Abstract
Developmental processes can have major impacts on the correlations in behaviour across contexts (contextual generality) and across time (temporal consistency) that are the hallmarks of animal personality. Personality can and does change: at any given age or life stage it is contingent upon a wide range of experiential factors that occurred earlier in life, from prior to conception through adulthood. We show how developmental reaction norms that describe the effects of prior experience on a given behaviour can be used to determine whether the effects of a given experience at a given age will affect contextual generality at a later age, and to illustrate how variation within individuals in developmental plasticity leads to variation in contextual generality across individuals as a function of experience. We also show why niche-picking and niche-construction, behavioural processes which allow individuals to affect their own developmental environment, can affect the contextual generality and the temporal consistency of personality. We conclude by discussing how an appreciation of developmental processes can alert behavioural ecologists studying animal personality to critical, untested assumptions that underlie their own research programmes, and outline situations in which a developmental perspective can improve studies of the functional significance and evolution of animal personality.
Keywords: gene–environment correlations, gene–environment interactions, developmental plasticity, differential consistency, contextual reaction norms, structural consistency
1. Introduction and definition of concepts
In the past few years, researchers have demonstrated that personality (individual differences in behaviour that are consistent both across time and across contexts) occurs in a wide range of animal taxa. Personality is a special case of a more general concept, behavioural syndrome, which refers to any correlation across individuals in behaviour, i.e. correlations that are consistent across time and/or across contexts (Sih et al. 2004; Sih & Bell 2008). Now that researchers have detected personality and behavioural syndromes in many animal species, attention is turning to questions about the ecological correlates, functional significance and evolution of these phenomena, as evidenced by other articles in this issue.
The general message of this article is that developmental perspectives are essential for framing and answering questions about the function and evolution of personality and syndromes. Our more specific message is that a ‘snapshot’ view of personality, which is based on descriptions of behaviour at a single age or life stage, provides an inadequate foundation for studies of personality across ecological and evolutionary scales of time and space. This is because the correlations in behaviour across contexts and across time that are the key criteria for animal personality depend on experiential factors, where ‘experiential' here refers to any external stimulus or event that affects gene expression in an individual, resulting in changes in its phenotype (see glossary, appendix A, for this and other definitions). A developmental perspective that explicitly considers how experiential factors across the lifetime affect the development of behavioural phenotypes can not only shed light on possible reasons for variation in animal personality across ecological and evolutionary scales of space and time, but also help reveal proximate mechanisms that contribute to that variation.
Because including development adds another level of complexity to an already confusing topic, we begin by discussing the terms and concepts required to understand personality development. We then use simple graphical models to illustrate why and how developmental processes can affect the correlations across individuals across contexts and across time that are the key components of animal personality. Finally, we identify situations in which ecological and evolutionary studies of animal personality are most likely to benefit from a developmental perspective.
Many of the conceptual issues concerning the development of animal personality have recently been reviewed elsewhere (Stamps & Groothuis 2010), so here we briefly discuss those most relevant to questions about its function and evolution. These concepts are not just relevant to the types of behaviour (e.g. boldness, aggressiveness) that are currently the focus of animal personality research (cf. Sih & Bell 2008). They are also relevant to many other behavioural and physiological traits that are correlated across individuals across contexts and/or across time, and whose expression depends upon the external stimuli that surround an individual at the time of trait expression. Conversely, these concepts were not required to study the development of individual differences in morphological traits (e.g. limb length in adult insects) whose expression is fixed once animals have reached a given life stage. In addition, animal personality provides a useful framework within which to discuss recent advances in behavioural development, some of which may be unfamiliar to researchers who focus on the adaptive significance or evolution of behavioural traits.
In order to study personality development, clear discrimination between variation in behaviour across contexts and variation in behaviour across time is essential (Caspi et al. 2005). The word ‘context' itself has been used in various ways in the animal personality literature. Early students of animal personality used context to refer to the environmental conditions surrounding an animal when it expressed behaviour (e.g. Wilson 1998). In a subsequent review of behavioural syndromes, Sih et al. (2004) discriminated between context, which they used to refer to ‘a functional category of behaviour', and ‘situation', used to refer to ‘a given set of conditions at one point in time'. However, this approach requires researchers to assign a single function to each of the behaviour patterns expressed by their subjects, a procedure that may be difficult or impractical (Stamps & Groothuis 2010). Modifications to definitions of context in the animal personality literature continue to the present day: Dingemanse et al. (2010) used context to refer to environmental stimuli that vary across a gradient. Despite this variation, a common element in these usages is that they all include stimuli exterior to the animal at the time it expresses behaviour. Hence, following traditional use of the term, we have defined context as all of the external stimuli, including stimuli from conspecifics and other animals, that impinge on an individual when it expresses a given behaviour (Stamps & Groothuis 2010).
Along the same lines, ‘contextual generality’ (and its inverse, ‘contextual specificity’) refers to the extent to which scores for behaviour expressed in one context are correlated across individuals or across genotypes with scores in behaviour expressed in one or more other contexts, where high contextual generality indicates that the rank order of scores is maintained across contexts (Stamps & Groothuis 2010). This definition is based on the history of the usage of these terms in the literature on animal personality and behavioural syndromes (e.g. Coleman & Wilson 1998; Sinn et al. 2008). Of course, contextual generality is one of the major criteria for animal personality, as it applies both within and across personality traits. That is, the statement that ‘aggressiveness' is a personality trait implies that scores on one behavioural assay expressed in one context (e.g. latency to attack an image in a mirror in a home cage) will not only be correlated across individuals with scores on the same assay at a later time, but also with scores on the same or a different assay in a different context at the same time (e.g. number of bites directed at a same-sex conspecific intruder in a neutral arena). Similarly, the statement that aggressiveness and boldness are correlated with one another implies that scores for one behavioural assay (e.g. attack latency) expressed in one context (e.g. proximity to a same-sex, same-size conspecific) will be correlated across individuals with scores on another behavioural assay (e.g. latency to leave a shelter) expressed in a different context (e.g. presence of odour cues from a predator).
Temporal consistency in behaviour is a second critical criterion of personality. Fortunately, researchers interested in studying temporal change and stability in animal personality need not ‘reinvent the wheel', but can profit from the many years of research that developmental psychologists have devoted to this topic. These psychologists have identified at least four different ways that one can describe temporal change and stability in personality (e.g. Roberts et al. 2001), two of which, differential consistency and structural consistency, are most useful for current purposes. ‘Differential consistency' refers to the extent to which differences across individuals in a certain behaviour measured in a single context are maintained over time, while ‘structural consistency' refers to the extent to which relationships between the behaviour expressed in different contexts at a given age are similar to the relationships between those same behaviours if the same individuals are measured at a different age. Differential consistency can be estimated a number of ways, one of which (repeatability as defined by population geneticists, Lessells & Boag 1987) is often used by behavioural ecologists. Differential and structural consistencies are examples of one category of behavioural syndromes, since they describe correlations across individuals in behaviour across time (Sih et al. 2004; Sih & Bell 2008).
In contrast to the surge of studies on the evolution, ecology and physiology of animal personality, studies of personality development are still in their infancy (Stamps & Groothuis 2010). There are several possible reasons for this neglect. First, one of the criteria for personality is temporal stability, whereas researchers interested in development often focus on changes in behaviour across different ages and life stages. However, temporal stability over the short term does not preclude changes in personality over the long term. Indeed, recent studies indicate that the differential consistency of behavioural traits, including personality traits, tends to decline as a function of duration of inter-test intervals (Roberts & DelVecchio 2000; Dingemanse et al. 2002; Bell et al. 2009). Hence, even though temporal stability is a criterion for personality, it is still important to describe change and stability of personality across the lifetime. Second, there is often a tendency, especially among those studying birds or mammals, to view development as a preparation for adulthood. This focus on adult personality overlooks the importance of ontogenetic adaptations that enhance the growth and survival of juveniles, who often experience a very different selective regime than that of adults in the same population. Hence, juveniles may have personality traits, albeit different from those expressed as adults, in response to their own set of selective pressures (e.g. Galef 1981).
