Abstract
BACKGROUND AND PURPOSE
Quercetin lowers plasma glucose, normalizes glucose tolerance tests and preserves pancreatic β-cell integrity in diabetic rats. However, its mechanism of action has never been explored in insulin-secreting β-cells. Using the INS-1 β-cell line, the effects of quercetin were determined on glucose- or glibenclamide-induced insulin secretion and on β-cell dysfunctions induced by hydrogen peroxide (H2O2). These effects were analysed along with the activation of the extracellular signal-regulated kinase (ERK)1/2 pathway. N-acetyl-L-cysteine (NAC) and resveratrol, two antioxidants also known to exhibit some anti-diabetic properties, were used for comparison.
EXPERIMENTAL APPROACH
Insulin release was quantified by the homogeneous time resolved fluorescence method and ERK1/2 activation tested by Western blot experiments. Cell viability was estimated by the [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide] (MTT) colorimetric assay.
KEY RESULTS
Quercetin (20 µmol·L−1) potentiated both glucose (8.3 mmol·L−1)- and glibenclamide (0.01 µmol·L−1)-induced insulin secretion and ERK1/2 phosphorylation. The ERK1/2 (but not the protein kinase A) signalling pathway played a crucial role in the potentiation of glucose-induced insulin secretion by quercetin. In addition, quercetin (20 µmol·L−1), protected β-cell function and viability against oxidative damage induced by 50 µmol·L−1 H2O2 and induced a major phosphorylation of ERK1/2. In the same conditions, resveratrol or NAC were ineffective.
CONCLUSION AND IMPLICATIONS
Quercetin potentiated glucose and glibenclamide-induced insulin secretion and protected β-cells against oxidative damage. Our study suggested that ERK1/2 played a major role in those effects. The potential of quercetin in preventing β-cell dysfunction associated with diabetes deserves further investigation.
Keywords: quercetin, pancreatic β-cells, insulin secretion, cell viability, ERK1/2, oxidative stress, diabetes
Introduction
Glucose-induced insulin secretion in pancreatic β-cells depends on its metabolism, which leads to an increase in the ATP/ADP ratio. Insulin secretion occurs after closure of the ATP-dependent potassium channels (KATP), β-cell membrane depolarization and subsequent entry of calcium through the L-type voltage-dependent calcium channels (Cav1.x) (Docherty and Steiner, 1996; channel nomenclature follows Alexander et al., 2009). Glucose-induced calcium entry activates various kinases in β-cells, including PKA via calcium-dependent cAMP production (Briaud et al., 2003), Ca2+ and calmodulin-dependent protein kinase II (CaMKII) (Wenham et al., 1994), PKC (Knutson and Hoenig, 1994) and extracellular signal-regulated kinase (ERK) 1/2 (Frödin et al., 1995; Benes et al., 1998; Briaud et al., 2003), an enzyme that is involved in many cellular events, including proliferation, differentiation and survival. These kinases have been shown to participate in the PKA-mediated phosphorylation of cAMP-responsive element-binding protein, induced by glucose and required for β-cell survival (Costes et al., 2006). Moreover, the ERK1/2 pathway has been shown to participate in the regulation of glucose-induced insulin secretion in a β-cell line (Longuet et al., 2005).
Oxidative stress, caused by an increase in intracellular reactive oxygen species (ROS), plays a central role in insulin resistance and in pancreatic β-cell death during the progressive deterioration in glucose tolerance and development of type 2 diabetes (Evans et al., 2003; Kaneto et al., 2005a,b; Lenzen, 2008; Li et al., 2008; Poitout and Robertson, 2008).
The protection of pancreatic β-cells is essential if the natural history of diabetes after the onset of the disease is to be changed but may also be crucial in preventing or delaying the onset of the disease in at-risk subjects. Several trials have shown that various drugs such as sulphonylureas, acarbose, metformin and peroxisome proliferator-activated receptor γ agonists are able to reduce the incidence of type 2 diabetes (Sartor et al., 1980; Chiasson et al., 2002; Knowler et al., 2002; 2005;). One of the mechanisms potentially involved in the prevention of diabetes in these trials could be the protection of β-cells from insults induced by inflammation and oxidative stress (Bonora, 2008).
Compounds with antioxidant properties such as aminoguanidine, R-α lipoic acid or gliclazide (a sulfonylurea with free radical scavenger properties) have been found to provide some protection of β-cells both in vitro and in vivo (Tanaka et al., 1999; Shen et al., 2008). More specifically, it has been shown that treatment using N-acetyl-L-cysteine (NAC) can improve the control of glycaemia with preservation of pancreatic β-cell function in diabetic mice (Kaneto et al., 1999) and reduce oxidative stress and restore β-cell function in diabetic hamsters (Takatori et al., 2004).
Recent data have also suggested that polyphenols, the most abundant antioxidants in the diet and widespread constituents of fruit, vegetables, cereals, dry legumes, chocolate or beverages such as tea, coffee or wine, display some anti-diabetic properties (Zhang et al., 2004; Scalbert et al., 2005; Xiong et al., 2006; Meghana et al., 2007). Among polyphenols, resveratrol (5-[(E)-2-(4-hydroxyphenyl)-ethenyl]benzene-1,3-diol) was shown to normalize hyperglycaemia in diabetic rats (Palsamy and Subramanian, 2008). Quercetin (3,5,7,3′,4′-pentahydroxyflavone) was shown to induce a dose-dependent decrease in plasma glucose levels, in addition to regenerating pancreatic islets, preserving the integrity of pancreatic β-cells and normalizing glucose tolerance tests. It was also shown in streptozotocin-induced diabetic rats to reduce oxidative stress markers (plasma thiobarbituric acid reactive substances and hydroperoxides) and regulate the detoxifying enzymes, superoxide dismutase and catalase (Vessal et al., 2003; Mahesh and Menon, 2004; Coskun et al., 2005). Quercetin was also recently shown to alleviate diabetic symptoms and reduce the disturbance of hepatic gene expression in streptozotocin-induced diabetic mice (Kobori et al., 2009).
Although most of the biological actions of polyphenols have been attributed to their antioxidant properties, mounting evidence suggests that the cellular effects of polyphenols could be mediated by their interactions with specific proteins of intracellular signalling cascades, especially the MAPK signalling pathways (Williams et al., 2004).
Using the INS-1 β-cell line, we investigated the in vitro potential of three antioxidant compounds known to display antidiabetic activities (quercetin, resveratrol and NAC) to modulate insulin secretion or protect β-cells against the impairment of insulin secretion or viability induced by oxidative stress (hydrogen peroxide, H2O2). The effects of the three compounds were also studied on ERK1/2 pathway activation. The results of this study demonstrated that quercetin (but not resveratrol or NAC) amplified both glucose and glibenclamide-induced insulin secretion, and protected β-cell function and viability against oxidative damage. They also suggested that ERK1/2 activation plays a major role in the insulin-secreting and β-cell-protecting effects of quercetin. In addition, quercetin was found to potentiate glucose-induced insulin secretion in a more physiological model, rat pancreatic islets.
Methods
INS-1 cells culture
The insulin-secreting cell line INS-1 (a gift from Professor C. B. Wollheim) was cultured in RPMI-1640, supplemented with 10% fetal calf serum (FCS), 100 U·mL−1 penicillin, 100 µg·mL−1 streptomycin, 2 mmol·L−1 L-glutamine, 10 mmol·L−1 HEPES, 1 mmol·L−1 sodium pyruvate, and 50 mmol·L−1 2-mercaptoethanol, in a humidified atmosphere (5% CO2, 37°C), according to the method of Asfari et al. (1992). Cells were plated in 24-well plates (4 × 105 cells per well) for insulin secretion and Western blotting studies and in 96-well plates (105 cells per well) for viability assays. After 5 days of culture, cells were used for the various experiments.
Rat pancreatic islets preparation
All animal care and experimental procedures were performed according to the rules of the Centre National de la Recherche Scientifique Animal Care and were approved by the local Ethics Committee. Pancreatic islets were isolated from fed male Wistar rats (Janvier, France) weighing 280–320 g when killed. Islets were isolated after collagenase digestion of whole pancreas, according to a technique previously described (Lacy and Kostianovsky, 1967). Briefly, rats were sacrified by decapitation and the pancreas was filled by injection of collagenase P solution (15 mL, 1 mg·mL−1), excised, digested at 37°C. Islets were separated by Ficoll gradient, selected by hand-picking under a microscope and stabilized as described next.
INS-1 cells and rat pancreatic islets treatment
When quercetin, resveratrol or NAC were used, INS-1 cells were pre-incubated with the indicated concentrations of each compound for 1 h (5% CO2, 37°C) in RPMI medium and washed twice in HEPES-balanced Krebs-Ringer bicarbonate buffer (123 mmol·L−1 NaCl, 5.4 mmol·L−1 KCl, 1.3 mmol·L−1 KH2PO4, 2.7 mmol·L−1 MgSO4, 2.9 mmol·L−1 CaCl2, 5 mmol·L−1 NaHCO3 and 20 mmol·L−1 HEPES, pH 7.5) containing 2 g·L−1 bovine serum albumin (KRB/BSA) before any subsequent experiment. When the inhibitors of MEK (UO126) or PKA (H89) were used, INS-1 cells were pre-incubated with the indicated concentrations of each compound for 90 min (5% CO2, 37°C) in RPMI medium and washed twice in KRB/BSA before any subsequent experiment. For determination of the effects of quercetin, resveratrol or NAC, INS-1 cells were incubated for 1 h (5% CO2, 37°C) in KRB/BSA containing each compound (at the indicated concentrations) in the absence (0 mmol·L−1 secretagogue: basal condition) or the presence of a secretagogue (8.3 mmol·L−1 glucose or 0.01 µmol·L−1 glibenclamide). When used, indicated concentrations of H2O2 or pharmacological inhibitors (UO126, H89) were added at the beginning of the 1 h incubation period. For kinetic experiments, INS-1 cells were incubated in KRB/BSA for 1 h in the presence of 8.3 mmol·L−1 glucose and in the absence or presence of quercetin (20 µmol·L−1) added at various times (5, 10, 20, 40 and 60 min) before the end of the incubation period. Control experiments were performed in the absence of secretagogue, antioxidant or pharmacological inhibitor during the pre-incubation and incubation periods.
