied angiopoietin-like 4 (ANGPTL4), the top HIF target gene in an Illumina HumanWG-6 v3.0 48k array of normoxic vs. hypoxic osteoclasts differentiated from human CD14+ monocytes (14.3-fold induction, P<0.0004). ANGPTL4 mRNA and protein were induced by 24 h at 2% O2 in human primary osteoclasts, monocytes, and osteoblasts. ANGPTL4 protein was observed by immunofluorescence in osteoclasts and osteoblasts in vivo. Normoxic inducers of HIF (CoCl2, desferrioxamine, and l-mimosine) and 100 ng/ml ANGPTL4 stimulated osteoclastic resorption 2- to 3-fold in assays of lacunar dentine resorption, without affecting osteoclast viability. Isoform-specific HIF-1α small interfering RNA ablated hypoxic induction of ANGPTL4 and of resorption, which was rescued by addition of exogenous ANGPTL4 (P<0.001). In the osteoblastic Saos2 cell line, ANGPTL4 caused a dose-dependent increase in proliferation (P<0.01, 100 ng/ml) and, at lower doses (1–25 ng/ml), mineralization. These results demonstrate that HIF is sufficient to enhance osteoclast-mediated bone resorption and that ANGPTL4 can compensate for HIF-1α deficiency with respect to stimulation of osteoclast activity and also augments osteoblast proliferation and differentiation.—Knowles, H. J., Cleton-Jansen, A.-M., Korsching, E., and Athanasou, N.A. Hypoxia-inducible factor regulates osteoclast-mediated bone resorption: role of angiopoietin-like 4.
Keywords: osteoblast, proliferation, differentiation, osteoclastogenesis, pathological bone resorption
A central role for hypoxia and the hypoxia-inducible transcription factor (HIF) is emerging in bone biology. HIF comprises a hypoxia-inducible α subunit and a constitutively expressed β subunit. Under normoxia, HIFα is post-translationally hydroxylated by the prolyl hydroxylase domain (PHD) enzymes, targeting it for proteasomal degradation (1, 2). A limitation of PHD enzyme activity under hypoxia allows stabilization of HIFα and transactivation of genes involved in processes such as angiogenesis, apoptosis, and metabolic adaptation (3).
Mice with osteoblast-specific deletion of the von Hippel-Lindau (Vhl) gene overexpress HIF-1α and HIF-2α and show enhanced angiogenesis-dependent osteogenesis (4) and bone repair (5) due to elevated levels of VEGF. With use of a Cre-loxP method, specific disruption of either HIF-1α or HIF-2α in osteoblasts revealed similar roles for both isoforms in VEGF-dependent promotion of bone vascularization, whereas only HIF-1α promoted bone formation by direct stimulation of osteoblast proliferation (4–6). However, studies in which manipulation of HIF is not cell-restricted have shown conflicting results. HIF stabilization induced by introduction of PHD enzyme inhibitors into a fracture site has shown increased angiogenesis and improved bone regeneration after fracture (5, 7). Conversely, fracture calluses in HIF-1α+/− mice were larger, stronger, and stiffer than those in wild-type littermates, at least partially due to reduced osteoblastic and chondrocytic apoptosis in the heterozygous animals (8). The reason for the discrepancy between these observations is unclear but may relate to either systemic effects of HIF manipulation or contributory effects of HIF activation in multiple cell types within the bone microenvironment.
Osteoclasts are monocyte-derived multinucleated cells that resorb bone. Hypoxia-reoxygenation is known to enhance osteoclast differentiation (9–11), and HIF-1α has been identified as part of the Fos-related protein-driven pathway whereby hypoxia initiates increased osteoclastogenesis in vivo (12). We have recently demonstrated that hypoxia also increases the resorptive capacity of human osteoclasts in an HIF-1α-dependent manner (9).
Angiopoietin-like 4 (ANGPTL4) is a recently identified adipokine, also described as FIAF (fasting-induced adipose factor) (13), PGAR [peroxisome proliferator-activated receptor (PPAR)γ-angiopoietin-related] (14), and HFARP (hepatic fibrinogen/angiopoietin-related protein) (15). ANGPTL4 transcription is regulated by PPARα, γ, and β/δ (13, 14, 16) and specifically by the HIF-1α isoform of HIF (17, 18). It is predominantly expressed in adipose tissue, liver, lung, kidney, and placenta (13–15). Hypoxia-inducible expression has been described in adipocytes (19), endothelial cells (20), cardiomyocytes (17), and articular chondrocytes, in which it also stimulates expression of the matrix metalloproteinases MMP1 and MMP3 (21). In vivo, ANGPTL4 is overexpressed in critical leg ischemia and in the hypoxic, perinecrotic regions of tumors of the lung, kidney, and brain (20). It also shows high levels of expression in conventional renal cell carcinoma (20), potentially due to the prevalence of Vhl mutations leading to constitutive HIF activation in this disease.
We present data supporting a role for ANGPTL4 in the HIF-1α-dependent stimulation of osteoclast resorptive activity and suggest enhancement of pathological bone resorption as a novel function for this adipokine.
MATERIALS AND METHODS
Reagents
Tissue culture reagents were from Lonza (Wokingham, UK), except FBS (Invitrogen, Paisley, UK). Macrophage colony-stimulating factor (M-CSF) and ANGPTL4 were from R&D Systems (Abingdon, UK); receptor activator for NF-κB ligand (RANKL) and osteoprotegerin (OPG) were from PeproTech (London, UK). Unless otherwise stated, other reagents were from Sigma-Aldrich (Poole, UK). This study was approved by the Oxford Clinical Research Ethics Committee (C01.071).
Osteoclast culture
Osteoclasts were differentiated and maintained in α-minimal essential medium (MEM) (without ribonucleosides/deoxyribonucleosides) with 10% FBS, l-glutamine (2 mM), penicillin (50 IU/ml), and streptomycin sulfate (50 μg/ml) in a humidified atmosphere (37°C, 5% CO2 in air). Peripheral blood mononuclear cells (PBMCs) were isolated from buffy coat blood using Histopaque. CD14+ monocytes were selected using an AutoMACS cell separator (Miltenyi Biotech, Surrey, UK) and seeded onto dentine slices. Cultures were washed to remove nonadherent cells and supplemented with fresh medium containing M-CSF (25 ng/ml) + RANKL (50 ng/ml) every 3–4 d. On d 11–14, osteoclasts were treated with ANGPTL4 (1–100 ng/ml), OPG (500 ng/ml), or hypoxia (2% O2, 5% CO2, and balance of N2 in a MiniGalaxy incubator; RS Biotech, Irvine, UK) for 24 h as described. Primary human osteoclasts were derived from fresh giant cell tumor of bone (GCTB) tissue obtained from the Nuffield Orthopaedic Centre (Oxford, UK) or the Royal National Orthopaedic Hospital (Stanmore, UK). GCTB was curetted to release osteoclastic giant cells as described (22). Osteoclasts were seeded onto dentine slices, nonadherent cells were removed after 2 h, and dentine slices were transferred into fresh dishes for 48 h further culture.
Cell culture
THP1 (monocytic leukemia), MG-63, and Saos2 (osteoblastic osteosarcoma) cell lines were maintained in αMEM (without ribonucleosides/deoxyribonucleosides) or RPMI 1640 supplemented with 10% FBS, l-glutamine (2 mM), penicillin (50 IU/ml), and streptomycin sulfate (50 μg/ml). Primary human monocytes were separated from PBMCs as described above and selected for CD14 expression before culture in αMEM. Mononuclear GCTB stromal cells were isolated from GCTB as described previously (23). In brief, tissue was washed in sterile PBS, and small fragments were cultured in αMEM for 24 h. Medium was changed weekly, with cell outgrowth monitored and cultures passaged at 80% confluence. Cells were used experimentally up to passage 3.
Genome-wide expression profiling
Profiling was performed using six paired samples of normoxic vs. hypoxic (24 h, 2% O2) osteoclasts. RNA was extracted in TRI reagent and purified using the RNeasy Mini kit (Qiagen, Crawley, UK). RNA quality was assessed using an Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA). Hybridization and data analysis were performed as described (24, 25). In brief, 200 ng of total RNA was converted into biotin-labeled cRNA using the Illumina TotalPrep RNA Amplification Kit (Ambion, Warrington, UK), and 1.5 μg of cRNA was hybridized overnight onto the Illumina HumanWG-6 v3.0 Expression BeadChip containing >48,000 transcript probes. Streptavidin-Cy3 detection was quantified on an Illumina BeadArray Reader with image analysis performed using Illumina BeadStudio Application Software (Ambion). Signal values were exported into the statistics software package S-Plus 6.2, and the different experiments were scaled to the same interquartile range and median. Significant differences in gene expression between experimental groups were evaluated using the t test (2-sample, 2-sided, equal variances). Genes were defined as differentially expressed if P < 0.05, fold change >1.5, and difference between group means >10. A sampling procedure was used to analyze the stability of gene expression candidates. The applied variant is similar to the proven significance analysis of microarrays approach (controls the false discovery rate) (26). Subsampling was done on a per experiment level, preserving the interrelation structure of the array. A group size of n − 1 was applied in the sampling procedure. The group assignment was preserved. All permutations in this scenario were evaluated. The resulting candidates are the least common multiple comparing the two groups and therefore termed stable in this view.
