Abstract
Background
We investigated renal effects of nebivolol, a selective β1-receptor blocker with additional antioxidative ability, in spontaneously hypertensive rats (SHR) where increased salt intake induces oxidative stress and worsens renal function as a result of further activation of the renin-angiotensin and sympathetic nervous systems.
Methods
Male SHR were given an 8% salt diet (HS; n = 22) for 5 weeks; their age-matched controls (n = 9) received standard chow. Nebivolol was given at a dose of 10 mg/kg/day for 5 weeks in 11 HS rats.
Results
HS increased blood pressure, plasma renin concentration, urinary protein excretion, and renal nitroxidative stress while decreasing renal blood flow and angiotensin 1–7 receptor (mas) protein expression. There was no change in angiotensin II type 1 receptor expression among the experimental groups. Nebivolol did not alter the salt-induced increase in blood pressure but reduced urinary protein excretion, plasma renin concentration, and nitroxidative stress. Nebivolol also increased neuronal NOS expression while preventing the salt-induced decrease in renal blood flow and mas protein expression.
Conclusion
Nebivolol prevented salt-induced kidney injury and associated proteinuria in SHR through a blood pressure-independent mechanism. Its protective effects may be related to reduction in oxidative stress, increases in neuronal NOS and restoration of angiotensin II type 1/mas receptor balance.
Key Words: Salt, Hypertension, Kidney, Oxidative stress, Nitric oxide, β1-Receptor antagonism
Introduction
Recent studies from our group and other laboratories suggest that salt excess is an important determinant of cardiovascular and renal derangement in hypertension [1,2,3]. Besides its hemodynamic effects, dietary salt excess exerts additional non-pressure-related tissue maladaptive effects in different forms of experimental and human essential hypertension. Increased sympathetic (SNS) [4] and renin-angiotensin system (RAS) activity [5,6,7,8,9], along with increased production of superoxide and other reactive oxygen species [10,11,12,13], are among the mechanisms suggested to be involved in the salt-induced hypertension, proteinuria, and progression of early renal injury to nephrosclerosis. In this regard, renal NADPH oxidase activity has been implicated in superoxide production [14,15,16] and salt-loading increases expression of its subunits, gp91 phox and p47 phox [11,17].
On the other hand, nitric oxide (NO) exerts a protective role against renal damage in several animal models of kidney disease [18,19,20,21,22] as well as in human chronic renal failure [23,24]. NO promotes sodium excretion and increases renal blood flow (RBF) [25] and exerts antigrowth and antiproliferative effects on vascular smooth muscle and mesangial cells [26], all of which may counterbalance, at least in part, the detrimental effects of increased SNS and RAS in rats exposed to a high salt diet. Three NO synthase (NOS) isoforms, endothelial (eNOS), neuronal (nNOS) and inducible (iNOS) NOS mediate NO production; however, their regulation in response to excessive salt intake is still controversial. Thus, depending on the renal region and animal model studied, an increased [27,28], no change [27,28,29] or diminished [29,30,31,32] renal expression of various NOS isoforms in response to salt loading was reported. Importantly, there is no information on expression of NOS isoforms during progression of renal disease in spontaneously hypertensive rats (SHR) fed high salt diet, a recognized experimental model of human essential hypertension. Since superoxide scavenges NO, its reduced availability may also contribute to the development of salt-induced renal injury and dysfunction.
Nebivolol is a highly selective β1-receptor blocker with additional direct antioxidant and vasodilator/antiproliferative potential related to an increased NO bioavailability [33,34]. Nebivolol reduced blood pressure and decreased NADPH oxidase activity as a main source of superoxide production in the hypertensive kidney of mRen2 transgenic rats while preventing the reduction in eNOS protein expression [15,16]. In that way, nebivolol corrected NO/superoxide imbalance resulting in diminished proteinuria. The effects of nebivolol on the other NOS isoforms, nNOS and iNOS, have not been investigated, and its renal effects under the conditions of high salt intake in the hypertensives have yet to be determined. Therefore, it is of interest to examine whether nebivolol improves renal dysfunction and remodeling in salt-loaded SHR in view of its potential to not only decrease hemodynamic burden but also to correct renal tissue NO/superoxide imbalance due to high salt intake. Since β-antagonists suppress not only SNS but also RAS, and angiotensin II (Ang II) and angiotensin-(1–7) [Ang-(1–7)] acting upon their receptors [Ang II type 1 (AT1) and mas receptor, respectively] exert opposing actions on oxidative stress and renal hemodynamics and injury [35,36,37,38], we also examined the effects of nebivolol on renal receptor protein expression of these counterbalancing peptides.
Methods
Animals
Male SHR (Charles River), weighing 180–200 g were randomly divided into two groups to receive either a control (1% NaCl; n = 9) or a high salt (8% NaCl; n = 22) diet for the ensuing 5 weeks. The salt-loaded SHR were randomized to one of two groups: vehicle (n = 11) and nebivolol-treated (n = 11) SHR. Nebivolol was given once a day (10 mg/kg) in suspension of 5% gum arabica by gastric gavage. All rats were permitted free access to chow and tap water and were maintained in a temperature and humidity-controlled room with a 12-hour light/dark cycle. Three untreated rats on high salt diet died before the end of the study period. None of the control or nebivolol-treated rats died during the course of the experiment. Systolic blood pressure was measured by tail-cuff plethysmography (Narco Bio System, Houston, Tex., USA) during a baseline period followed by weekly measurements in rats trained beforehand. After 5 weeks on the respective treatment, animals were placed in the metabolic cages for 24-hour urine collections and assessment of protein and creatinine excretion. After urine collection, the animals were decapitated, and trunk blood was collected for measurements of plasma renin concentration (PRC), serum nitrate and nitrite concentrations (NOx), and serum creatinine. Kidney cortex was collected for analysis of NOS, gp91phox, AT1, and mas protein expression, NOx as well as fibrosis and immunostaining for nitrotyrosine. Additional groups of rats kept under the same experimental conditions (n = 5–6 rats/group) were subjected at the end of the experiments to tracheal intubation and right carotid artery cannulation with a transducer-tipped catheter (Micro-Tip 2F, Millar Instruments) for direct measurement of mean arterial pressure (MAP) under pentobarbital anesthesia (50 mg/kg) using a digital data acquisition system (EMKA Technologies, Falls Church, Va., USA). RBF was measured in the same animals as described previously [39] using Transonic flow probe connected to a flowmeter (Transonic System Inc., Ithaca, N.Y., USA) after careful dissection of renal artery and 1-hour adjustment period with saline infusion via PE tubing in the right femoral vein at a rate of 0.4 ml/100 g–1/h–1. The rats were handled in accordance with National Institute of Health guidelines; our Institutional Animal Care and Use Committee approved the study in advance.