Rat isolated islets were stabilized in the absence or presence of quercetin (20 µmol·L−1) for 1 h at 37°C in a buffer containing: 115 mmol·L−1 NaCl; 5 mmol·L−1 KCl; 24 mmol·L−1 NaHCO3; 1 mmol·L−1 MgCl2, 6H2O; 2.5 mmol·L−1 CaCl2; 20 mmol·L−1 HEPES-Na, pH 7.3 supplemented with 0.5% BSA and 2.8 mmol·L−1 glucose. Then batches of 60 islets were incubated in 2.8 mmol·L−1 or 8.3 mmol·L−1 glucose in the absence or presence of quercetin (20 µmol·L−1) for 1 h at 37°C.
Insulin secretion
At the end of the 1 h incubation period, the medium was sampled and kept at −20°C until insulin assay by the homogeneous time resolved fluorescence technology (HTRF) according to the manufacturer's instructions. Two anti-insulin antibodies were used; one labelled with Eu3+-Cryptate and one labelled with XL665 that recognize distinct epitopes. When these two fluorophores bind to insulin molecules, the two antibodies come into close proximity, allowing fluorescence resonance energy transfer (FRET) to occur between the Eu3+-Cryptate and the XL665. This FRET increases proportionally with the insulin concentrations.
SDS-PAGE and Western blotting experiments
At the end of the 1 h incubation period, after removal of incubation media, INS-1 cells were washed with ice-cold phosphate buffer saline (PBS) and lysed in a buffer containing: 1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS, 0.2 mmol·L−1 sodium orthovanadate, 1 mmol·L−1 DTT, 10 mmol·L−1 HEPES, 5 mmol·L−1 EDTA, 50 mmol·L−1 NaF, 40 mmol·L−1β-glycerolphosphate, protease inhibitors cocktail (1:100 dilution) in 20 mmol·L−1 Tris, 150 mmol·L−1 NaCl (pH 7.5). After centrifugation (15 000 g, 30 min, 4°C), protein content of the supernatant was determined using the Bradford assay. Proteins (25 µg protein/lane) were separated by SDS-PAGE (10%) and transferred onto nitrocellulose membranes. Membranes were incubated in a blocking buffer (20 mmol·L−1 Tris, pH 7.5, 137 mmol·L−1 NaCl; TBS) containing 0.1% (v/v) Tween (TTBS) and 5% (w/v) skimmed milk for 1 h at room temperature followed by three 5 min washes in TTBS and incubated overnight with anti-phospho-ERK1/2 antibody (Thr202/Tyr204) or anti-ERK1/2 (diluted 1:4000) in TTBS containing 5% (w/v) BSA. After three washings in TTBS, the membrane was finally incubated in TTBS containing 5% (w/v) skimmed milk for 1 h with a horseradish peroxidase–conjugated anti-rabbit antibody (diluted 1:2000). Immunoreactivity was detected using an enhanced chemiluminescence reaction with ECL reagent (Amersham, GE Healthcare, Buckinghamshire, UK). Visualization of bands was achieved using a ChemiSmart 5000 (Vilbert Lourmat, Marne-la-Vallée, France) apparatus and quantification performed using the Gene Tools software (Ozyme, Saint Quentin en Yvelines, France).
Viability of INS-1 cells
Cell viability was determined using the 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. At the end of the 1 h incubation period, cells were washed with KRB/BSA and KRB/BSA containing 5 mg·mL−1 MTT was added to each well. Plates were then incubated for 3 h in the dark in a humidified atmosphere (5% CO2, 37°C). Cells were washed with PBS and precipitates were dissolved in 50 µL dimethyl sulfoxide (DMSO). The absorbance of the reduced intracellular formazan product was read at 492 nm on a microtiter plate reader (Tecan, Lyon, France). Experiments were performed in quadruplicate.
Image analysis of cytoplasmic Ca2+ in INS-1 cells
The intracellular Ca2+ concentration ([Ca2+]i) was measured using the ratiometric fluorescent Ca2+ indicator fura-2. Briefly, cells sub-cultured for 4 days in Lab-Tek® (Nunc, Naperville, IL, USA) chambers were incubated with 2.5 µmol·L−1 fura-2AM plus 0.02% pluronic acid F-127 as described (Gysembergh et al., 1999). Cells were rinsed with KRB and mounted on a microscope stage (Axiovert, Zeiss, Iena, Germany; ×20 objective lens) for 15 min to allow fura-2AM de-esterification, in presence of quercetin (20 µmol·L−1). Then, drugs were applied onto the cells using a perfusion system. Images were captured digitally, every 2 s, with a cooled CCD camera (Photometrics, Roper Scientific, Evry, France). Cells loaded with fura-2 were illuminated by dual excitation with a UV light source at 340 nm (Ca2+-bound) and 380 nm (Ca2+-free) using a lambda-DG4 excitation system (Sutter Instrument Company, Novato, CA, USA). Fluorescence emission was measured at 510 nm and analysed (Metafluor Software, Universal Imaging Corporation, Downingtown, PA, USA). The changes in [Ca2+]i were deduced from the variations of the F340/F380 ratio after correction for background and dark currents. Data were averaged (at least 100 cells per recorded field/coverglass with n representing the number of fields).
Statistical analysis
Statistical analysis was performed using a Statgraphics, Manugistics (Rockville, MA, USA) program. Multiple-group comparison was based on one-way anova followed by the Fischer's protected least significant difference test. For data obtained from Ca2+ measurements, two-group comparison was based on the Student's t-test. The limit of statistical significance was set at P < 0.05.
Materials
Rabbit anti-ERK1/2 antibody, rabbit anti-phospho-ERK1/2 antibody which selectively recognizes the doubly phosphorylated (Thr202/Tyr204) active form of this kinase and horseradish peroxidase-linked anti-rabbit antibody were purchased from Cell Signaling (New England Biolabs, Beverly, MA, USA). Nitrocellulose transfer membranes (Hybond ECL) and ECL plus Western blotting detection system were obtained from Amersham (GE Healthcare). RPMI-1640 media, FCS and PBS were purchased from Lonza (Levallois Perret, France). UO126 was purchased from Calbiochem (La Jolla, CA, USA). Bio-Rad protein assay was purchased from Bio-Rad Laboratories (Marnes-la-Coquette, France). Quercetin and all other reagents were obtained from Sigma-Aldrich (St. Louis, MO, USA). Insulin concentrations in cell supernatants were determined using the HTRF Insulin assay kit (Cis Bio International, Bagnols-sur-Cèze, France). Quercetin, resveratrol and UO126 were dissolved in DMSO (10 mmol·L−1) and stored at −20°C. When using compounds dissolved in DMSO, control cells were treated with the solvent in the same conditions. The final concentration of DMSO did not exceed 0.1% and did not affect insulin secretion or cell viability (data not shown). Fura-2AM was purchased from Tef-labs (Austin, TX, USA). Pluronic acid F-127 was purchased from Molecular Probes (Invitrogen, Cergy-Pontoise, France). Collagenase P was obtained from Roche Diagnostics (Mannheim, Germany).
Results
Effects of quercetin, resveratrol and NAC on β-cell functionality
Effects of quercetin, resveratrol and NAC on insulin secretion in INS-1 cells
Increasing concentrations of quercetin, resveratrol or NAC (0.2, 2, 20, 200 µmol·L−1) were tested in the absence or presence of glucose (Figure 1). In the absence of glucose, quercetin or resveratrol (but not NAC) induced a modest (about 1.3–1.5-fold) increase in insulin secretion (Figure 1A–C, respectively). Glucose (8.3 mmol·L−1) provoked a fourfold increase in basal insulin secretion (n = 8, P < 0.05). Quercetin (20 µmol·L−1) significantly potentiated 8.3 mmol·L−1 glucose-induced insulin secretion, inducing a 2.5-fold increase (n = 8, P < 0.05) (Figure 1A). However, potentiation did not occur at the highest quercetin concentration (200 µmol·L−1), glucose-induced insulin secretion being reduced to a value close to the basal value. Under the same conditions, resveratrol or NAC (0.2–200 µmol·L−1) did not modify glucose-induced insulin secretion (Figure 1B and C, respectively). Kinetic experiments showed that in INS-1 cells, quercetin (20 µmol·L−1) caused a time-dependent increase in glucose-induced insulin secretion, becoming significant after 40 min incubation (Figure 2).
Figure 1.