Vitronectin receptor (VNR) staining
Osteoclasts on dentine slices were fixed in acetone, and VNR staining was visualized immunohistochemically using a monoclonal antibody against CD51/61 (Serotec, Oxford, UK). Multinucleated cells containing ≥3 nuclei were considered osteoclasts.
Resorption assay
Dentine slices were analyzed for lacunar resorption as evidence of osteoclast function. Adherent cells were removed with 1 N NH4OH, and resorption pits were visualized using 0.5% toluidine blue in 0.5% boric acid. Dentine slices were photographed, resorbed areas were highlighted, and percentage resorption was quantified using ImageJ.
Western blot
Cells were homogenized in lysis buffer (6.2 M urea, 10% glycerol, 5 M DTT, 1% SDS, and protease inhibitors), and whole cell extract was separated by SDS-PAGE and transferred to PVDF membrane. Primary antibodies were against HIF-1α (BD Biosciences, Oxford, UK), HIF-2α (EP190b; Abcam, Cambridge, UK), ANGPTL4 (Prestige Antibody; Sigma-Aldrich), and β-tubulin. Immunoreactivity was visualized with horseradish peroxidase-linked goat serum and chemiluminescence. Quantification was performed by densitometry using ImageJ.
Real-time PCR
Total RNA was extracted in TRI reagent and treated with DNase I, and 1 μg was reverse-transcribed into cDNA using the SuperScript VILO cDNA Synthesis Kit (Invitrogen). Real-time PCR was performed using Express SYBR GreenER qPCR Supermix Universal (Invitrogen) and predesigned intron-spanning QuantiTect primers for ANGPTL4, HIF-1α, HIF-2α, and ACTB (Qiagen) on a RotorGene RG-3000 (Corbett Research UK, Cambridge, UK). Comparative quantification was performed using RotorGene v6, with ANGPTL4 expression levels normalized to ACTB.
ANGPTL4 ELISA
ANGPTL4 secretion was measured using the human ANGPTL4 DuoSet (R&D Systems).
HIF and ANGPTL4 small interfering RNA (siRNA)
siRNA sequences against HIF-1α, HIF-2α, or an HIF-1α scrambled control (27) were obtained preannealed from Eurogentec (Southampton, UK). Silencer Select predesigned siRNAs targeting ANGPTL4 [s27509 (A1) and s27511 (A2)] were from Applied Biosystems (Warrington, UK). In vitro differentiated osteoclasts were transfected with 50 nM siRNA duplexes using RNAiMAX (Invitrogen). Duplexes were removed after 16 h, and osteoclasts were incubated for a further 8 h before hypoxic stimulation.
Osteoblast proliferation and differentiation
For proliferation assays, osteoblasts were seeded at 1 × 103 cells/well in 96-well plates in media containing 0.1% FBS plus 1, 5, 25, or 100 ng/ml ANGPTL4. Fresh medium was added every 3–4 d, and the cell number was assayed using CellTiter-Blue (Promega, Southampton, UK). For differentiation experiments, cells were grown in 24-well plates until confluent and then treated with 2 nM β-glycerophosphate and 50 μg/ml ascorbic acid plus 1, 5, 25, or 100 ng/ml ANGPTL4 for 3 wk, changing the medium every 3–4 d. Mineralization was visualized by Alizarin red staining. Quantification was achieved by extraction in 10% acetic acid, neutralization in 10% NH4OH, and assessment of absorbance at 405 nm (28).
Fluorescent immunohistochemistry
Formalin-fixed, paraffin-embedded GCTB tissue was from the Oxford Musculoskeletal Biobank (90/H0606/11). Antigen retrieval was performed on deparaffinized sections by microwaving in 1 mM EDTA (pH 8). Sections were exposed to anti-ANGPTL4 (sc-66806; Santa Cruz Biotechnology, Heidelberg, Germany), anti-HIF-1α (clone 54; BD Biosciences), anti-CD51 (VNR) (NCL-CD51; Novocastra, Newcastle, UK), or a serum control. Secondary antibodies were DyLight 488 or 594 conjugates (Thermo Scientific, Northumberland, UK). Image acquisition was performed using an Olympus BX40 microscope, Olympus DP70 camera, and CellF (Olympus, Tokyo, Japan).
Statistics
Results are expressed as mean ± sd of ≥3 independent experiments. Statistical analysis comprised 1-way ANOVA using Bonferroni's multiple comparison as a post hoc test (except for experiments with only 2 conditions, for which a t test was applied), with results considered significant at values of P ≤ 0.05.
RESULTS
HIF is sufficient to stimulate osteoclast-mediated bone resorption
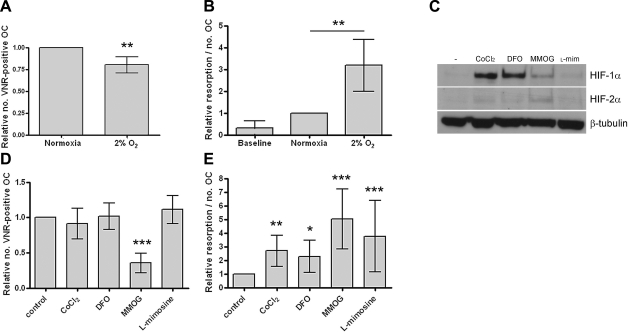
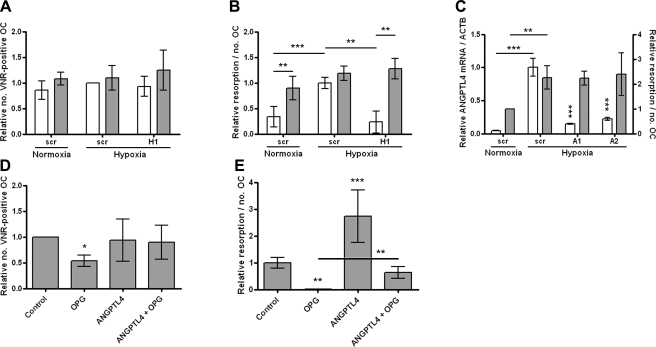
Based on our previous results that hypoxia stimulates osteoclast-mediated bone resorption (9), a microarray was performed to identify potential hypoxia-inducible genes mediating this effect. Osteoclasts were differentiated in vitro from CD14-selected monocytes to enhance the specificity of the array. RNA was hybridized from 6 paired samples of normoxic vs. hypoxic (24 h, 2% O2) osteoclasts, with confirmation that hypoxic induction of resorption had occurred in each case (Fig. 1A, B). A 3-fold increase in resorptive activity was observed under hypoxic conditions. Parallel experiments were performed using pharmacological PHD enzyme inhibitors to stabilize HIF expression under normoxic culture conditions (Fig. 1C). A 24-h exposure to these inhibitors also enhanced osteoclastic bone resorption 2- to 5-fold (Fig. 1D, E) showing that induction of HIF, in the absence of a hypoxic stimulus, is sufficient to increase osteoclastic bone resorption. Toxic effects were evident with dimethyloxalyl glycine (MMOG), which was therefore not used in subsequent experiments.
Figure 1.
HIF stimulates osteoclast-mediated bone resorption. A, B) Osteoclast cultures used for the microarray were exposed to normoxic or hypoxic conditions for 24 h and assessed for comparative osteoclast (OC) survival by counting the number of VNR-positive, multinucleated cells (A) and relative resorption by quantifying area of resorption and normalizing to osteoclast number (B). C) Western blot showing induction of HIF-1α and HIF-2α (5× overexposed compared with HIF-1α) in osteoclasts after 16 h of exposure to CoCl2 (100 μM), desferrioxamine (DFO, 100 μM), MMOG (1 mM), or l-mimosine (l-mim, 100 μM). D, E) Quantification of osteoclast survival (D) and relative resorption (E) observed in osteoclasts exposed to these agents for 24 h. *P < 0.05; **P < 0.01; ***P < 0.001.
On the basis of these results, the top 60 hypoxia-inducible genes from the microarray (based on fold induction, level of significance, and detection by multiple probes) were sorted into known HIF target genes (Table 1) and those genes not known to be regulated by HIF (Table 2). Further analysis focused on the HIF target genes.
Table 1.