Biochemical Assays
Urinary protein was measured by a Bradford protein assay (Bio-Rad, Hercules, Calif., USA). Serum and urinary creatinine were measured with a Quantichrom Creatinine Assay Kit (Bioassay Systems, Hayward, Calif., USA). Creatinine clearance as a measure of glomerular filtration rate (GFR) was calculated from its urinary excretion rate divided by serum concentration, and the data were expressed as milliliters per minute. Blood was collected in EDTA-containing tubes for the assay of PRC. PRC was defined as the rate of Ang I generation from renin in the sample incubated at pH 6.5 for 90 min with excess exogenous substrate provided from nephrectomized rat plasma. Ang I generated in the sample was quantified by radioimmunoassay (Diosarin Corp, Stillwater, Minn., USA). NOx in serum and tissue samples was determined using a Griess assay (nitrate/nitrite colorimetric assay kit, Alexis Biomedicals, Calif., USA).
Western Blotting
As described previously in detail [40], kidneys were removed and cortical tissue samples were snap frozen in liquid nitrogen and stored at −80°C. Frozen tissue was homogenized in a buffer containing 10 mM HEPES (pH 7.4), 125 mM NaCl, 1 mM EDTA, 1 mM NaF, 10 μg/ml leupeptin, 10 μg/ml pepstatin A, and 1 mM PMSF final concentrations. Homogenates were centrifuged at 2,000 and then at 100,000 g for 60 min at 4°C. Protein concentration in both fractions (pellet and supernatant), was determined by Bradford method using BioRad kit (Bio-Rad). Samples of pellets for eNOS, gp91phox, AT1, and mas and supernatant for nNOS and iNOS analysis were separated by gel electrophoresis, and then proteins were eluted from the gels to Hybond PVDF membranes (Bio-Rad) for 1 h at 100 V, except for NOS which was transferred for 3 h at 80 V. Nonspecific binding was blocked in 5% nonfat dried milk in 0.1% Tween 20 in TBS for 60 min at room temperature except for the AT1 receptor which was blocked by Starting Block™ Blocking Buffer (Thermo Scientific, Rockford, Ill., USA). The blots were incubated with a monoclonal anti-β-actin (1:2,000; Sigma, St. Louis, Mo., USA), monoclonal anti-α-tubulin (1:1,000, Abcam, Cambridge, Mass., USA), monoclonal anti-eNOS (1:500; Transduction Laboratories, Lexington, Ky., USA), monoclonal anti-nNOS (1:2,000; BD Transduction Laboratories), monoclonal anti-iNOS (1:2,000; Transduction Laboratories), monoclonal anti-gp91phox (1:1,000; BD Transduction Laboratories), polyclonal anti-AT1 (1:250; Alomone Labs, Israel), and polyclonal anti-mas (1:200; Alomone Labs). The blots were then incubated with either the secondary anti-rabbit antibody (1:5,000; Amersham Biosciences, Piscataway, N.J., USA) or anti-mouse antibodies (1:3,000 except for α-tubulin for which 1:1,000 was used; Amersham Biosciences). Immunoblots were then resolved with Pierce Super Signal West Pico Chemiluminescent substrates as described by the manufacturer, and exposed to Amersham Hyperfilm-enhanced chemiluminescence (Amersham Biosciences). As positive controls, human endothelial cell lysate for eNOS, rat cerebrum cell lysate for nNOS, mouse macrophage IFN/LPS lysate for iNOS were used. Signal quantification was performed using an image analysis program (MCID), and optical densities were expressed as the ratio between corresponding protein and β-actin or α-tubulin for membrane and cystosolic fraction, respectively. The data were reported as the percentage of the controls.
Histological Analysis
Fixed paraffin sections of kidneys were evaluated for 3-nitrotyrosine (3-NT) immunostaining as a marker for nitroxidative stress as described previously [16]. Fibrosis was evaluated on slides stained with Verhoeff-van Gieson, which is specific for fibrosis and stains elastin (black), nuclei (blue black), collagen (red), and connective tissue (yellow) [41]. Slides were evaluated under a bright-field microscope (model 50i, Nikon), and ×40 images (for 3-NT) or ×10 (for fibrosis) were captured with a Cool Snap camera. Images were analyzed, and signal intensities were measured with MetaVue software (Boyce Scientific, Gary Summit, Mo., USA).
Statistics
All values are expressed as the mean ± 1 SEM. Data were analyzed by use of ANOVA followed by Newman-Keuls’ post-test. A value of p < 0.05 was considered to be of statistical significance.
Results
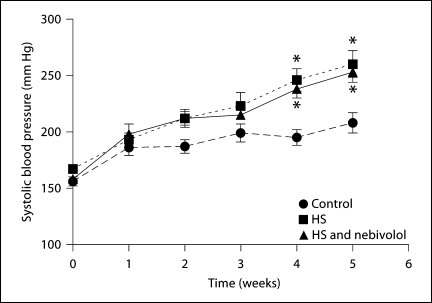
As already showed in our previous studies, tail-cuff blood pressure progressively increased in untreated salt-loaded SHR when compared to the rats fed control diet. Importantly, nebivolol failed to prevent or to ameliorate the salt-induced increase in blood pressure (fig. 1). Direct measures of MAP at the completion of the treatment regimen averaged 164 ± 7 mm Hg in control, 200 ± 11 mm Hg in untreated salt-loaded rats, and 195 ± 7 mm Hg in salt-loaded rats treated with nebivolol; these data confirmed the absence of an antihypertensive effect of nebivolol.
Fig. 1.
Effects of high salt diet and nebivolol on time course of systolic tail-cuff blood pressure in SHR. * p < 0.05 vs. control.
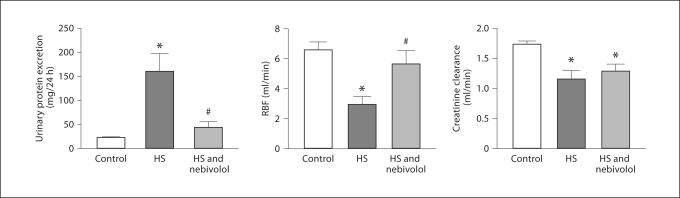
Renal studies revealed that high salt intake for 5 weeks induced extensive urinary protein excretion in SHR associated with decreased RBF and creatinine clearance (fig. 2). While nebivolol treatment significantly ameliorated excessive urinary protein loss and increased RBF, it failed to improve creatinine clearance.
