Abstract
HIV-1 neuropathology results from collective effects of viral proteins and inflammatory mediators on several cell types. Significant damage is mediated indirectly through inflammatory conditions promulgated by glial cells, including microglia that are productively infected by HIV-1, and astroglia. Neural and glial progenitors exist in both developing and adult brains. To determine whether progenitors are targets of HIV-1, a multi-plex assay was performed to assess chemokine/cytokine expression after treatment with viral proteins Tat or gp120. In the initial screen, ten analytes were basally released by murine striatal progenitors. The beta-chemokines CCL5/RANTES, CCL3/MIP-1α, and CCL4/MIP-1β were increased by 12 h exposure to HIV-1 Tat. Secreted factors from Tat-treated progenitors were chemoattractive towards microglia, an effect blocked by 2D7 anti-CCR5 antibody pretreatment. Tat and opiates have interactive effects on astroglial chemokine secretion, but this interaction did not occur in progenitors. gp120 did not affect chemokine/cytokine release, although both CCR5 and CXCR4, which serve as gp120 co-receptors, were detected in progenitors. We postulate that chemokine production by progenitors may be a normal, adaptive process that encourages immune inspection of newly generated cells. Pathogens such as HIV might usurp this function to create a maladaptive state, especially during development or regeneration, when progenitors are numerous.
Keywords: striatum, neuroAIDs, microglia, RANTES, CCL5, MIP-1α, CCL3, MIP-1β, CCL4, CCR5
Introduction
Human immunodeficiency virus (HIV) infection within the central nervous system (CNS) causes a variety of neuropathological changes. Neurons are not generally thought to be infected by HIV, although latent neuron infection has been reported (Trillo-Pazos et al. 2003, Torres-Munoz et al. 2001). Neuropathology is instead mediated by direct neurotoxic actions of released viral proteins, or secondarily, through toxic effects orchestrated by glial cells (Kaul et al. 2001, Gendelman et al. 1994, Persidsky & Gendelman 2003, Hauser et al. 2007, Brack-Werner 1999, Kramer-Hammerle et al. 2005b). HIV-infected macrophages/microglia reaching the brain create a reservoir of viral infection, and lay the groundwork for inflammation leading to neuropathology and cognitive changes. Although there is little evidence that macroglial cells in vivo are productively infected by HIV (Kramer-Hammerle et al. 2005b, Brack-Werner 1999, Gorry et al. 2003), activation of astroglia by viral proteins, or by substances released from reactive microglia, can amplify brain inflammation and neurotoxic sequelae, and also promote infiltration of infected monocytes from the periphery. Thus, HIV neuropathology results from collective effects of viral proteins and inflammatory mediators on several cell types.
Astroglia from humans and rodents secrete chemokine/cytokines in response to HIV-1 transactivator of transcription (Tat) protein (Nath et al. 1999, El-Hage et al. 2005, Kutsch et al. 2000, McManus et al. 2000, Rappaport et al. 1999, Conant et al. 1998). We have shown that Tat-induced [Ca2+]i responses mediate CCL2/MCP-1, CCL5/RANTES and interleukin-6 (IL-6) release, resulting in downstream signaling through NFκB-dependent pathways (El-Hage et al. 2005, El-Hage et al. 2008b). Concurrent exposure to morphine exacerbates Tat-induced chemokine/cytokine production and microglial activation through CCL5/RANTES-driven amplification of CCL2/MCP-1 (El-Hage et al. 2008a, El-Hage et al. 2006a, El-Hage et al. 2006b, Bruce-Keller et al. 2008), an observation that may partly explain relatively high incidences of microglial activation, neuropathology and cognitive disturbance among HIV patients who abuse opiates (Bell et al. 2006, Arango et al. 2004, Anthony et al. 2008, Bouwman et al. 1998, Dougherty et al. 2002). Astroglia are also sensitive to gp120, which can elevate [Ca2+]i (Codazzi et al. 1996, Holden et al. 1999), and alter gene expression (Wang et al. 2004, Galey et al. 2003) leading to chemokine/cytokine secretion (Buriani et al. 1999, Kong et al. 1996, Ronaldson & Bendayan 2006, Yeung et al. 1995), with some evidence for exacerbation by opioids (Mahajan et al. 2005). In our hands, Tat generally elicits more chemokine/cytokine secretion than gp120, and the responsivity varies with brain regional (Fitting et al. 2010). Responses of astroglia to other HIV-1 proteins have been less well studied (Kramer-Hammerle et al. 2005a, Lehmann et al. 2006).
We were intrigued by the possibility that less differentiated CNS cells, in addition to microglia and astroglia, might secrete inflammatory mediators. This would parallel situations in other tissues. Unstimulated bone marrow or cord-derived mesenchymal stem cells secrete a spectrum of chemokine/cytokines and growth factors, including multiple FGFs, interleukins, IGF-1, leukemia inhibitory factor, CCL2/MCP-1, MIP-1α, MIP-1β, SDF-1, and VEGF (Rafei et al. 2008, Croitoru-Lamoury et al. 2007, Schinkothe et al. 2008, Chen et al. 2008, Liu & Hwang 2005, Wagner et al. 2007). As mesenchymal stem cells differentiate, the balance of factors released varies with cell fate (Molloy et al. 2009, Kilroy et al. 2007). Neural progenitor cells (NPCs), which derive from undifferentiated neuroepithelial cells, are a self-renewing and multipotential source of neurons and macroglial cells. Common markers for NPCs include the intermediate filament nestin and the transcription factor Sox2 (sex determining region of Y (SRY)-related HMG-box gene 2). As NPCs differentiate, they become largely restricted to either neuronal or glial fates. Differentiating glial-restricted progenitors (GPCs) express markers typical of oligodendrocytes (e.g. Olig1, Olig2, Sox10, myelin proteins) or astroglia (e.g. GFAP, EAAT2). Nestin+ and Sox2+ cells continue to be found in the mature CNS, although in more restricted germinal zones (Komitova & Eriksson 2004, Ellis et al. 2004). There is evidence that neural progenitors may have a secretory role. For example, human NPCs expressing nestin and A2B5 release IP-10/CXCL10 and MCP-1/CCL2 after exposure to TNF-α (Sheng et al. 2005). NPCs also secrete neurotrophins and other growth regulators (Llado et al. 2004, Benoit et al. 2001, Shingo et al. 2001, Taupin et al. 2000), and transplantation of stem cells and/or NPCs increases their own survival (autocrine effects), as well as promoting neuron survival after injury (paracrine effects) (Llado et al. 2004, Chang et al. 2003).
Effects of HIV proteins on NPCs or GPCs are relatively unexplored, and are likely different from effects on mature glia. They may be critically important to pediatric patients, who frequently present with early and with more pathological forms of neuroAIDS (Drotar et al. 1997, Van Rie et al. 2007, Lobato et al. 1995). The majority of these patients are infected at birth, when progenitors are numerous and glial populations are still developing. The present study was designed to determine whether NPC/GPCs from the striatum have the capacity to produce chemokines/cytokines, and if so, whether exposure to HIV proteins can modify production, with potential functional consequences.
Methods
Experimental protocols conformed to local Institutional Animal Care and Use Committee (IACUC) and national (PHS) guidelines on the care and ethical use of animals. Experiments were designed to minimize the total number of animals used and their discomfort.