HIF target genes induced by hypoxia in the osteoclast microarray
| Gene symbol | Gene name | Fold induction | P value | Accession no. |
|---|---|---|---|---|
| ANGPTL4 | Angiopoietin-like 4 | 14.3 | 0.0004 | NM_139314.1 |
| PLOD2 | Procollagen-lysine, 2-oxoglutarate 5-dioxygenase 2 | 7.4 | 0.0003 | NM_182943.2 |
| STC2 | Stanniocalcin 2 | 7 | 0.00001 | NM_003714.2 |
| CA12 | Carbonic anhydrase XII | 6.6 | 0.002 | NM_001218.3 |
| MT3 | Metallothionein 3 | 6.2 | 0.00003 | NM_005954.2 |
| CXCR4 | Chemokine (C-X-C motif) receptor 4 | 5.1 | 0.02 | NM_003467.2 |
| NM_001008540.1 | ||||
| DDIT4 | DNA-damage-inducible transcript 4 | 4.5 | 0.002 | NM_019058.2 |
| ANG | Angiogenin, ribonuclease, RNase A family, 5 | 3.6 | 0.003 | NM_001145.2 |
| BNIP3 L | BCL2/adenovirus E1B 19-kDa interacting protein 3-like (NIX) | 3.2 | 0.0002 | NM_004331.2 |
| PFKFB4 | 6-Phosphofructo-2-kinase/fructose-2,6-biphosphatase 4 | 3.2 | 0.0003 | NM_004567.2 |
| ERO1L | ERO1-like (Streptomyces cerevisiae) | 3 | 0.00002 | NM_014584.1 |
| BNIP3 | BCL2/adenovirus E1B 19-kDa interacting protein 3 | 2.7 | 0.0002 | NM_004052.2 |
| VEGF | Vascular endothelial growth factor A | 2.7 | 0.003 | NM_001025366.1 |
| NM_003376.4 | ||||
| MXI1 | MAX interactor 1 | 2 | 0.0001 | NM_001008541.1 |
| PGK1 | Phosphoglycerate kinase 1 | 2 | 0.0002 | NM_000291.2 |
| P4HA2 | Procollagen-proline, 2-oxoglutarate 4-dioxygenase (proline 4-hydroxylase), α polypeptide II | 2 | 0.006 | NM_001017974.1 |
Table 2.
Top hypoxia-inducible genes in the osteoclast microarray; not HIF target genes
| Gene symbol | Gene name | Fold change | P value | Accession no. |
|---|---|---|---|---|
| ZNF395 | Zinc finger protein 395 | 7.2 | 0.0009 | NM_018660.2 |
| PNCK | Pregnancy upregulated nonubiquitously expressed CaM kinase | 6.2 | 0.00005 | NM_198452.1 |
| TMEM45A | Transmembrane protein 45A | 5.6 | 0.001 | NM_018004.1 |
| VLDLR | Very low density lipoprotein receptor | 5.3 | 0.00001 | NM_001018056.1 |
| SPAG4 | Sperm associated antigen 4 | 5 | 0.00001 | NM_0063116.1 |
| PPP1R3C | Protein phosphatase 1, regulatory (inhibitor) subunit 3C | 5 | 0.0002 | NM_005398.3 |
| FGF11 | Fibroblast growth factor 11 | 4.7 | 0.00001 | NM_004112.2 |
| CCL20 | Chemokine (C-C motif) ligand 20 (MIP3A) | 4.5 | 0.00063 | NM_004591.1 |
| NM_199165.1 | ||||
| ADSSL1 | Adenylosuccinate synthase like 1 | 4.5 | 0.006 | NM_152328.3 |
| ENO2 | Enolase 2 | 4.4 | 0.0005 | NM_001975.2 |
| SULF2 | Sulfatase 2 | 4.2 | 0.001 | NM_018837.2 |
| PSAT1 | Phosphoserine aminotransferase 1 | 4.1 | 0.00002 | NM_021154.3 |
| PPFIA4 | Protein tyrosine phosphatase, receptor type, f polypeptide (PTPRF), interacting protein α 4 | 4 | 0.00003 | NM_015053.1 |
| CD300A | CD300a molecule | 4 | 0.0005 | NM_007261.2 |
| EEF1A2 | Eukaryotic translation elongation factor 1 α 2 | 3.8 | 0.00001 | NM_001958.2 |
| ADFP | Adipose differentiation-related protein | 3.8 | 0.02 | NM_001122.2 |
| PTGS2 | Prostaglandin-endoperoxide synthase 2 (prostaglandin G/H synthase and cyclooxygenase, COX2) | 3.7 | 0.0001 | NM_000963.1 |
| RSNL2 | CAP-GLY domain containing linker protein family, member 4 | 3.6 | 0.0004 | NM_024692.3 |
| LOC652879 | Similar to phosphodiesterase 4D interacting protein isoform 2 | 3.6 | 0.0005 | XM_942594.1 |
| NM_194430.1, | ||||
| RNASE4 | Ribonuclease, RNase A family, 4 | 3.5 | 0.001 | NM_002937.3 |
| TMEM71 | Transmembrane protein 71 | 3.4 | 0.00001 | NM_144649.1 |
| DPYSL4 | Dihydropyrimidinase-like 4 | 3.4 | 0.0003 | NM_006426.1 |
| KISS1R | KISS1 receptor | 3.2 | 0.0005 | NM_032551.3 |
| BTG1 | B-cell translocation gene 1 | 3.2 | 0.0008 | NM_001731.1 |
| PDE2A | Phosphodiesterase 2A, cGMP-stimulated | 3 | 0.00001 | NM_002599.1 |
| TSC22D3 | TSC22 domain family, member 3 | 2.9 | 0.002 | NM_004089.3 |
| ACOT11 | Acyl-CoA thioesterase 11 | 2.7 | 0.00001 | NM_147161.2 |
| LOC653853 | Similar to hypothetical protein FLJ40722, transcript variant 2 | 2.7 | 0.0004 | XM_936029.1 |
| ADORA2B | Adenosine A2b receptor | 2.7 | 0.002 | NM_000676.2 |
| SORL1 | Sortilin-related receptor, L (DLR class) A repeats-containing | 2.6 | 0.001 | NM_003105.3 |
| C1orf165 | Chromosome 1 open reading frame 165 | 2.4 | 0.0003 | NM_024603.1 |
| ICAM5 | Intercellular adhesion molecule 5, telencephalin | 2.4 | 0.0005 | NM_003259.2 |
| LOC146439 | Hypothetical LOC146439 | 2.3 | 0.00002 | XM_940000.1 |
| LOC644782 | Similar to implantation-associated protein | 2.3 | 0.0001 | XM_927878.1 |
| NM_133280.1 | ||||
| FCAR | Fc fragment of IgA, receptor for | 2.3 | 0.002 | NM_133271.1 |
| ANKZF1 | Ankyrin repeat and zinc finger domain containing 1 | 2.2 | 0.0002 | NM_018089.1 |
| MGC17330 | Phosphoinositide-3-kinase interacting protein 1 | 2.2 | 0.0005 | NM_052880.3 |
| FER1L4 | Fer-1-like 4 (Caenorhabditis elegans) | 2.1 | 0.00001 | NR_001442.2 |
| CCL28 | Chemokine (C-C motif) ligand 28 (CCK1) | 2.1 | 0.0001 | NM_019846.3 |
| PPP1R3G | Protein phosphatase 1, regulatory (inhibitor) subunit 3G | 2.1 | 0.0004 | XM_371796.2 |
| FLJ40722 | Hypothetical protein FLJ40722, transcript variant 4 | 2 | 0.00005 | XM_945696.1 |
| LRRC6 | Leucine rich repeat containing 6 | 2 | 0.0001 | NM_012472.3 |
| NM_001018021.1 | ||||
| MUC1 | Mucin 1 | 2 | 0.0003 | NM_001044390.1 |
ANGPTL4 is an HIF-dependent hypoxia-inducible gene in osteoclasts
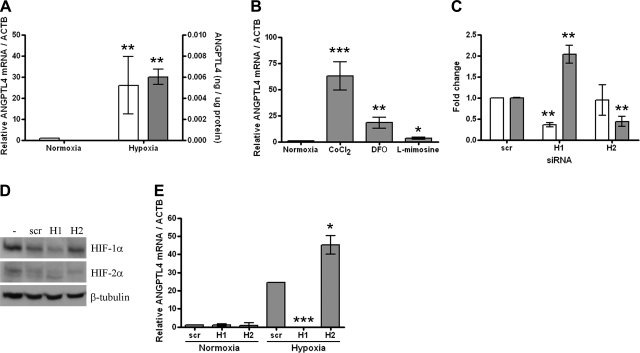
ANGPTL4 was the most hypoxia-inducible gene on the array (14.3-fold induction, P<0.0004) and is a known HIF target gene, although its expression has not previously been described in osteoclasts. Induction of ANGPTL4 by 24 h exposure to 2% O2 was confirmed in in vitro differentiated osteoclasts at both the mRNA and protein levels (Fig. 2A). ANGPTL4 was also induced by exposure to the panel of PHD enzyme inhibitors (Fig. 2B). HIF siRNA transfection procedures were optimized to achieve up to 70% knockdown of target gene mRNA 48 h after transfection using 50 nM siRNA and RNAiMAX (Fig. 2C). No associated osteoclast toxicity was observed (data not shown). This result translated to 59–85% inhibition of HIF-1α and 31–58% inhibition of HIF-2α protein (range of inhibition from 3 independent experiments) (Fig. 2D). Interestingly, at the mRNA level HIF-1α siRNA resulted in a significant increase in expression of HIF-2α (Fig. 2C). This was not mirrored at the protein level, however, in keeping with the predominantly post-translational mechanism of HIF regulation (29). Analysis of ANGPTL4 expression in siRNA-treated osteoclasts revealed it to be induced exclusively by HIF-1α (Fig. 2E). However, HIF-2α siRNA increased hypoxic induction of ANGPTL4 further, suggesting a potential inhibitory effect of HIF-2α on ANGPTL4 expression.