Fig. 2.
Effects of high salt diet and nebivolol treatment on renal function. * p < 0.05 vs. control; # p < 0.05 vs. untreated salt-loaded group.
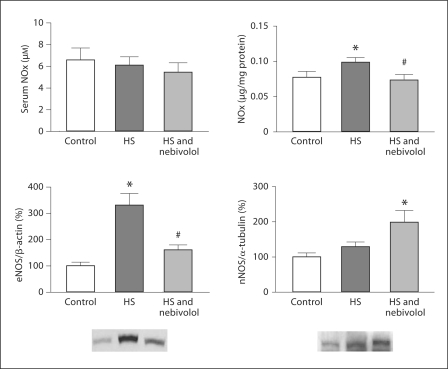
Serum NOx levels were measured as an index of NO production in the whole body as opposed to tissue NOx levels which evaluated only the local kidney cortex production. As shown in figure 3, there was no difference in serum NOx between the three experimental groups. In contrast, kidney cortex NOx was increased in untreated SHR fed the high salt diet and nebivolol prevented the salt-induced increase in NO production in the kidneys. Consistent with local NOx production, high salt diet increased eNOS protein expression while nebivolol reduced it to levels not different from those found in the control SHR. In contrast, high salt diet did not affect the nNOS expression, but nebivolol treatment increased it when compared to the control group. We did not detect iNOS isoform in any of the three experimental groups (data not shown).
Fig. 3.
Serum and renal cortical NOx, eNOS and nNOS in the control and untreated and nebivolol-treated SHR fed high salt diet. * p < 0.05 vs. control; # p < 0.05 vs. untreated salt-loaded group.
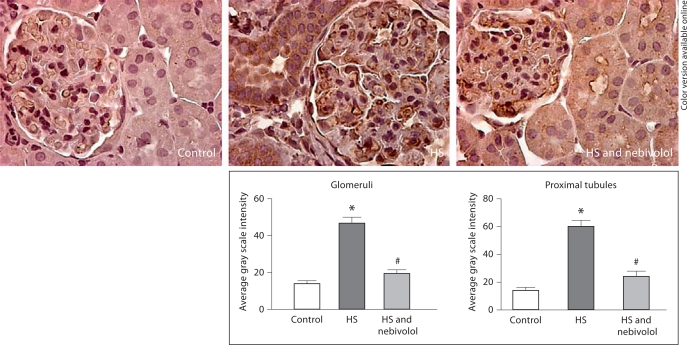
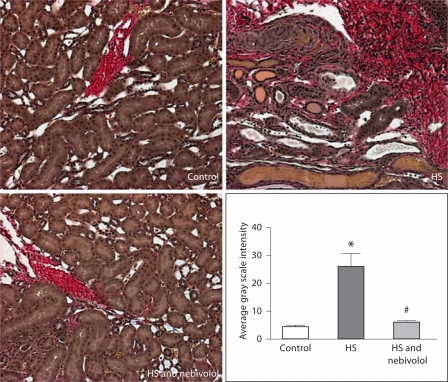
The protein expression of gp91phox component of NADPH oxidase was enhanced in renal cortical tissue of untreated SHR fed a high salt diet when compared to the controls (224 ± 27%, p < 0.05); nebivolol treatment reduced gp91phox protein expression to levels not different from the control animals (139 ± 30%, p > 0.05). Consistent with parallel changes in renal gp91phox protein expression and NOx production, we demonstrated an increased 3-NT immunostaining and fibrosis in salt-loaded SHR, and nebivolol reduced these changes (fig. 4, 5).
Fig. 4.
Effects of nebivolol on 3-nitrotyrosine staining in glomeruli and proximal tubule of salt-loaded SHR. * p < 0.05 vs. control; # p < 0.05 vs. untreated salt-loaded group.
Fig. 5.
Renal intertubular fibrosis in SHR fed high salt diet and effects of nebivolol treatment. * p < 0.05 vs. control; # p < 0.05 vs. untreated salt-loaded group.
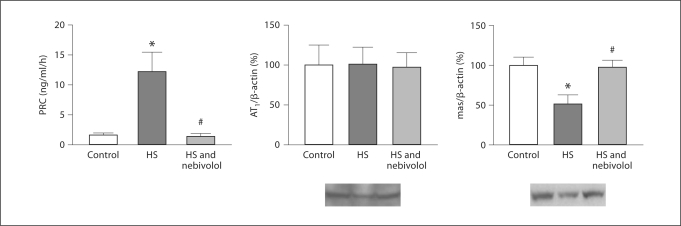
We confirmed our previous findings that salt loading in SHR increased PRC [9]. We now extended these studies including analysis of renal protein expression for AT1 and mas receptor. Salt loading decreased mas protein expression, whereas nebivolol prevented this decrease while suppressing salt-induced increase in PRC (fig. 6). There were no changes in AT1 receptor expression between the three experimental groups.
Fig. 6.
PRC and renal AT1 and mas receptor protein expression in control, salt-loaded, and nebivolol-treated SHR. AT1 = AT1 receptor; mas = mas receptor. * p < 0.05 vs. control; # p < 0.05 vs. untreated salt-loaded group.
Discussion
Our results demonstrated that salt-induced renal dysfunction and remodeling in SHR are associated with enhanced nitroxidative stress reflecting an increase in kidney protein expression of eNOS and gp91phox component of NADPH oxidase. In agreement with these results, increased PRC in untreated salt-loaded rats indicates activation of the circulatory Ang II [9], whereas reduced mas protein expression suggests suppressed Ang-(1–7)signaling. Nebivolol treatment of high salt diet-fed SHR did not prevent the salt-induced increase in blood pressure; however, it improved RBF and prevented excessive urinary protein loss, collagen deposition and associated nitroxidative stress while suppressing PRC and increasing renal mas and nNOS protein expression.
A large body of evidence suggests that dietary salt excess promotes renal injury in hypertensive subjects [2,42,43], although the underling mechanisms are still not well defined. Increased blood pressure may be implicated in the detrimental renal effects of high salt diet, and we posited that nebivolol could decrease the hemodynamic burden imposed by high salt diet [44]. However, in this study nebivolol did not prevent the salt-induced increase in arterial pressure and did not increase serum NO metabolites. These data suggest that in this experimental model nebivolol did not facilitate systemic vascular NO production. However, in agreement with the concept that detrimental renal effects of dietary salt excess are partly mediated by non-pressure related mechanisms, nebivolol ameliorated salt-related renal functional and structural derangements.
