Abstract
Pre-pulse inhibition (PPI) of the acoustic startle reflex is deficient in patients with schizophrenia. This deficiency is mimicked in mice by the use of the psychotomimetic drugs d-amphetamine and dizolcipine. Antipsychotic drugs such as clozapine are used to treat schizophrenic patients and are also administered to mice to prevent PPI disruption. Neurotensin (NT) produces antipsychotic-like effects when injected into rodent brain through its effects at NT subtype 1 (NTS1) and 2 (NTS2) receptors. We hypothesized that the NT receptor agonist (NT69L) would prevent PPI disruption in mice challenged with d-amphetamine (10 mg kg–1) and dizocilpine (1 mg kg–1). We investigated the role of NTS1 and NTS2 in PPI using wild-type (WT), NTS1 (NTS1–/–) and NTS2 (NTS2–/–) knockout mice, via its disruption by psychotomimetic drugs, as well as the ability of clozapine and NT69L to block these PPI disruptions. There were no differences in baseline PPI across the three genotypes. d-Amphetamine and dizocilpine disrupted PPI in WT and NTS2–/– mice but not in NTS1–/– mice. In WT mice, clozapine (1 mg kg–1) and NT69L (1 mg kg–1) significantly blocked d-amphetamine-induced disruption of PPI. Similarly, in WT mice, clozapine significantly blocked dizocilpine-induced PPI disruption, but NT69L did not. In NTS2–/– mice clozapine blocked d-amphetamine-but not dizocilpine-induced PPI disruption, while NT69L blocked both d-amphetamine- and dizocilpine-induced PPI disruption. Our results indicate that NTS1 seems essential for d-amphetamine and dizocilpine disruption of PPI. Additionally, this report provides support to the hypothesis that NT analogs could be used as novel antipsychotic drugs.
Keywords: schizophrenia, pre-pulse inhibition, neurotensin, NT69L, d-amphetamine, NTS1, NTS2, clozapine, dopamine
INTRODUCTION
Schizophrenia is a debilitating neuropsychiatric disease affecting approximately 0.4% of individuals in the adult population worldwide (Saha et al., 2005). Individuals with this disorder appear unable to filter out intrusive sensory, cognitive or motor information, as a result of a malfunction of sensorimotor gating mechanisms. Evidence for this malfunction was first reported in humans as deficits in pre-pulse inhibition (PPI) of the startle response, which serves as an operational measure of sensorimotor gating (Braff et al., 1992). PPI tests the ability of a barely detectable pre-stimulus to suppress the acoustic startle reflex in response to an intense acoustic startling stimulus. The reduction of the amplitude of the startle response reflex due to startle habituation or pre-pulse intensity reflects the ability of the nervous system to learn and to adapt temporarily to strong sensory stimuli, making these responses some of the simplest forms of learning (Geyer and Swerdlow, 2001). Additionally, because of the homology of PPI between humans and rodents, it has proved to be an effective cross-species measure of sensorimotor gating (Swerdlow et al., 1999).
Dopamine (DA) has been heavily implicated in PPI as evidenced by the disruption of PPI observed in rats and mice after amphetamine and apomorphine treatment (Mansbach et al., 1998; Varty et al., 2001). Similarly, serotonin (5-HT) agonists and non-competitive N-methyl-d-aspartate (NMDA) antagonists can effectively disrupt PPI (Feifel et al., 2003; Mansbach and Geyer, 1989; Rigdon and Weatherspoon, 1992). The dopamine 2 receptor (D2R) appears to be specifically involved in the modulation of the disruptive effects of the indirect DA agonist amphetamine, as amphetamine does not disrupt PPI in mice lacking the D2R (Ralph et al., 1999). Blockade of the disruption of PPI caused by amphetamine, apomorphine and dizocilpine (non-competitive NMDA receptor antagonist) has been achieved with systemic administration of well-established typical and atypical antipsychotics such as haloperidol and clozapine, making PPI in rodents an excellent tool to screen novel compounds for similar neuroleptic properties (Bubenikova et al., 2005; Ouagazzal et al., 2001; Swerdlow et al., 1998).
Consistent with these reports, neurotensin (NT) a 13 amino acid neuropeptide found naturally in the central nervous system and gastrointestinal tract (Carraway and Leeman, 1976), and its NT (8–13) analogs NT69L and PD149163 behave like atypical antipsychotic drugs by preventing disruption of PPI by amphetamine, dizocilpine and apomorphine in rats (Feifel et al., 1999; Shilling et al., 2003). PD149163 increases PPI in mice and in the Brattleboro strain of rat, which has naturally occurring PPI deficits (Feifel et al., 2004; Feifel et al., 2010b). The NT antagonist SR142948A blocks the ability of haloperidol and the atypical antipsychotic quetiapine to restore normal PPI levels in isolation-reared Long Evans rats (Binder et al., 2001). Mice with a deletion of hexapeptide NT/N gene have reduced PPI compared with wild-type (WT) controls (Kinkead et al., 2005). Amphetamine does not disrupt PPI in these mice. These reports provide evidence that NT, a hypothesized endogenous antipsychotic, plays a significant role in the neurobiology of PPI and schizophrenia, since an intact NT system is important in the normal functioning of sensorimotor gating (Kinkead et al., 2005; Nemeroff et al., 1989; Nemeroff et al., 1983; Radke et al., 1998). The effects of NT are hypothesized to be mediated via the G-protein-coupled neurotensin receptor subtype 1 (NTS1) and neurotensin receptor subtype 2 (NTS2) (Hwang et al., 2010; Le et al., 1996). Recently, Feifel and colleagues (Feifel et al., 2010a; Feifel et al., 2010b) tested PPI in mice lacking NTS1 and NTS2. According to their studies, endogenous NT does not seem to regulate baseline PPI or the PPI-disruptive effects of amphetamine and dizocilpine at NTS1. Regulation of baseline PPI by endogenous NT may be acting via NTS2, as in comparison to WT mice, baseline PPI was significantly elevated (Feifel et al., 2010a; Feifel et al., 2010b).
In an attempt to elucidate the NT receptor subtype involved in this sensorimotor gating mechanism and the effect of NT analogs, we tested the ability of d-amphetamine and dizocilpine to disrupt PPI in WT, NTS1–/– and NTS2–/– mice. Here we provide evidence supporting the hypothesis that NT receptor agonists may have antipsychotic-like activity. These effects seemed dependent on which genotype and pyschotomimetic drug was used to disrupt PPI. NT69L did not block dizocilpine-induced PPI disruption in WT mice and clozapine was not successful in blocking dizocilpine disruption of PPI in NTS2–/– mice. We report the effects of clozapine and NT69L alone on PPI and, in a secondary analysis, examine the effects of d-amphetamine, dizocilpine, clozapine and NT69L on the startle pulse alone acoustic startle response (ASR) for the three genotypes.
MATERIALS AND METHODS
All animal protocols were approved by the Mayo Foundation Institutional Animal Use and Care Committee. The principles of laboratory animal care were followed according to the Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research (National Research Council 2003).