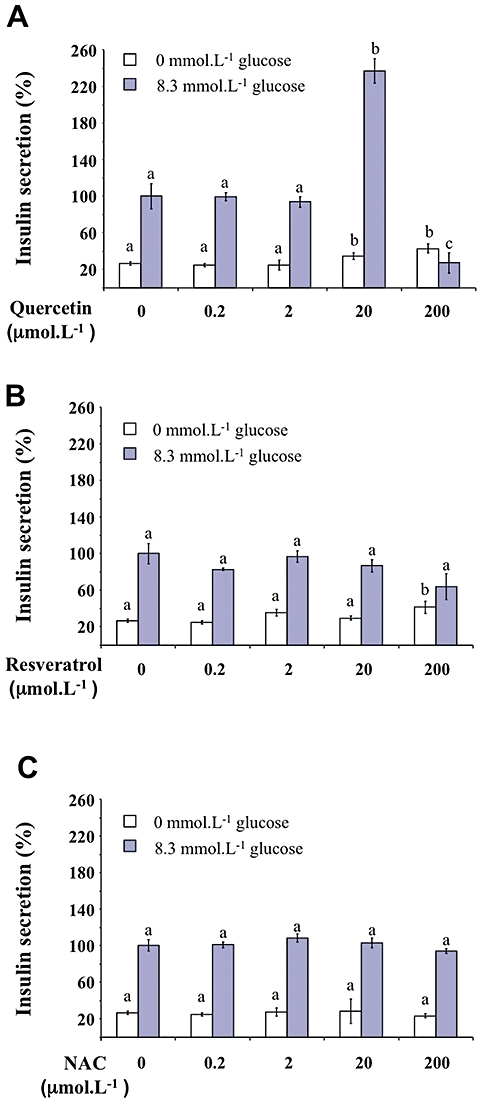
Effects of quercetin, resveratrol and N-acetyl-L-cysteine (NAC) on insulin secretion in INS-1 cells. The effects of increasing concentrations (0.2–200 µmol·L−1) of quercetin (A), resveratrol (B) and NAC (C) were determined in the absence (0 mmol·L−1 glucose) or the presence of 8.3 mmol·L−1 of glucose. Insulin secretion was quantified by the homogeneous time resolved fluorescence method and data are expressed relative to the response obtained with 8.3 mmol·L−1 glucose, taken as 100%. Results are presented as mean ± SEM of 4–6 separate experiments. A multiple comparison analysis of data was performed for each experimental condition (absence or presence of glucose). Bars with the same letter do not differ significantly (P > 0.05).
Figure 2.
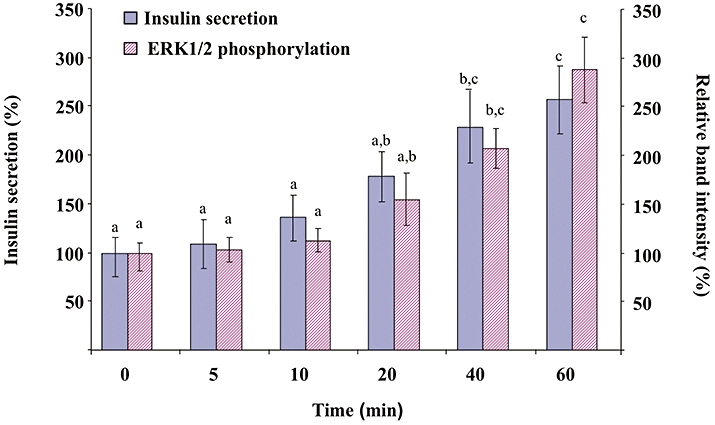
Kinetics of the effect of quercetin on glucose-induced insulin secretion and extracellular signal-regulated kinase (ERK)1/2 phosphorylation in INS-1 cells. INS-1 cells were incubated in the presence of 8.3 mmol·L−1 glucose and in the absence or presence of 20 µmol·L−1 quercetin for various periods, quercetin being added 5, 10, 20, 40 or 60 min before the end of incubation. Insulin secretion was quantified by the homogeneous time resolved fluorescence method. Western blotting experiments were performed; intensity of each band was quantified using a densitometric imager and relative band intensity was calculated as the ratio (phospho ERK1/2/ERK1/2). Data are expressed relative to the response obtained in the absence of quercetin (time = 0), taken as 100%. Results are presented as mean ± SEM (n = 4). A multiple comparison analysis of data was performed for each kind of experiment (insulin secretion or ERK1/2 phosphorylation). Bars with the same letter do not differ significantly (P > 0.05).
Effects of quercetin, resveratrol and NAC on ERK1/2 phosphorylation in INS-1 cells
Because ERK1/2 was shown to participate in the regulation of insulin secretion in β-cells (Longuet et al., 2005), we tested whether the potentiating effect of quercetin on glucose-induced insulin secretion could involve this signalling pathway. Figure 3 presents data obtained from Western blotting experiments carried out on cell lysates prepared after exposure of INS-1 cells to different conditions for 1 h. The dose-response curve for the effect of quercetin (0.2, 2, 5, 20, 50, 200 µmol·L−1) on ERK1/2 phosphorylation in the presence of 8.3 mmol·L−1 glucose showed no significant effect of quercetin from 0.2 to 5 µmol·L−1, a maximal potentiating effect for 20 µmol·L−1 which then decreased to a non-significant effect for 200 µmol·L−1 quercetin (Figure 3A,B). Based on these results, we chose to work with 20 µmol·L−1 quercetin in all subsequent studies. Resveratrol was tested under the same concentration whereas NAC concentration was 200 µmol·L−1, a concentration previously shown to prevent in vitro cellular oxidative stress damages (Pawlas and Małecki, 2009).
Figure 3.
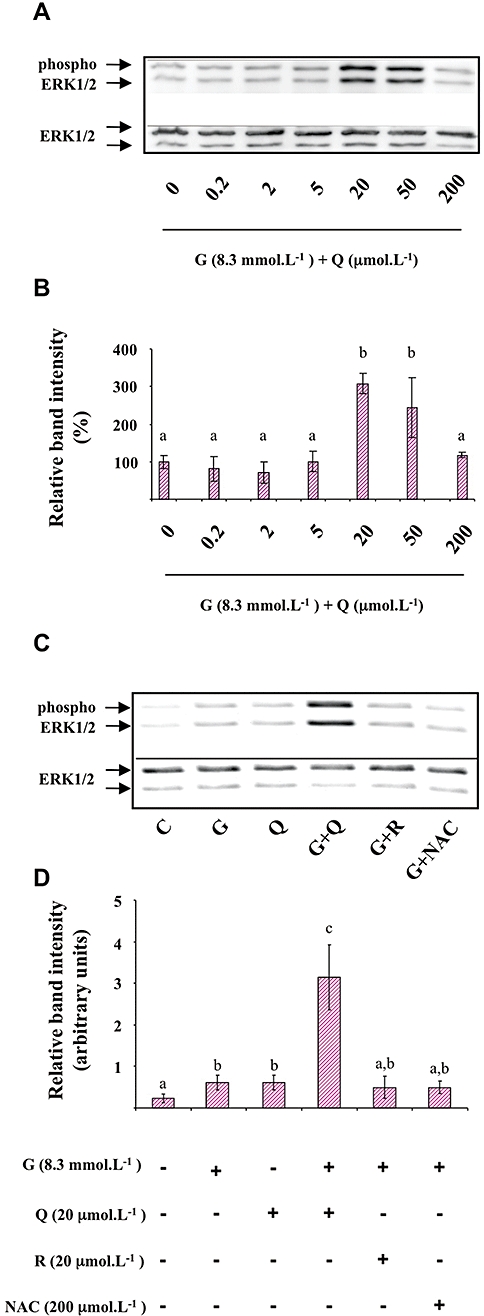
Effects of quercetin, resveratrol and N-acetyl-L-cysteine (NAC) on glucose induced-extracellular signal-regulated kinase (ERK)1/2 phosphorylation in INS-1 cells. INS-1 cells were incubated in the presence of 8.3 mmol·L−1 glucose and increasing concentrations (0.2–200 µmol·L−1) of quercetin (Figure 3A and B). INS-1 cells were incubated in the absence (C) or presence of 8.3 mmol·L−1 glucose (G) and in the absence or presence of quercetin (Q), resveratrol (R) or NAC used at the concentrations indicated (Figure 3C and D). Western blotting experiments were performed; intensity of each band was quantified using a densitometric imager and relative band intensity was calculated as the ratio (phospho ERK1/2/ERK1/2). Typical results are illustrated (Figure 3A and C). Data are expressed relative to the response obtained in the absence of quercetin (0), taken as 100% (B) or as arbitrary units (Figure 3D). Data are mean ± SEM from four separate experiments. A multiple comparison analysis of data was performed. Bars with the same letter do not differ significantly (P > 0.05).
As depicted in Figure 3C,D, glucose (8.3 mmol·L−1) induced a significant ERK1/2 phosphorylation (2.5-fold increase over basal level). When tested in the absence of glucose, quercetin (20 µmol·L−1) induced a significant ERK1/2 phosphorylation (2.5-fold increase over basal level). Resveratrol (20 µmol·L−1) or NAC (200 µmol·L−1) did not significantly modify ERK1/2 phosphorylation in the absence (not shown) or the presence of glucose (Figure 3C,D).
Kinetic experiments presented in Figure 2 indicate that 20 µmol·L−1 quercetin caused a time-dependent increase in glucose-induced ERK1/2 phosphorylation, becoming significant after 40 min incubation (same pattern of kinetics than that observed with insulin secretion).
Effects of quercetin on insulin secretion in rat pancreatic islets
To confirm, in a more physiological model, the potentiating effect of quercetin on glucose-induced insulin secretion demonstrated in INS-1 cells, we used rat isolated islets of Langerhans. Treatment of islets with glucose (8.3 mmol·L−1) stimulated insulin secretion (n = 3, P < 0.05). In the presence of 2.8 mmol·L−1 glucose (a non-stimulant concentration), quercetin (20 µmol·L−1) induced a slight but not statistically significant increase in insulin secretion (n = 3, ns). On the other hand, quercetin potentiated 8.3 mmol·L−1 glucose-induced insulin secretion (n = 3, P < 0.05) (Figure 4).
Figure 4.
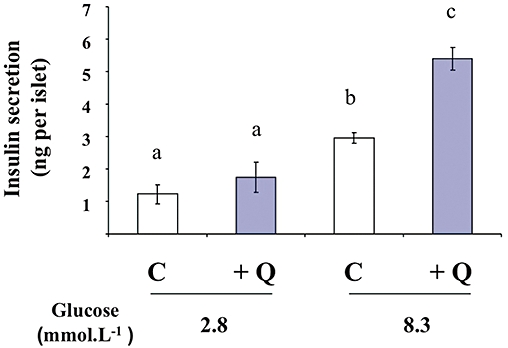
Effects of quercetin on glucose-induced insulin secretion in rat pancreatic islets. Islets were incubated in the absence (C) or presence of 20 µmol·L−1 quercetin (Q), in the presence of glucose used at the concentrations indicated. Insulin secretion was quantified by the homogeneous time resolved fluorescence method and data are expressed in ng per islet. Mean ± SEM from three separate experiments are presented. A multiple comparison analysis of data was performed. Bars with the same letter do not differ significantly (P > 0.05).