A third possible reason for the neglect of development in the animal personality literature is the current focus on the effects of genes on personality. This focus is understandable, given the key roles played by additive genetic variation (narrow-sense heritability) and genetic correlations in the evolution of any phenotypic trait (Falconer & Mackay 1996; Kruuk et al. 2008; Dochtermann & Roff (2010)), and the important contributions made by selected lines to the study of animal personality. However, a developmental perspective argues that the expression of phenotypic traits is always affected by experiential factors, as well as by interactions (G × E) and correlations (rGE) between genes and experiential factors. In support of this perspective, empirical studies have demonstrated that the heritability of a variety of traits, including personality traits, varies as a function of conditions that individuals experienced before those traits were measured (Charmantier & Garant 2005; Dingemanse et al. 2009). Similarly, genetic correlations between traits also change as a function of variation in developmental conditions (Sgro & Hoffmann 2004; Robinson et al. 2009). In addition, there are indications that rGE may play an important role in the generation and maintenance of animal personality (Stamps & Groothuis 2010, see also §§2 and 3b, below). Hence, detecting relationships between genes and personality under one set of developmental conditions is but the first step in predicting how personality will be expressed in different localities or generations, or how personality will evolve over time. We discuss this topic in greater detail in §3, below.
2. Effects of experience on personality development
Experiential factors with strong effects on personality development can occur throughout the lifetime. At one extreme, such factors can occur prior to birth or hatching, precluding control over them using simple cross-fostering experimental designs. In mammals, for instance, proximity to male embryos in utero affects the aggressiveness, activity rates, exploratory and other behaviour of both sexes later in life (Ryan & Vandenbergh 2002). In birds, steroid hormones that females deposit in their eggs have profound effects on the aggressiveness, boldness and exploratory behaviour of the offspring that hatch from them (Groothuis et al. 2005). Recent studies indicate that concentrations of maternal hormones in eggs differ across lines of great tits (Parus major) artificially selected for differences in exploratory behaviour, suggesting that correlations between maternal genotypes and experiential factors (hormone concentrations) provided by parents that affect offspring behaviour begin very early in the development of this species (Groothuis et al. 2008). In fact, this is just one of many situations in which maternal and paternal effects can encourage correlations between genotypes and experiential factors that affect behavioural development (e.g. Narusyte et al. 2008; Price & Jaffee 2008).
Moreover, experiential factors with pronounced effects on the personality development of a given individual can occur before that individual was even conceived. For instance, handling mother rats during a pregnancy changes the maternal behaviour of those females not only following that pregnancy, but also following subsequent pregnancies. In turn, rat maternal behaviour has enduring effects on the exploratory behaviour and physiology of their offspring later in life (Champagne & Meaney 2006). In zebra finches, Taeniopygia guttata, a female's diet prior to egg-laying affects the within-clutch distribution of maternal yolk androgens (Sandell et al. 2007), which in other birds affect the development of personality traits later in life (e.g. Daisley et al. 2005). Reaching even further back in time, factors affecting the maternal behaviour of grandmother mice and rats can, via behavioural epigenetic inheritance, affect the exploratory behaviour and other behavioural traits of their grand-offspring (Curley et al. 2008; Champagne & Curley 2009). Finally, there is mounting evidence that events and experiences that occurred to the individuals in one generation can, via cellular epigenetic inheritance, have strong and enduring effects on gene expression in their descendants (reviewed in Jablonka & Raz 2009). When the effects of experience on behavioural development reach across generations, experiences (e.g. food shortages, encounters with predators) that occurred within the lifetime of an individual's direct ancestors may affect the personality of that individual.
At the other extreme, experiences individuals themselves have as juveniles or adults may have strong and lasting effects on their own personality and other behavioural traits (review in Stamps & Groothuis 2010, see also Alleva & Francia 2009; Dingemanse et al. 2009). Thus far, most experimental studies of personality development have manipulated experiential factors at the juvenile stage, and then measured behaviour later in life. For instance, Carere et al. (2005) manipulated the amount of food provided to nestling great tits, and showed that the effects of the same manipulation on adult exploratory and aggressive behaviour differed for individuals from two selected lines. To date, relatively few researchers have looked at the effects of adult experience on adult personality (but see below). However, this topic has recently attracted considerable attention from psychologists, based on abundant evidence that stressful events in adulthood do have enduring effects on human personality (Beltran et al. 2009; Jovanovic & Ressler 2010).
Experiential factors can also affect correlations between behavioural traits, or between behavioural and physiological traits, leading to changes in contextual generality and structural consistency. For instance, Bell & Sih (2007) exposed juvenile three-spined stickleback, Gasterosteus aculeatus, to predators and found that contextual generality for the relationship between aggressiveness and boldness changed from r = 0.18 prior to exposure to r = 0.46 afterwards for those individuals that survived exposure to predators. Similarly, Ruiz-Gomez et al. (2008) found that relationships between physiological stress responses and boldness dramatically changed and were thereafter maintained for at least a year after adult rainbow trout, Oncorhynchus mykiss, experienced the trauma associated with transportation to a new laboratory. The latter example is particularly striking because it was previously assumed that levels of boldness, and relationships between physiological stress responses and boldness, were fixed once animals reached adulthood (e.g. Overli et al. 2007).
3. Genes, experience and contextual generality
It is a truism that the behaviour of an individual at any given point in time is the outcome of interactions between stimuli in the exterior world and that individual's physiological and morphological state at that point in time. In turn, an individual's state at a given point in time is the result of its unique developmental history: a series of complicated, reciprocal interactions between genes, cellular epigenetic factors, internal stimuli and external experiential factors that may have begun prior to conception, and that have continued up to the present time (Bateson 2001; West-Eberhardt 2003; Rutter 2007; Jablonka & Raz 2009). The challenge is how to conceptualize and study these developmental processes at the individual level, since it is impossible for any individual to have more than one developmental history.
One way out of this dilemma is to imagine ‘replicate individuals’ who are identical to one another not only with respect to their genetic makeup, but also with respect to the type and the timing of every experiential factor that might have affected their behavioural development up to a given age and time. In that case, we can conduct an experiment in which we can expose different members of each replicate (hereafter, genotype) to different experiential conditions of interest at one age, and then measure their behaviour at a later age.
In practice, one can use clones, inbred lines, F1 crosses between inbred lines or (more much approximately) full-sibs as approximations of genotypes for this sort of experiment. This is because individuals with the same genotype not only share genes, but also share a variety of factors, including cellular and behavioural epigenetic factors, maternal and paternal effects and sibling effects, that affect development before a researcher exposes the experimental subjects to the experiential conditions of interest (Crews 2008; Champagne & Curley 2009; Stamps & Groothuis 2010). For instance, if siblings are held in family groups before being allocated across experimental treatments, then consistent differences among genotypes in family size or offspring behaviour can lead to consistent differences across genotypes in the social environments their members experienced earlier in life. Here, we use the term ‘prior experiential factor' (PEF) to refer to any experiential factor that occurs to an individual prior to a specified age, and that can affect its phenotype at that age. Of course, PEFs are not identical for every individual with the same genotype, e.g. even in highly inbred strains of mice, uterine position affects aggressive behaviour later in life (vom Saal & Bronson 1978). However, to the extent that PEFs vary more across than within genotypes, one can use genotypes as approximations of ‘replicate individuals' for developmental studies.
In contrast, consistent differences among genotypes in PEFs at a given age make it difficult to determine how much of the phenotypic variation across genotypes at that age can be attributed to differences in their genetic makeup. Hence, if the goal of an experiment is to estimate how genes (G), a specific experience of interest (E) and interactions and correlations between them (G × E, rGE) affect the expression of behaviour at a given age, one must also control for associations between genotypes and the large array of PEFs that occurred prior to that age and that can affect the expression of behaviour at that age. In laboratory studies, these procedures include the use of paternal-half sib designs to control for maternal effects, cross-fostering subjects from birth or hatching to control for effects of maternal and/or paternal behaviour on offspring development, raising offspring in mixed-family groups from birth or hatching, and at the same densities, to control for sibling and other early social effects on the development of behaviour, and using subjects whose ancestors have been raised for multiple generations under constant, benign conditions, to reduce the contributions of cellular epigenetic inheritance to variation in trait expression in the current generation. In field studies, controlling for potential correlations between PEFs and genes is even more of a challenge, given the large array of experiential factors that vary more across than within related individuals, and that can inflate estimates of additive genetic variance (e.g. Kruuk 2004).
In contrast to the ‘permanent environmental effects' (PE) of classical quantitative genetics, which traditionally refer to experiential factors that occur during a specific period in life (typically early in life), and that thereafter have enduring effects on the expression of a particular phenotypic trait (Nussey et al. 2007; Brommer et al. 2008), PEFs are defined as experiential factors that occur prior to a specified age, and that affect the individual's phenotype at that age. The effects of PEFs on phenotypic traits can be ephemeral as well as enduring, they can occur at any age from conception to death, and there is no reason why the effects of PEFs on behaviour at one age can not be reversed by experiential factors at later ages.