Progenitor Cell Cultures
The striatum is particularly vulnerable to both HIV-induced and opioid-induced degenerative changes, and is a region of great interest for the synergistic effects of these two insults. Progenitor cultures were prepared from striata of embryonic day 15 (E15) mice using modifications of a previously published technique (Khurdayan et al. 2004). In brief, embryos were aseptically removed from the uterus of timed pregnant ICR dams (Charles River, Boston, MA) following cervical dislocation. Striata from 10–12 embryos were dissected from the rest of the cortex, finely minced, and incubated with DNase (0.015 mg/ml, Sigma-Aldrich Co., St. Louis, MO) in Dulbecco's Modified Eagle's Medium (DMEM) (15 min, 37 C). Tissue was triturated through a 5 ml pipette, centrifuged, and resuspended in 5 ml of defined, progenitor maintenance medium containing 1:1 DMEM/F12 (Invitrogen Corp., Carlsbad, CA) and 10% Knockout SR (Invitrogen), a commercial serum replacement product that does not contain serum or growth factors. Additives included insulin (25 μg/ml), transferrin (100 μg/ml), progesterone (20 nM), putresceine (60 μM), Na-HCO3 (6mM), and sodium selenite (30 nM) (all from Sigma-Aldrich Co.). Resuspended cells were filtered through a 70 μm nylon Cell strainer (BD Falcon, Bedford, MA), counted, centrifuged as above, and resuspended in an appropriate volume of the same medium to obtain desired plating densities. Cells were plated on either poly-L-lysine coated 24 well plates for multi-plex assay (105 cells per well) or poly-L-lysine coated 15 mm glass coverslips for immunostaining (5×104 cells in 150 μl), and medium was changed at 2 days. Cells were grown at 37°C, in a humidified 5% CO2 atmosphere. At 5 days the medium was refreshed again and cells were treated with Tat1-86 (100 nM; ImmunoDiagnostics, Woburn, MA), and/or gp120 (MN strain; 500 pM; ImmunoDiagnostics), and/or morphine sulphate (500nM; NIDA Drug Supply System, Rockville, MD), and/or naloxone (1.5 μM; Sigma-Aldrich, St. Louis, MO). Morphine, the major bioactive product of heroin in the brain, is a potent μ-opioid receptor agonist, and naloxone is a broad spectrum opioid receptor antagonist. These are concentrations of HIV proteins and opioids that have elicited functional effects in astroglia, oligodendrocytes, and neural progenitors in our earlier studies (Hauser et al. 2009, El-Hage et al. 2005, Buch et al. 2007, Khurdayan et al. 2004). In studies with naloxone, the inhibitor was applied 30 min prior to other treatments, which were applied simultaneously in pre-warmed medium.
Primary Microglial Cultures
Primary cultures enriched in microglia were prepared from mixed murine glial cultures using modifications of rat culture protocols (Levesque et al. 2010, Kong et al. 1997). Briefly, meninges and blood vessels were removed from whole brains of 1 day postnatal pups. Tissues were gently triturated in microglia-enrichment media and seeded (4 brains or > 5×107 cells) in poly-L-lysine-coated 150 cm3 flasks. Microglia-enrichment medium consists of 1:1 DMEM/F12 containing 10% heat-inactivated fetal bovine serum, 2 mM L-glutamine, 1 mM sodium pyruvate, 100 μM nonessential amino acids, 50 U/ml penicillin, and 50 μg/ml streptomycin (Invitrogen). Cell suspensions were filtered through nylon mesh with 70 μm pores before plating onto 150 cm3 flasks. Cultures were maintained at 37°C in a humidified, 5% CO2 atmosphere. Medium was replenished every third day after the initial seeding. 10~12 days after seeding, when the glia had formed a confluent monolayer, microglia were mechanically dislodged and the detached cells were panned on uncoated tissue culture dishes for 1–2 h to remove more adherent cells such as astrocytes and progenitors. Microglia were pelleted at 100g for 5 min, then resuspended in fresh medium. We confirmed that the primary microglia were >95% pure by immunostaining with the specific marker, Iba-1 (not shown).
Immunohistochemistry and Quantification
Progenitor cell cultures were immunostained at 5 days post-plating, using cell-specific and lineage-specific markers including the O4 antibody which detects sulfatide (Sommer & Schachner 1982), and antibodies to glial fibrillary acidic protein (GFAP; 1:2,000, Chemicon/Millipore, Billerica, MA), CD11b (1:100, Millipore, Billerica MA), Olig2 (1:100,Immuno-Biological Laboratories, Minneapolis, MN), Sox2 (1:100, R&D Systems; Millipore), nestin (1:5,000, Aves Labs, Inc., Tigard, OR), and doublecortin (DCX) (goat-anti-DCX; 1:100; Santa Cruz Biotechnology Inc., Santa Cruz, CA). In some experiments, and based upon the multi-plex data, cells were double-immunostained with CCL5/RANTES (1:100, Santa Cruz), CXCR4 (clone 12G5, 1:50, Abcam Inc., Cambridge, MA), and CCR5 (clone 45523, 25μg/ml, R&D Systems; clone 2D7, 1:100, BD Pharmingen TM, San Jose, CA) to examine whether precursor subtypes could express particular chemokines and their receptors. In all cases, cells were fixed with 4% paraformaldehyde, and for intracellular markers, cells were permeabilized with 0.1% Triton X-100 in PBS containing 5% normal goat serum for 30 min. Double-staining was performed sequentially, with staining for chemokines and receptors followed by immunostaining for precursor markers. Antibodies were applied overnight at 4 C, followed by visualization with appropriate fluorescent-conjugated second antibodies (Molecular Probes/Invitrogen). Immunostained coverslips were finally incubated with Hoechst 33342 dye (8 min) to identify nuclei (Molecular Probes/Invitrogen), then mounted in ProLong Gold antifade reagent (Molecular Probes/Invitrogen) and dried for 8h in the dark. To assess the proportion of different cell types in the cultures, 350–500 Hoechst+ cells were selected randomly per coverslip and assessed for nestin, Olig2, Sox2, O4, GFAP, DCX, or CD11b immunostaining (N=3–5 different experiments) under oil immersion at 63X using a Zeiss AxioObserver system with an integrated MRM camera system (Carl Zeiss, Inc., Thornwood, NY). Similar staining methods were applied to 10 μm tissue sections from adult mouse striatum.
Immunoblotting
CXCR4 and CCR5 expression was examined in 5 day cultures. Cells were harvested by scraping into ice-cold RIPA buffer (50 mM Tris, pH 7.4; 150 mM NaCl; 1% Nonidet; 0.5% deoxycholic acid; 0.1% SDS; protease inhibitor cocktail, Roche, Indianapolis, IN). Cell lysates were pelleted and stored at 80°C until use. Protein was assayed using the BCA protein assay (Pierce, Rockford IL). Duplicate samples of 20 μg total protein were loaded per well onto 4–20% Tris–HCl Ready Gels (Bio-Rad Laboratories, Hercules, CA) using Precision Plus Protein Dual Color Standards (Bio-Rad; MW range: 10–250 kDa) to monitor protein transfer and molecular weight. Proteins were transferred to Hybond-P PVDF membranes (Amersham Biosciences, Piscataway NJ), probed with monoclonal antibodies to either CXCR4 (ab2074, 1:1,000, Abcam) or CCR5 (clone 45523, 1:1,000, R&D Systems), and visualized using chemiluminescent substrate (Super Signal West Femto substrate, Pierce). Protein bands were detected on a Kodak Image Station 440CF and analyzed using 1D software.
Multi-plex Assay
After 12 h of treatment, 450 μl of media were harvested from each well and stored at -80 C. 50 μl of media from each treatment was analyzed for chemokine/cytokine content using the Bio-Plex 200 System (Bio-Rad) and a standard mouse 23-plex assay kit or a custom 12-plex assay kit, according to the manufacturer’s instruction. Concentrations were determined using Bio-Plex Manager Software (Version 5.0), based upon a standard curve calculated for each individual chemokine/cytokine.