Generation of WT, NTS1–/– and NTS2–/– mice
NTS1–/– (C57BL/6J) and NTS2–/– mice (C57BL/Ola29) were obtained from Roche Laboratories (Palo Alto, CA, USA) and colonies were established at our AAALAC accredited animal facility at Mayo Clinic (Jacksonville, FL, USA) as previously described (Liang et al., 2010). Mice were genotyped prior to PPI sessions as previously described (Liang et al., 2010).
Animals and housing
Mice genotypes described above were housed under a 12 h light/dark cycle (lights on at 07:00 h; off at 19:00 h) with food and water available ad libitum except during PPI testing. Sessions were run between 08:00 and 16:00 h. In total, 312 mice were utilized. Groups of mice tested included males between 3 and 8 months of age, divided as follows: 117 WT, 99 NTS1–/– and 96 NTS2–/–.
Drugs
d-Amphetamine sulfate, dizocilpine and clozapine were obtained from Sigma Aldrich (St Louis, MO, USA). NT69L was synthesized by the Mayo Peptide Synthesis Facility (Rochester, MN, USA). d-Amphetamine sulfate, dizocilpine and NT69L were dissolved in 0.9% saline, while clozapine was dissolved in a minimal volume of 10% acetic acid and brought up to volume in a ratio of 1 μl of acetic acid per 1 ml of saline as described previously (Duncan et al., 2006). The drug doses used in this study for successful PPI disruption and for effective blockade of PPI disruption were determined based on previous reports (Kinkead et al., 2005; McCaughran et al., 1997; Ouagazzal et al., 2001; Ralph-Williams et al., 2002; Ralph et al., 1999; Shilling et al., 2003; Varty et al., 2001; Yee et al., 2004). The following nine drug treatment groups were utilized in this study: saline/saline (S/S), saline/amphetamine (S/A), saline/dizocilpine (S/D), NT69L/saline (NT69L/S), NT69L/amphetamine (NT69L/A), NT69L/dizocilpine (NT69L/D), clozapine/saline (C/S), clozapine/amphetamine (C/A) and clozapine/dizocilpine (C/D). Baseline PPI was determined using the S/S treatment group for each respective genotype. WT, NTS1–/– and NTS2–/– mice were subjected to each of the nine drug treatments separately. Every mouse in each drug treatment group was naive to its respective drug treatment and was naive to PPI testing at the time of testing. All mice in each drug treatment group underwent drug treatment and PPI testing once and were subsequently killed.
Startle paradigm
On test days WT, NTS1–/– and NTS2–/– mice were allowed to acclimate for 1 h in the PPI experimental room. Following this acclimation period, mice were subjected to the PPI experimental protocol as previously described (Shilling et al., 2003). Briefly, mice were first treated with NT69L (1 mg kg–1), clozapine (1 or 2 mg kg–1) or saline in an equal volume. Thirty minutes after the initial treatment, mice received a second injection of 10 mg kg–1 d-amphetamine, 1 mg kg–1 dizocilpine or saline. Twenty minutes following the second treatment, each animal was individually placed in a startle response chamber (San Diego Instruments, San Diego, CA, USA). All drugs were administered intra-peritoneally in a volume of 4 ml kg–1.
Four startle response chambers were used, consisting of a Plexiglas cylinder 12.7 cm long×1.5 cm in diameter resting on a 12.7 cm×20.3 cm Plexiglas frame and located within a ventilated and white light illuminated enclosure measuring 38.1 cm× 40.6 cm×58.4 cm. Startle amplitudes were detected and measured by a piezoelectric accelerometer mounted directly below the midline of the animal on the underside of the Plexiglas frame. Mice were allowed to acclimate to the startle response chamber for 5 min prior to the onset of a PPI session.
PPI sessions had a duration of 13 min and consisted of 58 trials presented in pseudo-random order. Each trial was followed by an inter-trial interval (ITI) with a duration averaging 15 s. The PPI session consisted of the following eight distinct trial types, pseudo-randomly presented five different times during the session: no stimulus, startle pulse alone, pre-pulse alone with pre-pulse intensities of 4, 8 and 16 dB (PP4, PP8, PP16) above background, and pre-pulse + startle pulse trials (with pre-pulse intensities of 4, 8 and 16 dB above background). Additionally, four startle pulse alone trials not included in the %PPI calculation were presented at the beginning and end of the PPI session (Bakshi and Geyer, 1998). Background noise intensity was 65 dB, while startle pulse intensity was 120 dB (Geyer and Swerdlow, 2001). All pre-pulses and startle pulses had a duration of 20 ms and 40 ms, respectively. For pre-pulse + startle pulse trials, the interval between onset of the pre-pulse and onset of the startle pulse was 100 ms. The startle response recording window was over 65 ms and began at the onset of the startle pulse for pre-pulse + startle pulse trials. Similarly, for pre-pulse alone trials, the recording window was over 65 ms but began at the onset of the pre-pulse (Geyer and Swerdlow, 2001). The data used to measure the pulse alone ASR were gathered during the PPI session and consisted of the embedded startle pulse alone amplitudes recorded by the computer during the PPI session.
PPI calculation and statistical analysis
Startle responses were collected for each individual trial throughout the PPI session for each individual mouse subject. The startle responses for each pre-pulse + startle pulse trial yielded a %PPI value, and these were averaged into one %PPI value for each mouse. PPI was calculated as a percentage of the pulse alone startle amplitude using the following formula: PPI=[1–(startle magnitude after pre-pulse–startle pulse pair/startle magnitude after startle pulse alone)]×100.
Pre-pulse alone trials were not used to calculate PPI and only served as controls to ascertain that startle responses were not the result of the pre-pulse stimulus alone. Because of the variability in mean PPI, pre-pulse intensity PPI and ASR between drug treatment groups and considering the nature of the distribution of these measures, we used non-parametric (Hollander and Wolfe, 1999) and permutation-based methods (Davidson and Hinkley, 1997) in making all statistical comparisons. Pre-specified interactions of pre-pulse intensity and genotype with drug treatment and genotype were assessed using a bootstrap method based on two-factor ANOVA; a random effect was included for each mouse when assessing the interaction of pre-pulse intensity with drug treatment. Although these tests of interaction were performed, all analyses were stratified by genotype and pre-pulse intensity regardless of the results of interaction tests as genotype-specific and pre-pulse intensity-specific comparisons were of interest. Kruskal–Wallis rank-sum tests were used make pre-specified comparisons of mean PPI, pre-pulse intensity PPI and ASR between drug treatment groups within each genotype, and given significant evidence of an overall difference (P≤0.05), pair-wise comparisons were made using a Wilcoxon rank-sum test with adjusted P-values calculated using Bonferroni correction. All statistical analyses were performed using S-Plus (version 8.0.1; Insightful Corporation, Seattle, WA, USA).