Effects of MEK and PKA inhibitors on glucose-induced insulin secretion and ERK1/2 phosphorylation in the presence of quercetin in INS-1 cells
As shown in Figure 5, UO126 (10 µmol·L−1; a pharmacological inhibitor of MEK) significantly diminished glucose-induced insulin secretion and completely abolished the potentiation by quercetin of glucose-induced insulin secretion. The PKA inhibitor H89 (2 µmol·L−1) did not modify either glucose- or glucose plus quercetin-induced insulin secretion (Figure 5). As shown in Figure 6, UO126 (10 µmol·L−1) completely abolished ERK1/2 phosphorylation induced by both glucose alone and by glucose plus quercetin (20 µmol·L−1). In the same experimental conditions, H89 (2 µmol·L−1) did not modify glucose-induced ERK1/2 phosphorylation but significantly reduced the ERK1/2 phosphorylation provoked by glucose plus quercetin.
Figure 5.
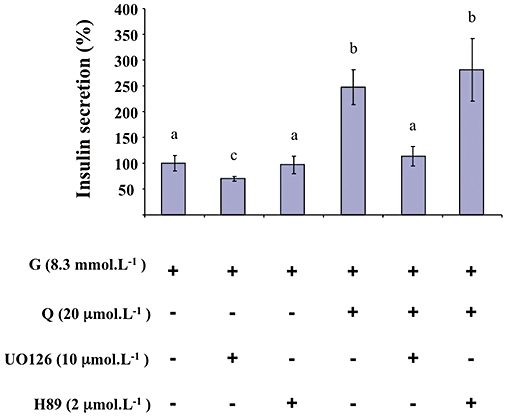
Effects of MEK and PKA inhibitors on glucose-induced insulin secretion potentiated by quercetin in INS-1 cells. INS-1 cells were incubated in the presence of 8.3 mmol·L−1 glucose (G), and in the absence or presence of quercetin (Q), UO126 (MEK inhibitor) and H89 (PKA inhibitor) used at the concentrations indicated. Insulin secretion was quantified by the homogeneous time resolved fluorescence method. Data were expressed relative to the response obtained with 8.3 mmol·L−1 glucose, taken as 100%. Results are presented as mean ± SEM (n = 5). A multiple comparison analysis of data was performed. Bars with the same letter do not differ significantly (P > 0.05).
Figure 6.
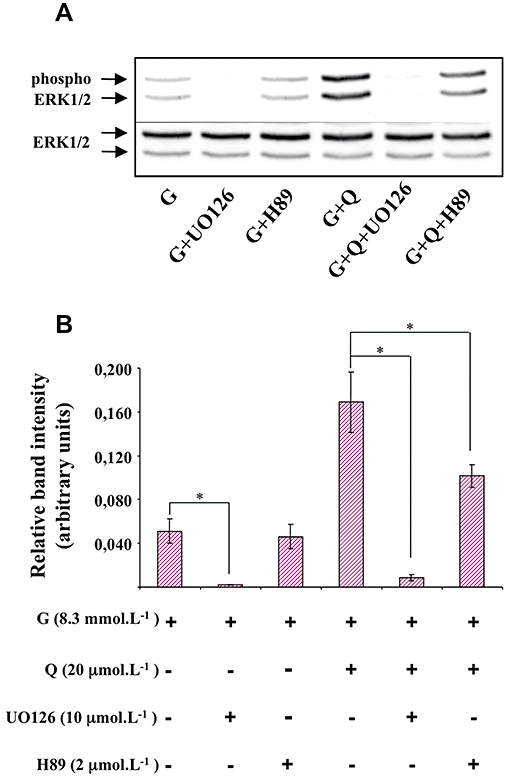
Effects of MEK and PKA inhibitors on extracellular signal-regulated kinase (ERK)1/2 phosphorylation in the presence of glucose and quercetin in INS-1 cells. INS-1 cells were incubated in the presence of 8.3 mmol·L−1 glucose (G), and in the absence or presence of quercetin (Q), UO126 (MEK inhibitor) and H89 (PKA inhibitor) used at the concentrations indicated. Western blotting experiments were performed; intensity of each band was quantified using a densitometric imager and relative band intensity was calculated as the ratio (phospho ERK1/2/ERK1/2). A typical experiment is illustrated (A). Data are mean ± SEM from four separate experiments (B). A multiple comparison analysis of data was performed for each experimental condition (glucose or glucose + quercetin). *P < 0.05, significant difference between the means indicated.
Effects of quercetin, resveratrol and NAC on insulin secretion and ERK1/2 phosphorylation induced by glibenclamide in INS-1 cells
The sulfonylurea glibenclamide (0.01 µmol·L−1) caused the stimulation of both insulin secretion and ERK1/2 phosphorylation (corresponding in both cases to approximately 70% of that induced by 8.3 mmol·L−1 glucose; data not shown). Quercetin (20 µmol·L−1) potentiated both glibenclamide-induced insulin secretion and ERK1/2 phosphorylation (approximately a threefold increase in both cases; Figure 7). Under the same experimental conditions, resveratrol (20 µmol·L−1) slightly amplified glibenclamide-induced insulin secretion while NAC (200 µmol·L−1) was ineffective. None of those compounds were able to significantly modify glibenclamide-induced ERK1/2 phosphorylation (Figure 7).
Figure 7.
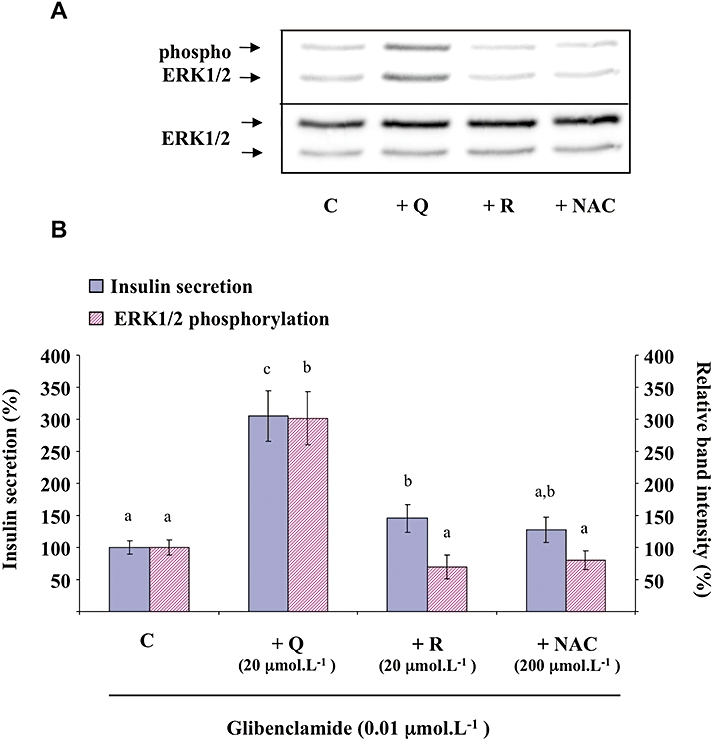
Effects of quercetin, resveratrol and N-acetyl-L-cysteine (NAC) on glibenclamide-induced insulin secretion and extracellular signal-regulated kinase (ERK)1/2 phosphorylation in INS-1 cells. INS-1 cells were incubated in the presence of 0.01 µmol·L−1 glibenclamide and in the absence (C) or presence of quercetin (Q), resveratrol (R) or NAC used at the concentrations indicated. Insulin secretion was quantified by the homogeneous time resolved fluorescence method. Western blotting experiments were performed, intensity of each band was quantified using a densitometric imager and relative band intensity was calculated as the ratio (phospho ERK1/2/ERK1/2). A typical experiment is illustrated (A). For insulin secretion and ERK1/2 phosphorylation, data are expressed relative to the response obtained with 0.01 µmol·L−1 glibenclamide, taken as 100%. Results are presented as mean ± SEM from four separate experiments (B). A multiple comparison analysis of data was performed for each type of experiment (insulin secretion or ERK1/2 phosphorylation). Bars with the same letter do not differ significantly (P > 0.05).
Effects of quercetin on depolarization-induced rise in intracellular calcium in INS-1 cells
Glucose and glibenclamide are known to close KATP channels inducing membrane depolarization, which in turn causes the opening of voltage-gated L-type Ca2+ channels, allowing subsequent Ca2+ entry triggering the exocytosis of insulin-containing granules (Tian et al., 1998; Henquin et al., 2009). To investigate whether the potentiation of insulin secretion by quercetin involves a change in [Ca2+]i, we explored the effect of 20 µmol·L−1 quercetin on INS-1 cells challenged with the depolarizing agent KCl. Fluorescence imaging with the Ca2+ indicator fura-2 enabled us to visualize changes in [Ca2+]i as exemplified in Figure 8A. Application of 15 mmol·L−1 KCl induced a reproducible transient rise in [Ca2+]i as illustrated with two subsequent tests in Figure 8B. In the presence of 20 µmol·L−1 quercetin, an application of KCl induced similar reproducible effect (Figure 8B) but the maximal increase was potentiated (Figure 8C). Quercetin induced a substantial increase (57 ± 12%) which persisted overtime during the response to KCl.
Figure 8.