Microglial Chemotaxis Assay and CCR5 Antibody Neutralization
An 8.0 μm transwell system (Costar, Corning, NY) was used to measure changes in the motility of BV-2 microglia-derived cells or primary microglia in response to Tat- or gp120-treated glial precursor cells in 24-well plates. BV-2 cells were cultured in T-75 flasks in medium consisting of Dulbecco's Minimal Essential Medium (DMEM; Gibco/Invitrogen) supplemented with glucose (27 mM), Na2HCO3 (6 mM), 10% defined fetal bovine serum (Hyclone Laboratories, Inc., Logan, UT) and antibiotics. Cells were harvested at 90% confluency using a non-enzymatic cell dissociation solution (Sigma-Aldrich Co.), washed and resuspended in DMEM supplemented with glucose (27 mM), Na2HCO3 (6mM), and 1% fetal bovine serum, and then plated onto transwell inserts (105 BV-2 cells per insert). Primary microglia were grown in microglia-enrichment medium and mechanically harvested as described earlier. They were washed, resuspended and plated onto inserts similarly to BV-2 cells. Prior to the addition of transwell inserts containing microglia or BV-2 cells, 106 progenitors were cultured in each well of a poly-L-lysine coated 24 well plate for 5 days, then treated with Tat or gp120 ± opioids for 12 h. A transwell insert containing BV-2 cells or microglia was then placed into each well. The transwell inserts were incubated for an additional 3 h and then removed for analysis. Cells remaining on the upper surface of the insert were carefully aspirated, leaving those which had migrated to the surface underneath in place. The insert was then transferred to a clean well containing 225 μL of non-enzymatic cell dissociation solution (Sigma-Aldrich Co.) and incubated for 30 min, at 37°C, during which time each insert was gently tilted several times to completely detach cells. The inserts were discarded and 75 μL of 4X Lysis Buffer/CyQuantR GR dye solution (Millipore) were applied to each well and incubated per manufacturer’s instructions. 200 μL of dye/buffer/lysate solution were transferred to a 96-well plate, and the fluorescence intensity of each sample was measured using a PHERAstar FS plate reader (BMG LABTECH Inc. Cary, NC) with a 485 nM excitation filter and 520 nM emission filter. In experiments to assess the role of CCR5 in migration, the BV-2 cells or microglia were incubated with anti-CCR5 (clone 2D7, without sodium azide; BD Pharmingen ) or control IgG (mouse, IgG2a, BD Pharmingen) at a dose of 2 μg per 106 cells for 1h at 4°C, prior to the placement of the inserts.
Viability Assay
NPC/GPCs were cultured on poly-L-lysine pre-coated 48 well plates and treated at 5 days after plating with morphine, Tat, gp120, and naloxone either alone or in combination as in experiments described above. After 12 h, medium containing treatments was replaced with sterile, tissue culture–grade D-PBS containing the LIVE/DEADR reagents (Invitrogen; calcein AM, 2 μM and EthD-1, 4 μM) and 5 μg/mL Hoechst 33342. The working solution was added directly to cells and incubated for 15 mins, at 37°C. The fluorescence intensity of each color was measured using a PHERAstar FS plate reader (BMG LABTECH) in N=8 different cultures using appropriate excitation and emission filters (calcein AM: 495/515 nm; EthD-1: 495/635 nM; Hoechst: 350/510 nM).
Statistical Analysis
Statistical analyses were done by analysis of variance (ANOVA) followed by Bonferroni’s post-hoc testing using StatSoft software (Statistica, Tulsa, OK).
Results
Progenitors from E15 striatum secrete chemokines: Effects of viral proteins and opioids
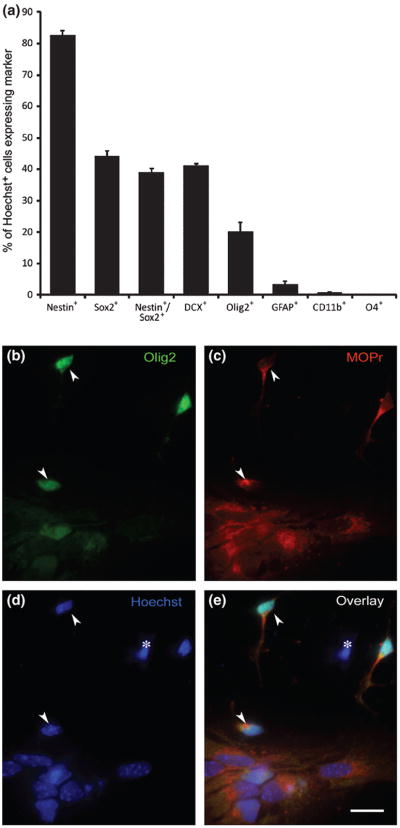
After 5 days growth in defined medium, progenitor cultures consisted of a mixture of immature cell types (Fig. 1A), containing both NPCs and GPCs at various states of differentiation. The majority of the cells were immunostained for nestin (82%) and/or Sox2 (44%), markers of relatively undifferentiated CNS progenitors (Ellis et al. 2004, Komitova & Eriksson 2004, Lendahl et al. 1990, Tanaka et al. 2004). Of the nestin+ cells, 47% also showed Sox2 immunoreactivity. Approximately 20% of the cells had entered the oligodendrocyte lineage, as indicated by expression of Olig2 (Woodruff et al. 2001, Ligon et al. 2006). Another ~40% expressed doublecortin (DCX), a microtubule-associated protein found almost exclusively in neuronal precursors. Somewhat surprisingly, very few of the cells expressed the astrocyte marker GFAP (<3%). Many DCX+, Olig2+ and GFAP+ cells also were nestin+ or Sox2+, suggesting relatively recent entry into a specific lineage. Less than 0.8% of cells stained with the CD11b microglial marker. As reported previously, over 70% of the Olig2+ cells in these cultures expressed the μ-opioid receptor (MOPr) (Hauser et al. 2009). Most cells that were Olig2− or DCX− were also MOPr+, indicating that MOPr was also expressed in uncommitted precursors (Fig. 1B and C). Thus, multiple types of progenitors are potential targets of MOPr agonists and antagonists such as morphine and naloxone.
Figure 1.
Characterization of progenitor cultures from E15 striatum. Cultures were immunostained after 5 days for typical markers of neural and glial precursors, and for MOPr.
A. Most cells stained for nestin (>80%) and/or Sox2 (>40%), indicating that they were relatively immature precursors. Approximately 40% were DCX+ and 20% were Olig2+, indicating entry into the path leading towards a neuronal or oligodendrocyte fate, respectively, while less than 3% of cells were GFAP+. Many cells that expressed DCX, Olig2 or GFAP also expressed nestin or Sox2, suggesting very recent entry into a specific lineage. CD11b+ microglia were <0.8% of the population. None of the cells stained for O4, an marker of more mature OLs.
B-E. Triple-labeling shows that most precursors in these cultures, regardless of differentiation state, express MOPr (red, B and D). The Hoechst+ nucleus of a cell that is MOPr- is indicated by (*) in panel D. Two Olig2+ cells the express MOPr are indicated by arrowheads in each panel. Scale bar = 10μM.
Progenitors were treated for 12 h with HIV-1 proteins and/or opioids, after which their conditioned medium was harvested and screened for expression of an array of chemokines and cytokines significant in the pathobiology of inflammatory disease and/or displaying cross-sensitization to opioids. The 12 h time-point was chosen because there was a consistently detectable accumulation of secreted products. Based on our earlier studies with other types of glia (El-Hage et al. 2005), longer time-points may result in higher accumulation, but they also increase the likelihood that autocrine and paracrine effects of the chemokines/cytokines will influence cell secretions. Of the 23 factors initially screened, only 10 were released at detectable levels by untreated striatal progenitors. The concentrations of the individual chemokines/cytokines detected after 12 h (pg/ml) are given in Table 1. Exposure of progenitors to viral proteins and/or opioids did not change the factors that were secreted, but did affect the accumulated amounts of several. The secretion of β-chemokines CCL3/MIP-1α, CCL4/MIP-1β and CCL5/RANTES were all significantly increased by Tat exposure, but not by gp120 exposure. Unlike prior observations in astrocytes, concurrent exposure to Tat and morphine did not have an interactive effect on chemokine/cytokine release, even though the majority of cells in the cultures expressed MOPr. Neither was there an interactive effect of Tat and gp120. CCL2/MCP-1 showed a trend towards a Tat response, but the effect was not significant using ANOVA analysis. It is likely that cells at multiple stages of differentiation contributed to both baseline and Tat-induced secretion, since CCL5/RANTES was detected by immunostaining in a subset of both Sox2+ and Olig2+ cells in vitro (Fig. 3A-C). CCL5/RANTES staining was also evident in Sox2+ cells in vivo (Fig. 3D), but it was quite difficult to detect in Olig2+ cells in vivo (not shown), suggesting downregulation in oligodendroglial precursors in the brain. We also examined expression of the β-chemokine receptors CXCR4 and CCR5, known to be co-receptors for gp120 binding and HIV entry into cells. Immunohistochemistry showed that both CCR5 and CXCR4 were present on Olig2+ and Sox2+ progenitors, as well as on neighboring Olig2− and Sox2− cells. Both receptors were also readily detected on western blots from progenitor cultures (Fig. 4E; 20 μg load), as well as astroglia and striatal homogenates. Major bands were found at ~40 kD, the reported molecular weight of both CCR5 and CXCR4.