RESULTS
Comparison of baseline PPI and ASR between genotypes
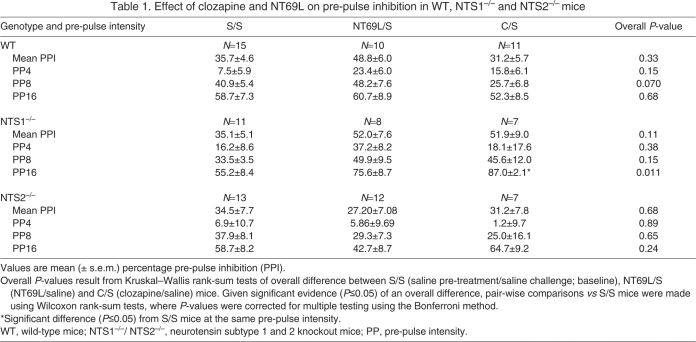
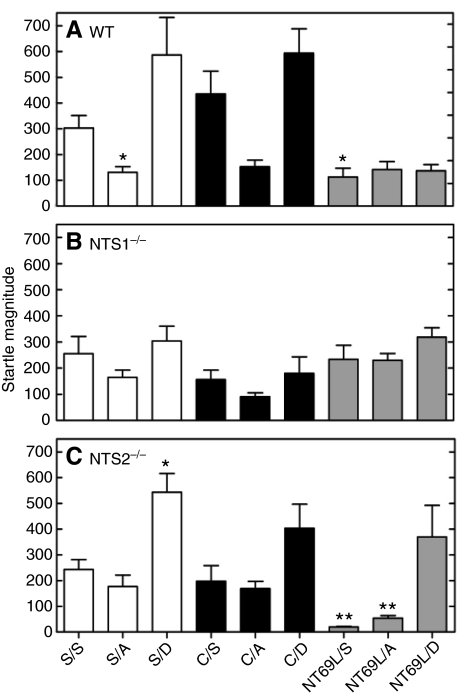
Baseline PPI for the genotypes was not significantly different between WT, NTS1–/– and NTS2–/– mice for mean PPI and PPI at PP4, PP8 or PP16, as shown in the S/S column of Table 1. There were also no significant differences in baseline (S/S treatment) startle pulse alone ASR (Fig. 1) between the three genotypes.
Table 1.
Effect of clozapine and NT69L on pre-pulse inhibition in WT, NTS1–/– and NTS2–/– mice
Fig. 1.
Effect of drug treatment on pulse alone acoustic startle response (ASR) for wild-type mice (WT, A), and neurotensin subtype 1 (NTS1–/–, B) and 2 (NTS2–/–, C) receptor knockout mice. In WT mice, pulse alone ASR magnitude for the drug treatment groups was significantly different from that of the saline/saline (S/S) group (*P<0.05). In NTS2–/– mice, pulse alone ASR magnitude for the drug treatment groups was significantly different from that of the S/S group (*P<0.01 and **P<0.001). For NTS1–/– mice, there were no significant differences in percentage pre-pulse inhibition (%PPI) or pulse alone ASR. S, saline; A, amphetamine; D, dizocilpine; C, clozapine; NT69L, neurotensin analog.
Effect of clozapine and NT69L alone on PPI
Table 1 shows a summary of the effects of either clozapine or NT69L alone on mean PPI and for PPI at PP4, PP8 and PP16 for the three genotypes. Results of the effects of genotype and drug treatment (S/S, NT69L/S, C/S) on mean PPI did not reveal a significant interaction. Analysis of mean PPI and PPI for each pre-pulse intensity separately for each genotype did not reveal any significant differences between the drug treatment groups, except in NTS1–/– mice where PPI for C/S mice was significantly higher at PP16 (P=0.006) when compared with that for S/S-treated mice (Table 1). No interaction between pre-pulse intensity and drug treatment (S/S, NT69L/S, C/S) was observed for any of the three genotypes (all interactions, P≥0.19).
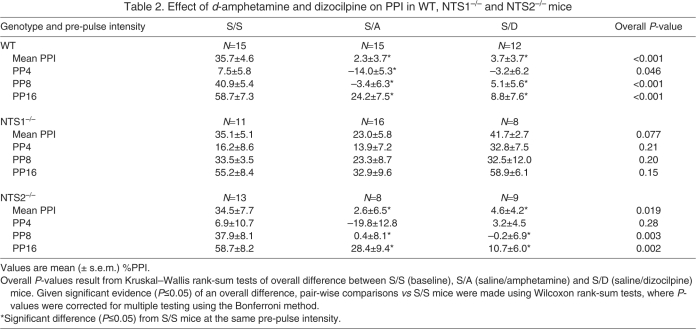
Effect of amphetamine and dizocilpine alone on PPI
Table 2 shows a summary of the effects of d-amphetamine and dizocilpine alone for mean PPI and PPI at PP4, PP8, and PP16 for each genotype. For mean PPI there was a significant interaction (P<0.001) between genotype and drug treatment (S/S, S/A, S/D), likely due to d-amphetamine and dizocilpine significantly disrupting PPI in WT and in NTS2–/– mice, but not in NTS1–/– mice. In WT mice, post hoc pair-wise comparisons revealed that mean PPI values were significantly lower in S/A (P<0.001) and S/D (P<0.001) drug treatment groups when compared with S/S-treated mice. Results were similar in NTS2–/– mice, where post hoc pair-wise comparisons revealed that mean PPI values were significantly lower in S/A (P=0.049) and S/D (P=0.028) drug treatment groups in comparison to S/S-treated mice. In contrast to WT and NTS2–/– mice, NTS1–/– mice did not show a significant difference in mean PPI between S/S, S/A and S/D treatment groups. Results were fairly similar when examining pre-pulse intensity PPI, though there was a significant interaction between drug treatment and pre-pulse intensity in the WT mice (P=0.021) and in the NTS2–/– mice (P=0.019), but not in the NTS1–/– mice (P=0.63). These interactions appear to be due to the greater differences in PPI for S/D and S/A drug treatments in comparison to S/S mice at PP8 and PP16 than at PP4. PPI was significantly lower in S/A mice than in S/S mice at all pre-pulse intensities in WT mice (all P≤0.029), and at PP8 and PP16 in NTS2–/– mice (both P≤0.040). In WT mice and NTS2–/– mice, PPI was lower in S/D mice compared with S/S mice at PP8 and PP16 (all P≤0.008).
Table 2.
Effect of d-amphetamine and dizocilpine on PPI in WT, NTS1–/– and NTS2–/– mice
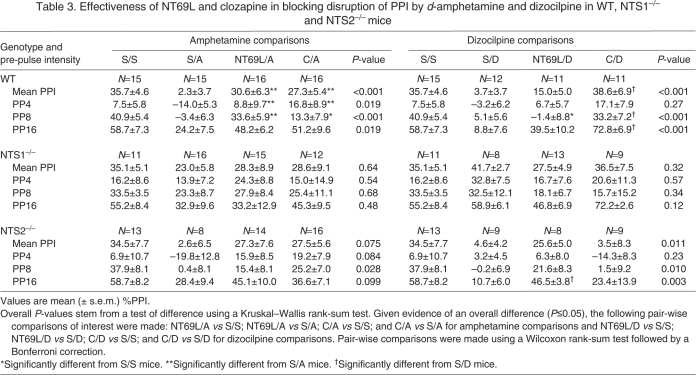
Effect of clozapine and NT69L pre-treatment vs amphetamine and dizocilpine challenge
Table 3 shows the effects of pre-treatment with either clozapine or NT69L in combination with d-amphetamine or dizocilpine challenge on mean PPI and for PPI at PP4, PP8 and PP16 for the three genotypes. Results presented in Table 3 are provided separately for ‘amphetamine comparisons’ and ‘dizocilpine comparisons’. Amphetamine comparisons consisted of comparing %PPI between S/S, S/A, NT69L/A and C/A treatment groups, while dizocilpine comparisons consisted of comparing %PPI between S/S, S/D, NT69L/D and C/D treatment groups. Comparisons of S/A mice and S/D mice with S/S mice are presented in Table 2. When considering mean PPI, there was not a significant interaction detected between genotype and drug treatment for amphetamine comparisons (S/S, S/A, NT69L/A, C/A). There was, however, a significant interaction detected (P<0.001) between genotype and drug treatment when considering dizocilpine comparisons (S/S, S/D, NT69L/D, C/D). When testing the effects of clozapine pre-treatment on dizocilpine challenge, clozapine was adjusted to 2 mg kg–1 to block 1 mg kg–1 dizocilpine PPI disruption in the three genotypes tested.