(a). Developmental reaction norms and contextual generality
Imagine that individuals with the same genotype are exposed to two or more different sets of experiential conditions at one age, and then their behaviour is measured at a later age. When it is possible to arrange a set of experiential conditions along a continuum, the results of such an experiment generate a ‘developmental reaction norm'. Reaction norm is a general term that describes the range of phenotypes that can be generated by individuals with a given genotype (see also below); developmental reaction norm is a more specific term that describes how the phenotype of a given genotype varies as a function of the experiential factors to which those individuals were exposed earlier in life (Stamps & Groothuis 2010). Developmental reaction norms also provide a way to describe and measure ‘developmental plasticity', which refers to the extent to which a genotype's phenotype at a given age varies as a function of an experiential factor to which it was exposed earlier in life.
Developmental reaction norms belong to a family of reaction norms that can be used to describe how variables that fall along a gradient affect the expression of phenotypic traits in individuals or genotypes (Nussey et al. 2007; Dingemanse et al. 2010; Stamps & Groothuis 2010). For instance, students of animal personality sometimes measure behaviour in different contexts that can be arranged along a gradient (e.g. activity rate as a function of the presence or absence of nearby conspecifics, Webster et al. 2007). In this situation, one can describe a ‘contextual reaction norm' for an individual or for a genotype that describes how levels of a given behaviour (e.g. activity) change as a function of the current context (e.g. number of nearby conspecifics) (Stamps & Groothuis 2010). In contrast to developmental reaction norms, which describe how experience in the past affects the behaviour expressed in a single test or assay at a given age, contextual reaction norms describe how the behaviour expressed at a given age varies as a function of the current external stimulus situation. Contextual reaction norms can be viewed as a special case of ‘behavioural reaction norms', a term that can describe the behaviour of an individual as a function of many different types of gradients, not only variation in the external stimulus situation, but also variation in the individual's internal state or condition, age, time or prior experiences (Dingemanse et al. 2010).
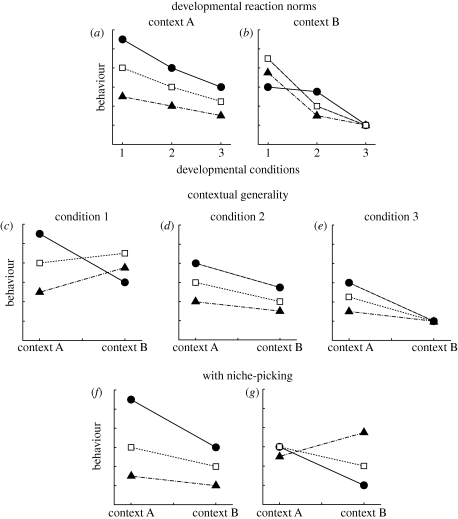
When genotypes are exposed to a given type of experience at one age, and then their behaviour is measured in two or more contexts at a later age, one can readily see how developmental reaction norms affect contextual generality. We illustrate this point here using a hypothetical situation in which a set of three genotypes are exposed to conditions 1, 2 or 3 at one age, where conditions 1 to 3 vary along a continuum. Then, at a later age, their behaviour is measured in two different contexts (A or B; figure 1). In this situation, we can construct two developmental reaction norms, one that describes the effect of conditions 1 to 3 on the behaviour expressed in context A, while the other describes the effects of those same conditions on the behaviour expressed in context B (figure 1a,b). Similarly, since the behaviour expressed in contexts A and B was measured at the same age for the same set of genotypes, we can also measure contextual generality at that age for three sets of individuals: those who experienced conditions 1, 2 or 3 earlier in life (figure 1c,d,e). At that point, we are in a position to see why and how experiences earlier in life can affect the contextual generality of personality traits.
Figure 1.
(a,b) Developmental reaction norms and (c–g) contextual generality diagrams for three genotypes (I = filled circles, II = open squares, III = filled triangles). Developmental reaction norms (a,b) show the behaviour expressed at one age in context A and in context B, respectively, if individuals with these genotypes had been exposed to conditions 1, 2 or 3 before their behaviour was measured. Contextual generality diagrams (c–e) indicate the relationship between standardized scores for the behaviour expressed in context A and in context B at one age for animals who had been exposed to conditions 1, 2 or 3, respectively, before their behaviour was later measured in both contexts. Niche-picking at a given age can encourage high contextual generality at a later age (figure 1f: genotype I developed in condition 1, genotype II in condition 2 and genotype III in condition 3) or low contextual generality at a later age (figure 1g: genotype I developed in condition 3, genotype II in condition 2 and genotype III in condition 1), depending on the conditions each genotype chose for development.
We can use contextual generality diagrams, which show the standardized scores of each genotype on different behavioural tests, to illustrate the strength of correlations across contexts in behaviour. In the example illustrated in figure 1, if genotypes I, II and III were exposed to condition 2 earlier in life, there would be a strong correlation across those genotypes between the behaviour expressed in context A and the behaviour expressed in context B (indicated by the parallel lines in figure 1d). However, this strong correlation would not be maintained if the same three genotypes had been exposed to conditions 1 (figure 1c) or 3 (figure 1e) earlier in life. Instead, prior exposure to condition 1 would generate a negative association between the behaviour expressed in context A and context B, and after exposure to condition 3 there would be no association between the behaviour expressed in the two contexts, because all of the genotypes would express the same level of behaviour in context B.
Comparison of the developmental reaction norms for the behaviour expressed in context A (figure 1a) and the behaviour expressed in context B (figure 1b) reveals why contextual generality changes so dramatically as a function of developmental conditions for these genotypes. In particular, contextual generality is not maintained across these developmental conditions because for some of the genotypes, the developmental reaction norms for the behaviour expressed in context A and context B do not have the same elevation and shape (e.g. compare the two developmental reaction norms for genotype I for the behaviour expressed in context A and context B). Conversely, this graphical model indicates that the maintenance of similar correlations in behaviour across contexts following different developmental conditions requires that, for each genotype, the developmental reaction norm for the behaviour expressed in one context be similar in elevation and shape to the developmental reaction norm for the behaviour expressed in the other context(s).
Of course, in many personality studies, the question of interest is not simply whether contextual generality is maintained across developmental conditions, but also whether the rank-order of the scores of the different genotypes is stable across different developmental conditions. For instance, in addition to asking whether strong correlations between ‘boldness' and ‘aggressiveness' across genotypes are maintained when animals are raised in different social environments, an investigator might also want to know whether genotypes that are highly bold and aggressive after being raised in groups are also highly bold and aggressive after being raised in isolation. The maintenance of both contextual generality and rank-order stability in the relative scores of different genotypes when those genotypes are exposed to different sets of developmental conditions requires an even more stringent set of conditions, namely that the developmental reaction norms not only be comparable for each genotype for the behaviour expressed in different contexts but also that the developmental reaction norms of different genotypes do not cross one another.
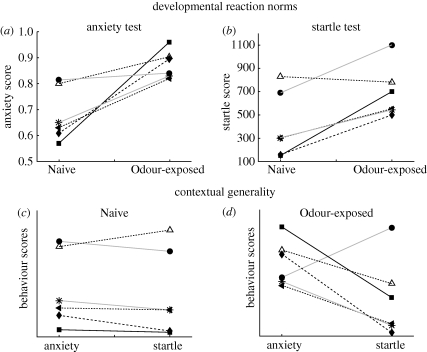
Although empirical studies of the effects of developmental reaction norms on contextual generality are still quite rare, a recent study of the effects of prior exposure to predator odour on the subsequent behaviour of inbred strains of house mice illustrates the value of this approach (Cohen et al. 2008). Adult male mice from six inbred strains were either exposed to the odour of a cat (Odour-exposed) or were not exposed to that odour (Naive). One week later they were given scores on two behavioural assays, an ‘anxiety score', based on their movement patterns on an elevated maze, and a ‘startle score', based on their responses to brief, loud sounds.