Table 1.
Multiplex analysis of chemokines/cytokines secreted by E15 striatal progenitors (pg/ml)
| IL-1β | GM-CSF | TNF-α | IL-6 | IL-9 | KC | RANTES | MIP-1α | MIP-1β | MCP-1 | |
|---|---|---|---|---|---|---|---|---|---|---|
| C | <0.85# | <1.17# | <0.3# | 1.78±1.06 | <20.4# | 6.70±2.42 | 5.74±0.56 | 57.86±15.59 | 135.5±36.09 | 68.77±26.9 |
| M | 1.10±0.25 | <1.17# | <0.3# | <0.72# | 23.21±2.57 | 6.21±2.52 | 5.09±0.35 | 26.94±0.02 | 98.07±22.08 | 57.03±24.12 |
| T | 5.54±1.52 | 2.43±0.83 | 0.68±0.28 | 5.10±2.88 | 28.85±3.15 | 25.40±10.81 | 336±26.8 | 312.5±25.11 | 957±102.5 | 183.6±78.2 |
| G | 1.35±0.32 | 1.64±0.47 | 0.44±0.09 | <0.72# | 27.54±6.53 | 7.63±2.29 | 6.34±1.03 | 119.5±62.8 | 273±185.1 | 94.26±38.65 |
| N | <0.85# | <1.17# | 1.27±0.21 | <0.72# | <20.4# | 6.11±2.39 | 3.59±0.81 | 26.95±0.0 | 75.17±16.64 | 58.83±23.72 |
| MT | 5.76±2.31 | 1.64±0.47 | 1.14±0.36 | 4.81±3.00 | 26.03±3.15 | 20.61±9.52 | 292±59.7 | 307.1±62.06 | 714.5±127.8 | 170.15±76.58 |
| MG | <0.85# | 1.96±0.79 | 0.34±0.04 | <0.72# | <20.4# | 5.74±2.03 | 5.06±1.09 | 48.63±21.69 | 94.12±17.35 | 47.85±16.08 |
| MTN | 7.46±4.47 | 2.46±1.29 | 0.76±0.23 | 6.72±4.74 | 23.21±2.57 | 32.62±15.89 | 397±105 | 376.8±104.6 | 1009.1±194 | 237.2±131.6 |
| MTG | 6.99±2.77 | 3.22±0.96 | 1.20±0.81 | 3.09±1.06 | 26.03±3.15 | 24.12±9.13 | 271.7±32.33 | 321.9±61.6 | 945±165.9 | 167.9±75.83 |
| MTGN | 9.87±4.02 | 1.81±0.64 | 0.51±0.14 | 1.78±1.61 | <20.4# | 24.81±9.51 | 336.3±28.31 | 376.0±46.9 | 1063±116.3 | 124.8±75.26 |
| MN | <0.85# | <1.17# | <0.3# | <0.72# | <20.4# | 5.85±2.58 | 4.08±0.58 | 26.94±0.0 | 66.93±18.06 | 55.79±21.8 |
C=control; M=morphine; Tat=Tat; G=gp120; N=naloxone.
The following proteins were not routinely detected in control or treated cells: IL1α, IL-2, IL-3, IL-4, IL-5, IL-10, IL-12(p40), IL-12(p70), IL-13, IL-17, G-CSF, Eotaxin, IFNγ
< x.xx designates that sample concentration was determined to be below the accuracy limit of the assay software, based on the standard curve generated for the individual chemokine/cytokine. In those cases, we have indicated the lower limit value in pg/ml. For all samples, N=6.
Figure 3.

Localization of CCL5/RANTES in NPC/GPCs in vitro and in vivo. Cultured cells were immunostained (A-C) at 5 days in vitro. In vivo staining (D-E) was done in adult striatum. A and B: NPC/GPCs were immunostained with an antibody to Olig2 (A) to identify oligodendroglial precursors, and an antibody to CCL-5/RANTES (B) to determine which cells are producing this chemokine. Of the five Olig2+ cells in this field, two show CCL-5/RANTES expression in their cytoplasm (arrows indicate double-labeled cells). Three other cells that are Olig2+ (asterisks) do not express detectable levels of CCL5/RANTES. For (C-E), Z stacked images (AxioVision software, version 4, Carl Zeiss, Inc.) were taken through the entire depth of the cell layer (C) or section (D-E), then optimized for deconvolution microscopy (AutoQuant X Version 2, Media Cybernetics, Bethesda, MD) in order to better demonstrate sub-cellular details of Sox2 and CCL5/RANTES co-localization. C. Cells in the same NPC/GPC cultured stained for Sox2 (green) and CCL5/RANTES (red). The Sox2+ cells are a mixture of CCL5+ (asterisks) and CCL5− (arrows). Note also that many CCL5+ cells are Sox2−(arrowheads). Hoechst staining (blue) identifies the nuclei of all cells in the field. D-E: Co-localization of Sox2 (green) and CCL5/RANTES (red) in the adult mouse striatum in situ. Hoechst staining (blue) identifies nuclei of all cells in the field. Since CCL5 is a secreted, soluble chemokine, it is difficult to associate specific cell boundaries with CCL5 immunostaining. However, it can be appreciated from panel D that Sox2+ cells (asterisks) in the striatum are frequently associated with significant CCL5 immunostaining. The region shown in E has a high density of Sox2- cells, as indicated by the Hoechst staining, and little CCL5 signal.
Figure 4.
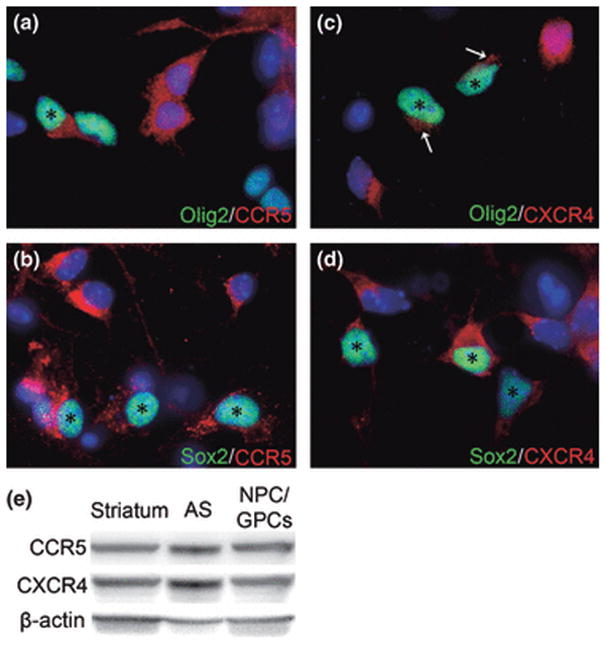
NPC/GPCs express both CCR5 and CXCR4 in vitro. Olig2+ (green) oligodendroglial precursors show both CCR5 (A) and CXCR4 (C) immunoreactivity. The signal for CXCR4 was generally light in these cells, and is indicated by arrows in (C). Undifferentiated Sox2+ NPC/GPCs (green) also are immuno-positive for CCR5 (B) and CXCR4 (D). Asterisks in A-D indicate double-labeled cells. Hoechst 33342 nuclear staining (blue) shows that some cells positive for CCR5 or CXCR4 do not express Olig2 or Sox2. (E) A major band at approximately 40 kD was noted on immunoblots of NPC/GPCs harvested at 5 DIV using antibodies to both CCR5 and CXCR4. Actin detection is included as a loading control. These bands are also detected in homogenates of cultured astroglia (AS) and whole striatum, preparations that are known to express both receptors.