Figure 2.
ANGPTL4 is regulated by HIF-1α in osteoclasts. A) Hypoxic induction of ANGPTL4 mRNA (open bars, n=7) and protein (shaded bars, n=4). B) Induction of ANGPTL4 mRNA by 24 h exposure to CoCl2 (100 μM), desferrioxamine (DFO, 100 μM), and l-mimosine (100 μM). C) Effect of siRNA targeting HIF-1α (H1), HIF-2α (H2), or HIF-1α scrambled control (scr) on expression of HIF-1α (open bars) or HIF-2α (shaded bars) mRNA; relative expression under hypoxia, normalized to ACTB. D) Western blot showing hypoxic expression of HIF-1α and HIF-2α (5× overexposed compared with HIF-1α) in response to siRNA targeting HIF-1α, HIF-2α, or HIF-1α scrambled control. E) Effect of HIF siRNA on ANGPTL4 mRNA expression in normoxic and hypoxic osteoclasts. *P < 0.05; **P < 0.01; ***P < 0.001.
ANGPTL4 stimulates osteoclast-mediated bone resorption
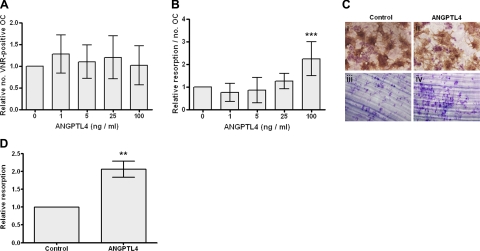
To determine whether ANGPTL4 contributes to the hypoxic increase in osteoclast-mediated bone resorption, mature osteoclasts derived from CD14-selected monocytes were exposed to 1–100 ng/ml ANGPTL4 for 24 h. ANGPTL4 at 100 ng/ml increased lacunar bone resorption 2- to 3-fold, with no evidence of toxic effects (Fig. 3A–C). The same result was observed with primary human osteoclasts from GCTB (Fig. 3D).
Figure 3.
ANGPTL4 stimulates osteoclast resorption. A, B) Effect of 24 h exposure to ANGPTL4 (1–100 ng/ml) on osteoclast (OC) viability (comparative number of VNR-positive, multinucleated cells; A) and relative resorption (area of resorption normalized to osteoclast number; B). C) 100 ng/ml ANGPTL4 has no effect on osteoclast number (VNR-positive multinucleated cells; i, ii) and stimulates resorption (iii, iv). D) Effect of 24 h exposure to ANGPTL4 (100 ng/ml) on resorptive capacity of primary human osteoclasts. **P < 0.01; ***P < 0.001.
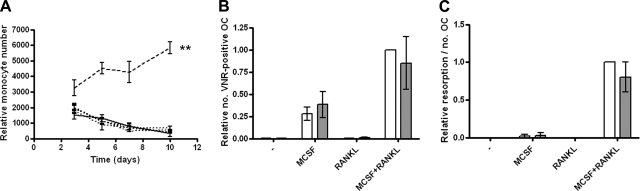
In contrast, ANGPTL4 had no effect on the proliferation of CD14+ monocytic osteoclast precursors at any concentration (Fig. 4A). In addition, ANGPTL4 did not influence monocyte-osteoclast differentiation in either the presence or absence of M-CSF or RANKL at any concentration tested (shown for 100 ng/ml ANGPTL4) (Fig. 4B, C).
Figure 4.
ANGPTL4 has no effect on osteoclast differentiation. A) CD14-positive monocytes in 1% FCS medium were exposed to 1, 5, 25, or 100 ng/ml ANGPTL4 (dotted lines, all concentrations) for 10 d, and cell numbers were compared with the untreated control (solid line). Positive control (25 ng/ml M-CSF; dashed line); **P < 0.01. B, C) Monocyte-osteoclast differentiation in the presence of M-CSF and/or RANKL (open bars) was unaffected by the addition of 100 ng/ml ANGPTL4 (shaded bars) with respect to either osteoclast number (B) or resorption (C). Experiments were performed in normoxic culture conditions.
Further analysis using isoform-specific HIF siRNA demonstrated that ANGPTL4 was able to rescue the inhibitory effect of HIF-1α siRNA on hypoxic induction of osteoclast resorption (Fig. 5A, B). HIF-2α siRNA had no significant effect on hypoxic osteoclast activity, which remained unaffected by the presence of supplementary ANGPTL4 (data not shown). However, hypoxic induction of osteoclast activity was not ANGPTL4 dependent. ANGPTL4 siRNA reduced hypoxic induction of ANGPTL4 by ≥79% but had no effect on the level of hypoxic resorption (Fig. 5C). Potential mechanisms of ANGPTL4-mediated induction of osteoclast activity were investigated using OPG, the soluble decoy receptor for RANKL. ANGPTL4 prevented the reduction in osteoclast number/viability induced by exposure to OPG (Fig. 5D). Conversely, ANGPTL4-mediated induction of osteoclast activity was inhibited in the presence of OPG (Fig. 5E). However, comparison of resorption with OPG alone and with OPG + ANGPTL4 revealed that ANGPTL4 was still able to promote resorption in the absence of RANKL (no OPG, 2.9-fold induction; OPG, 90-fold induction), although the total amount of resorption achieved was reduced. This was not due to induction of RANKL by ANGPTL4, which had no effect on expression of RANKL mRNA or protein by either monocytes or osteoclasts (data not shown), nor did ANGPTL4 induce expression of MMP1 or MMP3 in these cell types, as has previously been reported in human chondrocytes (21).
Figure 5.
Mechanisms of ANGPTL4-mediated resorption. A, B) Effect of 24 h exposure to ANGPTL4 (100 ng/ml, shaded bars) on osteoclast viability (A) and relative resorption (B) in comparison with the untreated control (open bars) after treatment with HIF-1α (H1) or scrambled control (scr) siRNA. C) Effect of ANGPTL4 siRNA (A1 and A2) on hypoxic induction of ANGPTL4 mRNA (open bars) and resorption (shaded bars) in comparison with scrambled hypoxic control siRNA. D) Effect of ANGPTL4 (100 ng/ml) on osteoclast viability during 24 h exposure to OPG (500 ng/ml). E) Effect of ANGPTL4 (100 ng/ml) on resorption during 24 h exposure to OPG (500 ng/ml). *P < 0.05; **P < 0.01; ***P < 0.001.
ANGPTL4 is expressed by osteoclasts in vivo
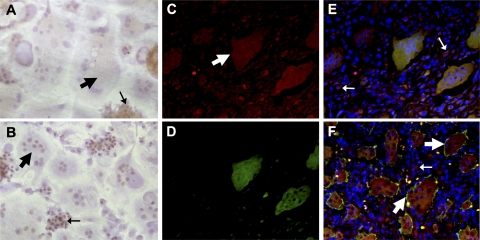
To determine whether ANGPTL4 might be a physiologically relevant mechanism for driving osteoclastic bone resorption, expression of ANGPTL4 was investigated using GCTB as an osteoclast-rich model of a condition demonstrating high levels of pathological bone resorption, which we have previously shown to express HIF (30). Preliminary investigation of hypoxic osteoclasts in vitro demonstrated ANGPTL4 reactivity to be diffuse and cytoplasmic in osteoclasts with confirmed nuclear expression of HIF-1α (Fig. 6A, B; broad arrows). Diffuse cytoplasmic expression of ANGPTL4 was also evident in VNR-positive osteoclasts in GCTB (Fig. 6C, F; broad arrows). Colocalization with HIF-1α was apparent in osteoclasts with the strongest ANGPTL4 immunoreactivity, although ANGPTL4 was also expressed in HIF-1α-negative cells (Fig. 6C–E). However, osteoclasts were not the only cell type to express ANGPTL4. Undifferentiated hypoxic CD14+ monocytes (Fig. 6A, narrow arrows) and numerous cells comprising the osteoblastic mononuclear stromal component of GCTB (Fig. 6E, F; narrow arrows) also exhibited expression of ANGPTL4.