First, we can compare contextual generality for the two groups with different types of prior experience, by comparing the scores of each of the six strains on each of the behavioural assays, using contextual generality diagrams (figure 2c,d). For naive animals, contextual generality based on the mean scores for each strain on the anxiety test and on the startle test was very high, as illustrated by the nearly parallel lines in figure 2c (Naive: r = 0.97, p < 0.001, n = 6 genotypes). In contrast, for odour-exposed animals contextual generality for the same two tests was extremely low (Odour exposed: r = −0.002, p = 0.996, n = 6 genotypes, figure 2d). Comparison of the developmental reaction norms for the two tests reveals the reason for the dramatic impact of prior experience on contextual generality: for several of the strains, the slopes of their developmental reaction norms were different for the anxiety test and for the startle test (compare figure 2a,b). For instance, developmental plasticity was low for strain BALB/CJ for the anxiety test but significantly positive for the startle test; conversely, for strain 129J developmental plasticity was significantly positive for the anxiety test but non-significantly negative for the startle test. As a result of this variation in the effects of the same prior experience (cat odour) on the behaviour expressed by the same genotypes in the two behavioural assays, the strong positive correlation between scores on the anxiety test and the startle test observed across the six strains when animals were naive was nowhere in evidence when animals from the same strains had been exposed to cat odour earlier in life. In addition, because of extensive crossing of the developmental reaction norms for the anxiety test, the rank-order scores on that test were unstable across developmental conditions (rs = −0.314). By contrast, less crossing of the developmental reaction norms for the startle test resulted in higher rank-order stability across developmental conditions for that test (rs = 0.580) (figure 2a,b).
Figure 2.
(a,b) Developmental reaction norms and (c,d) contextual generality diagrams for adult male mice from six inbred strains: symbols indicate the mean value for each strain: filled diamonds, DBA/2J; filled squares, C57BL/6J; filled circles, BALB/CJ; asterisks, NZB; filled triangles, SJL; open triangles, 129J. Individuals were either not exposed (Naive) or were exposed (Odour-exposed) to cat odour a week before being tested on assays for ‘anxiety' and for a ‘startle response'. For several strains, developmental plasticity (slope of the developmental reaction norm) for the anxiety test was different from developmental plasticity for the startle test (e.g. compare slopes of BALB/CJ in figure 2a,b). As a result of these differences in developmental plasticity within strains, contextual generality was high for Naive animals (cf. the parallel lines in figure 2c) but low for Odour-exposed animals (figure 2d). Figure 2a,b redrawn from Cohen et al. (2008), with permission of Cambridge University Press.
This example also helps illustrate the familiar principle that ‘genetic' correlations between phenotypic traits vary as a function of conditions to which individuals were exposed before those traits were measured. That is, to the extent that the differences in behaviour across these inbred strains of mice can be attributed to genetic differences (as opposed to PEFs that also varied among those strains), these results imply that a genetic correlation between ‘anxiety' and the ‘startle response' in mice depends on whether or not they had been exposed to cat odour after reaching adulthood.
Thus far, we lack comparably detailed studies of the effects of experiential factors on the development of behaviour in non-domesticated animals, using behavioural assays that are common in animal personality research. However, a study by Carere et al. (2005) using two selected lines of great tits indicates that variation in food regimes for nestlings can affect their scores on assays of aggressiveness and exploratory behaviour after independence. More importantly, this study reports variation within each of the two lines with respect to the effects of the same food treatments on the development of aggressiveness versus exploratory behaviour, i.e. the sort of pattern that would, if expressed by a wider range of genotypes, lead to variation in contextual generality as a function of food conditions earlier in life.
(b). The effects of niche-picking and niche-construction on personality development
Developmental reaction norms can also be used to illustrate two other important principles in personality development, the concepts of niche-picking and niche-construction. These concepts have received extensive attention from developmental psychologists, who noted years ago that humans have considerable control over the environments in which they develop (Plomin et al. 1977, see also Narusyte et al. 2008; Price & Jaffee 2008), and from biologists interested in how these processes affect patterns of ecology and evolution (e.g. Laland et al. 1999; Bonduriansky & Day 2009). Niche-picking occurs when individuals seek out particular social or physical environments, leading to non-random associations between an individual's phenotype (and potentially, also its genotype) and the environment in which it lives and develops. For instance, variation among dispersers in preferences for habitats and social environments will, if those dispersers have a choice of areas in which to settle, lead to systematic variation among dispersers in the conditions they experience for a period of time after that dispersal event. Several authors have reported broad-sense heritable variation in preferences for habitat features (Leibold et al. 1994; Barker & Starmer 1999), and social group sizes (Brown & Brown 2000; Serrano & Tella 2007), supporting the notion that animals with different genotypes and any PEFs associated with those genotypes may, at a given age or life stage, prefer different types of environments, potentially leading to rGE (Plomin et al. 1977).
Similarly, individuals may, by their own behaviour, modify the social or physical environment in which they develop (niche-construction). Niche-construction obviously applies to physical structures built by individuals, e.g. intraspecific variation in web design in the common house spider, Achaearanea tepidariorum, is related to the type of prey they are likely to capture in the future (Boutry & Blackledge 2008). However, niche-construction can also occur in many other situations, notably including social environments that are generated and maintained as a result of the behaviour of a focal individual. For instance, when aggressive behaviour by territorial animals discourages conspecifics from returning to the area where they were attacked, a focal individual who was highly aggressive at the time of territory establishment would have lower ongoing rates of social interactions for the rest of that season than a focal individual who was less aggressive during that same period (Stamps & Krishnan 2001; Switzer et al. 2001).
Niche-picking and niche-construction can affect both the contextual generality and the temporal stability of animal personality, as compared with situations in which every genotype is exposed to the same conditions during a given period of their lives. The effects of niche-picking and niche-construction on contextual generality at a given age not only depend on the developmental reaction norms for each genotype, but also on the developmental conditions that each genotype chose or created for themselves prior to that age. For instance, imagine that instead of forcing individuals to develop under certain conditions during a particular period of their lives (as is typical of most experimental studies of development), we instead allow them some control over the social or physical conditions in which they develop. Further imagine that when allowed this choice, genotype I develops under condition 1, genotype II under condition 2 and genotype III under condition 3. In that situation, contextual generality across these three genotypes for the behaviour expressed at a later age in context A and context B would be high (cf. the parallel lines in figure 1f). Conversely, consider what would happen if genotype I had developed under condition 3, genotype II under condition 2 and genotype III under condition 1. In that situation, contextual generality for the same three genotypes would be low, since they would all express comparable levels of behaviour in Context A (figure 1g). Hence, describing the developmental reaction norms for a group of genotypes is only half the story: contextual generality for behaviour expressed at a given age depends on the developmental conditions that those genotypes chose or constructed for themselves prior to that age.
With respect to the temporal stability of personality, niche-picking and niche-construction are likely to increase both differential and structural consistency, when behaviour is measured at ages or life stages when animals are able to engage in these processes. This is because niche-picking and niche-construction increase the chances that a given individual is able to maintain itself in the same set of environmental conditions for extended periods of time, and conversely, increase the chances that different individuals are able to maintain themselves under different sets of developmental conditions for extended periods of time. Hence, even if environmental conditions in the field vary widely across spatial scales that could easily be traversed by individual animals, niche-picking and niche-construction may allow individuals to buffer themselves from variation in environmental and experiential factors that affect the temporal stability of their behaviour.
It is currently unclear whether niche-picking and niche-construction themselves vary as a function of animal personality. In humans, some authors have suggested that individuals with extremely high or extremely low scores on certain personality traits may be more likely to engage in niche-picking or niche-construction than individuals with intermediate personality trait scores (Buss & Plomin 1984). However, even if only a portion of the individuals in a group or population engage in niche-picking or niche-construction, these processes would still tend to increase the contextual generality and differential and structural consistency for the group as a whole, as compared with situations in which all of the individuals were either forced to develop or live under the same set of environmental conditions, or were randomly distributed across the entire range of available environmental conditions.
4. Implications for ecological and evolutionary studies of animal personality
(a). Estimating the functional significance of personality when personality changes over time
Given evidence that the differential consistency (repeatability) of behavioural traits declines as a function of inter-test intervals, and that experiences throughout the lifetime can affect correlations between behavioural and other traits, we cannot assume that personality traits are necessarily stable across long periods of time. If they are not, then empirical studies of the functional significance and fitness consequences of personality are more likely to produce valid results if personality traits and factors associated with them are measured over relatively short periods. Thus, when studying personality traits associated with natal dispersal (e.g. Dingemanse et al. 2003; Cote & Clobert 2007; Duckworth 2009; Cote et al. (2010)), one might reasonably assume that scores on behavioural assays taken just before dispersers leave their natal habitat might be stable across the days to weeks of the dispersal period. In that case, one could look for relationships between those scores and dispersal distance, survival during dispersal, condition upon arrival or the quality of a new habitat. By contrast, it would be more dangerous to assume that behavioural traits measured once or twice for either juveniles or adults are necessarily stable across their entire lives.