Table 3.
Effectiveness of NT69L and clozapine in blocking disruption of PPI by d-amphetamine and dizocilpine in WT, NTS1–/– and NTS2–/– mice
In WT mice, for amphetamine comparisons with respect to S/A drug-treated mice, pair-wise comparisons revealed mean PPI values that were significantly higher in both C/A (P=0.009) and NT69L/A (P=0.002) drug treatment groups. No significant differences were observed for mean PPI between S/S-treated mice when compared with C/A or NT69L/A mice, suggesting that clozapine and NT69L blocked the disruption of PPI caused by d-amphetamine challenge. There was no significant interaction detected between pre-pulse intensity and drug treatment. PPI at PP4 and PP8 was significantly higher in C/A- and NT69L/A-treated mice compared with S/A mice (all P≤0.048). No other significant differences were detected.
For dizocilpine, pair-wise comparisons in WT mice revealed that mean PPI values were significantly higher in the C/D (P<0.001) drug treatment group in comparison to S/D mice, but not when compared with S/S mice. There was a significant pre-pulse intensity × drug treatment interaction (P=0.001). Specifically, in comparison to S/D mice, PPI was significantly higher in C/D mice at PP8 and PP16 (both P≤0.006). There was no significant difference in mean PPI between NT69L/D-treated mice and S/D-treated mice, which seems primarily driven by the lack of effect of NT69L at PP8. Additionally, the results of NT69L at PP8 seem to influence the significantly lower mean PPI value of the NT69L/D-treated group relative to S/S mice. For the NT69L/D drug treatment group in comparison to S/S, PPI was much higher at PP16 than at PP4 or PP8, though this difference was not significant. These results suggest that clozapine was effective at blocking disruption of PPI caused by dizocilpine in WT mice at the higher pre-pulses but NT69L was not, primarily due to the low PPI at PP8.
For NTS1–/– mice there were no significant differences in mean PPI between S/S-, S/A-, C/A- and NT69L/A-treated mice and no significant differences in mean PPI between S/S, S/D, C/D and NT69L/D mice. These results were not unexpected, as mean PPI in NTS1–/– mice challenged with either d-amphetamine or dizocilpine was not significantly different from mean PPI for saline alone treatment. Analysis of PPI for each pre-pulse intensity across the drug treatments for NTS1–/– mice revealed similar results (Table 3), with no significant interactions detected between pre-pulse intensity and drug treatment for amphetamine comparisons or dizocilpine comparisons, and no significant differences between drug treatment groups at PP4, PP8 and PP16.
In NTS2–/– mice, while there were no statistically significant differences in mean PPI between S/A, NT69L/A and C/A drug treatment groups (P=0.075), values were higher in the C/A and NT69L/A drug treatment groups when compared with S/A-treated mice, and likewise in the C/A and NT69L/A groups compared with S/S mice. Similar results were observed at each pre-pulse intensity, with no significant interaction detected between pre-pulse intensity and drug treatment for amphetamine comparisons in NTS2–/– mice.
For dizocilpine comparisons in NTS2–/– mice, there was a significant overall difference in mean PPI between treatment groups; however, pair-wise comparisons were not significant after adjustment for multiple comparisons (all P≥0.060), which seems to be a result of the observed inability of clozapine to prevent PPI disruptions caused by dizocilpine. There was a significant interaction between drug treatment and pre-pulse intensity (P=0.036) that may have been due to the lack of effect of clozapine against dizocilpine challenge at all pre-pulse intensities. PPI was significantly higher at PP16 in the NT69L/D treatment group when compared with S/D-treated mice (P=0.007). Significant differences were not observed at PP4 or PP8. No other differences were detected.
Pulse alone ASR analysis
In a secondary analysis (Fig. 1), for each genotype separately, we compared pulse alone ASR values for each drug treatment group with those for mice treated with saline alone (S/S). There was a significant interaction between genotype and drug treatment when considering pulse alone ASR values (P<0.001). In WT mice (Fig. 1A), there were significant differences in pulse alone ASR between S/A (P=0.046) and NT69L/S (P=0.035) drug treatment groups when compared with that for S/S-treated mice. No other differences in pulse alone ASR were detected. For NTS1–/– mice there were no significant differences (all P>0.05) detected between the drug treatment groups in comparison to S/S-treated mice for pulse alone ASR (Fig. 1B). For NTS2–/– mice, in comparison to the S/S group (Fig. 1C), there was a significant difference in pulse alone ASR for S/D, NT69L/S and NT69L/A groups (all P<0.01). It is evident that 1 mg kg–1 NT69L treatment had profound effects on ASR response in the NTS2–/– mice as they were the lowest values for this genotype and for the experiment in general.
DISCUSSION
In this study we set out to investigate the involvement of NT receptor subtypes in sensorimotor gating using WT, NTS1–/– and NTS2–/– mice. We found no significant differences in baseline (S/S) PPI and ASR between males of the three genotypes tested. Based on these data, it appears that NTS1 and NTS2 do not play a role in the modulation of baseline PPI. A recent report by Feifel and collaborators examined baseline PPI in NTS2–/– mice (Feifel et al., 2010a). The authors found that NTS2–/– mice show higher baseline PPI as well as a lower ASR in comparison to WT mice and conclude that endogenous NT plays a role in regulating PPI via activation of NTS2. Additional reports from other research groups on baseline PPI measured during test weeks 1 and 2 of life for NT knockout mice were significantly lower than that for WT mice (Kinkead et al., 2005). This difference became less evident as the mice aged, suggesting a developmental effect on baseline PPI. We tested our mice at least at post-natal day 90, when the mice had reached adulthood and our results support those of Kinkead and collaborators as we found no significant differences in PPI between genotypes at this age. Our results with NTS1–/– are consistent with reports by others (Feifel et al., 2010b), who also found no significant differences in baseline PPI between NTS1–/– mice and their respective WT controls.
There is significant evidence implicating NTS1 in the sensorimotor gating mechanism of PPI (Binder et al., 2001; Caceda et al., 2005; Feifel et al., 2007; Feifel et al., 2004; Kinkead et al., 2005). Our results indicate that NTS1 is necessary for normal disruption of PPI by the indirect DA agonist d-amphetamine and the non-competitive NMDA receptor antagonist dizocilpine. Our results are consistent with previous reports on the important role of NTS1 in the modulations of PPI, as found by pharmacological interventions (Caceda et al., 2005; Kinkead et al., 2005). Analysis of startle pulse alone ASR data showed a significant decrease in startle magnitude with amphetamine alone disruption for WT mice, a trend often reported in other studies that have used amphetamine to disrupt PPI (Dulawa and Geyer, 1996; Feifel et al., 2010b; Ralph-Williams et al., 2002; Ralph et al., 2001; Ralph et al., 1999; Varty et al., 2001).