The RAS have been also implicated in the harmful renal effects of high salt diet [7,12]. The observed increased PRC in the present study confirmed our previous findings on paradoxical activation of RAS in salt-loaded rats [9]. In agreement, AT1 receptor-blocking agents improved renal hemodynamics and function, reduced excessive proteinuria and fibrosis and ameliorated progressive proliferative glomerular changes in salt-sensitive hypertension [7,43,45]. Consistent with its β1 receptor antagonism, nebivolol prevented renin release in rats fed the high salt diet. Further, reduced renal mas protein expression in untreated SHR fed high salt diet was associated with unfavorable changes in RBF and GFR and extensive fibrosis and proteinuria; nebivolol prevented the salt-induced reduction in mas expression along with improvement in RBF, urinary protein excretion, and collagen deposition but not reduced GFR. The critical role of Ang-(1–7) in the regulation of renal hemodynamics and tubuloglomerular structure and function is consistent with mas expression in afferent arterioles, glomerular mesangial cells, and tubular epithelium [38,46,47]. Ang-(1–7) not only induced robust vasodilation in isolated renal afferent arterioles [48] but also abolished Ang II-induced renal vasoconstriction in isolated kidney from both Wistar-Kyoto rats and SHR [49]. Additionally, increases in circulating Ang-(1–7) by acute infusion or chronically in transgenic rats expressing an Ang-(1–7) producing fusion protein did not affect blood pressure but profoundly decreased renal vascular resistance [50,51]; the effect was partially abolished by the specific mas antagonist [D-Ala7]-Ang-(1–7) (A-779) [51]. Moreover, mas-deficient mice developed extensive tubulointerstitial and glomerular fibrosis as well as renal dysfunction [37]. Consistent with these reports, results of the current investigation suggest deficient mas-mediated renal effects of Ang-(1–7) in response to high salt intake that was corrected by nebivolol treatment. The inability of nebivolol to prevent the salt-related reduction in GFR may be attributed to the lack of its antihypertensive action and corresponding persistent structural changes in glomerular capillary basement membranes. Additionally, it is not clear from this study how nebivolol regulates mas expression. In view of the recent report revealing a direct interaction between β-adrenergic and AT1 receptors [52], further studies are warranted to explore the potential interaction between adrenergic and mas receptors as well.
This investigation assessed the expression of three NOS isoforms in order to elucidate the effects of dietary salt excess on NO system in SHR keeping in mind the important role of NO in regulation of RBF and growth of vascular smooth muscle and mesangial cells [26,53,54]. Interestingly, we demonstrated that the cortical eNOS expression was increased in salt-loaded SHR while there were no changes in the expression of nNOS. Although we did not determine directly the renal NOS-dependent production of NO, cortical NOx levels paralleled the changes in the eNOS expression, suggesting a greater eNOS activity. The enhanced activity of NO system in response to high salt diet may be compensatory in order to prevent the excessive renal vasoconstriction and growth-promoting effects of activated sympathetic nervous system and RAS.
On the other hand, the functionality of the NO system depends not only on the NO production but also on its bioavailability that may be decreased due to enhanced superoxide formation. Increased oxidative stress, detrimental in many forms of salt-sensitive hypertension [11,16,55,56,57], is frequently associated with increased expression or activity of NADPH oxidase as the primary source of superoxide in the kidneys [11]. Thus, increased renal superoxide level in response to high salt intake may react with locally produced NO diminishing bioavailability of NO while producing peroxynitrite [58] that may also contribute to renal injury [59,60]. Indeed, in salt-loaded rats we demonstrated an enhanced 3-NT staining associated with increased expression of gp91phox, one of the critical components of NADPH oxidase, indicative of this enhanced interaction between NO and superoxide anion and subsequent peroxynitrite reaction with tyrosine residues on proteins. Increased eNOS expression may reflect the low levels of bioactive NO and its diminished negative regulatory role on eNOS [61,62]. Alternatively, increased oxidative stress may promote oxidizing of tetrahydrobiopterin, a critical cofactor for the NOS, which becomes uncoupled and produces itself superoxide instead of NO [63]. However, this possibility seems less likely since increased renal NOx paralleled the increase in renal eNOS expression mirroring NO formation rather than deficiency. These results imply that, despite the beneficial effects of NO on renal hemodynamics and function, high NO formation is not necessarily always advantageous for optimal renal protection. Importantly, nebivolol prevented excessive formation of peroxynitrite reflective in reduced 3-NT staining, gp91phox, and eNOS expression. Unlike other conventional β-blockers, nebivolol has strong antioxidative properties [33,34,64], and treatment with nebivolol decreased renal fibrosis and glomerular injury and improved endothelial dysfunction associated with increased NO bioavailability in different experimental models [15,16,65,66,67]. Indeed, in addition to the effects on the enzymatic source of superoxide and its intrinsic direct superoxide scavenger properties [34], nebivolol may have reduced oxidative injury in salt-loaded SHR by reducing the renal effects of Ang II while improving Ang-(1–7) signaling. Evidence from the literature testified for pro-oxidative action of Ang II [68], while recent studies revealed a potential of Ang-(1–7) to reduce NADPH oxidase activity in diabetic SHR kidney [35]. Moreover, Ang-(1–7) buffered the Ang II-induced superoxide production in nuclei isolated from the sheep kidneys [36]. Further studies are needed to elucidate the underling mechanism of renal protection of nebivolol with respect to its potential interaction with mas signaling.
Finally, nebivolol-induced increase in nNOS expression may also contribute to its beneficial renal effects against salt-induced injury. Previous reports showed that the neuronal isoform could be detected in macula densa and arterioles as well as interlobular arteries [69,70,71], reflecting its input in regulating renal hemodynamics [72,73]. Thus, it is plausible that NO derived from nNOS may mediate renoprotective effects of nebivolol to prevent excessive renal vasoconstriction. To our knowledge, this is the first report on the effects of nebivolol on renal nNOS, although the relationship between β3 adrenergic receptor and nNOS has been explored in the heart [74]. Nebivolol has additional β3-adrenergic receptor agonist effects, but the exact mechanisms as to how nebivolol regulates renal nNOS expression and its role in mediating renoprotection under the condition of dietary salt excess remain to be explored in future studies.
In summary, these studies showed that nebivolol, when given concomitantly with dietary salt excess, prevented kidney injury in SHR through a blood pressure-independent mechanism that may be related to reduction in oxidative stress, increased nNOS, and restoration of AT1/mas receptor balance.