Progenitor cells induce a chemotactic response in microglial cells
A transwell apparatus was used to test whether secreted factors from progenitors could influence the movement of BV-2 and primary microglial cells. All tests were conducted over a 3 h period. Some cells moved randomly across the membrane during the test period (Fig. 5A-C, medium only). Addition of Tat or gp120 to the medium in the absence of NPC/GPCs did not change the migration of either BV-2 cells or primary microglia (Fig. 5A-C, medium+Tat, medium+gp120). When NPC/GPCs were plated in a 24 well plate and transwell inserts were added after 5 days, secretions from NPC/GPCs did not alter BV-2 or primary microglial migration (Fig. 5A-C, NPC/GPC). However, when the progenitors were pre-treated for 12 h with HIV-1 Tat the result was quite different (Fig. 5A-C, NPC/GPC+Tat). In that case, there was a significant increase in migration of both BV-2 cells and primary microglia across the transwell membrane towards the Tat-treated progenitors. NPC/GPCs treated with gp120 did not elicit a similar enhancement of migration in either type of microglial cell.
Figure 5.
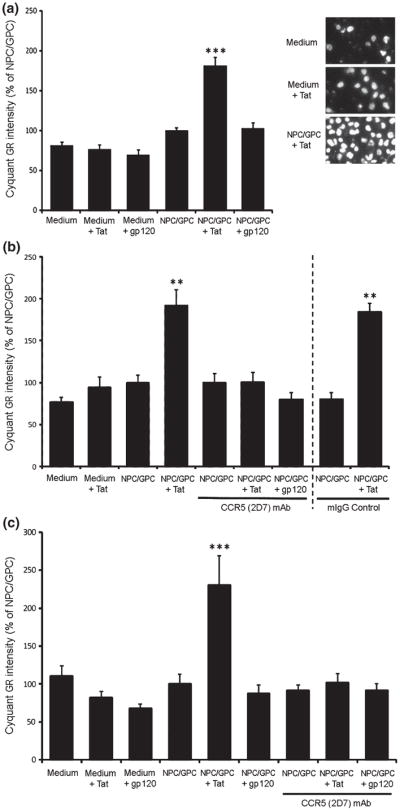
Chemotaxis of BV-2 cells and primary microglia in response to NPC/GPCs in transwell cultures. A. NPC/GPCs grown on a 24-well plate were treated with Tat or gp120 for 12 h prior to the addition of transwell inserts containing BV-2 cells. Transwell inserts were removed after 3 h, and cells crossing the insert membrane were labeled with CyQuantR GR dye and quantified on a PHERAstar FS plate reader with appropriate filters. Migration of BV-2 cells was not affected when either Tat or gp120 alone was added to the medium. Untreated NPC/GPCs also had no effect on BV-2 migration. However, the number of BV-2 cells moving across the transwell membrane was significantly enhanced when NPC/GPCs were pretreated with Tat for 12 h (*** p<0.001 vs. all other groups; N=6 independent cultures). Pretreatment of NPC/GPCs with gp120 did not affect BV-2 migration. The inset image shows Hoechst staining of BV-2 cells, and illustrates the effect of NPC/GPC treated with Tat to enhance migration. B. Antibody neutralization of CCR5 on BV-2 cells significantly attenuated Tat-enhanced migration. BV-2 cells on transwell inserts were pre-incubated with either 2D7 anti-CCR5 monoclonal antibody or control IgG2a for 1 h at 4°C, then placed into wells containing treated or untreated NPC/GPCs. Incubation with 2D7 antibody, but not control IgG2a, reduced BV-2 cell migration in the NPC/GPC+Tat treatment group back to basal levels. This suggests that the enhanced microglial migration caused by secretions from Tat-treated NPC/GPC is entirely mediated by CCR5-signaling. Antibody neutralization did not reduce migration in other treatment groups to below basal levels. (** p<0.01 vs. all other groups; N=6 independent cultures). C. Studies in A and B were repeated on primary murine microglia with similar results. Migration of primary microglia was significantly enhanced by the presence of NPC/GPCs pre-treated with Tat, although there was no effect of gp120 pre-treatment. Incubation with 2D7 antibody reduced migration in the NPC/GPC+Tat treatment group back to basal levels, but did not affect migration in other treatment groups. (*** p<0.001 vs. all other group; N=4 independent cultures).
Since microglia can be attracted by signals from dying cells, we examined cell death using a fluorescent LIVE/DEAD assay kit (Invitrogen). Approximately 10% of the total Hoechst-labeled nuclei belonged to dead cells, but there were no significant differences among treatments that might account for the additional BV-2 cell migration induced by HIV-1 Tat (n=8; Fig. 6). This suggests that the microglial chemotaxis observed in our experiments is most likely to result from enhanced chemotactic signals released from progenitors stimulated by viral proteins.
Figure 6.
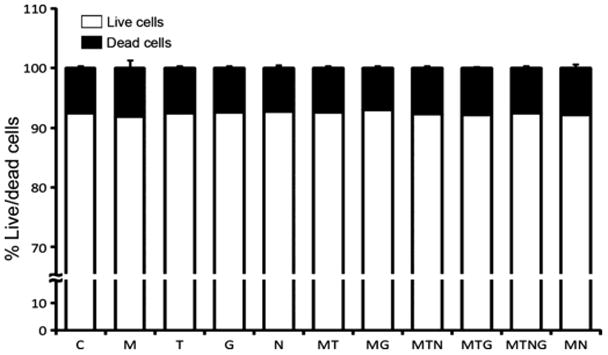
A fluorescent viability assay reveals that 12h exposure to HIV-1 Tat1-86 or gp120 alone or in combination with opiates did not increase the rate of NPC/GPC cell death in vitro. Cells were treated at 5 days after plating with morphine (M), Tat (T), gp120 (G), naloxone (N) either alone or in the combinations indicated. There was no significant increase in cell death in any treatment group. N=8 independent cultures.
CCR5 is the common receptor for the β-chemokines that were upregulated by Tat in progenitor cultures. To further confirm that chemokines upregulated by Tat were contributing to the enhanced migration of microglial cells, we conducted experiments to neutralize CCR5. Both BV-2 cells and primary microglia were incubated for 1 h with the 2D7 antibody (sodium azide-free), which has been shown by others to be suitable for receptor blockade studies (Lee et al. 1999, De Clercq 2009, Jayasuriya et al. 2004). Fig. 5B and C show, respectively, that treatment of both BV-2 and primary microglia with the 2D7 monoclonal CCR5 antibody prior to their addition to the transwell insert completely abolished the enhanced migration elicited by NPC/GPCs.
Discussion
HIV-associated neuropathology in the CNS is generally thought to result from the combined effects of direct viral protein toxicity, and inflammatory/toxic conditions propagated by surrounding glial cells. Because of our interests in the effects of co-morbidity between opiate drugs of abuse and HIV exposure in the CNS, we chose to examine the striatum, an area which is both rich in opiate receptors and extremely vulnerable to HIV-induced neuropathology. Our data show that progenitors from embryonic striatum secrete a variety of chemokines/cytokines in culture, and that exposure to HIV-1 Tat1-86 selectively increases secretion of a subset of these. The concept that NPC/GPCs in the normal brain may secrete factors to regulate either their own function or fate, or that of surrounding cells, has been previously established (Chang et al. 2003, Benoit et al. 2001, Taupin et al. 2000, Shingo et al. 2001). Further, it is known that progenitors or stem cells from other regions have this ability. Mesenchymal stem cells, for instance, secrete multiple chemokine/cytokines and growth factors, including multiple FGFs, IGF-1, IL-1β, IL-6, CCL2/MCP-1, CCL3/MIP-1α, CCL4/MIP-1β, SDF-1, VEGF, and others (Rafei et al. 2008, Croitoru-Lamoury et al. 2007, Schinkothe et al. 2008, Chen et al. 2008, Liu & Hwang 2005, Wagner et al. 2007). The profile of released factors appears to differ markedly among classes of immature cells, suggesting unique signatures that may also depend on environment. Importantly, this is the first study to show that secretions from a neural progenitor population have a chemotactic effect on microglial cells, and that these secretions and their effects can be enhanced by exposure to HIV-1 Tat.
A previous study on E15 striatal progenitors grown as proliferating neurospheres (Benoit et al. 2001) examined some of the cytokines that we screened. In agreement, we both found a complete lack of IL-3, IL-4 and IL-1α secretion. However, our cells secreted detectable levels of GM-CSF, IL-1β, IL-6, IL-9 and TNF-α, as well as 5 other chemokines/cytokines that were not assayed in the previous study. Our cells were grown in monolayers, and assayed at an earlier in vitro age using a different methodology, so these differences are perhaps not surprising. There are likely to be additional chemokine/cytokines secreted by striatal progenitors, since our initial screening process used the manufacturer’s standard kit which assays for a group of 23 selected analytes.