Schizophrenia patients are reported to show deficits in PPI (Braff et al., 1992; Kumari et al., 2000; Oranje et al., 2002), and one current method of treatment for these patients is with antipsychotic drugs that target the DA neurotransmitter system (Galletly et al., 2000; Hoff et al., 1996; Kumari et al., 2000; Meltzer and McGurk, 1999; Oranje et al., 2002). Recent evidence supports a complex interaction between NTS1 and D2R. NTS1 located on cortical and midbrain DA neurons internalizes in response to overstimulation by NT, while NT via NTS1 enhances DA release by modulating or inhibiting the function of inhibitory D2 auto-receptors (Fawaz et al., 2009; Fuxe et al., 1992; Legault et al., 2002; Nouel et al., 1997). This evidence suggests an interaction between DA and NT with respect to PPI, adding further support to the hypothesis that for successful amphetamine-induced PPI disruption, as well as the mechanism of action of antipsychotic drugs, intact DA and NT systems are essential (Binder et al., 2001; Caceda et al., 2005; Kinkead et al., 2005; Ralph-Williams et al., 2002; Ralph et al., 1999).
Recent data from our laboratory provide further evidence for the importance of this interaction between DA and NT. Basal extracellular DA levels are higher in NTS1–/– and NTS2–/– mice than in WT mice, with significantly less D2R receptor binding by the D2R antagonist [3H]raclopride in the striatal area of NTS1–/– mice (Liang et al., 2010). These data suggest an involvement of the striatal region and a possible malfunction of the D2R in the NTS1–/– mice, both which are reported by others to interact via the cortex, striatum, pallidum and pontine tegmentum (CSPP) circuit that seems to largely regulate PPI (Kodsi and Swerdlow, 1994; Kodsi and Swerdlow, 1997; Seaman, 2000; Swerdlow et al., 2001; Swerdlow et al., 1995). The interaction between DA and NT may be a significant factor in our observed lack of PPI disruption in these mice, as a similar phenotype to that observed in the NTS1–/– mice can be achieved in WT mice by blockade of the D2R by the DA antagonist clozapine, prior to amphetamine administration. Further examination using these NT receptor knock-out mice in combination with other receptor-specific DA compounds is necessary, as clozapine also actively binds to dopamine 1 and 4 receptors, serotonin type 2 (5HT2) receptors and histaminergic receptors (Broderick et al., 2004; Farde et al., 1989; Nguyen et al., 2001; Oda and Matsumoto, 2001; Van Tol et al., 1991).
The report by Liang and colleagues gives evidence of significantly higher baseline extracellular DA levels in the striatum of NTS1–/– and NTS2–/– in comparison to WT mice (Liang et al., 2010); however, we do not report any deficits in baseline PPI as a result of this ‘overflow’ of DA. Previously, Zhang and collaborators quantified and reported marked decreases of PPI in rat, concomitant with an overflow of DA due to systemic amphetamine administration (Zhang et al., 2000). Overflow of DA in the nucleus accumbens (NAcc) increased 100% over baseline levels causing a significant PPI disruption, followed by a 450% DA overflow increase over baseline levels and near-total PPI disruption (Zhang et al., 2000). Other groups have reported similar increases in DA transmission after amphetamine administration with human cohorts (Abi-Dargham et al., 1998; Laruelle et al., 1996). While it is evident that deficits in PPI are concomitant with overflow of DA, the report by Liang and colleagues (Liang et al., 2010) shows a natural 78% overflow of striatal DA in NTS1–/– mice and a 63.8% overflow in NTS2–/– mice over WT levels. It is likely that a much higher DA overflow is necessary for the NTS1–/– and NTS2–/– mice to express natural PPI deficits. As we report here, we found no difference in baseline PPI levels between the genotypes, and, with the exception of NTS1–/– mice, decreased PPI can be achieved via systemic administration of amphetamine and dizocilpine. It should be noted that for a thorough understanding of how DA overflow affects PPI in the genotypes examined here, DA levels in the NAcc and other CSPP areas need to be examined.
While our study examined baseline and psychotomimetically induced disruptions of PPI in WT, NTS1–/– and NTS2–/– mice, we investigated whether treatment with the atypical antipsychotic drug clozapine and the NT receptor agonist NT69L would block these disruptions. Clozapine and NT69L successfully blocked amphetamine-induced disruption of PPI in WT and in NTS2–/– mice. These results are consistent with previous reports (Bubenikova et al., 2005; Caceda et al., 2005; Feifel et al., 2004; Feifel et al., 1999; Kinkead et al., 2005; Ouagazzal et al., 2001; Russig et al., 2004; Shilling et al., 2003; Swerdlow et al., 1998). Our results for WT mice were mixed for dizocilpine-induced disruption of PPI, as clozapine significantly blocked this disruption at PP8 and PP16, the very same pre-pulse intensities where dizocilpine alone, without clozapine pre-treatment, significantly disrupted PPI. Interestingly, NT69L pre-treatment followed by dizocilpine challenge did not significantly block PPI, an effect seemingly caused by the lack of effect of NT69L at PP8 and thus directly affecting mean PPI comparisons between the two drug treatment groups. PPI levels in NTS1–/– mice that were pretreated with either clozapine or NT69L followed by challenges with amphetamine or dizocilpine were not significantly different from that for S/S-, S/A- and S/D-treated mice.
In NTS2–/– mice PPI disruption by dizocilpine was significantly blocked at PP16 by NT69L but not by clozapine, while, as previously mentioned, clozapine was effective against dizocilpine in WT mice. These results suggest that clozapine may mediate its effects via NTS2. This report shows that clozapine and NT69L were able to block PPI disruption by d-amphetamine. Similar results were not achieved with dizolcipine disruption of PPI in WT and NTS2–/– mice. Because of the different nature of the psychotomimetic drugs (d-amphetamine is an indirect DA agonist and dizocilpine is a noncompetitive NMDA glutamate receptor antagonist) used in this report, it may be that our results with dizocilpine stem from a complex interaction between the psychostimulant used in conjunction with the presence or absence of a neurotensin receptor subtype. We are investigating these observations further using a dose–response study for the drugs tested in this report.
There were no significant differences in mean PPI or pre-pulse PPI between the NT69L/S-treated group and the saline controls for WT, NTS1–/– or NTS2–/– mice, although there were small nonsignificant trends toward increases in PPI in WT and NTS1–/– mice. These data are consistent with previous reports showing that NT and NT agonists tend to increase PPI (Feifel et al., 2004; Feifel et al., 1997; Feifel et al., 2010b; Shilling et al., 2003). C/S treatment did not significantly affect PPI levels in WT and NTS2–/– mice. In contrast, C/S treatment in NTS1–/– mice showed a tendency to increase PPI, with a significant increase observed at PP16, suggesting again that NTS2 may be more important than NTS1 in modulating the effects of clozapine on PPI. Analysis of startle pulse alone ASR results showed that NT69L alone affected the ASR for NTS2–/– mice more profoundly than that for WT or NTS1–/– mice, although mean PPI remained similar to that of S/S-treated mice for all three genotypes. It is possible the significantly lower ASR magnitudes exhibited by the absence of NTS2 in NTS2–/– mice may be due to an imbalance of stimulation by NT69L at the NTS1 receptors located on DA neurons. NT69L may be inhibiting the function of dopamine auto-receptors, prolonging synaptic DA overflow (Fawaz et al., 2009; Legault et al., 2002; Nouel et al., 1997), thus causing the marked decreases in ASR in the NTS2–/– mice.