This is not to say that we cannot look for correlations between behaviour expressed at one age and fitness consequences of that behaviour at later ages. However, such correlations do not imply that the behaviour is itself stable across time, because behaviour expressed at one age or life stage can have strong effects on components of fitness at later ages or life stages. Habitat selection by natal dispersers is one such example: the behaviour expressed by individuals during the relatively brief period when they are searching or competing for space in a new habitat can, by affecting their chances of settling in a high-quality habitat, influence their growth, survival and/or fecundity for the rest of their lives (e.g. Stamps 2006).
Similarly, the assumption that personality is fixed for life may be convenient for building tractable theoretical models of the functional significance of personality, but it might be useful to determine if the predictions of these models are robust if this assumption is relaxed. Otherwise, these models will only apply to species in which high differential and structural consistency of personality across the lifetime have been documented for free-living animals, or to species in which social and environmental factors that might affect personality are constant across the lifetimes of the subjects.
(b). Variation and stability in personality across generations and across localities
As was described above, contextual generality (correlations across individuals between the behaviour expressed in different contexts) is quite likely to vary as a function of a wide range of conditions that those individuals experienced prior to testing. By extension, temporal or spatial variation in conditions affecting behavioural development could lead to substantial variation in personality across generations and across localities, even in the absence of any genetic variation across time or space. This might help account for results from dumpling squid, Euprymna tasmanica, indicating that correlations between ‘boldness' measured in two different contexts varied across successive generations of the same population (e.g. from r = −0.35 in 1 year to r = 0.09 the following year for adults at one locality, although the difference between these two correlation coefficients was not quite statistically significant after correction for multiple tests, Sinn et al. 2010). Similarly, variation in developmental conditions across large spatial scales (i.e. distances too far to be travelled by most dispersers) could also encourage variation in contextual generality across populations. Currently, researchers may be tempted to assume that variation in personality across generations or across localities has a genetic basis; a developmental perspective argues that this need not necessarily be the case.
(c). Effects of niche-picking on the temporal stability and contextual generality of personality
With respect to the temporal stability of personality, variation in developmental conditions over relatively small spatial scales (i.e. distances routinely travelled by members of a species) may provide different individuals with the opportunity to maintain themselves in different sets of environmental conditions for extended periods of time. As a result, if behaviour is measured at ages or life stages when animals are able to engage in niche-picking, this process is likely to increase the differential and structural consistency of personality in spatially heterogeneous situations, as compared with situations in which every individual lives in the same set of conditions (see §3b). In that case, when niche-picking is an option, differential and structural consistency both would be stronger under spatially heterogeneous than under spatially homogeneous environmental conditions. Indirect support for this prediction comes from a recent meta-analysis, which indicates that the repeatability of behaviour tends to be higher for field than for laboratory studies (Bell et al. 2009).
With respect to the contextual generality of personality, variation in habitat heterogeneity during one period of life can affect the development of correlations in the behaviour expressed in different contexts later in life. By extension, natural habitats that are heterogeneous over small spatial scales for individuals at a given age or life stage may provide a wider array of microhabitats and social environments, and hence be more likely to generate higher levels of contextual generality later in life, than homogeneous habitats. Thus, niche-picking might encourage higher levels of contextual generality for populations whose members develop in heterogeneous than in homogeneous habitats, and also, via correlations between genotypes and the conditions in which they develop, encourage the maintenance of higher levels of genetic variation in the former than in the latter. Data from three-spined stickleback provide indirect support for these ideas. Heterogeneity in the biotic, abotic and social microhabitats available for the development of three-spined stickleback is probably higher in large than in small bodies of freshwater (e.g. Nosil & Reimchen 2005; Dingemanse et al. 2007), and across stickleback populations, both contextual generality (Dingemanse et al. 2007) and genetic variation in personality traits (Dingemanse et al. 2009) were higher for fishes from large ponds or lakes than for fishes from small ponds.
(d). Robust contextual generality
The concept of robust contextual generality (i.e. contextual generality that is maintained across a wide range of developmental conditions) is relevant to many topics in the animal personality literature. These include (i) measuring personality in populations in which food levels, predation risk, population density, etc. vary across generations or localities; (ii) questions about the maintenance of personality following range expansions, invasions, translocations or other situations in which animals from one type of habitat live and produce offspring in a different type of habitat; and (iii) the issue of whether estimates of contextual generality based on laboratory-reared animals are likely to apply to free-living members of the same population.
As was outlined above, when conditions affecting personality development substantially change over space or time, robust contextual generality is expected only under a rather restricted set of conditions. In particular, robust contextual generality requires that the patterns of developmental plasticity across a range of developmental conditions be comparable within genotypes for the behaviour that they express in different contexts. Given the current lack of empirical information on this point, it seems inadvisable to design theoretical or empirical studies whose findings depend on an untested assumption that contextual generality is necessarily robust across time or space. In addition, given the many ways that developmental conditions in the laboratory differ from those of any natural habitat, it is important to validate estimates of contextual generality based on laboratory-reared animals using individuals from the same population who developed in nature.
(e). Developmental plasticity and the heritability of personality traits
A developmental reaction norm perspective helps show why genetic variation in developmental plasticity can lead to situations in which heritability changes as a function of variation in developmental conditions. Theoreticians have demonstrated that heritability will change as a function of developmental conditions if genotypes vary with respect to the slopes or shapes of their developmental reaction norms (Gavrilets & Scheiner 1993; Hoffmann & Merila 1999). This point can be readily illustrated using figure 1b, under the simplifying assumption that for each genotype, variance in behaviour is comparable across each of the three developmental conditions. In that case, the broad-sense heritability of the behaviour expressed in context B would be higher if individuals with genotypes I, II and III had been raised under condition 1 than if that same set of individuals had been raised under condition 3 (figure 1b). To date, there is empirical evidence of significant variation among genotypes in the developmental plasticity of behavioural traits, including personality traits, and of resulting changes in the heritability of behaviour as a result of prior experience (e.g. Cohen et al. 2008; Zhou et al. 2008; Dingemanse et al. 2009). Hence, we should be cautious in assuming that estimates of heritability for a given species measured in the laboratory or for a single locality or season in the field necessarily apply across their entire range, or across multiple generations.
(f). Correlations between personality, physiological and life-history traits
A developmental perspective argues that correlations across individuals between behavioural traits and physiological traits, including life-history traits such as growth, fecundity or age at maturity, could easily vary across localities or across cohorts, for the same reasons that correlations between the behaviour expressed in different contexts are likely to vary across space and time (see also Réale et al. (2010)). By extension, if relationships between behavioural traits and life-history traits are contingent upon developmental conditions, then studies of these relationships should, as much as possible, be conducted using animals exposed to conditions comparable to those in the field. For instance, we might expect positive relationships between boldness in a foraging context and growth rate (cf. Stamps 2007; Biro & Stamps 2008) to be stronger if subjects had been reared under conditions in which they had to forage for limited food under perceived predation risk than if they had been reared with access to food ad libitum in the absence of cues from predators. In fact, given the many ways that developmental conditions in the laboratory differ from those of any natural population, it might be advisable to begin studies of relationships between behavioural and physiological traits using experimental subjects that developed in natural or in semi-natural environments, as opposed to subjects reared under standard laboratory conditions.
5. Conclusions
Although studies of animal personality development are still rare, they demonstrate that personality is less temporally stable, and more dependent upon experiential factors, than is often assumed. This article demonstrates how a developmental perspective can help behavioural ecologists identify implicit, untested assumptions about developmental processes that underlie their own research, and thus avoid designing studies whose conclusions would be compromised if those assumptions are invalid. Examples of insights provided by developmental perspectives include suggestions about appropriate time-frames for studying the fitness consequences of personality, appreciation of the conditions required for contextual generality to be maintained across a wide range of developmental conditions, caveats in extrapolating results from laboratory-raised animals to free-living members of the same population, and understanding why the heritability of personality traits may vary across generations and localities as a function of variation in developmental conditions. More generally, the concepts outlined in this article show how a developmental perspective can provide a way forward to understanding how genes and a wide variety of experiential factors interact with one another across the lifetime to affect the correlations in behaviour across time and across contexts that form the basis of animal personality.
However, it should also be apparent that what we do not know about the development of animal personality is currently much more imposing than what we do.