Disclosure Statement
J.V. received investigator-initiated support from the Forest Research Institute.
Acknowledgment
This study was supported by the grants from Forest Research Institute (J.V.), American Heart Association (2300114 to J.V.), and NIH (2PO1 HL-051952 to C.M.F.). We also acknowledge partial support provided by the Farley-Hudson Foundation, Jacksonville, N.C., USA. In addition, the authors acknowledge the excellent technical assistance of Ms. Jessica VonCannon.
References
- 1.Ahn J, Varagic J, Slama M, Susic D, Frohlich ED. Cardiac structural and functional responses to salt loading in SHR. Am J Physiol Heart Circ Physiol. 2004;287:H767–H772. doi: 10.1152/ajpheart.00047.2004. [DOI] [PubMed] [Google Scholar]
- 2.Matavelli LC, Zhou X, Varagic J, Susic D, Frohlich ED. Salt loading produces severe renal hemodynamic dysfunction independent of arterial pressure in spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 2007;292:H814–H819. doi: 10.1152/ajpheart.00671.2006. [DOI] [PubMed] [Google Scholar]
- 3.Yu HC, Burrell LM, Black MJ, Wu LL, Dilley RJ, Cooper ME, Johnston CI. Salt induces myocardial and renal fibrosis in normotensive and hypertensive rats. Circulation. 1998;98:2621–2628. doi: 10.1161/01.cir.98.23.2621. [DOI] [PubMed] [Google Scholar]
- 4.Leenen FH, Yuan B. Dietary-sodium-induced cardiac remodeling in spontaneously hypertensive rat versus Wistar-Kyoto rat. J Hypertens. 1998;16:885–892. doi: 10.1097/00004872-199816060-00020. [DOI] [PubMed] [Google Scholar]
- 5.Hodge G, Ye VZ, Duggan KA. Dysregulation of angiotensin II synthesis is associated with salt sensitivity in the spontaneous hypertensive rat. Acta Physiol Scand. 2002;174:209–215. doi: 10.1046/j.1365-201x.2002.00937.x. [DOI] [PubMed] [Google Scholar]
- 6.Kreutz R, Fernandez-Alfonso MS, Liu Y, Ganten D, Paul M. Induction of cardiac angiotensin I-converting enzyme with dietary NaCl-loading in genetically hypertensive and normotensive rats. J Mol Med. 1995;73:243–248. doi: 10.1007/BF00189924. [DOI] [PubMed] [Google Scholar]
- 7.Nishiyama A, Yoshizumi M, Rahman M, Kobori H, Seth DM, Miyatake A, Zhang GX, Yao L, Hitomi H, Shokoji T, Kiyomoto H, Kimura S, Tamaki T, Kohno M, Abe Y. Effects of AT1 receptor blockade on renal injury and mitogen-activated protein activity in Dahl salt-sensitive rats. Kidney Int. 2004;65:972–981. doi: 10.1111/j.1523-1755.2004.00476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stier CT, Jr, Chander P, Gutstein WH, Levine S, Itskovitz HD. Therapeutic benefit of captopril in salt-loaded stroke-prone spontaneously hypertensive rats is independent of hypotensive effect. Am J Hypertens. 1991;4:680–687. doi: 10.1093/ajh/4.8.680. [DOI] [PubMed] [Google Scholar]
- 9.Varagic J, Ahmad S, Brosnihan KB, Groban L, Chappell MC, Tallant EA, Gallagher PE, Ferrario CM. Decreased cardiac Ang-(1–7) is associated with salt-induced cardiac remodeling and dysfunction. Ther Adv Cardiovasc Dis. 2010;4:17–25. doi: 10.1177/1753944709353337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen X, Touyz RM, Park JB, Schiffrin EL. Antioxidant effects of vitamins C and E are associated with altered activation of vascular NADPH oxidase and superoxide dismutase in stroke-prone SHR. Hypertension. 2001;38:606–611. doi: 10.1161/hy09t1.094005. [DOI] [PubMed] [Google Scholar]
- 11.Kitiyakara C, Chabrashvili T, Chen Y, Blau J, Karber A, Aslam S, Welch WJ, Wilcox CS. Salt intake, oxidative stress, and renal expression of NADPH oxidase and superoxide dismutase. J Am Soc Nephrol. 2003;14:2775–2782. doi: 10.1097/01.asn.0000092145.90389.65. [DOI] [PubMed] [Google Scholar]
- 12.Nagase M, Matsui H, Shibata S, Gotoda T, Fujita T. Salt-induced nephropathy in obese spontaneously hypertensive rats via paradoxical activation of the mineralocorticoid receptor: role of oxidative stress. Hypertension. 2007;50:877–883. doi: 10.1161/HYPERTENSIONAHA.107.091058. [DOI] [PubMed] [Google Scholar]
- 13.Welch WJ, Mendonca M, Blau J, Karber A, Dennehy K, Patel K, Lao YS, Jose PA, Wilcox CS. Antihypertensive response to prolonged tempol in the spontaneously hypertensive rat. Kidney Int. 2005;68:179–187. doi: 10.1111/j.1523-1755.2005.00392.x. [DOI] [PubMed] [Google Scholar]
- 14.Chabrashvili T, Tojo A, Onozato ML, Kitiyakara C, Quinn MT, Fujita T, Welch WJ, Wilcox CS. Expression and cellular localization of classic NADPH oxidase subunits in the spontaneously hypertensive rat kidney. Hypertension. 2002;39:269–274. doi: 10.1161/hy0202.103264. [DOI] [PubMed] [Google Scholar]
- 15.Hayden MR, Habibi J, Whaley-Connell A, Sowers D, Johnson M, Tilmon R, Jain D, Ferrario C, Sowers JR. Nebivolol attenuates maladaptive proximal tubule remodeling in transgenic rats. Am J Nephrol. 2010;31:262–272. doi: 10.1159/000278757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Whaley-Connell AT, Chowdhury NA, Hayden MR, Stump CS, Habibi J, Wiedmeyer CE, Gallagher PE, Tallant EA, Cooper SA, Link CD, Ferrario C, Sowers JR. Oxidative stress and glomerular filtration barrier injury: role of the renin-angiotensin system in the Ren2 transgenic rat. Am J Physiol Renal Physiol. 2006;291:F1308–F1314. doi: 10.1152/ajprenal.00167.2006. [DOI] [PubMed] [Google Scholar]