The limitations to this work are those of any study that uses mice lacking a gene function from the embryonic stage of development. Knocking out one gene can affect the expression of other genes (e.g. decreases in receptor binding of the D2R in NTS1–/– mice) as a result of important interactions between genes or gene products that exist naturally in WT mice (Liang et al., 2010). Some changes in knockout mice can be compensatory in nature. Recent reports by Feifel and collaborators do not implicate NTS1 in the disruption of PPI by d-amphetamine and dizocilpine, and attribute the regulation of baseline PPI to NTS2 (Feifel et al., 2010a; Feifel et al., 2010b). There are limitations that occur as a result of differences in environmental conditions between animal facilities and different experimental parameters. These factors are likely to account for the differences observed in results between studies. For example, in one study, Feifel and colleagues challenged a combined group of male and female mice (WT or NTS1–/–) with amphetamine and dizocilpine (Feifel et al., 2010b), while in a study of NTS2–/– they examined locomotor activity immediately prior to PPI experimentation (Feifel et al., 2010a). In our study, we used only males and allowed 1 h of undisturbed acclimation to the experimental room prior to PPI experimentation. Dose–response curves, additional developmental studies and more congruent experimental parameters should assist in reconciling the differences in results observed between laboratories.
In conclusion, this report provides data supporting the growing body of evidence that gives NT receptor agonists, such as NT69L, a possible role and use as novel antipsychotic drugs. We show that clozapine blocked amphetamine-induced PPI disruption in WT and NTS2–/– mice. Our results also showed that NT69L blocked amphetamine-induced disruption in WT and NTS2–/– mice. We observed different PPI results with dizocilpine, as clozapine and NT69L showed differences in their ability to block PPI disruption by dizocilpine in WT and NTS2–/– mice. Additionally, this report gives evidence for the role played by NTS1 in PPI, as our results suggest that the presence of NTS1 is necessary to disrupt PPI. Amphetamine and dizocilpine disrupted PPI in WT and NTS2–/– mice but not in NTS1–/– mice. We found no differences in baseline PPI in WT, NTS1–/– and NTS2–/– mice, suggesting that neither NTS1 nor NTS2 modulates normal functioning of sensorimotor gating mechanisms.
- 5-HT
- serotonin
- 5-HT2
- serotonin type 2
- ASR
- acoustic startle response
- C/A
- clozapine/amphetamine
- C/D
- clozapine/dizocilpine
- C/S
- clozapine/saline
- CSPP
- cortex, striatum, pallidum and pontine tegmentum
- D2R
- dopamine 2 receptor
- DA
- dopamine
- Nacc
- nucleus accumbens
- NMDA
- N-methyl-d-aspartate
- NT
- neurotensin
- NT69L/A
- NT69L/amphetamine
- NT69L/D
- NT69L/dizocilpine
- NT69L/S
- NT69L/saline
- NTS1
- NT subtype 1 receptor
- NTS1–/–
- neurotensin subtype 1 knockout
- NTS2
- NT subtype 2 receptor
- NTS2–/–
- neurotensin subtype 2 knockout
- PP4
- pre-pulse 4
- PP8
- pre-pulse 8
- PP16
- pre-pulse 16
- PPI
- pre-pulse inhibition
- S/A
- saline/amphetamine
- S/D
- saline/dizocilpine
- S/S
- saline/saline
- WT
- wild-type
This work was funded by NIMH grant no. MH71241 and by the Mayo Foundation for Medical Education and Research. We would like to acknowledge the comments and insightful additions to our manuscript by other members of the neuropsychopharmacology laboratory and the neuroscience department at the Mayo Clinic, Jacksonville. All authors, excluding E.R., have no disclosures or conflicts of interests to report. E.R. has served as a consultant for Ortho-McNeil-Janssen, Takeda, and Lundbeck; received grant/research support from Ortho-McNeil-Janssen; and is co-inventor on issued patents that relate to NT69L. Deposited in PMC for release after 12 months.
REFERENCES
- Abi-Dargham A., Gil R., Krystal J., Baldwin R. M., Seibyl J. P., Bowers M., van Dyck C. H., Charney D. S., Innis R. B., Laruelle M. (1998). Increased striatal dopamine transmission in schizophrenia: confirmation in a second cohort. Am. J. Psychiatry 155, 761-767 [DOI] [PubMed] [Google Scholar]
- Bakshi V. P., Geyer M. A. (1998). Multiple limbic regions mediate the disruption of prepulse inhibition produced in rats by the noncompetitive NMDA antagonist dizocilpine. J. Neurosci. 18, 8394-8401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder E. B., Kinkead B., Owens M. J., Kilts C. D., Nemeroff C. B. (2001). Enhanced neurotensin neurotransmission is involved in the clinically relevant behavioral effects of antipsychotic drugs: evidence from animal models of sensorimotor gating. J. Neurosci. 21, 601-608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braff D. L., Grillon C., Geyer M. A. (1992). Gating and habituation of the startle reflex in schizophrenic patients. Arch. Gen. Psychiatry 49, 206-215 [DOI] [PubMed] [Google Scholar]
- Broderick P. A., Hope O., Okonji C., Rahni D. N., Zhou Y. (2004). Clozapine and cocaine effects on dopamine and serotonin release in nucleus accumbens during psychostimulant behavior and withdrawal. Prog. Neuropsychopharmacol. Biol. Psychiatry 28, 157-171 [DOI] [PubMed] [Google Scholar]
- Bubenikova V., Votava M., Horacek J., Palenicek T., Dockery C. (2005). The effect of zotepine, risperidone, clozapine and olanzapine on MK-801-disrupted sensorimotor gating. Pharmacol. Biochem. Behav. 80, 591-596 [DOI] [PubMed] [Google Scholar]
- Caceda R., Kinkead B., Owens M. J., Nemeroff C. B. (2005). Virally mediated increased neurotensin 1 receptor in the nucleus accumbens decreases behavioral effects of mesolimbic system activation. J. Neurosci. 25, 11748-11756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carraway R., Leeman S. E. (1976). Characterization of radioimmunoassayable neurotensin in the rat. Its differential distribution in the central nervous system, small intestine, and stomach. J. Biol. Chem 251, 7045-7052 [PubMed] [Google Scholar]
- Davidson A. C., Hinkley (1997). Bootstrap Methods and their Application. Cambridge: Cambridge University Press; [Google Scholar]
- Dulawa S. C., Geyer M. A. (1996). Psychopharmacology of prepulse inhibition in mice. Chin. J. Physiol. 39, 139-146 [PubMed] [Google Scholar]