Studies of developmental reaction norms of personality traits are virtually non-existent, and, with a few notable exceptions, we have no idea how experiential factors at a given age affect contextual generality later in life (Stamps & Groothuis 2010). Hence, while the current article identifies assumptions about development that underlie many studies of the functional significance and evolution of animal personality, we currently have many more questions about these assumptions than answers. In addition to putting studies of the ecology and evolution of animal personality on a sound developmental footing, integrating developmental perspectives into the study of animal personality may prove useful insights to researchers in other disciplines who are currently attempting to understand how genetic and a wide variety of experiential factors in previous as well as the current generation combine to affect the development and expression of phenotypic traits that consistently differ across individuals (e.g. Lande 2009).
Acknowledgements
We are very grateful to Pete Biro and two anonymous referees for their comments on an earlier draft of this article, and to the editors (especially Niels Dingemanse and Anahita Kazem) for their extensive comments and suggestions on several previous versions of this article.
Appendix A. Glossary of Terms
- agent:
an individual animal, or a group of individuals with the same genotype
- behavioural reaction norm1:
the set of behavioural phenotypes that a single individual produces in a given set of environments, where ‘environmental context' includes any external or internal stimulus that can vary across a gradient, as well as age, time and prior experiences
- context:
all of the external stimuli that impinge on an individual when it expresses a given behaviour
- contextual generality:
the extent to which scores for behaviour expressed in one context are correlated across agents with scores for behaviour expressed in other contexts, when the behaviour in each context is measured at the same age
- contextual plasticity:
the extent to which the behaviour of an agent varies across contexts, relative to the behaviour of other agents in those same contexts, when the behaviour in each context is measured at the same age
- contextual reaction norm2:
a description of how the behaviour of an agent at a given age varies as a function of context, for contexts that can be arranged along a continuum
- developmental plasticity:
the extent to which the behaviour of an agent varies as a function of conditions experienced by that agent before that behaviour was expressed
- developmental reaction norm:
a description of the behaviour expressed by an agent in a given context at a given age, as a function of the conditions experienced by that agent before the behaviour was expressed, for conditions that can be arranged along a continuum.
- differential consistency:
the extent to which scores for a given behaviour in a given context at a given time are correlated across individuals with scores for the same behaviour in the same context at a later time.
- experiential factors:
any external stimulus or event that affects gene expression, leading to changes in the phenotype
- niche-construction:
processes by which individuals create or encourage experiential factors (including environments) that affect their own subsequent development
- niche-picking:
processes by which individuals choose experiential factors (including environments) that affect their own subsequent development
- prior experiential factors (PEF):
all experiential factors with effects on the phenotype at a specific age that occurred from conception until that age
- structural consistency:
the extent to which correlations among behaviour patterns expressed in two or more contexts at one time are preserved when the same set of behaviour patterns is measured in the same set of contexts at a different time.
Endnotes
Definition from Dingemanse et al. 2010. Behavioural reaction norm is a more general term than contextual reaction norm, as the former can describe variation in behaviour as a function of many variables besides variation in the current external stimulus situation.
This definition differs from the one provided in Stamps & Groothuis (2010) in stressing that it should be possible to arrange contextual stimuli along a continuum.
One contribution of 16 to a Theme Issue ‘Evolutionary and ecological approaches to the study of personality’.
References
- Alleva E., Francia N.2009Psychiatric vulnerability: suggestions from animal models and role of neurotrophins. Neurosci. Biobehav. Rev. 33, 525–536 10.1016/j.neubiorev.2008.09.004 (doi:10.1016/j.neubiorev.2008.09.004) [DOI] [PubMed] [Google Scholar]
- Barker J. S. F., Starmer W. T.1999Environmental effects and the genetics of oviposition site preference for natural yeast substrates in Drosophila buzzatii. Hereditas 130, 145–175 10.1111/j.1601-5223.1999.00145.x (doi:10.1111/j.1601-5223.1999.00145.x) [DOI] [PubMed] [Google Scholar]
- Bateson P.2001Behavioural development and Darwinian evolution. In Cycles of contingency (eds Oyama P., Griffiths P., Gray R. E.). Cambridge, MA: MIT Press [Google Scholar]
- Bell A. M., Sih A.2007Exposure to predation generates personality in three-spined sticklebacks (Gasterosteus aculeatus). Ecol. Lett. 10, 828–834 10.1111/j.1461-0248.2007.01081.x (doi:10.1111/j.1461-0248.2007.01081.x) [DOI] [PubMed] [Google Scholar]
- Bell A. M., Hankison S. J., Laskowski K. L.2009The repeatability of behaviour: a meta-analysis. Anim. Behav. 77, 771–783 10.1016/j.anbehav.2008.12.022 (doi:10.1016/j.anbehav.2008.12.022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltran R. O., Silove D., Llewellyn G. M.2009Comparison of ICD-10 diagnostic guidelines and research criteria for enduring personality change after catastrophic experience. Psychopathology 42, 113–118 10.1159/000204761 (doi:10.1159/000204761) [DOI] [PubMed] [Google Scholar]
- Biro P. A., Stamps J. A.2008Are animal personality traits linked to life-history productivity? Trends Ecol. Evol. 23, 361–368 10.1016/j.tree.2008.04.003 (doi:10.1016/j.tree.2008.04.003) [DOI] [PubMed] [Google Scholar]
- Bonduriansky R., Day T.2009Nongenetic inheritance and its evolutionary implications. Annu. Rev. Ecol. Evol. Syst. 40, 103–125 10.1146/annurev.ecolsys.39.110707.173441 (doi:10.1146/annurev.ecolsys.39.110707.173441) [DOI] [Google Scholar]
- Boutry C., Blackledge T. A.2008The common house spider alters the material and mechanical properties of cobweb silk in response to different prey. J. Exp. Zool. Part A Ecol. Genet. Physiol. 309A, 542–552 10.1002/jez.487 (doi:10.1002/jez.487) [DOI] [PubMed] [Google Scholar]
- Brommer J. E., Rattiste K., Wilson A. J.2008Exploring plasticity in the wild: laying date–temperature reaction norms in the common gull Larus canus. Proc. R. Soc. B 275, 687–693 10.1098/rspb.2007.0951 (doi:10.1098/rspb.2007.0951) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown C. R., Brown M. B.2000Heritable basis for choice of group size in a colonial bird. Proc. Natl Acad. Sci. USA 97, 14 825–14 830 10.1073/pnas.97.26.14825 (doi:10.1073/pnas.97.26.14825) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss A. H., Plomin R.1984Temperament: early developing personality traits. Hillsdale, NJ: Lawrence Erlbaum Associates [Google Scholar]
- Carere C., Drent P. J., Koolhaas J. M., Groothuis T. G. G.2005Epigenetic effects on personality traits: early food provisioning and sibling competition. Behaviour 142, 1329–1355 10.1163/156853905774539328 (doi:10.1163/156853905774539328) [DOI] [Google Scholar]