Figure 6.
Immunostaining for ANGPTL4. A, B) In vitro differentiated osteoclasts cultured on dentine slices were exposed to hypoxia (2% O2, 24 h) and stained with anti-ANGPTL4 (A) or anti-HIF-1α (B). C–F) Immunofluorescence of GCTB sections stained for ANGPTL4 (C), HIF-1α (D), and merge: DAPI (blue), ANGPTL4 (red), and HIF-1α (green) (E) and DAPI (blue), ANGPTL4 (red), and VNR (green) (F). Broad arrows indicate osteoclasts; narrow arrows indicate mononuclear cells.
Hypoxia-inducible expression of ANGPTL4 in osteoblasts
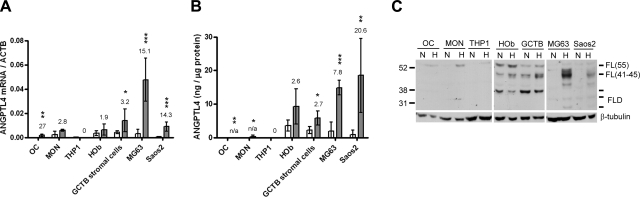
The relative level of ANGPTL4 expression in osteoclasts was compared with that in monocytes and osteoblasts in vitro, using both primary human cells and cell lines. At both the mRNA (Fig. 7A) and protein (Fig. 7B) levels, osteoblastic cells expressed considerably more ANGPTL4 than monocytes or osteoclasts, although the magnitude of hypoxic induction remained greatest in osteoclasts. ANGPTL4 can be proteolytically processed, in a cell- and species-specific manner, from the full-length protein into an N-terminal coiled-coil domain (CCD) and a C-terminal fibrinogen-like domain (FLD) fragment (16). The ELISA used is based on a polyclonal antibody raised against the full-length recombinant immunogen and is therefore unable to distinguish these forms. Western blotting with an antibody raised against the FLD enabled confirmation of hypoxic induction of full-length ANGPTL4 in osteoclasts, monocytes, and, most strongly, osteoblastic cells (Fig. 7C). The FLD fragment was also detected in osteoblastic cells, indicating that osteoblasts are capable of proteolytic processing of ANGPTL4, although expression of this cleaved form was not apparently hypoxia-regulated. An FLD fragment was not detected in either monocytes or osteoclasts, although this may be due to low levels of expression of ANGPTL4 in these cells.
Figure 7.
Osteoblasts and ANGPTL4. A, B) Effect of 24 h exposure to 2% O2 (shaded bars) on ANGPTL4 expression assessed by real-time PCR (A) and ELISA (B) vs. the normoxic (open bars) control. Mean fold hypoxic induction is indicated over the bars for each cell type (n/a, not calculated, normoxic levels undetectable). *P < 0.05; **P < 0.01; ***P < 0.001. C) Western blot showing hypoxic induction of ANGPTL4 in the same cell panel and detecting full-length ANGPTL4 of the predicted 41- to 45-kDa molecular mass [FL(41–45)], full-length ANGPTL4 of 50–55 kDa at which it normally runs in reducing conditions [FL(55)], and the FLD domain at 20–35 kDa (FLD). OC, in vitro differentiated osteoclasts; MON, CD14+ monocytes; THP1, monocytic cell line; HOb, primary human osteoblasts; GCTB stromal cells, mononuclear stromal component of GCTB; MG-63 and Saos2, osteoblastic cell lines.
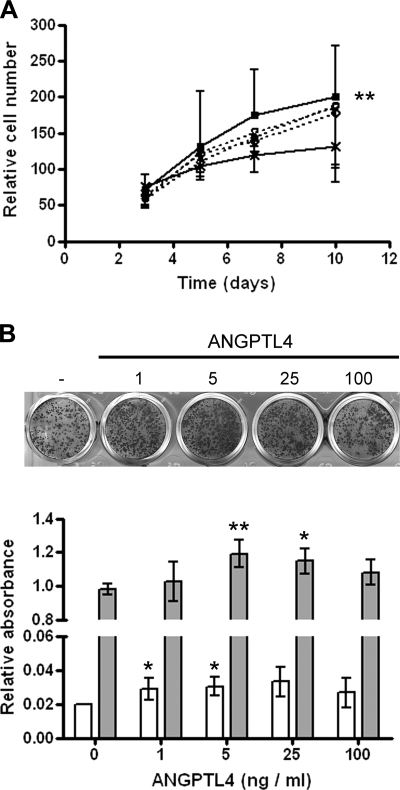
Effects of exogenous ANGPTL4 on the osteoblast phenotype were therefore investigated. ANGPTL4 at 100 ng/ml significantly enhanced osteoblast proliferation in comparison with that in untreated control cells (Fig. 8A). Lower concentrations of ANGPTL4 (1–25 ng/ml) induced osteoblast mineralization (Fig. 8B).
Figure 8.
ANGPTL4 and the osteoblastic phenotype. A) Proliferation of Saos2 cells over a 10-d period in the presence of 1–100 ng/ml ANGPTL4. ANGPTL4 at 100 ng/ml (■——■) induced significant osteoblast proliferation compared with the untreated control (×——×). ○– – –○, 1 ng/ml ANGPTL4; ▵– – –▵, 5 ng/ml ANGPTL4; ◊– – –◊, 25 ng/ml ANGPTL4. **P < 0.01 vs. untreated control. B) Visualization of Saos2 mineralization with Alizarin red. Quantification at d 7 (open bars) and d 14 (shaded bars) showed the most significant effect at 5 ng/ml ANGPTL4. *P < 0.05, **P < 0.01 vs. no ANGPTL4 control.
DISCUSSION
These data add to the accumulating evidence that hypoxia and HIF play important roles in conditions manifesting pathological levels of bone resorption. We have demonstrated that induction of HIF, in the absence of a hypoxic stimulus, enhances osteoclastic resorption 3- to 4-fold, broadening the implications of these results to include not only bony lesions in which microenvironmental hypoxia and osteoclasts coincide (e.g., arthritis and cancer metastases to bone) but also those manifesting HIF expression due to the presence of factors such as mechanical stress or induction of oncogenes, proinflammatory cytokines, and growth factors (3, 31). Situations encompassed would include aseptic loosening of prosthetic implants, in which accumulation of metal ions such as Co2+ has been shown to enhance osteolysis (32). It also offers a further explanation for the success of the antiangiogenic agent endostatin, which inhibits HIF-1α, in reducing arthritis scores and subchondral bone erosion in a murine model of collagen-induced arthritis (33). Furthermore, conditional deletion of HIF-1α from the myeloid lineage in a model of passively induced arthritis eliminated swelling of affected joints and significantly reduced joint destruction (34).
Our results also support a dominant role for the HIF-1α isoform of HIF in regulating osteoclast function. HIF-1α was substantially overexpressed in comparison with HIF-2α after stimulation with either hypoxia or PHD enzyme inhibitors, and whereas isoform-specific HIF-1α siRNA returned hypoxic osteoclast activity to normoxic levels, the effects of HIF-2α siRNA were variable and not significant. In keeping with a phenotypically dominant role for HIF-1α, we have shown that hypoxic induction of ANGPTL4 in osteoclasts is HIF-1α-dependent, in agreement with previous reports in cardiomyocytes (17) and Hep3B cells (18). However, we also report an inhibitory effect of HIF-2α on ANGPTL4 expression, which may provide a secondary level of regulation for fine-tuning the induction of ANGPTL4 by HIF.
In keeping with its HIF-1α-dependent regulation, hypoxic induction of ANGPTL4 was also observed in osteoblastic cells in vitro. The diffuse staining pattern observed in all cell types in vivo might be explained by the structural composition of the protein. ANGPTL4 is a 406-aa protein structurally similar to the angiopoietins in that it contains a signal peptide mediating its secretion, an N-terminal CCD, a linker, and a C-terminal FLD (15). Binding of ANGPTL4 to heparan and dermatan sulfate components of the extracellular matrix (ECM) via the CCD results in diffuse localization in vitro (35, 36). The only previous report of ANGPTL4 staining in vivo is in human articular chondrocytes, which showed strong cytoplasmic staining in rheumatoid arthritis, in which the cartilage is strongly hypoxic, and diffuse staining of both the cytoplasm and matrix in osteoarthritis, in which the hypoxic insult is less severe (21).