- Duncan G. E., Moy S. S., Lieberman J. A., Koller B. H. (2006). Effects of haloperidol, clozapine, and quetiapine on sensorimotor gating in a genetic model of reduced NMDA receptor function. Psychopharmacology (Berl) 184, 190-200 [DOI] [PubMed] [Google Scholar]
- Farde L., Wiesel F. A., Nordstrom A. L., Sedvall G. (1989). D1- and D2-dopamine receptor occupancy during treatment with conventional and atypical neuroleptics. Psychopharmacology (Berl) 99Suppl., S28-31 [DOI] [PubMed] [Google Scholar]
- Fawaz C. S., Martel P., Leo D., Trudeau L. E. (2009). Presynaptic action of neurotensin on dopamine release through inhibition of D(2) receptor function. BMC Neurosci. 10, 96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feifel D., Melendez G., Priebe K., Shilling P. D. (2007). The effects of chronic administration of established and putative antipsychotics on natural prepulse inhibition deficits in Brattleboro rats. Behav. Brain Res. 181, 278-286 [DOI] [PubMed] [Google Scholar]
- Feifel D., Melendez G., Shilling P. D. (2003). A systemically administered neurotensin agonist blocks disruption of prepulse inhibition produced by a serotonin-2A agonist. Neuropsychopharmacology 28, 651-653 [DOI] [PubMed] [Google Scholar]
- Feifel D., Melendez G., Shilling P. D. (2004). Reversal of sensorimotor gating deficits in Brattleboro rats by acute administration of clozapine and a neurotensin agonist, but not haloperidol: a potential predictive model for novel antipsychotic effects. Neuropsychopharmacology 29, 731-738 [DOI] [PubMed] [Google Scholar]
- Feifel D., Minor K. L., Dulawa S., Swerdlow N. R. (1997). The effects of intra-accumbens neurotensin on sensorimotor gating. Brain Res. 760, 80-84 [DOI] [PubMed] [Google Scholar]
- Feifel D., Pang Z., Shilling P. D., Melendez G., Schreiber R., Button D. (2010a). Effects of neurotensin-2 receptor deletion on sensorimotor gating and locomotor activity. Behav. Brain Res. 212, 174-178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feifel D., Pang Z., Shilling P. D., Melendez G., Schreiber R., Button D. (2010b). Sensorimotor gating in neurotensin-1 receptor null mice. Neuropharmacology 58, 173-178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feifel D., Reza T. L., Wustrow D. J., Davis M. D. (1999). Novel antipsychotic-like effects on prepulse inhibition of startle produced by a neurotensin agonist. J. Pharmacol. Exp. Ther. 288, 710-713 [PubMed] [Google Scholar]
- Fuxe K., O’Connor W. T., Antonelli T., Osborne P. G., Tanganelli S., Agnati L. F., Ungerstedt U. (1992). Evidence for a substrate of neuronal plasticity based on pre- and postsynaptic neurotensin-dopamine receptor interactions in the neostriatum. Proc. Natl. Acad. Sci. USA 89, 5591-5595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galletly C. A., Clark C. R., McFarlane A. C., Weber D. L. (2000). The effect of clozapine on the speed and accuracy of information processing in schizophrenia. Prog. Neuropsychopharmacol Biol. Psychiatry 24, 1329-1338 [DOI] [PubMed] [Google Scholar]
- Geyer M. A., Swerdlow N. R. (2001). Measurement of startle response, prepulse inhibition, and habituation. Curr. Protoc. Neurosci., Chapter 8, Unit 8.7 [DOI] [PubMed] [Google Scholar]
- Hoff A. L., Faustman W. O., Wieneke M., Espinoza S., Costa M., Wolkowitz O., Csernansky J. G. (1996). The effects of clozapine on symptom reduction, neurocognitive function, and clinical management in treatment-refractory state hospital schizophrenic inpatients. Neuropsychopharmacology 15, 361-369 [DOI] [PubMed] [Google Scholar]
- Hollander M., Wolfe D. A. (1999). Nonparametric Statistical Methods. New York: Wiley; [Google Scholar]
- Hwang J. R., Baek M. W., Sim J., Choi H. S., Han J. M., Kim Y. L., Hwang J. I., Kwon H. B., Beaudet N., Sarret P., et al. (2010). Intermolecular cross-talk between NTR1 and NTR2 neurotensin receptor promotes intracellular sequestration and functional inhibition of NTR1 receptors. Biochem. Biophys. Res. Commun. 391, 1007-1013 [DOI] [PubMed] [Google Scholar]
- Kinkead B., Dobner P. R., Egnatashvili V., Murray T., Deitemeyer N., Nemeroff C. B. (2005). Neurotensin-deficient mice have deficits in prepulse inhibition: restoration by clozapine but not haloperidol, olanzapine, or quetiapine. J. Pharmacol. Exp. Ther. 315, 256-264 [DOI] [PubMed] [Google Scholar]
- Kodsi M. H., Swerdlow N. R. (1994). Quinolinic acid lesions of the ventral striatum reduce sensorimotor gating of acoustic startle in rats. Brain Res. 643, 59-65 [DOI] [PubMed] [Google Scholar]
- Kodsi M. H., Swerdlow N. R. (1997). Mitochondrial toxin 3-nitropropionic acid produces startle reflex abnormalities and striatal damage in rats that model some features of Huntington’s disease. Neurosci. Lett. 231, 103-107 [DOI] [PubMed] [Google Scholar]
- Kumari V., Soni W., Mathew V. M., Sharma T. (2000). Prepulse inhibition of the startle response in men with schizophrenia: effects of age of onset of illness, symptoms, and medication. Arch Gen Psychiatry 57, 609-614 [DOI] [PubMed] [Google Scholar]
- Laruelle M., Abi-Dargham A., van Dyck C. H., Gil R., D’Souza C. D., Erdos J., McCance E., Rosenblatt W., Fingado C., Zoghbi S. S., et al. (1996). Single photon emission computerized tomography imaging of amphetamine-induced dopamine release in drug-free schizophrenic subjects. Proc. Natl. Acad. Sci. USA 93, 9235-9240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le F., Cusack B., Richelson E. (1996). The neurotensin receptor: is there more than one subtype? Trends Pharmacol. Sci. 17, 1-3 [DOI] [PubMed] [Google Scholar]
- Legault M., Congar P., Michel F. J., Trudeau L. E. (2002). Presynaptic action of neurotensin on cultured ventral tegmental area dopaminergic neurones. Neuroscience 111, 177-187 [DOI] [PubMed] [Google Scholar]
- Liang Y., Boules M., Li Z., Williams K., Miura T., Oliveros A., Richelson E. (2010). Hyperactivity of the dopaminergic system in NTS1 and NTS2 null mice. Neuropharmacology 58, 1199-1205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansbach R. S., Brooks E. W., Sanner M. A., Zorn S. H. (1998). Selective dopamine D4 receptor antagonists reverse apomorphine-induced blockade of prepulse inhibition. Psychopharmacology (Berl) 135, 194-200 [DOI] [PubMed] [Google Scholar]
- Mansbach R. S., Geyer M. A. (1989). Effects of phencyclidine and phencyclidine biologs on sensorimotor gating in the rat. Neuropsychopharmacology 2, 299-308 [DOI] [PubMed] [Google Scholar]