- 17.Fujii S, Zhang L, Igarashi J, Kosaka H. L-arginine reverses p47phox and gp91phox expression induced by high salt in Dahl rats. Hypertension. 2003;42:1014–1020. doi: 10.1161/01.HYP.0000094557.36656.D0. [DOI] [PubMed] [Google Scholar]
- 18.Aiello S, Noris M, Todeschini M, Zappella S, Foglieni C, Benigni A, Corna D, Zoja C, Cavallotti D, Remuzzi G. Renal and systemic nitric oxide synthesis in rats with renal mass reduction. Kidney Int. 1997;52:171–181. doi: 10.1038/ki.1997.317. [DOI] [PubMed] [Google Scholar]
- 19.Baylis C, Mitruka B, Deng A. Chronic blockade of nitric oxide synthesis in the rat produces systemic hypertension and glomerular damage. J Clin Invest. 1992;90:278–281. doi: 10.1172/JCI115849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakamura Y, Ono H, Zhou X, Frohlich ED. Angiotensin type 1 receptor antagonism and ACE inhibition produce similar renoprotection in N(omega)-nitro-L>-arginine methyl ester/spontaneously hypertensive rats. Hypertension. 2001;37:1262–1267. doi: 10.1161/01.hyp.37.5.1262. [DOI] [PubMed] [Google Scholar]
- 21.Vaziri ND, Ding Y, Ni Z, Gonick HC. Altered nitric oxide metabolism and increased oxygen free radical activity in lead-induced hypertension: effect of lazaroid therapy. Kidney Int. 1997;52:1042–1046. doi: 10.1038/ki.1997.426. [DOI] [PubMed] [Google Scholar]
- 22.Vaziri ND, Ni Z, Wang XQ, Oveisi F, Zhou XJ. Downregulation of nitric oxide synthase in chronic renal insufficiency: role of excess PTH. Am J Physiol. 1998;274:F642–F649. doi: 10.1152/ajprenal.1998.274.4.F642. [DOI] [PubMed] [Google Scholar]
- 23.Blum M, Yachnin T, Wollman Y, Chernihovsky T, Peer G, Grosskopf I, Kaplan E, Silverberg D, Cabili S, Iaina A. Low nitric oxide production in patients with chronic renal failure. Nephron. 1998;79:265–268. doi: 10.1159/000045047. [DOI] [PubMed] [Google Scholar]
- 24.Schmidt RJ, Yokota S, Tracy TS, Sorkin MI, Baylis C. Nitric oxide production is low in end-stage renal disease patients on peritoneal dialysis. Am J Physiol. 1999;276:F794–F797. doi: 10.1152/ajprenal.1999.276.5.F794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Majid DS, Navar LG. Nitric oxide in the control of renal hemodynamics and excretory function. Am J Hypertens. 2001;14:74S–82S. doi: 10.1016/s0895-7061(01)02073-8. [DOI] [PubMed] [Google Scholar]
- 26.Raij L, Baylis C. Glomerular actions of nitric oxide. Kidney Int. 1995;48:20–32. doi: 10.1038/ki.1995.262. [DOI] [PubMed] [Google Scholar]
- 27.Mattson DL, Higgins DJ. Influence of dietary sodium intake on renal medullary nitric oxide synthase. Hypertension. 1996;27:688–692. doi: 10.1161/01.hyp.27.3.688. [DOI] [PubMed] [Google Scholar]
- 28.Kihara M, Sato K, Hashimoto T, Imai N, Toya Y, Umemura S. Expression of endothelial nitric oxide synthase is suppressed in the renal vasculature of angiotensinogen-gene knockout mice. Cell Tissue Res. 2006;323:313–320. doi: 10.1007/s00441-005-0058-3. [DOI] [PubMed] [Google Scholar]
- 29.Hayakawa H, Raij L. Nitric oxide synthase activity and renal injury in genetic hypertension. Hypertension. 1998;31:266–270. doi: 10.1161/01.hyp.31.1.266. [DOI] [PubMed] [Google Scholar]
- 30.Ni Z, Vaziri ND. Effect of salt loading on nitric oxide synthase expression in normotensive rats. Am J Hypertens. 2001;14:155–163. doi: 10.1016/s0895-7061(00)01234-6. [DOI] [PubMed] [Google Scholar]
- 31.Nishimoto Y, Tomida T, Matsui H, Ito T, Okumura K. Decrease in renal medullary endothelial nitric oxide synthase of fructose-fed, salt-sensitive hypertensive rats. Hypertension. 2002;40:190–194. doi: 10.1161/01.hyp.0000024267.71656.0d. [DOI] [PubMed] [Google Scholar]
- 32.Singh I, Grams M, Wang WH, Yang T, Killen P, Smart A, Schnermann J, Briggs JP. Coordinate regulation of renal expression of nitric oxide synthase, renin, and angiotensinogen mRNA by dietary salt. Am J Physiol. 1996;270:F1027–F1037. doi: 10.1152/ajprenal.1996.270.6.F1027. [DOI] [PubMed] [Google Scholar]
- 33.Mason RP, Kalinowski L, Jacob RF, Jacoby AM, Malinski T. Nebivolol reduces nitroxidative stress and restores nitric oxide bioavailability in endothelium of black Americans. Circulation. 2005;112:3795–3801. doi: 10.1161/CIRCULATIONAHA.105.556233. [DOI] [PubMed] [Google Scholar]
- 34.Mason RP, Kubant R, Jacob RF, Walter MF, Boychuk B, Malinski T. Effect of nebivolol on endothelial nitric oxide and peroxynitrite release in hypertensive animals: role of antioxidant activity. J Cardiovasc Pharmacol. 2006;48:862–869. doi: 10.1097/01.fjc.0000238593.67191.e2. [DOI] [PubMed] [Google Scholar]
- 35.Benter IF, Yousif MH, Dhaunsi GS, Kaur J, Chappell MC, Diz DI. Angiotensin-(1–7) prevents activation of NADPH oxidase and renal vascular dysfunction in diabetic hypertensive rats. Am J Nephrol. 2008;28:25–33. doi: 10.1159/000108758. [DOI] [PubMed] [Google Scholar]
- 36.Gwathmey TM, Pendergrass KD, Reid SD, Rose JC, Diz DI, Chappell MC. Angiotensin-(1–7)-angiotensin-converting enzyme 2 attenuates reactive oxygen species formation to angiotensin II within the cell nucleus. Hypertension. 2010;55:166–171. doi: 10.1161/HYPERTENSIONAHA.109.141622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pinheiro SV, Ferreira AJ, Kitten GT, da Silveira KD, da Silva DA, Santos SH, Gava E, Castro CH, Magalhaes JA, da Mota RK, Botelho-Santos GA, Bader M, Alenina N, Santos RA, Simoes e Silva AC. Genetic deletion of the angiotensin-(1–7) receptor Mas leads to glomerular hyperfiltration and microalbuminuria. Kidney Int. 2009;75:1184–1193. doi: 10.1038/ki.2009.61. [DOI] [PubMed] [Google Scholar]