We report novel roles for this adipokine in stimulation of osteoclastic bone resorption, osteoblast proliferation, and differentiation. These roles are potentially of clinical significance given that ANGPTL4 is overexpressed in rheumatoid arthritis (21, 37), various human cancers (20), and their distant metastases (38). An effect of ANGPTL4 to stimulate osteoblast differentiation at low local concentrations and osteoclast activity at higher concentrations might tip bone homeostatic mechanisms in favor of pathological resorption under conditions of severe local hypoxia and/or inflammation.
It is not surprising that the concentrations of ANGPTL4 that maximally stimulate differentiation exhibit no significant effects on preosteoblast proliferation. As with all cell types the osteoblastic cell cycle is suppressed during differentiation (39), a feature also evident on pharmaceutical intervention with bisphosphonates such as zoledronate, which dose dependently inhibits osteoblast proliferation while increasing the proportion of differentiated cells (40). One of the few cytokines to affect osteoclast activity directly, rather than indirectly enhancing resorption via stimulation of osteoclast differentiation, is RANKL (41). ANGPTL4-mediated induction of osteoclast activity could be achieved, albeit with reduced total levels of resorption, in the absence of RANKL. This result suggests that ANGPTL4 is activating distinct intracellular signaling pathways to stimulate osteoclast activity. ANGPTL4 is an orphan ligand, and the only reported effect of exogenous application of the adipokine on cell signaling is a suppressive effect on the Raf/MEK/ERK cascade in murine endothelial cells (42). However ANGPTL1 and 2 function via PI3K/Akt, ANGPTL3 activates αvβ3 integrin by direct binding, and ANGPTL6 stimulates ERK1/2 signaling (43, 44). Because ANGPTL3 is the most closely related to ANGPTL4, it is interesting to speculate that ANGPTL4 might also directly activate osteoclastic αvβ3/VNR to initiate the cytoskeletal reorganization and c-Src activation associated with polarization and resorption (45). Such an interaction might also explain the observed effects of ANGPTL4 on osteoblast function, integrin-ECM interactions being known to affect osteoblast differentiation and mineralization (46).
The primary known roles of ANGPTL4 to date are in regulation of lipid metabolism and angiogenesis. There is some controversy regarding the effect of ANGPTL4 on angiogenic processes. The ECM-bound form has been shown to inhibit endothelial cell adhesion, migration, and sprouting (35, 36), whereas soluble ANGPTL4 variably either inhibits (42, 47) or stimulates (20, 37) tube formation. In vivo, ANGPTL4 inhibits angiogenesis and vascular leakiness (47) as well as melanoma cell motility and invasiveness to prevent metastasis (48). The role of ANGPTL4 in lipid metabolism is mediated via inhibition of lipoprotein lipase activity, leading to increased levels of plasma triacylglycerol and nonesterified fatty acids, with subsequent depletion of adipose tissue stores (49, 50). The full-length and truncated forms of ANGPTL4 have again been proposed to function differentially in this respect (16, 51), in a fashion similar to that of other adipokines whose proteolytically processed forms are more biologically active [e.g., adiponectin (52), resistin (53), IL-6 (54), and TNF-α (55)]. These adipokines also exert bone homeostatic effects. IL-6 and TNF-α are able to substitute for RANKL in osteoclastogenesis (56). Resistin stimulates osteoblast proliferation, osteoclastogenesis, and bone resorption in murine culture systems (57, 58). Adiponectin enhances osteoblast proliferation and differentiation (59, 60) and modulates the RANKL/OPG ratio in osteoblasts to stimulate osteoclast formation (61), although direct effects to inhibit osteoclastogenesis and resorption have also been reported (60). Interestingly leptin, which inhibits ANGPTL4 expression, also exerts inhibitory effects on osteoclast formation and activity at the peripheral level (62). ANGPTL4 might therefore represent an additional connection between bone loss and both osteoporosis and diabetes, linked to accumulation of adipocytes in the bone marrow with age and therapeutic intervention with PPARγ-inducing agents, respectively (63, 64).
In summary, our data clearly demonstrate a role for HIF in regulation of osteoclast activity that is mediated, at least partially, by ANGPTL4. Given the additional effects of ANGPTL4 on the osteoblast phenotype, this could represent a mechanism whereby HIF coordinates osteoclastic and osteoblastic components of the osseous niche. If we take into account other effects of ANGPTL4, these results suggest a tripartite role for the adipokine in mechanisms coupling the regulation of bone, fat, and angiogenesis with potential implications in diseases ranging from senile osteoporosis and diabetes to rheumatoid arthritis and cancer.
Acknowledgments
This study was funded by EuroBoNeT, a European Commission-granted European Network of Excellence for studying the pathology and genetics of bone tumors (LSHCCT-2006-018 814). H.J.K. is also funded by Arthritis Research UK (MP/19200). H.J.K. and N.A.A. acknowledge support from the Oxford National Institute Health Research Biomedical Research Unit (NIHR BRU). E.K. is also funded by the German Bundesministerium für Bildung und Forschung (01GM0869).
REFERENCES
- 1.Epstein A. C., Gleadle J. M., McNeill L. A., Hewitson K. S., O'Rourke J., Mole D. R., Mukherji M., Metzen E., Wilson M. I., Dhanda A., Tian Y. M., Masson N., Hamilton D. L., Jaakkola P., Barstead R., Hodgkin J., Maxwell P. H., Pugh C. W., Schofield C. J., Ratcliffe P. J. (2001) C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell 107, 43–54 [DOI] [PubMed] [Google Scholar]
- 2.Bruick R. K., McKnight S. L. (2001) A conserved family of prolyl-4-hydroxylases that modify HIF. Science 294, 1337–1340 [DOI] [PubMed] [Google Scholar]
- 3.Harris A. L. (2002) Hypoxia—a key regulatory factor in tumour growth. Nat. Rev. Cancer 2, 38–47 [DOI] [PubMed] [Google Scholar]
- 4.Wang Y., Wan C., Deng L., Liu X., Cao X., Gilbert S. R., Bouxsein M. L., Faugere M. C., Guldberg R. E., Gerstenfeld L. C., Haase V. H., Johnson R. S., Schipani E., Clemens T. L. (2007) The hypoxia-inducible factor alpha pathway couples angiogenesis to osteogenesis during skeletal development. J. Clin. Invest. 117, 1616–1626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wan C., Gilbert S. R., Wang Y., Cao X., Shen X., Ramaswamy G., Jacobsen K. A., Alaql Z. S., Eberhardt A. W., Gerstenfeld L. C., Einhorn T. A., Deng L., Clemens T. L. (2008) Activation of the hypoxia-inducible factor-1α pathway accelerates bone regeneration. Proc. Natl. Acad. Sci. U. S. A. 105, 686–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shomento S. H., Wan C., Cao X., Faugere M. C., Bouxsein M. L., Clemens T. L., Riddle R. C. (2010) Hypoxia-inducible factors 1α and 2α exert both distinct and overlapping functions in long bone development. J. Cell. Biochem. 109, 196–204 [DOI] [PubMed] [Google Scholar]
- 7.Shen X., Wan C., Ramaswamy G., Mavalli M., Wang Y., Duvall C. L., Deng L. F., Guldberg R. E., Eberhart A., Clemens T. L., Gilbert S. R. (2009) Prolyl hydroxylase inhibitors increase neoangiogenesis and callus formation following femur fracture in mice. J. Orthop. Res. 27, 1298–1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Komatsu D. E., Bosch-Marce M., Semenza G. L., Hadjiargyrou M. (2007) Enhanced bone regeneration associated with decreased apoptosis in mice with partial HIF-1α deficiency. J. Bone Miner. Res. 22, 366–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knowles H. J., Athanasou N. A. (2009) Acute hypoxia and osteoclast activity: a balance between enhanced resorption and increased apoptosis. J. Pathol. 218, 256–264 [DOI] [PubMed] [Google Scholar]