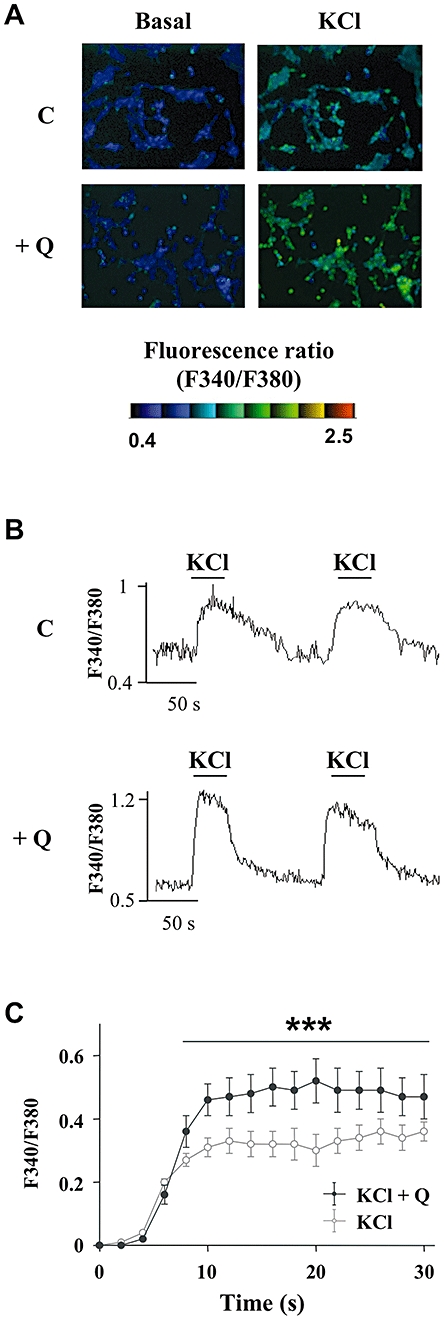
Effects of quercetin on the depolarization-induced rise in intracellular calcium in INS-1 cells. The intracellular Ca2+ concentration ([Ca2+]i) was measured using the ratiometric fluorescent Ca2+ indicator fura-2. Typical cell images illustrating the change in fluorescence induced after addition of 15 mmol·L−1 KCl (right panels) in absence (C) (upper panels) or presence (lower panels) of 20 µmol·L−1 quercetin (Q) are presented (Figure 8A). Typical recording of the rise in [Ca2+]i (fluorescence ratio F340/F380) after addition of 15 mmol·L−1 KCl either under basal KRB condition (C) (upper panel) or after a 15 min pre-incubation period with quercetin 20 µmol·L−1 (Q) (lower panel) is shown (Figure 8B). Time-course of the averaged response to 15 mmol·L−1 KCl in the absence (KCl) or presence of 20 µmol·L−1 quercetin (KCl + Q) is shown (Figure 8C). ***P < 0.001, significant difference between the values indicated.
Effects of quercetin, resveratrol and NAC on INS-1 cells function and viability in the presence of oxidative stress
Effects of oxidative stress on INS-1 cells function and viability
The effects of H2O2 were evaluated both on insulin secretion and β-cells viability. H2O2 (10, 25, 50, 100, 1000 µmol·L−1) did not change basal insulin secretion in the absence of glucose (data not shown). In the presence of 8.3 mmol·L−1 glucose, H2O2 (10–1000 µmol·L−1) inhibited insulin secretion in a concentration-dependent manner, inducing a 60% decrease for 50 µmol·L−1 (Figure 9). In the same conditions, H2O2 provoked a concentration-dependent decrease in cell viability, reaching approximately 50% inhibition for 50 µmol·L−1 (Figure 9). Therefore, in subsequent experiments, quercetin, resveratrol and NAC were tested on β-cell function and viability in the presence of 50 µmol·L−1 H2O2.
Figure 9.
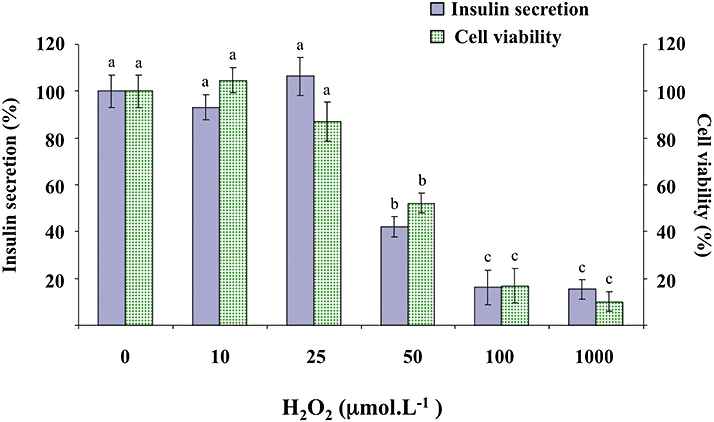
Effects of H2O2 on glucose-induced insulin secretion and viability in INS-1 cells. INS-1 cells were incubated in the presence of glucose (8.3 mmol·L−1) and in the absence or presence of increasing concentrations (10–1000 µmol·L−1) of H2O2. Insulin secretion was quantified by the homogeneous time resolved fluorescence method. Viability was evaluated using the MTT assay. Data are expressed relative to the responses determined in the absence of H2O2, taken as 100%. Results are presented as mean ± SEM (n = 4–6 separate experiments). A multiple comparison analysis of data was performed for each type of experiment (insulin secretion or cell viability). Bars with the same letter do not differ significantly (P > 0.05).
Effects of quercetin, resveratrol and NAC on INS-1 cells functional alterations induced by oxidative stress
As shown in Figure 10, in the presence of H2O2, insulin secretion induced by 8.3 mmol·L−1 glucose plus 20 µmol·L−1 quercetin reached values 2.5-fold higher than control values, a result similar to that obtained in the absence of H2O2 (see Figure 1). Regarding cell viability, quercetin (20 µmol·L−1), resveratrol (20 µmol·L−1) or NAC (200 µmol·L−1) did not modify INS-1 cell viability in the absence of oxidative stress (data not shown). However, quercetin (20 µmol·L−1) prevented H2O2-induced decrease in cell viability (Figure 10). In contrast, when tested in the same experimental conditions, resveratrol (20 µmol·L−1) or NAC (200 µmol·L−1) were devoid of any protective effect on H2O2-induced β-cell dysfunction or impairment of viability (Figure 10).
Figure 10.
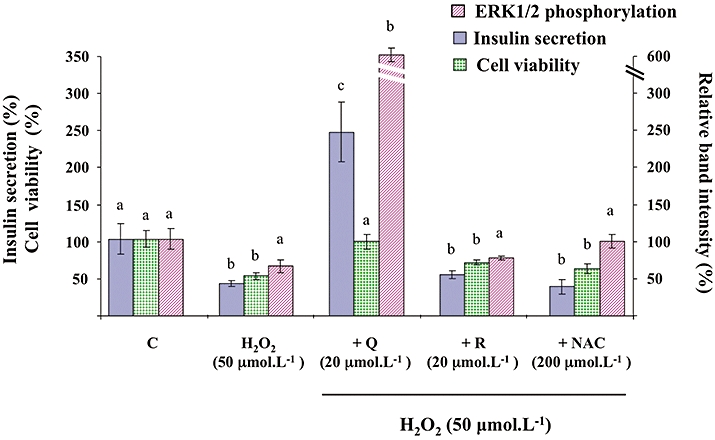
Effects of quercetin, resveratrol and N-acetyl-L-cysteine (NAC) on H2O2-induced changes of glucose-induced insulin secretion, viability and extracellular signal-regulated kinase (ERK)1/2 phosphorylation in INS-1 cells. INS-1 cells were incubated in the presence of glucose (8.3 mmol·L−1) and in the absence (C) or presence of H2O2 alone (H2O2) or with quercetin (+Q), resveratrol (+R) and N-acetyl-L-cysteine (+NAC) used at the concentrations indicated. Insulin secretion was quantified by the homogeneous time resolved fluorescence method. Viability was evaluated using the MTT assay. Western blotting experiments were performed; intensity of each band was quantified using a densitometric imager and relative band intensity was calculated as the ratio (phospho ERK1/2/ERK1/2). Data are expressed relative to the response obtained with 8.3 mmol·L−1 glucose, taken as 100%. In each case, results are presented as mean ± SEM (n = 4–6). A multiple comparison analysis of data was performed for each type of experiment (insulin secretion, cell viability or ERK1/2 phosphorylation). Bars with the same letter do not differ significantly (P > 0.05).
Effect of quercetin on INS-1 cells insulin secretion alteration induced by oxidative stress in the presence of a MEK inhibitor
As shown in Figure 11, in the presence of 10 µmol·L−1 UO126 and 50 µmol·L−1 H2O2, the potentiating effect of quercetin (20 µmol·L−1) on 8.3 mmol·L−1 glucose-induced insulin secretion in the INS-1 cells was not observed.
Figure 11.
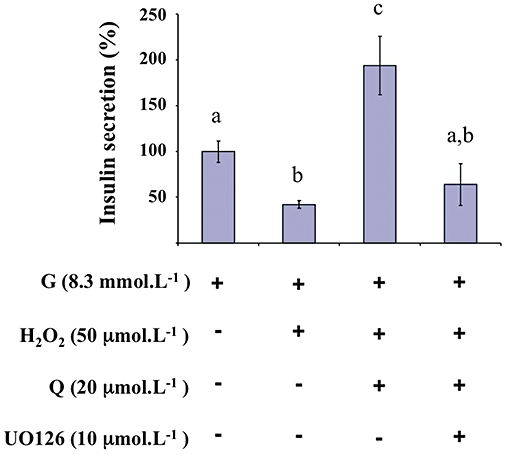
Effects of a MEK inhibitor on glucose-induced insulin secretion in the presence of H2O2 and quercetin in INS-1 cells. INS-1 cells were incubated in the presence of 8.3 mmol·L−1 glucose (G), and in the absence or presence of H2O2, quercetin (Q) and UO126 (MEK inhibitor) used at the concentrations indicated. Insulin secretion was quantified by the homogeneous time resolved fluorescence method. Data are expressed relative to the response obtained with 8.3 mmol·L−1 glucose, taken as 100%. A multiple comparison analysis of data was performed. Bars with the same letter do not differ significantly (P > 0.05).