- Caspi A., Roberts B. W., Shiner R. L.2005Personality development: stability and change. Annu. Rev. Psychol. 56, 453–484 10.1146/annurev.psych.55.090902.141913 (doi:10.1146/annurev.psych.55.090902.141913) [DOI] [PubMed] [Google Scholar]
- Champagne F. A., Curley J. P.2009Epigenetic mechanisms mediating the long-term effects of maternal care on development. Neurosci. Biobehav. Rev. 33, 593–600 10.1016/j.neubiorev.2007.10.009 (doi:10.1016/j.neubiorev.2007.10.009) [DOI] [PubMed] [Google Scholar]
- Champagne F. A., Meaney M. J.2006Stress during gestation alters postpartum maternal care and the development of the offspring in a rodent model. Biol. Psychiatry 59, 1227–1235 10.1016/j.biopsych.2005.10.016 (doi:10.1016/j.biopsych.2005.10.016) [DOI] [PubMed] [Google Scholar]
- Charmantier A., Garant D.2005Environmental quality and evolutionary potential: lessons from wild populations. Proc. R. Soc. B 272, 1415–1425 10.1098/rspb.2005.3117 (doi:10.1098/rspb.2005.3117) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen H., Geva A. B., Matar M. A., Zohar J., Kaplan Z.2008Post-traumatic stress behavioural responses in inbred mouse strains: can genetic predisposition explain phenotypic vulnerability? Int. J. Neuropsychopharmacol. 11, 331–349 [DOI] [PubMed] [Google Scholar]
- Coleman K., Wilson D. S.1998Shyness and boldness in pumpkinseed sunfish: individual differences are context-specific. Anim. Behav. 56, 927–936 10.1006/anbe.1998.0852 (doi:10.1006/anbe.1998.0852) [DOI] [PubMed] [Google Scholar]
- Cote J., Clobert J.2007Social personalities influence natal dispersal in a lizard. Proc. R. Soc. B 274, 383–390 10.1098/rspb.2006.3734 (doi:10.1098/rspb.2006.3734) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cote J., Clobert J., Brodin T., Fogarty S., Sih A.2010Personality-dependent dispersal: characterization, ontogeny and consequences for spatially structured populations. Phil. Trans. R. Soc. B 365, 4065–4076 10.1098/rstb.2010.0176 (doi:10.1098/rstb.2010.0176) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews D.2008Epigenetics and its implications for behavioral neuroendocrinologry. Front. Neuroendocrinol. 29, 344–357 10.1016/j.yfrne.2008.01.003 (doi:10.1016/j.yfrne.2008.01.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curley J. P., Champagne F. A., Bateson P., Keverne E. B.2008Transgenerational effects of impaired maternal care on behaviour of offspring and grandoffspring. Anim. Behav. 75, 1551–1561 10.1016/j.anbehav.2007.10.008 (doi:10.1016/j.anbehav.2007.10.008) [DOI] [Google Scholar]
- Daisley J. N., Bromundt V., Mostl E., Kotrschal K.2005Enhanced yolk testosterone influences behavioral phenotype independent of sex in Japanese quail chicks Coturnix japonica. Horm. Behav. 47, 185–194 [DOI] [PubMed] [Google Scholar]
- Dingemanse N. J., Both C., Drent P. J., Van Oers K., VAN Noordwijk A. J.2002Repeatability and heritability of exploratory behaviour in great tits from the wild. Anim. Behav. 64, 929–938 10.1006/anbe.2002.2006 (doi:10.1006/anbe.2002.2006) [DOI] [Google Scholar]
- Dingemanse N. J., Both C., van Noordwijk A. J., Rutten A. L., Drent P. J.2003Atal dispersal and personalities in great tits (Parus major). Proc. R. Soc. Lond. B 270, 741–747 10.1098/rspb.2002.2300 (doi:10.1098/rspb.2002.2300) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingemanse N. J., Wright J., Kazem A. J. N., Thomas D. K., Hickling R., Dawnay N.2007Behavioural syndromes differ predictably between 12 populations of three-spined stickleback. J. Anim. Ecol. 76, 1128–1138 10.1111/j.1365-2656.2007.01284.x (doi:10.1111/j.1365-2656.2007.01284.x) [DOI] [PubMed] [Google Scholar]
- Dingemanse N. J., van der Plas F., Wright J., Réale D., Schrama M., Roff D. A., van der Zee E., Barber I.2009Individual experience and evolutionary history of predation affect expression of heritable variation in fish personality and morphology. Proc. R. Soc. B 276, 1285–1293 10.1098/rspb.2008.1555 (doi:10.1098/rspb.2008.1555) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingemanse N. J., Kazem A. J. N., Réale D., Wright J.2010Behavioural reaction norms: animal personality meets individual plasticity. Trends Ecol. Evol. 25, 81–89 10.1016/j.tree.2009.07.013 (doi:10.1016/j.tree.2009.07.013) [DOI] [PubMed] [Google Scholar]
- Dochtermann N. A., Roff D. A.2010Applying a quantitative genetics framework to behavioural syndrome research. Phil. Trans. R. Soc. B 365, 4013–4020 10.1098/rstb.2010.0129 (doi:10.1098/rstb.2010.0129) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckworth R. A.2009Maternal effects and range expansion: a key factor in a dynamic process? Phil. Trans. R. Soc. B 364, 1075–1086 10.1098/rstb.2008.0294 (doi:10.1098/rstb.2008.0294) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falconer D. S., Mackay T. F. C.1996Introduction to quantitative genetics. New York, NY: Longman [Google Scholar]
- Galef B. G.1981The ecology of weaning: parasitism and the achievement of independence by altricial mammals. In Parental care in mammals (eds Gubernick D., Klopfer P. H.). New York, NY: Plenum [Google Scholar]
- Gavrilets S., Scheiner S. M.1993The genetics of phenotypic plasticity. 6. Theoretical predictions for directional selection. J. Evol. Biol. 6, 49–68 10.1046/j.1420-9101.1993.6010049.x (doi:10.1046/j.1420-9101.1993.6010049.x) [DOI] [Google Scholar]
- Groothuis T. G. G., Muller W., von Engelhardt N., Carere C., Eising C.2005Maternal hormones as a tool to adjust offspring phenotype in avian species. Neurosci. Biobehav. Rev. 29, 329–352 10.1016/j.neubiorev.2004.12.002 (doi:10.1016/j.neubiorev.2004.12.002) [DOI] [PubMed] [Google Scholar]
- Groothuis T. G. G., Carere C., Lipar J., Drent P. J., Schwabl H.2008Selection on personality in a songbird affects maternal hormone levels tuned to its effect on timing of reproduction. Biol. Lett. 4, 465–467 10.1098/rsbl.2008.0258 (doi:10.1098/rsbl.2008.0258) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann A. A., Merila J.1999Heritable variation and evolution under favourable and unfavourable conditions. Trends Ecol. Evol. 14, 96–101 10.1016/S0169-5347(99)01595-5 (doi:10.1016/S0169-5347(99)01595-5) [DOI] [PubMed] [Google Scholar]
- Jablonka E., Raz G.2009Transgenerational epigenetic inheritance: prevalence, mechanisms, and implications for the study of heredity and evolution. Q. Rev. Biol. 84, 131–176 10.1086/598822 (doi:10.1086/598822) [DOI] [PubMed] [Google Scholar]
- Jovanovic T., Ressler K. J.2010How the neurocircuitry and genetics of fear inhibition may inform our understanding of PTSD. Am. J. Psychiatry 167, 648–662 10.1176/appi.ajp.2009.09071074 (doi:10.1176/appi.ajp.2009.09071074) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruuk L. E. B.2004Estimating genetic parameters in natural populations using the ‘animal model'. Phil. Trans. R. Soc. Lond. B 359, 873–890 10.1098/rstb.2003.1437 (doi:10.1098/rstb.2003.1437) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruuk L. E. B., Slate J., Wilson A. J.2008New answers for old questions: the evolutionary quantitative genetics of wild animal populations. Annu. Rev. Ecol. Evol. Syst. 39, 525–548 10.1146/annurev.ecolsys.39.110707.173542 (doi:10.1146/annurev.ecolsys.39.110707.173542) [DOI] [Google Scholar]
- Laland K. N., Odling-Smee F. J., Feldman M. W.1999Evolutionary consequences of niche construction and their implications for ecology. Proc. Natl Acad. Sci. USA 96, 10 242–10 247 10.1073/pnas.96.18.10242 (doi:10.1073/pnas.96.18.10242) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lande R.2009Adaptation to an extraordinary environment by evolution of phenotypic plasticity and genetic assimilation. J. Evol. Biol. 22, 1435–1446 10.1111/j.1420-9101.2009.01754.x (doi:10.1111/j.1420-9101.2009.01754.x) [DOI] [PubMed] [Google Scholar]