The progenitor cultures from E15 striatum contain a mixture of cells at different stages of differentiation (Fig. 1). Immunocytochemistry showed that most expressed either nestin, Sox2, or both. While 20% expressed Olig2, a transcription factor that indicates commitment to an oligodendroglial fate, there was a complete absence of O4 staining, suggesting that the Olig2+ cells were still quite immature. Likewise, the 3% of cells that were GFAP+ seemed quite immature based on morphology and variable co-expression of nestin or Olig2. As expected in a striatal progenitor culture, many of the cells were young neural precursors expressing DCX (~40%), but the absence of NeuN reactivity suggested their relative immaturity. Individual chemokines/cytokines appear to be secreted by cells across several stages of development, and there is also variability of expression within specific populations. For example, although most CCL5/RANTES+ cells do not immunostain for Olig2, a subset are Olig2+ (Fig. 3); and among Olig2+ cells, only about 50% are CCL5/RANTES+ (data not shown).
Regardless of the stage at which progenitors release these factors, our results show that the accumulation of CCL5/RANTES, CCL3/MIP-1α and CCL4/MIP-1β, is significantly increased following a 12 h exposure to HIV-1 Tat (Fig. 2). In contrast, exposure to gp120 did not affect levels of any of the chemokines/cytokines. It is unlikely that the very small populations of microglia (<0.8%) or immature astroglia (3%) are responsible for either baseline or Tat-induced chemokine/cytokine secretion, especially since MIP-1α and MIP-1β were not been detected in our other studies. The earlier studies also showed that co-exposure to morphine enhanced Tat-induced CCL5/RANTES production by astroglia (El-Hage et al. 2005, El-Hage et al. 2008b). The lack of morphine-induced synergy in the present results also suggests that astroglia are not the primary source.
Figure 2.
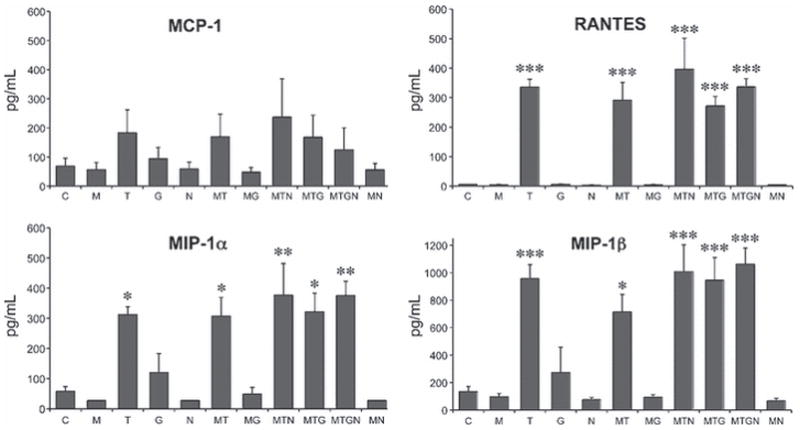
Exposure to HIV-1 Tat1-86 increases progenitor secretion of a select group of chemokines. Cells were treated at 5 days after plating with morphine (M), Tat (T), gp120 (G), and naloxone (N) either alone or in combinations as indicated. Of 23 factors tested, 10 were secreted by unstimulated cells (see Table 1). Only RANTES, MIP-1α, and MIP-1β showed a significant change in concentration after 12 h treatment. Cells responded to Tat, but not to gp120. There was no synergistic response of concurrent exposure to either morphine or gp120. MCP-1 showed a similar trend towards a Tat response, but the effect was not significant. * p<0.05; ** p<0.01; *** p<0.001 vs. untreated control cells (C), ANOVA and post-hoc Bonferroni’s test. N=6 independent cultures.
In striatal astroglia there is a synergistic effect of morphine on Tat-induced secretion of CCL2/MCP-1, CCL5/RANTES and IL-6. The lack of morphine-induced synergy in progenitor cultures is not due to a lack of appropriate receptors since most progenitors express MOPr (Fig. 1) (Hauser et al. 2009, Buch et al. 2007). However, the signaling pathways leading to synergistic secretory responses may not be functional in immature cells. Gp120 did not increase NPC/GPC secretion of the panel of analytes that we examined. This is similar to our observations in mature astroglia from several brain regions (Fitting et al. 2010), although gp120 from different HIV strains is variably reported to induce TNFα, IL-1α, IL-1β, and IL-6 in other astroglial preparations (Kong et al. 1996, Buriani et al. 1999, Ronaldson & Bendayan 2006, Yeung et al. 1995). CD4 is the major receptor for gp120 binding and virus entry, and binding is facilitated by expression of CD4 co-receptors such as CCR5 and CXCR4. The strain of gp120 that we used (MN) preferentially uses the CCR5 co-receptor. Progenitors expressed abundant CCR5 (Fig. 3), but do not express CD4 and therefore may have very limited ability to respond to gp120. Binding of chemokine ligand versus gp120 to a chemokine receptor may have fundamentally different consequences. For example, in a previous study, gp120 IIIb required “priming” by exposure to soluble CD4 in order to elicit a simple CXCR4-mediated Ca++ response from similar murine progenitors (Tran et al. 2005).
Since Tat exposure can clearly enhance chemokine secretion from progenitors, might this have functional consequences? A transwell assay showed that progenitors exposed to HIV-1 Tat for 12 h were able to enhance the directed movement of both BV-2 cells and primary microglia (Fig. 5). Gp120 did not enhance chemokine production, so it was not surprising that progenitors treated with gp120 did not elicit the same migratory response (Fig. 5). Importantly, blockade of the CCR5 receptor on microglial cells eliminated the enhanced migration (Fig. 5B-C), showing that secretion of the CCR5 ligands RANTES, MIP1α and MIP1β, from progenitors have the potential to direct microglial movement. We believe that our data suggest chemotaxis, not just enhanced, random motility, as there is only a modest exchange of medium over the short assay period through the small membrane plating area. The BV-2 cells are plated into a well containing their original culture medium and are moving towards the continually renewed source of chemokines.
What are the potential consequences of chemokine/cytokine secretion by NPC/GPCs, and the upregulation of β-chemokine production by HIV-1 Tat? These are best discussed considering the two very different contexts of interest – the normal brain and the HIV-infected brain. Ten of the chemokines/cytokines we examined were secreted at detectable levels by untreated progenitors, and several of these are chemoattractants for inflammatory cells. Under normal conditions, attracting monocytes/microglia may not be harmful. They serve diverse roles essential for normal brain function, independent of the innate immune response, which include providing trophic support, regulating neurogenesis and neuron development, and clearing toxic debris (Whitney et al. 2009, Polazzi & Contestabile 2002, Simard et al. 2006, Ziv et al. 2006, Aarum et al. 2003, Walton et al. 2006, Block et al. 2007). During HIV-infection there are different considerations. CCL5/RANTES, CCL3/MIP-1α and CCL4/MIP-1β are all known to act as endogenous suppressors of HIV infection and cell damage due to their ability to block gp120 binding with its CCR5 co-receptor (Cocchi et al. 1995, Catani et al. 2000), and both CCR5 and its ligands are the subject of ongoing investigation as therapeutic targets to reduce viral entry [reviewed in (Vangelista et al. 2008, DeVico & Gallo 2004)]. Thus, enhanced β-chemokine secretion might afford a degree of protection. However, all three β-chemokines are also implicated in HIV-neuropathology. Depending upon the severity of inflammation they may contribute to an environment that is detrimental to neurons. RANTES is a well known chemoattractant for T-cells, granulocytes, and monocytes, and is thought to recruit HIV-infected monocytes to the brain, either alone or through triggering MCP-1 production (Schall et al. 1990, Luo et al. 2003, El-Hage et al. 2005, Kelder et al. 1998, Conant et al. 1998). CCL3/MIP-1α and CCL4/MIP-1β are routinely produced by activated macrophages and attract monocytes as well as lymphocyte subpopulations (Menten et al. 2002). β-chemokines also stimulate synthesis and release of pro-inflammatory cytokines, are upregulated and expressed by astroglia and microglia in the HIV infected brain (Schmidtmayerova et al. 1996, Letendre et al. 1999, Kelder et al. 1998), and correlate positively with HIV dementia (Letendre et al. 1999). Thus, dysregulated β-chemokine expression by progenitors and glia might potentiate local inflammation, and enhance local trafficking of HIV-infected cells into the brain. Supporting this concept, multiple indicators of inflammation were reduced after intrastriatal injection of HIV-1 Tat into RANTES/CCL5 deficient mice compared to control (El-Hage et al. 2008a). The concept that chemokine/cytokine effects may differ in normal vs inflammatory situations is well-illustrated by considering CCL2/MCP-1. While CCL2/MCP-1 can be protective towards isolated neurons (Eugenin et al. 2003, Madrigal et al. 2009, Yao et al. 2009), in the context of HIV-1 infection, the negative effects of attracting infected monocytes and driving inflammation are likely to outweigh direct, neuroprotective effects observed in isolation. The complexity of these issues is clearly highlighted by disparate results in two studies using mice deficient in CCR2, showing either protection (El-Hage et al. 2006a) or exacerbation (Yao et al. 2009) after intrastriatal Tat injection, depending on timing, region examined, and outcome measure.