Effects of quercetin, resveratrol and NAC on glucose-induced ERK1/2 phosphorylation in the presence of oxidative stress
As illustrated in Figure 10, H2O2 (50 µmol·L−1) did not significantly affect ERK1/2 phosphorylation in the INS-1 cells induced by 8.3 mmol·L−1 glucose (P = 0.095). Surprisingly, in the presence of H2O2, glucose-induced ERK1/2 phosphorylation was dramatically amplified by 20 µmol·L−1 quercetin (sixfold increase above the control value, as compared with the threefold increase observed in the absence of H2O2; see Figure 3). When tested in the same experimental conditions, resveratrol (20 µmol·L−1) or NAC (200 µmol·L−1) were devoid of any significant effect on glucose-induced ERK1/2 phosphorylation, in the presence of H2O2.
Discussion and conclusions
As the antioxidant polyphenol quercetin is known to exert antidiabetic properties not yet explored on insulin secreting β-cells, the present work was undertaken to determine its effects on β-cell function and viability under normal and oxidative stress conditions.
We showed that quercetin (20 µmol·L−1) potentiated glucose-induced insulin secretion in a β-cell line, while having only a minor effect in the absence of stimulated insulin secretion. Furthermore, using a more physiological model (rat pancreatic islets), we confirmed the potentiating effect of quercetin on glucose-induced insulin secretion.
Moreover, in INS-1 cells, quercetin was also able to potentiate insulin secretion stimulated by glibenclamide, a KATP channel blocker which triggers membrane depolarization independently of any change in glucose metabolism.
In pancreatic β-cells, glucose or glibenclamide are known to activate the ERK1/2 pathway (Frödin et al., 1995; Briaud et al., 2003; Wang et al., 2008). In this context, we investigated a possible involvement of ERK1/2 in the potentiation by quercetin of glucose- or glibenclamide-induced insulin secretion. We confirmed the observation that glucose or glibenclamide induced ERK1/2 phosphorylation. In INS-1 cells, we also demonstrated that the MEK inhibitor, UO126, completely abolished glucose-induced ERK1/2 phosphorylation and significantly reduced glucose-induced insulin secretion, a result in accordance with previously published data regarding insulin secretion regulation by ERK1/2 (Longuet et al., 2005). Our major and original finding was that quercetin (at a concentration shown to potentiate insulin secretion) was able to phosphorylate ERK1/2 in the absence of glucose or glibenclamide, and to amplify ERK1/2 phosphorylation in their presence in INS-1 cells.
To date, limited data are available regarding the possible effect of quercetin on the ERK1/2 pathway. In accordance with our results, previous studies on cardiomyoblasts or lung cancer cells reported that quercetin was able to stimulate ERK1/2 phosphorylation (Nguyen et al., 2004; Angeloni et al., 2007) whereas, conversely, in a mouse epidermal cell line and in human glioma cells, quercetin inhibited ERK1/2 phosphorylation (Kim et al., 2008; Lee et al., 2008). In our study, the close correlation observed in the INS-1 cells between the time-courses of ERK1/2 phosphorylation and insulin secretion amplification strongly suggests that ERK1/2 plays a crucial role in the potentiation of glucose-induced insulin secretion by quercetin. This is confirmed by our data indicating that the amplification of glucose-induced insulin secretion by quercetin is totally inhibited when ERK1/2 activation is blocked by the MEK inhibitor UO126.
The existence of a cross-talk between the cAMP-PKA and ERK1/2 signalling pathways in β-cells has been reported and compounds which promote cAMP synthesis and PKA subsequent activation (forskolin or GLP-1) amplify both glucose-induced insulin secretion and ERK1/2 phosphorylation (Khoo and Cobb, 1997; Briaud et al., 2003; Longuet et al., 2005). We were therefore interested in exploring whether the cAMP-PKA pathway was involved in the potentiation by quercetin of both glucose-induced insulin secretion and ERK1/2 phosphorylation. In our experimental conditions, in the INS-1 cells, neither glucose-induced insulin secretion nor glucose-induced ERK1/2 phosphorylation were affected by the PKA inhibitor H89 (used at a concentration able to completely suppress glucagon-induced ERK1/2 activation; see Dalle et al., 2004). Apparent discrepancies with previously published data could possibly be explained by differences in the experimental procedures. Indeed, in our experimental conditions, we evaluated ERK1/2 phosphorylation after a 60 min incubation with glucose, as compared with the 5 to 10 min incubation in the previously cited studies. Moreover, H89 did not modify the quercetin-induced potentiation of glucose-induced insulin secretion, despite having some inhibitory effect on glucose plus quercetin-induced ERK1/2 phosphorylation. A major role for PKA in the quercetin potentiating effect of glucose-induced insulin secretion can also be excluded.
As Ca2+ plays a central role in the induction of insulin secretion, we hypothesize that quercetin, through an increase in basal [Ca2+i] and subsequent ERK1/2 activation, could sensitize β-cells to primary stimulants of insulin secretion and thereby amplify insulin response. Indeed, quercetin was shown to increase intracellular Ca2+ level in rat islets of Langerhans (Hii and Howell, 1985) or inhibit sarcoendoplasmic reticulum Ca2+-ATPase (SERCA) pump (responsible for cytosolic Ca2+ removal) (Ogunbayo et al., 2008), knowing that SERCA pump inhibition increased the magnitude of depolarization-triggered Ca2+ entry and subsequent insulin exocytosis in rat pancreatic β-cell (Hughes et al., 2006). In accordance with those data, our results obtained in the INS-1 cells using Ca2+ imaging indicated that quercetin amplifies depolarization-induced intracellular Ca2+ increase.
Finally, the fact that quercetin was able to inhibit glucose-induced insulin secretion in the INS-1 cells at a concentration 10-fold higher than that displaying insulin secretion potentiation (200 µmol·L−1) raises the possibility of a toxicity linked to pro-oxidant effects, as recently described on human non-small lung cancer cells A549 (Robaszkiewicz et al., 2007). In addition, our results are in agreement with data from Chen et al. (2007) showing that resveratrol (but not NAC) exerted only a slight effect on basal insulin secretion. In contrast, neither resveratrol nor NAC was able to affect glucose- (or glibenclamide)-induced insulin secretion or ERK1/2 phosphorylation.
A second part of our work was aimed at studying the effects of quercetin on H2O2-induced INS-1 cell damage, as H2O2 has been commonly used to study ROS-induced alterations in various cell types including β-cells (Xiong et al., 2006; Chen et al., 2009). Our results, obtained at a relatively low and physiologically relevant concentration of H2O2 (50 µmol·L−1) (Dröge, 2002), agree with previous studies showing that H2O2 dose-dependently decreased both β-cell viability and glucose-induced insulin secretion (Rasilainen et al., 2002; Sakai et al., 2003; Xiong et al., 2006). At this concentration, we also noted a trend towards a decrease of glucose-induced ERK1/2 phosphorylation. This result could be explained by recently published work showing that JNK and p38MAPK (two pro-apoptotic protein kinases known to be activated by H2O2; Hou et al., 2008) would activate specific phosphatases negatively regulating the ERK pathway (Junttila et al., 2008).
We also showed that quercetin not only protected viability of INS-1 cells against oxidative stress but also retained its insulin secretion potentiating effect in the presence of H2O2. Regarding the other antioxidants, although some studies have found that NAC protected β-cells against oxidative injury (a result previously obtained at a concentration 10 times higher than that used in our study; Chen et al., 2007; Hou et al., 2008), we found that NAC or resveratrol was inactive in protecting INS-1 cells function or viability against oxidative stress in our experimental conditions. The fact that quercetin, but not resveratrol or NAC, protects β-cells against oxidative damage induced by H2O2 suggests the existence of a mechanism for quercetin, in addition to its antioxidant activity.
Through the inhibition of protein tyrosine phosphatases and subsequent increase of tyrosine phosphorylation, H2O2 has been shown to activate, in different cell types, several signalling protein kinases (Akt/PKB, ERK1/2, PI3K, JNK, p38 MAPK …) regulating mitogenesis, migration, proliferation, survival or death responses (McCubrey et al., 2006; Mehdi et al., 2007; Ahn and Lee, 2008). We hypothesize that quercetin could modulate some of these pathways. Indeed, we found that in the presence of H2O2 and quercetin, ERK1/2 phosphorylation was dramatically amplified when compared with phosphorylation observed in the absence of H2O2, suggesting a crucial role of ERK1/2 in the mechanism of action of quercetin. This hypothesis was confirmed by our data showing that the potentiating effect of quercetin on glucose-induced insulin secretion in the presence of H2O2 was totally inhibited when the ERK1/2 activation was blocked by the MEK inhibitor UO126. Our data suggest that quercetin and H2O2 may act through different mechanisms on a common pathway leading to ERK1/2 phosphorylation. A possible explanation could be that quercetin may inhibit stress-activated protein kinases (such as JNK or p38 MAPK) described to induce ERK dephosphorylation (Junttila et al., 2008; see above). Indeed, quercetin was shown to inhibit JNK activation in various cell types (Ishikawa and Kitamura, 2000; Wang et al., 2002).
In summary, the present study allowed us to show that quercetin, but not the two other antioxidants (resveratrol or NAC), potentiated glucose-induced insulin secretion and protected β-cell function and viability against oxidative damages. Both effects were correlated with a major increase in ERK1/2 phosphorylation, suggesting that ERK1/2 activation was involved in the actions of quercetin. Whether quercetin activates ERK1/2 phosphorylation directly or indirectly by inhibiting its dephosphorylation remains to be determined. The potential of quercetin in preventing β-cell dysfunction deserves to be further studied.
Acknowledgments
Estelle Youl was supported in part by a grant from French Foreign Minister (Egide). The technical assistance of Michel Tournier is gratefully acknowledged.