- Leibold M. A., Tessier A. J., West C. T.1994Genetic, acclimatization, and ontogenic effects on habitat selection behavior in Daphnia pulicaria. Evolution 48, 1324–1332 10.2307/2410389 (doi:10.2307/2410389) [DOI] [PubMed] [Google Scholar]
- Lessells C. M., Boag P. T.1987Unrepeatable repeatabilities—a common mistake. Auk 104, 116–121 [Google Scholar]
- Narusyte J., Neiderhiser J. M., D'Onofrio B. M., Reiss D., Spotts E. L., Ganiban J., Lichtenstein P.2008Testing different types of genotype-environment correlation: an extended children-of-twins model. Dev. Psychol. 44, 1591–1603 10.1037/a0013911 (doi:10.1037/a0013911) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosil P., Reimchen T. E.2005Ecological opportunity and levels of morphological variance within freshwater stickleback populations. Biol. J. Linnean Soc. 86, 297–308 10.1111/j.1095-8312.2005.00517.x (doi:10.1111/j.1095-8312.2005.00517.x) [DOI] [Google Scholar]
- Nussey D. H., Wilson A. J., Brommer J. E.2007The evolutionary ecology of individual phenotypic plasticity in wild populations. J. Evol. Biol. 20, 831–844 10.1111/j.1420-9101.2007.01300.x (doi:10.1111/j.1420-9101.2007.01300.x) [DOI] [PubMed] [Google Scholar]
- Overli O., Sorensen C., Pulman K. G. T., Pottinger T. G., Korzan W., Summers C. H., Nilsson G. E.2007Evolutionary background for stress-coping styles: relationships between physiological, behavioral, and cognitive traits in non-mammalian vertebrates. Neurosci. Biobehav. Rev. 31, 396–412 10.1016/j.neubiorev.2006.10.006 (doi:10.1016/j.neubiorev.2006.10.006) [DOI] [PubMed] [Google Scholar]
- Plomin R., Defries J. C., Loehlin J. C.1977Genotype–environment interaction and correlation in analysis of human behavior. Psychol. Bull. 84, 309–322 10.1037/0033-2909.84.2.309 (doi:10.1037/0033-2909.84.2.309) [DOI] [PubMed] [Google Scholar]
- Price T. S., Jaffee S. R.2008Effects of the family environment: gene–environment interaction and passive gene–environment correlation. Dev. Psychol. 44, 305–315 10.1037/0012-1649.44.2.305 (doi:10.1037/0012-1649.44.2.305) [DOI] [PubMed] [Google Scholar]
- Réale D., Dingemanse N. J., Kazem A. J. N., Wright J.2010Evolutionary and ecological approaches to the study of personality. Phil. Trans. R. Soc. B 365, 3937–3946 10.1098/rstb.2010.0222 (doi:10.1098/rstb.2010.0222) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts B. W., Delvecchio W. F.2000The rank-order consistency of personality traits from childhood to old age: a quantitative review of longitudinal studies. Psychol. Bull. 126, 3–25 10.1037/0033-2909.126.1.3 (doi:10.1037/0033-2909.126.1.3) [DOI] [PubMed] [Google Scholar]
- Roberts B. W., Caspi A., Moffitt T. E.2001The kids are alright: growth and stability in personality development from adolescence to adulthood. J. Pers. Soc. Psychol. 81, 670–683 10.1037/0022-3514.81.4.670 (doi:10.1037/0022-3514.81.4.670) [DOI] [PubMed] [Google Scholar]
- Robinson M. R., Wilson A. J., Pilkington J. G., Clutton-Brock T. H., Pemberton J. M., Kruuk L. E. B.2009The impact of environmental heterogeneity on genetic architecture in a wild population of Soay sheep. Genetics 181, 1639–1648 10.1534/genetics.108.086801 (doi:10.1534/genetics.108.086801) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Gomez M. D., et al. 2008Behavioral plasticity in rainbow trout (Oncorhynchus mykiss) with divergent coping styles: when doves become hawks. Horm. Behav. 54, 534–538 10.1016/j.yhbeh.2008.05.005 (doi:10.1016/j.yhbeh.2008.05.005) [DOI] [PubMed] [Google Scholar]
- Rutter M.2007Gene-environment interdependence. Dev. Sci. 10, 12–18 10.1111/j.1467-7687.2007.00557.x (doi:10.1111/j.1467-7687.2007.00557.x) [DOI] [PubMed] [Google Scholar]
- Ryan B. C., Vandenbergh J. G.2002Intrauterine position effects. Neurosci. Biobehav. Rev. 26, 665–678 10.1016/S0149-7634(02)00038-6 (doi:10.1016/S0149-7634(02)00038-6) [DOI] [PubMed] [Google Scholar]
- Sandell M. I., Adkins-Regan E., Ketterson E. D.2007Pre-breeding diet affects the allocation of yolk hormones in zebra finches Taeniopygia guttata. J. Avian Biol. 38, 284–290 10.1111/j.2007.0908-8857.03640.x (doi:10.1111/j.2007.0908-8857.03640.x) [DOI] [Google Scholar]
- Serrano D., Tella J. L.2007The role of despotism and heritability in determining settlement patterns in the colonial lesser kestrel. Am. Nat. 169, E53–E67 10.1086/510598 (doi:10.1086/510598) [DOI] [PubMed] [Google Scholar]
- Sgro C. M., Hoffmann A. A.2004Genetic correlations, tradeoffs and environmental variation. Heredity 93, 241–248 10.1038/sj.hdy.6800532 (doi:10.1038/sj.hdy.6800532) [DOI] [PubMed] [Google Scholar]
- Sih A., Bell A. M.2008Insights for behavioral ecology from behavioral syndromes. Adv. Study Behav. 38, 227–281 10.1016/S0065-3454(08)00005-3 (doi:10.1016/S0065-3454(08)00005-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sih A., Bell A. M., Johnson J. C., Ziemba R. E.2004Behavioral syndromes: an integrative overview. Q. Rev. Biol. 79, 241–277 [DOI] [PubMed] [Google Scholar]
- Sinn D. L., Gosling S. D., Moltschaniwskyj N. A.2008Development of shy/bold behaviour in squid: context-specific phenotypes associated with developmental plasticity. Anim. Behav. 75, 433–442 10.1016/j.anbehav.2007.05.008 (doi:10.1016/j.anbehav.2007.05.008) [DOI] [Google Scholar]
- Sinn D. L., Moltschaniwskyj N. A., Wapstra E., Dall S. R. X.2010Are behavioral syndromes invariant? Spatiotemporal variation in shy/bold behavior in squid. Behav. Ecol. Sociobiol. 64, 693–702 10.1007/s00265-009-0887-2 (doi:10.1007/s00265-009-0887-2) [DOI] [Google Scholar]
- Stamps J. A.2006The silver spoon effect and habitat selection by natal dispersers. Ecol. Lett. 9, 1179–1185 10.1111/j.1461-0248.2006.00972.x (doi:10.1111/j.1461-0248.2006.00972.x) [DOI] [PubMed] [Google Scholar]
- Stamps J. A.2007Growth-mortality tradeoffs and ‘personality traits' in animals. Ecol. Lett. 10, 355–363 10.1111/j.1461-0248.2007.01034.x (doi:10.1111/j.1461-0248.2007.01034.x) [DOI] [PubMed] [Google Scholar]
- Stamps J. A., Groothuis T. G. G.2010The development of animal personality: relevance, concepts and perspectives. Biol. Rev. 85, 301–325 10.1111/j.1469-185X.2009.00103.x (doi:10.1111/j.1469-185X.2009.00103.x) [DOI] [PubMed] [Google Scholar]
- Stamps J. A., Krishnan V. V.2001How territorial animals compete for divisible space: a learning-based model with unequal competitors. Am. Nat. 157, 154–169 10.1086/318634 (doi:10.1086/318634) [DOI] [PubMed] [Google Scholar]
- Switzer P. V., Stamps J. A., Mangel M.2001When should a territory resident attack? Anim. Behav. 62, 749–759 10.1006/anbe.2001.1799 (doi:10.1006/anbe.2001.1799) [DOI] [Google Scholar]
- Vom Saal F. S., Bronson F. H.1978In-utero proximity of female mouse fetuses to males—effect on reproductive performance during later life. Biol. Reprod. 19, 842–853 10.1095/biolreprod19.4.842 (doi:10.1095/biolreprod19.4.842) [DOI] [PubMed] [Google Scholar]
- Webster M. M., Ward A. J. W., Hart P. J. B.2007Boldness is influenced by social context in threespine sticklebacks (Gasterosteus aculeatus). Behaviour 144, 351–371 10.1163/156853907780425721 (doi:10.1163/156853907780425721) [DOI] [Google Scholar]
- West-Eberhardt M. J.2003Developmental plasticity and evolution New York, NY: Oxford University Press [Google Scholar]
- Wilson D. S.1998Adaptive individual differences within single populations. Phil. Trans. R. Soc. Lond. B 353, 199–205 10.1098/rstb.1998.0202 (doi:10.1098/rstb.1998.0202) [DOI] [Google Scholar]
- Zhou Y. H., Kuster H. K., Pettis J. S., Danka R. G., Gleason J. M., Greenfield M. D.2008Reaction norm variants for male calling song in populations of Achroia grisella (Lepidoptera: Pyralidae): toward a resolution of the lek paradox. Evolution 62, 1317–1334 10.1111/j.1558-5646.2008.00371.x (doi:10.1111/j.1558-5646.2008.00371.x) [DOI] [PubMed] [Google Scholar]