- McCaughran J., Jr, Mahjubi E., Decena E., Hitzemann R. (1997). Genetics, haloperidol-induced catalepsy and haloperidol-induced changes in acoustic startle and prepulse inhibition. Psychopharmacology (Berl) 134, 131-139 [DOI] [PubMed] [Google Scholar]
- Meltzer H. Y., McGurk S. R. (1999). The effects of clozapine, risperidone, and olanzapine on cognitive function in schizophrenia. Schizophr. Bull. 25, 233-255 [DOI] [PubMed] [Google Scholar]
- Nemeroff C. B., Bissette G., Widerlov E., Beckmann H., Gerner R., Manberg P. J., Lindstrom L., Prange A. J., Jr, Gattaz W. F. (1989). Neurotensin-like immunoreactivity in cerebrospinal fluid of patients with schizophrenia, depression, anorexia nervosa-bulimia, and premenstrual syndrome. J Neuropsychiatry Clin. Neurosci. 1, 16-20 [DOI] [PubMed] [Google Scholar]
- Nemeroff C. B., Luttinger D., Hernandez D. E., Mailman R. B., Mason G. A., Davis S. D., Widerlov E., Frye G. D., Kilts C. A., Beaumont K., et al. (1983). Interactions of neurotensin with brain dopamine systems: biochemical and behavioral studies. J. Pharmacol. Exp. Ther. 225, 337-345 [PubMed] [Google Scholar]
- Nguyen T., Shapiro D. A., George S. R., Setola V., Lee D. K., Cheng R., Rauser L., Lee S. P., Lynch K. R., Roth B. L., et al. (2001). Discovery of a novel member of the histamine receptor family. Mol. Pharmacol. 59, 427-433 [DOI] [PubMed] [Google Scholar]
- Nouel D., Faure M. P., St Pierre J. A., Alonso R., Quirion R., Beaudet A. (1997). Differential binding profile and internalization process of neurotensin via neuronal and glial receptors. J. Neurosci. 17, 1795-1803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda T., Matsumoto S. (2001). [Identification and characterization of histamine H4 receptor]. NipponYakurigaku Zasshi 118, 36-42 [DOI] [PubMed] [Google Scholar]
- Oranje B., Van Oel C. J., Gispen-De Wied C. C., Verbaten M. N., Kahn R. S. (2002). Effects of typical and atypical antipsychotics on the prepulse inhibition of the startle reflex in patients with schizophrenia. J. Clin. Psychopharmacol. 22, 359-365 [DOI] [PubMed] [Google Scholar]
- Ouagazzal A. M., Jenck F., Moreau J. L. (2001). Drug-induced potentiation of prepulse inhibition of acoustic startle reflex in mice: a model for detecting antipsychotic activity? Psychopharmacology (Berl) 156, 273-283 [DOI] [PubMed] [Google Scholar]
- Radke J. M., Owens M. J., Ritchie J. C., Nemeroff C. B. (1998). Atypical antipsychotic drugs selectively increase neurotensin efflux in dopamine terminal regions. Proc. Natl. Acad. Sci. USA 95, 11462-11464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph R. J., Paulus M. P., Geyer M. A. (2001). Strain-specific effects of amphetamine on prepulse inhibition and patterns of locomotor behavior in mice. J. Pharmacol. Exp. Ther. 298, 148-155 [PubMed] [Google Scholar]
- Ralph R. J., Varty G. B., Kelly M. A., Wang Y.-M., Caron M. G., Rubinstein M., Grandy D. K., Low M. J., Geyer M. A. (1999). The dopamine D2, but not D3 or D4, receptor subtype is essential for the disruption of prepulse inhibition produced by amphetamine in mice. J. Neurosci. 19, 4627-4633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph-Williams R. J., Lehmann-Masten V., Otero-Corchon V., Low M. J., Geyer M. A. (2002). Differential effects of direct and indirect dopamine agonists on prepulse inhibition: a study in D1 and D2 receptor knock-out mice. J. Neurosci. 22, 9604-9611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigdon G. C., Weatherspoon J. K. (1992). 5-Hydroxytryptamine 1a receptor agonists block prepulse inhibition of acoustic startle reflex. J. Pharmacol. Exp. Ther. 263, 486-493 [PubMed] [Google Scholar]
- Russig H., Spooren W., Durkin S., Feldon J., Yee B. K. (2004). Apomorphine-induced disruption of prepulse inhibition that can be normalised by systemic haloperidol is insensitive to clozapine pretreatment. Psychopharmacology (Berl) 175, 143-147 [DOI] [PubMed] [Google Scholar]
- Saha S., Chant D., Welham J., McGrath J. (2005). A systematic review of the prevalence of schizophrenia. PLoS Med. 2, e141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaman R. L. (2000). Effects of acute systemic 3-nitropropionic acid administration on rat activity and acoustic startle. Neurosci. Lett. 280, 183-186 [DOI] [PubMed] [Google Scholar]
- Shilling P. D., Richelson E., Feifel D. (2003). The effects of systemic NT69L, a neurotensin agonist, on baseline and drug-disrupted prepulse inhibition. Behav. Brain Res. 143, 7-14 [DOI] [PubMed] [Google Scholar]
- Swerdlow N. R., Braff D. L., Geyer M. A. (1999). Cross-species studies of sensorimotor gating of the startle reflex. Ann. NY Acad. Sci. 877, 202-216 [DOI] [PubMed] [Google Scholar]
- Swerdlow N. R., Geyer M. A., Braff D. L. (2001). Neural circuit regulation of prepulse inhibition of startle in the rat: current knowledge and future challenges. Psychopharmacology (Berl) 156, 194-215 [DOI] [PubMed] [Google Scholar]
- Swerdlow N. R., Paulsen J., Braff D. L., Butters N., Geyer M. A., Swenson M. R. (1995). Impaired prepulse inhibition of acoustic and tactile startle response in patients with Huntington’s disease. J. Neurol. Neurosurg. Psychiatry 58, 192-200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow N. R., Varty G. B., Geyer M. A. (1998). Discrepant findings of clozapine effects on prepulse inhibition of startle: is it the route or the rat? Neuropsychopharmacology 18, 50-56 [DOI] [PubMed] [Google Scholar]
- Van Tol H. H., Bunzow J. R., Guan H. C., Sunahara R. K., Seeman P., Niznik H. B., Civelli O. (1991). Cloning of the gene for a human dopamine D4 receptor with high affinity for the antipsychotic clozapine. Nature 350, 610-614 [DOI] [PubMed] [Google Scholar]
- Varty G. B., Walters N., Cohen-Williams M., Carey G. J. (2001). Comparison of apomorphine, amphetamine and dizocilpine disruptions of prepulse inhibition in inbred and outbred mice strains. Eur. J. Pharmacol. 424, 27-36 [DOI] [PubMed] [Google Scholar]
- Yee B. K., Russig H., Feldon J. (2004). Apomorphine-induced prepulse inhibition disruption is associated with a paradoxical enhancement of prepulse stimulus reactivity. Neuropsychopharmacology 29, 240-248 [DOI] [PubMed] [Google Scholar]
- Zhang J., Forkstam C., Engel J. A., Svensson L. (2000). Role of dopamine in prepulse inhibition of acoustic startle. Psychopharmacology (Berl) 149, 181-188 [DOI] [PubMed] [Google Scholar]