- 38.Zhang J, Noble NA, Border WA, Huang Y. Infusion of angiotensin-(1–7) reduces glomerulosclerosis through counteracting angiotensin II in experimental glomerulonephritis. Am J Physiol Renal Physiol. 2010;298:F579–F588. doi: 10.1152/ajprenal.00548.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Varagic J, Jerkic M, Jovovic D, Nastic-Miric D, Adanja-Grujic G, Markovic-Lipkovski J, Lackovic V, Radujkovic-Kuburovic G, Kentera D. Regional hemodynamics after chronic nitric oxide inhibition in spontaneously hypertensive rats. Am J Med Sci. 2000;320:171–176. doi: 10.1097/00000441-200009000-00007. [DOI] [PubMed] [Google Scholar]
- 40.Yamaleyeva LM, Gallagher PE, Vinsant S, Chappell MC. Discoordinate regulation of renal nitric oxide synthase isoforms in ovariectomized mRen2. Lewis rats. Am J Physiol Regul Integr Comp Physiol. 2007;292:R819–R826. doi: 10.1152/ajpregu.00389.2006. [DOI] [PubMed] [Google Scholar]
- 41.Habibi J, Whaley-Connell A, Qazi MA, Hayden MR, Cooper SA, Tramontano A, Thyfault J, Stump C, Ferrario C, Muniyappa R, Sowers JR. Rosuvastatin, a 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitor, decreases cardiac oxidative stress and remodeling in Ren2 transgenic rats. Endocrinology. 2007;148:2181–2188. doi: 10.1210/en.2006-1355. [DOI] [PubMed] [Google Scholar]
- 42.Susic D, Zhou X, Frohlich ED, Lippton H, Knight M. Cardiovascular effects of prorenin blockade in genetically spontaneously hypertensive rats on normal and high-salt diet. Am J Physiol Heart Circ Physiol. 2008;295:H1117–H1121. doi: 10.1152/ajpheart.00055.2008. [DOI] [PubMed] [Google Scholar]
- 43.Susic D, Zhou X, Frohlich ED. Angiotensin blockade prevents salt-induced injury of the renal circulation in spontaneously hypertensive rats. Am J Nephrol. 2009;29:639–645. doi: 10.1159/000195633. [DOI] [PubMed] [Google Scholar]
- 44.Cosentino F, Bonetti S, Rehorik R, Eto M, Werner-Felmayer G, Volpe M, Luscher TF. Nitric-oxide-mediated relaxations in salt-induced hypertension: effect of chronic beta1 -selective receptor blockade. J Hypertens. 2002;20:421–428. doi: 10.1097/00004872-200203000-00017. [DOI] [PubMed] [Google Scholar]
- 45.Varagic J, Frohlich ED, Susic D, Ahn J, Matavelli L, Lopez B, Diez J. AT1 receptor antagonism attenuates target organ effects of salt excess in SHRs without affecting pressure. Am J Physiol Heart Circ Physiol. 2008;294:H853–H858. doi: 10.1152/ajpheart.00737.2007. [DOI] [PubMed] [Google Scholar]
- 46.Alenina N, Xu P, Rentzsch B, Patkin EL, Bader M. Genetically altered animal models for Mas and angiotensin-(1–7) Exp Physiol. 2008;93:528–537. doi: 10.1113/expphysiol.2007.040345. [DOI] [PubMed] [Google Scholar]
- 47.Su Z, Zimpelmann J, Burns KD. Angiotensin-(1–7) inhibits angiotensin II-stimulated phosphorylation of MAP kinases in proximal tubular cells. Kidney Int. 2006;69:2212–2218. doi: 10.1038/sj.ki.5001509. [DOI] [PubMed] [Google Scholar]
- 48.Ren Y, Garvin JL, Carretero OA. Vasodilator action of angiotensin-(1–7) on isolated rabbit afferent arterioles. Hypertension. 2002;39:799–802. doi: 10.1161/hy0302.104673. [DOI] [PubMed] [Google Scholar]
- 49.Dharmani M, Mustafa MR, Achike FI, Sim MK. Effects of angiotensin 1–7 on the actions of angiotensin II in the renal and mesenteric vasculature of hypertensive and streptozotocin-induced diabetic rats. Eur J Pharmacol. 2007;561:144–150. doi: 10.1016/j.ejphar.2007.01.037. [DOI] [PubMed] [Google Scholar]
- 50.Botelho-Santos GA, Sampaio WO, Reudelhuber TL, Bader M, Campagnole-Santos MJ, Souza dos Santos RA. Expression of an angiotensin-(1–7)-producing fusion protein in rats induced marked changes in regional vascular resistance. Am J Physiol Heart Circ Physiol. 2007;292:H2485–H2490. doi: 10.1152/ajpheart.01245.2006. [DOI] [PubMed] [Google Scholar]
- 51.Sampaio WO, Nascimento AA, Santos RA. Systemic and regional hemodynamic effects of angiotensin-(1–7) in rats. Am J Physiol Heart Circ Physiol. 2003;284:H1985–H1994. doi: 10.1152/ajpheart.01145.2002. [DOI] [PubMed] [Google Scholar]
- 52.Barki-Harrington L, Luttrell LM, Rockman HA. Dual inhibition of beta-adrenergic and angiotensin II receptors by a single antagonist: a functional role for receptor-receptor interaction in vivo. Circulation. 2003;108:1611–1618. doi: 10.1161/01.CIR.0000092166.30360.78. [DOI] [PubMed] [Google Scholar]
- 53.Craven PA, Studer RK, DeRubertis FR. Impaired nitric oxide-dependent cyclic guanosine monophosphate generation in glomeruli from diabetic rats. Evidence for protein kinase C-mediated suppression of the cholinergic response. J Clin Invest. 1994;93:311–320. doi: 10.1172/JCI116961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.DeRubertis FR, Craven PA. Activation of protein kinase C in glomerular cells in diabetes. Mechanisms and potential links to the pathogenesis of diabetic glomerulopathy. Diabetes. 1994;43:1–8. doi: 10.2337/diab.43.1.1. [DOI] [PubMed] [Google Scholar]