Importantly, we found robust expression of CCL5/RANTES associated with some Sox2+ cells in the striatum (Fig. 3D), suggesting that secretion of chemokines by NPCs does occur in vivo, and in the adult CNS. Progenitors are a relatively small population in the mature brain and will have less influence on overall inflammatory tone than microglia and astroglia. However, we speculate that they may have an important influence on the local milieu, and particularly in situations where progenitor populations are increased, such as regions of active CNS repair, or during development. In the context of HIV, progenitors may particularly contribute to the neuropathology that occurs in pediatric patients, where glial populations are still developing. HIV-1 associated progressive encephalopathy occurs in a relatively high percentage of pediatric HIV patients, where it develops aggressively and is frequently the earliest clinical manifestation of disease (Ensoli et al. 1997, Van Rie et al. 2007, Schwartz & Major 2006, Drotar et al. 1997). Chemokine production by progenitors might normally be an adaptive process, to encourage immune surveillance and approval of immature cells before they make add to neural or glial populations. Enhanced secretion of chemokines in the context of HIV might circumvent this adaptive strategy, by attracting HIV-infected microglia/monocytes to NPC/GPCs, which can be infected by HIV (Rothenaigner et al. 2007, Lawrence et al. 2004), and which appear to pass virus to their neuronal and glial progeny (Lawrence et al. 2004).
In summary, we have shown that HIV-1 Tat increases production β-chemokines by striatal progenitors, resulting in the CCR5-mediated movement of microglial cells. Since Tat mRNA and/or protein have been detected in CNS tissue from both patients with HIV-1 encephalitis and rhesus macaques infected with a chimeric HIV-SIV (SHIV) virus (Hudson et al. 2000), these findings have significant disease relevance. The effects of enhanced chemokine release will be contextual, due to complex interplay between multiple secretory and responder cell types in vivo, as well as temporal issues during normal and disease processes. Emerging evidence suggests that human neural progenitors may be cellular reservoirs of HIV infection (Schwartz & Major 2006, Lawrence et al. 2004, Rothenaigner et al. 2007), although the extent of NPC/GPC infection in the brain remains unclear. The potential contribution of infected progenitors to the chemokine milieu must also be considered.
Acknowledgments
We acknowledge generous NIH support from R01 DA024461 and P01 DA19398
Abbreviations
- AIDS
acquired immunodeficiency syndrome
- [Ca2+]i
intracellular calcium concentration
- CNS
central nervous system
- CCL
CC motif chemokine ligand
- CXCL
CXC motif chemokine ligand
- DCX
doublecortin
- EAAT
excitatory amino acid transporter
- FGF
fibroblast growth factor
- GFAP
glial fibrillary acidic protein
- gp120
glycoprotein 120
- GPC
glial precursor cell
- HIV
human immunodeficiency virus
- IGF-1
insulin like growth factor 1; IL-interleukin
- IP-10
interferon gamma-inducible protein of 10 kDa
- MCP-1
monocyte chemoattractant protein-1
- MIP
macrophage inflammatory protein
- MOPr
mu-opioid receptor
- NPC
neural precursor cell
- Olig
oligodendrocyte transcription factor
- RANTES
regulated upon activation, normal T cell expressed and secreted
- SDF-1
stromal cell derived factor 1
- SIV
simian immunodeficiency virus
- Sox2
Sex determining region of Y-related HMG-box gene 2
- Tat
transactivator of transcription
- VEGF
vascular endothelial growth factor
Literature Cited
- Aarum J, Sandberg K, Haeberlein SL, Persson MA. Migration and differentiation of neural precursor cells can be directed by microglia. Proc Natl Acad Sci U S A. 2003;100:15983–15988. doi: 10.1073/pnas.2237050100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony IC, Arango JC, Stephens B, Simmonds P, Bell JE. The effects of illicit drugs on the HIV infected brain. Front Biosci. 2008;13:1294–1307. doi: 10.2741/2762. [DOI] [PubMed] [Google Scholar]
- Arango JC, Simmonds P, Brettle RP, Bell JE. Does drug abuse influence the microglial response in AIDS and HIV encephalitis? AIDS. 2004;18:S69–74. [PubMed] [Google Scholar]
- Bell JE, Arango JC, Anthony IC. Neurobiology of multiple insults: HIV-1-associated brain disorders in those who use illicit drugs. J Neuroimmune Pharmacol. 2006;1:182–191. doi: 10.1007/s11481-006-9018-2. [DOI] [PubMed] [Google Scholar]
- Benoit BO, Savarese T, Joly M, Engstrom CM, Pang L, Reilly J, Recht LD, Ross AH, Quesenberry PJ. Neurotrophin channeling of neural progenitor cell differentiation. J Neurobiol. 2001;46:265–280. [PubMed] [Google Scholar]
- Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci. 2007;8:57–69. doi: 10.1038/nrn2038. [DOI] [PubMed] [Google Scholar]
- Bouwman FH, Skolasky RL, Hes D, Selnes OA, Glass JD, Nance-Sproson TE, Royal W, Pan GJD, McArthur JC. Variable progression of HIV-associated dementia. Neurology. 1998;50:1814–1820. doi: 10.1212/wnl.50.6.1814. [DOI] [PubMed] [Google Scholar]
- Brack-Werner R. Astrocytes: HIV cellular reservoirs and important participants in neuropathogenesis. AIDS. 1999;13:1–22. doi: 10.1097/00002030-199901140-00003. [DOI] [PubMed] [Google Scholar]
- Bruce-Keller AJ, Turchan-Cholewo J, Smart EJ, Geurin TM, Chauhan A, Ried R, Nath A, Knapp PE, Hauser KF. Morphine Increases astroglial and microglial activation in the brains of conditional HIV-Tat transgenic mice. Glia. 2008;56:1414–1427. doi: 10.1002/glia.20708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buch SK, Khurdayan VK, Lutz SE, Knapp PE, El-Hage N, Hauser KF. Glial-restricted precursors: Patterns of expression of opioid receptors and relationship to HIV-1 Tat and morphine susceptibility in vitro. Neuroscience. 2007;146:1546–1554. doi: 10.1016/j.neuroscience.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buriani A, Petrelli L, Facci L, Romano PG, Dal Tosso R, Leon A, Skaper SD. Human immunodeficiency virus type 1 envelope glycoprotein gp120 induces tumor necrosis factor-alpha in astrocytes. J NeuroAIDS. 1999;2:1–13. doi: 10.1300/J128v02n02_01. [DOI] [PubMed] [Google Scholar]
- Catani MV, Corasaniti MT, Navarra M, Nistico G, Finazzi-Agro A, Melino G. gp120 induces cell death in human neuroblastoma cells through the CXCR4 and CCR5 chemokine receptors. J Neurochem. 2000;74:2373–2379. doi: 10.1046/j.1471-4159.2000.0742373.x. [DOI] [PubMed] [Google Scholar]
- Chang MY, Park CH, Lee SH. Embryonic cortical stem cells secrete diffusible factors to enhance their survival. Neuroreport. 2003;14:1191–1195. doi: 10.1097/00001756-200307010-00001. [DOI] [PubMed] [Google Scholar]