- 10.Arnett T. R., Gibbons D. C., Utting J. C., Orriss I. R., Hoebertz A., Rosendaal M., Meghji S. (2003) Hypoxia is a major stimulator of osteoclast formation and bone resorption. J. Cell. Physiol. 196, 2–8 [DOI] [PubMed] [Google Scholar]
- 11.Muzylak M., Price J. S., Horton M. A. (2006) Hypoxia induces giant osteoclast formation and extensive bone resorption in the cat. Calcif. Tissue Int. 79, 301–309 [DOI] [PubMed] [Google Scholar]
- 12.Bozec A., Bakiri L., Hoebertz A., Eferl R., Schilling A. F., Komnenovic V., Scheuch H., Priemel M., Stewart C. L., Amling M., Wagner E. F. (2008) Osteoclast size is controlled by Fra-2 through LIF/LIF-receptor signalling and hypoxia. Nature 454, 221–225 [DOI] [PubMed] [Google Scholar]
- 13.Kersten S., Mandard S., Tan N. S., Escher P., Metzger D., Chambon P., Gonzalez F. J., Desvergne B., Wahli W. (2000) Characterization of the fasting-induced adipose factor FIAF, a novel peroxisome proliferator-activated receptor target gene. J. Biol. Chem. 275, 28488–28493 [DOI] [PubMed] [Google Scholar]
- 14.Yoon J. C., Chickering T. W., Rosen E. D., Dussault B., Qin Y. B., Soukas A., Friedman J. M., Holmes W. E., Spiegelman B. M. (2000) Peroxisome proliferator-activated receptor γ target gene encoding a novel angiopoietin-related protein associated with adipose differentiation. Mol. Cell. Biol. 20, 5343–5349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim I., Kim H. G., Kim H., Kim H. H., Park S. K., Uhm C. S., Lee Z. H., Koh G. Y. (2000) Hepatic expression, synthesis and secretion of a novel fibrinogen/angiopoietin-related protein that prevents endothelial-cell apoptosis. Biochem. J. 346, 603–610 [PMC free article] [PubMed] [Google Scholar]
- 16.Mandard S., Zandbergen F., Tan N. S., Escher P., Patsouris D., Koenig W., Kleemann R., Bakker A., Veenman F., Wahli W., Muller M., Kersten S. (2004) The direct peroxisome proliferator-activated receptor target fasting-induced adipose factor (FIAF/PGAR/ANGPTL4) is present in blood plasma as a truncated protein that is increased by fenofibrate treatment. J. Biol. Chem. 279, 34411–34420 [DOI] [PubMed] [Google Scholar]
- 17.Belanger A. J., Lu H. W., Date L., Liu L. X., Vincent K. A., Akita G. Y., Cheng S. H., Gregory R. J., Jiang C. W. (2002) Hypoxia up-regulates expression of peroxisome proliferator-activated receptor gamma angiopoietin-related gene (PGAR) in cardiomyocytes: role of hypoxia inducible factor 1 α. J. Mol. Cell. Cardiol. 34, 765–774 [DOI] [PubMed] [Google Scholar]
- 18.Warnecke C., Weidemann A., Volke M., Schietke R., Wu X. Q., Knaup K. X., Hackenbeck T., Bernhardt W., Willam C., Eckardt K. U., Wiesener M. S. (2008) The specific contribution of hypoxia-inducible factor-2α to hypoxic gene expression in vitro is limited and modulated by cell type-specific and exogenous factors. Exp. Cell Res. 314, 2016–2027 [DOI] [PubMed] [Google Scholar]
- 19.Wang B., Wood I. S., Trayhurn P. (2007) Dysregulation of the expression and secretion of inflammation-related adipokines by hypoxia in human adipocytes. Pflügers Arch. 455, 479–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Le Jan S., Amy C., Cazes A., Monnot C., Lamande N., Favier J., Philippe J., Sibony M., Gasc J. M., Corvol P., Germain S. (2003) Angiopoietin-like 4 is a proangiogenic factor produced during ischemia and in conventional renal cell carcinoma. Am. J. Pathol. 162, 1521–1528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murata M., Yudo K., Nakamura H., Chiba J., Okamoto K., Suematsu N., Nishioka K., Beppu M., Inoue K., Kato T., Masuko K. (2009) Hypoxia upregulates the expression of angiopoietin-like-4 in human articular chondrocytes: role of angiopoietin-like-4 in the expression of matrix metalloproteinases and cartilage degradation. J. Orthop. Res. 27, 50–57 [DOI] [PubMed] [Google Scholar]
- 22.Joyner C. J., Quinn J. M., Triffitt J. T., Owen M. E., Athanasou N. A. (1992) Phenotypic characterisation of mononuclear and multinucleated cells of giant cell tumour of bone. Bone Miner. 16, 37–48 [DOI] [PubMed] [Google Scholar]
- 23.Lau Y. S., Sabokbar A., Gibbons C. L., Giele H., Athanasou N. (2005) Phenotypic and molecular studies of giant-cell tumors of bone and soft tissue. Hum. Pathol. 36, 945–954 [DOI] [PubMed] [Google Scholar]
- 24.Hallor K. H., Staaf J., Bovee J., Hogendoorn P. C. W., Cleton-Jansen A. M., Knuutila S., Savola S., Niini T., Brosjo O., Bauer H. C. F., von Steyern F. V., Jonsson K., Skorpil M., Mandahl N., Mertens F. (2009) Genomic profiling of chondrosarcoma: chromosomal patterns in central and peripheral tumors. Clin. Cancer Res. 15, 2685–2694 [DOI] [PubMed] [Google Scholar]
- 25.Seitz S., Korsching E., Weimer J., Jacobsen A., Arnold N., Meindl A., Arnold W., Gustavus D., Klebig C., Petersen I., Scherneck S. (2006) Genetic background of different cancer cell lines influences the gene set involved in chromosome 8 mediated breast tumor suppression. Genes Chromosomes Cancer 45, 612–627 [DOI] [PubMed] [Google Scholar]
- 26.Tusher V. G., Tibshirani R., Chu G. (2001) Significance analysis of microarrays applied to the ionizing radiation response. Proc. Natl. Acad. Sci. U. S. A. 98, 5116–5121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sowter H. M., Raval R. R., Moore J. W., Ratcliffe P. J., Harris A. L. (2003) Predominant role of hypoxia-inducible transcription factor (Hif)-1α versus Hif-2α in regulation of the transcriptional response to hypoxia. Cancer Res. 63, 6130–6134 [PubMed] [Google Scholar]
- 28.Gregory C. A., Gunn W. G., Peister A., Prockop D. J. (2004) An alizarin red-based assay of mineralization by adherent cells in culture: comparison with cetylpyridinium chloride extraction. Anal. Biochem. 329, 77–84 [DOI] [PubMed] [Google Scholar]
- 29.Wiesener M. S., Turley H., Allen W. E., Willam C., Eckardt K. U., Talks K. L., Wood S. M., Gatter K. C., Harris A. L., Pugh C. W., Ratcliffe P. J., Maxwell P. H. (1998) Induction of endothelial PAS domain protein-1 by hypoxia: characterization and comparison with hypoxia-inducible factor-1α. Blood 92, 2260–2268 [PubMed] [Google Scholar]
- 30.Knowles H. J., Athanasou N. A. (2008) Hypoxia-inducible factor is expressed in giant cell tumour of bone and mediates paracrine effects of hypoxia on monocyte-osteoclast differentiation via induction of VEGF. J. Pathol. 215, 56–66 [DOI] [PubMed] [Google Scholar]
- 31.Gaber T., Dziurla R., Tripmacher R., Burmester G. R., Buttgereit F. (2005) Hypoxia inducible factor (HIF) in rheumatology: low O2! See what HIF can do! Ann. Rheum. Dis. 64, 971–980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patntirapong S., Habibovic P., Hauschka P. V. (2009) Effects of soluble cobalt and cobalt incorporated into calcium phosphate layers on osteoclast differentiation and activation. Biomaterials 30, 548–555 [DOI] [PubMed] [Google Scholar]
- 33.Kurosaka D., Yoshida K., Yasuda J., Yokoyama T., Kingetsu I., Yamaguchi N., Joh K., Matsushima M., Saito S., Yamada A. (2003) Inhibition of arthritis by systemic administration of endostatin in passive murine collagen induced arthritis. Ann. Rheum. Dis. 62, 677–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cramer T., Yamanishi Y., Clausen B. E., Forster I., Pawlinski R., Mackman N., Haase V. H., Jaenisch R., Corr M., Nizet V., Firestein G. S., Gerber H. P., Ferrara N., Johnson R. S. (2003) HIF-1α is essential for myeloid cell-mediated inflammation. Cell 112, 645–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cazes A., Galaup A., Chomel C., Bignon M., Brechot N., Le Jan S., Weber H., Corvol P., Muller L., Germain S., Monnot C. (2006) Extracellular matrix-bound angiopoietin-like 4 inhibits endothelial cell adhesion, migration, and sprouting and alters actin cytoskeleton. Circ. Res. 99, 1207–1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chomel C., Cazes A., Faye C., Bignon M., Gomez E., Ardidie-Robouant C., Barret A., Ricard-Blum S., Muller L., Germain S., Monnot C. (2009) Interaction of the coiled-coil domain with glycosaminoglycans protects angiopoietin-like 4 from proteolysis and regulates its antiangiogenic activity. FASEB J. 23, 940–949 [DOI] [PubMed] [Google Scholar]