- 55.Tian N, Moore RS, Phillips WE, Lin L, Braddy S, Pryor JS, Stockstill RL, Hughson MD, Manning RD., Jr NADPH oxidase contributes to renal damage and dysfunction in Dahl salt-sensitive hypertension. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1858–R1865. doi: 10.1152/ajpregu.90650.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Touyz RM. Reactive oxygen species, vascular oxidative stress, and redox signaling in hypertension: what is the clinical significance? Hypertension. 2004;44:248–252. doi: 10.1161/01.HYP.0000138070.47616.9d. [DOI] [PubMed] [Google Scholar]
- 57.Chandramohan G, Bai Y, Norris K, Rodriguez-Iturbe B, Vaziri ND. Effects of dietary salt on intrarenal angiotensin system, NAD(P)H oxidase, COX-2, MCP-1 and PAI-1 expressions and NF-kappaB activity in salt-sensitive and -resistant rat kidneys. Am J Nephrol. 2008;28:158–167. doi: 10.1159/000110021. [DOI] [PubMed] [Google Scholar]
- 58.Gryglewski RJ, Palmer RM, Moncada S. Superoxide anion is involved in the breakdown of endothelium-derived vascular relaxing factor. Nature. 1986;320:454–456. doi: 10.1038/320454a0. [DOI] [PubMed] [Google Scholar]
- 59.Lyall F, Gibson JL, Greer IA, Brockman DE, Eis AL, Myatt L. Increased nitrotyrosine in the diabetic placenta: evidence for oxidative stress. Diabetes Care. 1998;21:1753–1758. doi: 10.2337/diacare.21.10.1753. [DOI] [PubMed] [Google Scholar]
- 60.Thuraisingham RC, Nott CA, Dodd SM, Yaqoob MM. Increased nitrotyrosine staining in kidneys from patients with diabetic nephropathy. Kidney Int. 2000;57:1968–1972. doi: 10.1046/j.1523-1755.2000.00046.x. [DOI] [PubMed] [Google Scholar]
- 61.Vaziri ND, Wang XQ. cGMP-mediated negative-feedback regulation of endothelial nitric oxide synthase expression by nitric oxide. Hypertension. 1999;34:1237–1241. doi: 10.1161/01.hyp.34.6.1237. [DOI] [PubMed] [Google Scholar]
- 62.Vaziri ND, Ni Z, Oveisi F, Trnavsky-Hobbs DL. Effect of antioxidant therapy on blood pressure and NO synthase expression in hypertensive rats. Hypertension. 2000;36:957–964. doi: 10.1161/01.hyp.36.6.957. [DOI] [PubMed] [Google Scholar]
- 63.Landmesser U, Dikalov S, Price SR, McCann L, Fukai T, Holland SM, Mitch WE, Harrison DG. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest. 2003;111:1201–1209. doi: 10.1172/JCI14172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Troost R, Schwedhelm E, Rojczyk S, Tsikas D, Frolich JC. Nebivolol decreases systemic oxidative stress in healthy volunteers. Br J Clin Pharmacol. 2000;50:377–379. doi: 10.1046/j.1365-2125.2000.00258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Georgescu A, Pluteanu F, Flonta ML, Badila E, Dorobantu M, Popov D. The cellular mechanisms involved in the vasodilator effect of nebivolol on the renal artery. Eur J Pharmacol. 2005;508:159–166. doi: 10.1016/j.ejphar.2004.11.043. [DOI] [PubMed] [Google Scholar]
- 66.Pires MJ, Rodriguez-Pena AB, Arevalo M, Cenador B, Evangelista S, Esteller A, Sanchez-Rodriguez A, Colaco A, Lopez-Novoa JM. Long-term nebivolol administration reduces renal fibrosis and prevents endothelial dysfunction in rats with hypertension induced by renal mass reduction. J Hypertens. 2007;25:2486–2496. doi: 10.1097/HJH.0b013e3282efeecb. [DOI] [PubMed] [Google Scholar]
- 67.Toprak O, Cirit M, Tanrisev M, Yazici C, Canoz O, Sipahioglu M, Uzum A, Ersoy R, Sozmen EY. Preventive effect of nebivolol on contrast-induced nephropathy in rats. Nephrol Dial Transplant. 2008;23:853–859. doi: 10.1093/ndt/gfm691. [DOI] [PubMed] [Google Scholar]
- 68.Gill PS, Wilcox CS. NADPH oxidases in the kidney. Antioxid Redox Signal. 2006;8:1597–1607. doi: 10.1089/ars.2006.8.1597. [DOI] [PubMed] [Google Scholar]
- 69.Bachmann S, Bosse HM, Mundel P. Topography of nitric oxide synthesis by localizing constitutive NO synthases in mammalian kidney. Am J Physiol. 1995;268:F885–F898. doi: 10.1152/ajprenal.1995.268.5.F885. [DOI] [PubMed] [Google Scholar]
- 70.Bennai F, Morsing P, Paliege A, Ketteler M, Mayer B, Tapp R, Bachmann S. Normalizing the expression of nitric oxide synthase by low-dose AT1 receptor antagonism parallels improved vascular morphology in hypertensive rats. J Am Soc Nephrol. 1999;(suppl 11):S104–S115. [PubMed] [Google Scholar]
- 71.Mundel P, Bachmann S, Bader M, Fischer A, Kummer W, Mayer B, Kriz W. Expression of nitric oxide synthase in kidney macula densa cells. Kidney Int. 1992;42:1017–1019. doi: 10.1038/ki.1992.382. [DOI] [PubMed] [Google Scholar]
- 72.Tojo A, Onozato ML, Fukuda S, Asaba K, Kimura K, Fujita T. Nitric oxide generated by nNOS in the macula densa regulates the afferent arteriolar diameter in rat kidney. Med Electron Microsc. 2004;37:236–241. doi: 10.1007/s00795-004-0263-2. [DOI] [PubMed] [Google Scholar]
- 73.Wilcox CS, Welch WJ, Murad F, Gross SS, Taylor G, Levi R, Schmidt HH. Nitric oxide synthase in macula densa regulates glomerular capillary pressure. Proc Natl Acad Sci USA. 1992;89:11993–11997. doi: 10.1073/pnas.89.24.11993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Moens AL, Yang R, Watts VL, Barouch LA. Beta 3-adrenoreceptor regulation of nitric oxide in the cardiovascular system. J Mol Cell Cardiol. 2010;48:1088–1095. doi: 10.1016/j.yjmcc.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]