Glossary
Abbreviations
- BSA
bovine serum albumin
- [Ca2+]i
intracellular Ca2+ concentration
- CaMKII
Ca2+ and calmodulin-dependent protein kinase II
- DMSO
dimethyl sulfoxide
- ERK1/2
extracellular signal-regulated kinases 1/2
- FCS
fetal calf serum
- HTRF
homogeneous time resolved fluorescence
- JNK
c-jun N-terminal kinase
- KRB
Krebs-Ringer bicarbonate buffer
- MTT
[3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide]
- NAC
N-acetyl-L-cysteine
- p38 MAPK
p38 mitogen-activated protein kinase
- PKA
protein kinase A
- PBS
phosphate buffered saline
- PKC
protein kinase C
- ROS
reactive oxygen species
- SERCA
sarcoendoplasmic reticulum Ca2+-ATPase
Conflicts of interest
None.
Supplemental material
References
- Ahn JH, Lee M. Tyrosine phosphorylation and Ras activation is required for hydrogen peroxide-mediated Raf-1 kinase activation. Mol Cell Biochem. 2008;317:121–129. doi: 10.1007/s11010-008-9839-9. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 4th edn. Br J Pharmacol. 2009;158(Suppl. 1):S1–S254. doi: 10.1111/j.1476-5381.2009.00499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angeloni C, Spencer JP, Leoncini E, Biagi PL, Hrelia S. Role of quercetin and its in vivo metabolites in protecting H9c2 cells against oxidative stress. Biochimie. 2007;89:73–82. doi: 10.1016/j.biochi.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Asfari M, Janjic D, Meda P, Li G, Halban PA, Wollheim CB. Establishment of 2-mercaptoethanol-dependent differentiated insulin-secreting cell lines. Endocrinology. 1992;130:167–178. doi: 10.1210/endo.130.1.1370150. [DOI] [PubMed] [Google Scholar]
- Benes C, Roisin MP, Van Tan H, Creuzet C, Miyazaki J, Fagard R. Rapid activation and nuclear translocation of mitogen-activated protein kinases in response to physiological concentration of glucose in the MIN6 pancreatic ß cell line. J Biol Chem. 1998;273:15507–15513. doi: 10.1074/jbc.273.25.15507. [DOI] [PubMed] [Google Scholar]
- Bonora E. Protection of pancreatic beta-cells: is it feasible? Nutr Metab Cardiovasc Dis. 2008;18:74–83. doi: 10.1016/j.numecd.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Briaud I, Lingohr MK, Dickson LM, Wrede CE, Rhodes CJ. Differential activation mechanisms of Erk-1/2 and p70(S6K) by glucose in pancreatic beta-cells. Diabetes. 2003;52:974–983. doi: 10.2337/diabetes.52.4.974. [DOI] [PubMed] [Google Scholar]
- Chen L, Liu L, Yin J, Luo Y, Huang S. Hydrogen peroxide-induced neuronal apoptosis is associated with inhibition of protein phosphatase 2A and 5, leading to activation of MAPK pathway. Int J Biochem Cell Biol. 2009;41:1284–1295. doi: 10.1016/j.biocel.2008.10.029. [DOI] [PubMed] [Google Scholar]
- Chen WP, Chi TC, Chuang LM, Su MJ. Resveratrol enhances insulin secretion by blocking K(ATP) and K(V) channels of beta cells. Eur J Pharmacol. 2007;568:269–277. doi: 10.1016/j.ejphar.2007.04.062. [DOI] [PubMed] [Google Scholar]
- Chiasson JL, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M, STOP-NIDDM Trail Research Group Acarbose for prevention of type 2 diabetes mellitus: the STOP-NIDDM randomised trial. Lancet. 2002;359:2072–2077. doi: 10.1016/S0140-6736(02)08905-5. [DOI] [PubMed] [Google Scholar]
- Coskun O, Kanter M, Korkmaz A, Oter S. Quercetin, a flavonoid antioxidant, prevents and protects streptozotocin-induced oxidative stress and beta-cell damage in rat pancreas. Pharmacol Res. 2005;51:117–123. doi: 10.1016/j.phrs.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Costes S, Broca C, Bertrand G, Lajoix AD, Bataille D, Bockaert J, et al. ERK1/2 control phosphorylation and protein level of cAMP-responsive element-binding protein: a key role in glucose-mediated pancreatic beta-cell survival. Diabetes. 2006;55:2220–2230. doi: 10.2337/db05-1618. [DOI] [PubMed] [Google Scholar]
- Dalle S, Longuet C, Costes S, Broca C, Faruque O, Fontés G, et al. Glucagon promotes cAMP-response element-binding protein phosphorylation via activation of ERK1/2 in MIN6 cell line and isolated islets of Langerhans. J Biol Chem. 2004;279:20345–20355. doi: 10.1074/jbc.M312483200. [DOI] [PubMed] [Google Scholar]
- Docherty K, Steiner DF. Molecular and cellular biology of the beta cell. In: Portes D, Sherwin R, editors. Ellenberg and Rifkin's Diabetis Mellitus. 5th edn. Stamford, CT: Appleton & Lange; 1996. pp. 29–48. [Google Scholar]
- Dröge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- Evans JL, Goldfine ID, Maddux BA, Grodsky GM. Are oxidative stress-activated signaling pathways mediators of insulin resistance and beta-cell dysfunction? Diabetes. 2003;52:1–8. doi: 10.2337/diabetes.52.1.1. [DOI] [PubMed] [Google Scholar]
- Frödin M, Sekine N, Roche E, Filloux C, Prentki M, Wollheim CB, et al. Glucose, other secretagogues, and nerve growth factor stimulate mitogen-activated protein kinase in the insulin-secreting beta-cell line, INS-1. J Biol Chem. 1995;270:7882–7889. doi: 10.1074/jbc.270.14.7882. [DOI] [PubMed] [Google Scholar]
- Gysembergh A, Lemaire S, Piot C, Sportouch C, Richard S, Kloner RA, et al. Pharmacological manipulation of Ins(1,4,5)P3 signaling mimics preconditioning in rabbit heart. Am J Physiol. 1999;277:H2458–H2469. doi: 10.1152/ajpheart.1999.277.6.H2458. [DOI] [PubMed] [Google Scholar]
- Henquin JC, Nenquin M, Ravier MA, Szollosi A. Shortcomings of current models of glucose-induced insulin secretion. Diabetes Obes Metab. 2009;11:168–179. doi: 10.1111/j.1463-1326.2009.01109.x. [DOI] [PubMed] [Google Scholar]
- Hii CS, Howell SL. Effects of flavonoids on insulin secretion and 45Ca2+ handling in rat islets of Langerhans. J Endocrinol. 1985;107:1–8. doi: 10.1677/joe.0.1070001. [DOI] [PubMed] [Google Scholar]
- Hou N, Torii S, Saito N, Hosaka M, Takeuchi T. Reactive oxygen species-mediated pancreatic beta-cell death is regulated by interactions between stress-activated protein kinases, p38 and c-Jun N-terminal kinase, and mitogen-activated protein kinase phosphatases. Endocrinology. 2008;149:1654–1665. doi: 10.1210/en.2007-0988. [DOI] [PubMed] [Google Scholar]
- Hughes E, Lee AK, Tse A. Dominant role of sarcoendoplasmic reticulum Ca2+-ATPase pump in Ca2+ homeostasis and exocytosis in rat pancreatic beta-cells. Endocrinology. 2006;147:1396–1407. doi: 10.1210/en.2005-1023. [DOI] [PubMed] [Google Scholar]
- Ishikawa Y, Kitamura M. Anti-apoptotic effect of quercetin: intervention in the JNK- and ERK-mediated apoptotic pathways. Kidney Int. 2000;58:1078–1087. doi: 10.1046/j.1523-1755.2000.00265.x. [DOI] [PubMed] [Google Scholar]
- Junttila MR, Li SP, Westermarck J. Phosphatase-mediated crosstalk between MAPK signaling pathways in the regulation of cell survival. FASEB J. 2008;22:954–965. doi: 10.1096/fj.06-7859rev. [DOI] [PubMed] [Google Scholar]
- Kaneto H, Kajimoto Y, Miyagawa J, Matsuoka T, Fujitani Y, Umayahara Y, et al. Beneficial effects of antioxidants in diabetes: possible protection of pancreatic beta-cells against glucose toxicity. Diabetes. 1999;48:2398–2406. doi: 10.2337/diabetes.48.12.2398. [DOI] [PubMed] [Google Scholar]
- Kaneto H, Matsuoka TA, Nakatani Y, Kawamori D, Matsuhisa M, Yamasaki Y. Oxidative stress and the JNK pathway in diabetes. Curr Diabetes Rev. 2005a;1:65–72. doi: 10.2174/1573399052952613. [DOI] [PubMed] [Google Scholar]
- Kaneto H, Nakatani Y, Kawamori D, Miyatsuka T, Matsuoka TA, Matsuhisa M, et al. Role of oxidative stress, endoplasmic reticulum stress, and c-Jun N-terminal kinase in pancreatic beta-cell dysfunction and insulin resistance. Int J Biochem Cell Biol. 2005b;37:1595–1608. doi: 10.1016/j.biocel.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Khoo S, Cobb MH. Activation of mitogen-activating protein kinase by glucose is not required for insulin secretion. Proc Natl Acad Sci U S A. 1997;94:5599–5604. doi: 10.1073/pnas.94.11.5599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim EJ, Choi CH, Park JY, Kang SK, Kim YK. Underlying mechanism of quercetin-induced cell death in human glioma cells. Neurochem Res. 2008;33:971–979. doi: 10.1007/s11064-007-9416-8. [DOI] [PubMed] [Google Scholar]
- Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Diabetes Prevention Program Research Group Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowler WC, Hamman RF, Edelstein SL, Barrett-Connor E, Ehrmann DA, Walker EA, et al. Diabetes Prevention Program Research Group Prevention of type 2 diabetes with troglitazone in the Diabetes Prevention Program. Diabetes. 2005;54:1150–1156. doi: 10.2337/diabetes.54.4.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson KL, Hoenig M. Identification and subcellular characterization of protein kinase C isoforms in insulinoma ß-cells and whole islets. Endocrinology. 1994;135:881–886. doi: 10.1210/endo.135.3.8070382. [DOI] [PubMed] [Google Scholar]
- Kobori M, Masumoto S, Akimoto Y, Takahashi Y. Dietary quercetin alleviates diabetic symptoms and reduces streptozotocin-induced disturbance of hepatic gene expression in mice. Mol Nutr Food Res. 2009;53:859–868. doi: 10.1002/mnfr.200800310. [DOI] [PubMed] [Google Scholar]
- Lacy PE, Kostianovsky M. Method for the isolation of intact islets of Langerhans from the rat pancreas. Diabetes. 1967;16:35–39. doi: 10.2337/diab.16.1.35. [DOI] [PubMed] [Google Scholar]
- Lee KW, Kang NJ, Heo YS, Rogozin EA, Pugliese A, Hwang MK, et al. Raf and MEK protein kinases are direct molecular targets for the chemopreventive effect of quercetin, a major flavonol in red wine. Cancer Res. 2008;68:946–955. doi: 10.1158/0008-5472.CAN-07-3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenzen S. Oxidative stress: the vulnerable beta-cell. Biochem Soc Trans. 2008;36:343–347. doi: 10.1042/BST0360343. [DOI] [PubMed] [Google Scholar]
- Li N, Frigerio F, Maechler P. The sensitivity of pancreatic beta-cells to mitochondrial injuries triggered by lipotoxicity and oxidative stress. Biochem Soc Trans. 2008;36:930–934. doi: 10.1042/BST0360930. [DOI] [PubMed] [Google Scholar]
- Longuet C, Broca C, Costes S, Hani EH, Bataille D, Dalle S. Extracellularly regulated kinases 1/2 (p44/42 mitogen-activated protein kinases) phosphorylate synapsin I and regulate insulin secretion in the MIN6 beta-cell line and islets of Langerhans. Endocrinology. 2005;146:643–654. doi: 10.1210/en.2004-0841. [DOI] [PubMed] [Google Scholar]
- McCubrey JA, Lahair MM, Franklin RA. Reactive oxygen species-induced activation of the MAP kinase signaling pathways. Antioxid Redox Signal. 2006;8:1775–1789. doi: 10.1089/ars.2006.8.1775. [DOI] [PubMed] [Google Scholar]
- Mahesh T, Menon VP. Quercetin allievates oxidative stress in streptozotocin-induced diabetic rats. Phytother Res. 2004;18:123–127. doi: 10.1002/ptr.1374. [DOI] [PubMed] [Google Scholar]
- Meghana K, Sanjeev G, Ramesh B. Curcumin prevents streptozotocin-induced islet damage by scavenging free radicals: a prophylactic and protective role. Eur J Pharmacol. 2007;577:183–191. doi: 10.1016/j.ejphar.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Mehdi MZ, Azar ZM, Srivastava AK. Role of receptor and nonreceptor protein tyrosine kinases in H2O2-induced PKB and ERK1/2 signaling. Cell Biochem Biophys. 2007;47:1–10. doi: 10.1385/cbb:47:1:1. [DOI] [PubMed] [Google Scholar]
- Nguyen TT, Tran E, Nguyen TH, Do PT, Huynh TH, Huynh H. The role of activated MEK-ERK pathway in quercetin-induced growth inhibition and apoptosis in A549 lung cancer cells. Carcinogenesis. 2004;25:647–659. doi: 10.1093/carcin/bgh052. [DOI] [PubMed] [Google Scholar]
- Ogunbayo OA, Harris RM, Waring RH, Kirk CJ, Michelangeli F. Inhibition of the sarcoplasmic/endoplasmic reticulum Ca2+-ATPase by flavonoids: a quantitative structure-activity relationship study. IUBMB Life. 2008;60:853–858. doi: 10.1002/iub.132. [DOI] [PubMed] [Google Scholar]
- Palsamy P, Subramanian S. Resveratrol, a natural phytoalexin, normalizes hyperglycemia in streptozotocin-nicotinamide induced experimental diabetic rats. Biomed Pharmacother. 2008;62:598–605. doi: 10.1016/j.biopha.2008.06.037. [DOI] [PubMed] [Google Scholar]
- Pawlas N, Małecki A. Neuroprotective effect of N-acetylcysteine in neurons exposed to arachidonic acid during simulated ischemia in vitro. Pharmacol Rep. 2009;61:743–750. doi: 10.1016/s1734-1140(09)70129-x. [DOI] [PubMed] [Google Scholar]
- Poitout V, Robertson RP. Glucolipotoxicity: fuel excess and beta-cell dysfunction. Endocr Rev. 2008;29:351–366. doi: 10.1210/er.2007-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasilainen S, Nieminen JM, Levonen AL, Otonkoski T, Lapatto R. Dose-dependent cysteine-mediated protection of insulin-producing cells from damage by hydrogen peroxide. Biochem Pharmacol. 2002;63:1297–1304. doi: 10.1016/s0006-2952(02)00864-x. [DOI] [PubMed] [Google Scholar]
- Robaszkiewicz A, Balcerczyk A, Bartosz G. Antioxidative and prooxidative effects of quercetin on A549 cells. Cell Biol Int. 2007;31:1245–1250. doi: 10.1016/j.cellbi.2007.04.009. [DOI] [PubMed] [Google Scholar]
- Sakai K, Matsumoto K, Nishikawa T, Suefuji M, Nakamaru K, Hirashima Y, et al. Mitochondrial reactive oxygen species reduce insulin secretion by pancreatic beta-cells. Biochem Biophys Res Commun. 2003;300:216–222. doi: 10.1016/s0006-291x(02)02832-2. [DOI] [PubMed] [Google Scholar]
- Sartor G, Scherstén B, Carlström S, Melander A, Nordén A, Persson G. Ten-year follow-up of subjects with impaired glucose tolerance: prevention of diabetes by tolbutamide and diet regulation. Diabetes. 1980;29:41–49. doi: 10.2337/diab.29.1.41. [DOI] [PubMed] [Google Scholar]
- Scalbert A, Manach C, Morand C, Rémésy C, Jiménez L. Dietary polyphenols and the prevention of diseases. Crit Rev Food Sci Nutr. 2005;45:287–306. doi: 10.1080/1040869059096. [DOI] [PubMed] [Google Scholar]
- Shen W, Liu K, Tian C, Yang L, Li X, Ren J, et al. Protective effects of R-alpha-lipoic acid and acetyl-L-carnitine in MIN6 and isolated rat islet cells chronically exposed to oleic acid. J Cell Biochem. 2008;104:1232–1243. doi: 10.1002/jcb.21701. [DOI] [PubMed] [Google Scholar]
- Takatori A, Ishii Y, Itagaki S, Kyuwa S, Yoshikawa Y. Amelioration of the beta-cell dysfunction in diabetic APA hamsters by antioxidants and AGE inhibitor treatments. Diabetes Metab Res Rev. 2004;20:211–218. doi: 10.1002/dmrr.428. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Gleason CE, Tran PO, Harmon JS, Robertson RP. Prevention of glucose toxicity in HIT-T15 cells and Zucker diabetic fatty rats by antioxidants. Proc Natl Acad Sci U S A. 1999;96:10857–10862. doi: 10.1073/pnas.96.19.10857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian YA, Johnson G, Ashcroft SJ. Sulfonylureas enhance exocytosis from pancreatic beta-cells by a mechanism that does not involve direct activation of protein kinase C. Diabetes. 1998;47:1722–1726. doi: 10.2337/diabetes.47.11.1722. [DOI] [PubMed] [Google Scholar]
- Vessal M, Hemmati M, Vasei M. Antidiabetic effects of quercetin in streptozocin-induced diabetic rats. Comp Biochem Physiol C. 2003;135C:357–364. doi: 10.1016/s1532-0456(03)00140-6. [DOI] [PubMed] [Google Scholar]
- Wang L, Matsushita K, Araki I, Takeda M. Inhibition of c-Jun N-terminal kinase ameliorates apoptosis induced by hydrogen peroxide in the kidney tubule epithelial cells (NRK-52E) Nephron. 2002;91:142–147. doi: 10.1159/000057616. [DOI] [PubMed] [Google Scholar]
- Wang Q, Heimberg H, Pipeleers D, Ling Z. Glibenclamide activates translation in rat pancreatic beta cells through calcium-dependent mTOR, PKA and MEK signalling pathways. Diabetologia. 2008;51:1202–1212. doi: 10.1007/s00125-008-1026-8. [DOI] [PubMed] [Google Scholar]
- Wenham RM, Landt M, Easom RA. Glucose activates the multifunctional calcium/calmodulin dependent protein kinase II in isolated rat pancreatic islets. J Biol Chem. 1994;269:4947–4952. [PubMed] [Google Scholar]
- Williams RJ, Spencer JP, Rice-Evans C. Flavonoids: antioxidants or signalling molecules? Free Radic Biol Med. 2004;36:838–849. doi: 10.1016/j.freeradbiomed.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Xiong FL, Sun XH, Gan L, Yang XL, Xu HB. Puerarin protects rat pancreatic islets from damage by hydrogen peroxide. Eur J Pharmacol. 2006;529:1–7. doi: 10.1016/j.ejphar.2005.10.024. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Jayaprakasam B, Seeram NP, Olson LK, DeWitt D, Nair MG. Insulin secretion and cyclooxygenase enzyme inhibition by cabernet sauvignon grape skin compounds. J Agric Food Chem. 2004;52:228–233. doi: 10.1021/jf034616u. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