- 37.Hermann L. M., Pinkerton M., Jennings K., Yang L., Grom A., Sowders D., Kersten S., Witte D. P., Hirsch R., Thornton S. (2005) Angiopoietin-like-4 is a potential angiogenic mediator in arthritis. Clin. Immunol. 115, 93–101 [DOI] [PubMed] [Google Scholar]
- 38.Hu Z. Y., Fan C., Livasy C., He X. P., Oh D. S., Ewend M. G., Carey L. A., Subramanian S., West R., Ikpatt F., Olopade O. I., van de Rijn M., Perou C. M. (2009) A compact VEGF signature associated with distant metastases and poor outcomes. BMC Med. 7, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xia K., Xue H. L., Dong D., Zhu S. S., Wang J. M., Zhang Q. P., Hou L., Chen H., Tao R., Huang Z., Fu Z., Chen Y. G., Han J. D. J. (2006) Identification of the proliferation/differentiation switch in the cellular network of multicellular organisms. PLoS Comput. Biol. 2, 1482–1497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pan B. Q., To L. B., Farrugia A. N., Findlay D. M., Green J., Gronthos S., Evdokiou A., Lynch K., Atkins G. J., Zannettino A. C. W. (2004) The nitrogen-containing bisphosphonate, zoledronic acid, increases mineralisation of human bone-derived cells in vitro. Bone 34, 112–123 [DOI] [PubMed] [Google Scholar]
- 41.Fuller K., Kirstein B., Chambers T. J. (2007) Regulation and enzymatic basis of bone resorption by human osteoclasts. Clin. Sci. 112, 567–575 [DOI] [PubMed] [Google Scholar]
- 42.Yang Y. H., Wang Y., Lam K. S., Yau M. H., Cheng K. K., Zhang J., Zhu W., Wu D., Xu A. (2008) Suppression of the Raf/MEK/ERK signaling cascade and inhibition of angiogenesis by the carboxyl terminus of angiopoietin-like protein 4. Arterioscler. Thrombosis Vasc. Biol. 28, 835–840 [DOI] [PubMed] [Google Scholar]
- 43.Camenisch G., Pisabarro M. T., Sherman D., Kowalski J., Nagel M., Hass P., Xie M. H., Gurney A., Bodary S., Liang X. H., Clark K., Beresini M., Ferrara N., Gerber H. P. (2002) ANGPTL3 stimulates endothelial cell adhesion and migration via integrin αvβ3 and induces blood vessel formation in vivo. J. Biol. Chem. 277, 17281–17290 [DOI] [PubMed] [Google Scholar]
- 44.Hato T., Tabata M., Oike Y. (2008) The role of angiopoietin-like proteins in angiogenesis and metabolism. Trends Cardiovasc. Med. 18, 6–14 [DOI] [PubMed] [Google Scholar]
- 45.Teitelbaum S. L., Ross F. P. (2003) Genetic regulation of osteoclast development and function. Nat. Rev. Genet. 4, 638–649 [DOI] [PubMed] [Google Scholar]
- 46.Franceschi R. T. (1999) The developmental control of osteoblast-specific gene expression: role of specific transcription factors and the extracellular matrix environment. Crit. Rev. Oral Biol. Med. 10, 40–57 [DOI] [PubMed] [Google Scholar]
- 47.Ito Y., Oike Y., Yasunaga K., Hamada K., Miyata K., Matsumoto S., Sugano S., Tanihara H., Masuho Y., Suda T. (2003) Inhibition of angiogenesis and vascular leakiness by angiopoietin-related protein 4. Cancer Res. 63, 6651–6657 [PubMed] [Google Scholar]
- 48.Galaup A., Cazes A., Le Jan S., Philippe J., Connault E., Le Coz E., Mekid H., Mir L. M., Opolon P., Corvol P., Monnot C., Germain S. (2006) Angiopoietin-like 4 prevents metastasis through inhibition of vascular permeability and tumor cell motility and invasiveness. Proc. Natl. Acad. Sci. U. S. A. 103, 18721–18726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mandard S., Zandbergen F., van Straten E., Wahli W., Kuipers F., Muller M., Kersten S. (2006) The fasting-induced adipose factor/angiopoietin-like protein 4 is physically associated with lipoproteins and governs plasma lipid levels and adiposity. J. Biol. Chem. 281, 21575–21575 [DOI] [PubMed] [Google Scholar]
- 50.Adachi H., Fujiwara Y., Kondo T., Nishikawa T., Ogawa R., Matsumura T., Ishii N., Nagai R., Miyata K., Tabata M., Motoshima H., Furukawa N., Tsuruzoe K., Kawashima J., Takeya M., Yamashita S., Koh G. Y., Nagy A., Suda T., Oike Y., Araki E. (2009) Angptl 4 deficiency improves lipid metabolism, suppresses foam cell formation and protects against atherosclerosis. Biochem. Biophys. Res. Commun. 379, 806–811 [DOI] [PubMed] [Google Scholar]
- 51.Li C. (2006) Genetics and regulation of angiopoietin-like proteins 3 and 4. Curr. Opin. Lipidol. 17, 152–156 [DOI] [PubMed] [Google Scholar]
- 52.Pajvani U. B., Du X. L., Combs T. P., Berg A. H., Rajala M. W., Schulthess T., Engel J., Brownlee M., Scherer P. E. (2003) Structure-function studies of the adipocyte-secreted hormone Acrp30/adiponectin - Implications for metabolic regulation and bioactivity. J. Biol. Chem. 278, 9073–9085 [DOI] [PubMed] [Google Scholar]
- 53.Patel S. D., Rajala M. W., Rossetti L., Scherer P. E., Shapiro L. (2004) Disulfide-dependent multimeric assembly of resistin family hormones. Science 304, 1154–1158 [DOI] [PubMed] [Google Scholar]
- 54.Aderka D., Le J. M., Vilcek J. (1989) IL-6 inhibits lipopolysaccharide-induced tumour necrosis factor production in human monocytes, U937 cells, and in mice. J. Immunol. 143, 3517–3523 [PubMed] [Google Scholar]
- 55.Gearing A. J. H., Beckett P., Christodoulou M., Churchill M., Clements J., Davidson A. H., Drummond A. H., Galloway W. A., Gilbert R., Gordon J. L., Leber T. M., Mangan M., Miller K., Nayee P., Owen K., Patel S., Thomas W., Wells G., Wood L. M., Woolley K. (1994) Processing of tumour necrosis factor alpha precursor by metalloproteinases. Nature 370, 555–557 [DOI] [PubMed] [Google Scholar]
- 56.Knowles H. J., Athanasou N. A. (2009) Canonical and non-canonical pathways of osteoclast formation. Histol. Histopathol. 24, 337–346 [DOI] [PubMed] [Google Scholar]
- 57.Thommesein L., Stunes A. K., Monjo M., Grosvik K., Tamburstuen M. V., Kjobli E., Lyngstadaas S. P., Reseland J. E., Syversen U. (2006) Expression and regulation of resistin in osteoblasts and osteoclasts indicate a role in bone metabolism. J. Cell. Biochem. 99, 824–834 [DOI] [PubMed] [Google Scholar]
- 58.Cornish J., Callon K. E., Watson M., Lin J. M., Reid I. R. (2006) Resistin, an adipocytokine, stimulates osteoblast and osteoclast proliferation. Bone 38, S9–S9 [Google Scholar]
- 59.Berner H. S., Lyngstadaas S. P., Spahr A., Monjo M., Thommesen L., Drevon C. A., Syversen U., Reseland J. E. (2004) Adiponectin and its receptors are expressed in bone-forming cells. Bone 35, 842–849 [DOI] [PubMed] [Google Scholar]
- 60.Oshima K., Nampei A., Matsuda M., Iwaki M., Fukuhara A., Hashimoto J., Yoshikawa H., Shimomura I. (2005) Adiponectin increases bone mass by suppressing osteoclast and activating osteoblast. Biochem. Biophys. Res. Commun. 331, 520–526 [DOI] [PubMed] [Google Scholar]
- 61.Luo X. H., Guo L. J., Xie H., Yuan L. Q., Wu X. P., Zhou H. D., Liao E. Y. (2006) Adiponectin stimulates RANKL and inhibits OPG expression in human osteoblasts through the MAPK signaling pathway. J. Bone Miner. Res. 21, 1648–1656 [DOI] [PubMed] [Google Scholar]
- 62.Reid I. R. (2008) Relationships between fat and bone. Osteoporos. Int. 19, 595–606 [DOI] [PubMed] [Google Scholar]
- 63.Wan Y. H., Chong L. W., Evans R. M. (2007) PPAR-γ regulates osteoclastogenesis in mice. Nat. Med. 13, 1496–1503 [DOI] [PubMed] [Google Scholar]
- 64.Grey A. (2008) Skeletal consequences of thiazolidinedione therapy. Osteoporos. Int. 19, 129–137 [DOI] [PubMed] [Google Scholar]