- Chen L, Tredget EE, Wu PY, Wu Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS ONE. 2008;3:e1886. doi: 10.1371/journal.pone.0001886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocchi F, DeVico AL, Garzino-Demo A, Arya SK, Gallo RC, Lusso P. Identification of RANTES, MIP-1 alpha, and MIP-1 beta as the major HIV-suppressive factors produced by CD8+ T cells. Science. 1995;270:1811–1815. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- Codazzi F, Racchetti G, Grohovaz F, Meldolesi J. Transduction signals induced in rat brain cortex astrocytes by the HIV-1 gp120 glycoprotein. Federation of European Biochemical Society Letters. 1996;384:135–137. doi: 10.1016/0014-5793(96)00301-8. [DOI] [PubMed] [Google Scholar]
- Conant K, Garzino-Demo A, Nath A, McArthur JC, Halliday W, Power C, Gallo RC, Major EO. Induction of monocyte chemoattractant protein-1 in HIV-1 Tat-stimulated astrocytes and elevation in AIDS dementia. Proceedings of the National Academy of Science, USA. 1998;95:3117–3121. doi: 10.1073/pnas.95.6.3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croitoru-Lamoury J, Lamoury FM, Zaunders JJ, Veas LA, Brew BJ. Human mesenchymal stem cells constitutively express chemokines and chemokine receptors that can be upregulated by cytokines, IFN-beta, and Copaxone. J Interferon Cytokine Res. 2007;27:53–64. doi: 10.1089/jir.2006.0037. [DOI] [PubMed] [Google Scholar]
- De Clercq E. The AMD3100 story: the path to the discovery of a stem cell mobilizer (Mozobil) Biochem Pharmacol. 2009;77:1655–1664. doi: 10.1016/j.bcp.2008.12.014. [DOI] [PubMed] [Google Scholar]
- DeVico AL, Gallo RC. Control of HIV-1 infection by soluble factors of the immune response. Nat Rev Microbiol. 2004;2:401–413. doi: 10.1038/nrmicro878. [DOI] [PubMed] [Google Scholar]
- Dougherty RH, Skolasky RL, Jr, McArthur JC. Progression of HIV-associated dementia treated with HAART. AIDS Read. 2002;12:69–74. [PubMed] [Google Scholar]
- Drotar D, Olness K, Wiznitzer M, et al. Neurodevelopmental outcomes of Ugandan infants with human immunodeficiency virus type 1 infection. Pediatrics. 1997;100:E5. doi: 10.1542/peds.100.1.e5. [DOI] [PubMed] [Google Scholar]
- El-Hage N, Bruce-Keller AJ, Knapp PE, Hauser KF. CCL5/RANTES gene deletion attenuates opioid-induced increases in glial CCL2/MCP-1 immunoreactivity and activation in HIV-1 Tat-exposed mice. J Neuroimmune Pharmacol. 2008a;3:275–285. doi: 10.1007/s11481-008-9127-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Hage N, Bruce-Keller AJ, Yakovleva T, Bazov I, Bakalkin G, Knapp PE, Hauser KF. Morphine exacerbates HIV-1 Tat-induced cytokine production in astrocytes through convergent effects on [Ca(2+)](i), NF-kappaB trafficking and transcription. PLoS ONE. 2008b;3:e4093. doi: 10.1371/journal.pone.0004093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Hage N, Gurwell JA, Singh IN, Knapp PE, Nath A, Hauser KF. Synergistic increases in intracellular Ca2+, and the release of MCP-1, RANTES, and IL-6 by astrocytes treated with opiates and HIV-1 Tat. Glia. 2005;50:91–106. doi: 10.1002/glia.20148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Hage N, Wu G, Ambati J, Bruce-Keller AJ, Knapp PE, Hauser KF. CCR2 mediates increases in glial activation caused by exposure to HIV-1 Tat and opiates. Journal of Neuroimmunology. 2006a;178:9–16. doi: 10.1016/j.jneuroim.2006.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Hage N, Wu G, Wang J, Ambati J, Knapp PE, Reed J, Bruce-Keller A, Hauser KF. HIV Tat1–72 and opiate-induced changes in astrocytes promote chemotaxis of microglia through the expression of MCP-1 and alternative chemokines. Glia. 2006b;53:132–146. doi: 10.1002/glia.20262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis P, Fagan BM, Magness ST, Hutton S, Taranova O, Hayashi S, McMahon A, Rao M, Pevny L. SOX2, a persistent marker for multipotential neural stem cells derived from embryonic stem cells, the embryo or the adult. Dev Neurosci. 2004;26:148–165. doi: 10.1159/000082134. [DOI] [PubMed] [Google Scholar]
- Ensoli F, Wang H, Fiorelli V, Zeichner SL, De Cristofaro MR, Luzi G, Thiele CJ. HIV-1 infection and the developing nervous system: lineage-specific regulation of viral gene expression and replication in distinct neuronal precursors. J Neurovirol. 1997;3:290–298. doi: 10.3109/13550289709029470. [DOI] [PubMed] [Google Scholar]
- Eugenin EA, D'Aversa TG, Lopez L, Calderon TM, Berman JW. MCP-1 (CCL2) protects human neurons and astrocytes from NMDA or HIV-tat-induced apoptosis. Journal of Neurochemistry. 2003;85:1299–1311. doi: 10.1046/j.1471-4159.2003.01775.x. [DOI] [PubMed] [Google Scholar]
- Fitting S, Hauser KF, Chen W, Zou S, Vo P, Bruce-Keller AJ, Knapp PE. Multiplex analysis of HIV-1 Tat, gp120 and interactive morphine effects on chemokine/cytokine production by astroglia: regional differences. Journal of Proteome Research. 2010 doi: 10.1021/pr900926n. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galey D, Becker K, Haughey N, Kalehua A, Taub D, Woodward J, Mattson MP, Nath A. Differential transcriptional regulation by human immunodeficiency virus type 1 and gp120 in human astrocytes. J Neurovirol. 2003;9:358–371. doi: 10.1080/13550280390201119. [DOI] [PubMed] [Google Scholar]
- Gendelman HE, Genis P, Jett M, Zhai QH, Nottet HS. An experimental model system for HIV-1-induced brain injury. Advances in Neuroimmunology. 1994;4:189–193. doi: 10.1016/s0960-5428(06)80256-1. [DOI] [PubMed] [Google Scholar]
- Gorry PR, Ong C, Thorpe J, Bannwarth S, Thompson KA, Gatignol A, Vesselingh SL, Purcell DF. Astrocyte infection by HIV-1: mechanisms of restricted virus replication, and role in the pathogenesis of HIV-1-associated dementia. Curr HIV Res. 2003;1:463–473. doi: 10.2174/1570162033485122. [DOI] [PubMed] [Google Scholar]
- Hauser KF, El-Hage N, Stiene-Martin A, Maragos WF, Nath A, Persidsky Y, Volsky DJ, Knapp PE. HIV-1 neuropathogenesis: glial mechanisms revealed through substance abuse. J Neurochem. 2007;100:567–586. doi: 10.1111/j.1471-4159.2006.04227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser KF, Hahn YK, Adjan VV, Zou S, Buch SK, Nath A, Bruce-Keller AJ, Knapp PE. HIV-1 Tat and morphine have interactive effects on oligodendrocyte survival and morphology. Glia. 2009;57:194–206. doi: 10.1002/glia.20746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden CP, Haughey NJ, Nath A, Geiger JD. Role of Na+/H+ exchangers, excitatory amino acid receptors and voltage-operated Ca2+ channels in human immunodeficiency virus type 1 gp120-mediated increases in intracellular Ca2+ in human neurons and astrocytes. Neuroscience. 1999;91:1369–1378. doi: 10.1016/s0306-4522(98)00714-3. [DOI] [PubMed] [Google Scholar]
- Hudson L, Liu J, Nath A, Jones M, Raghavan R, Narayan O, Male D, Everall I. Detection of the human immunodeficiency virus regulatory protein tat in CNS tissues. J Neurovirol. 2000;6:145–155. doi: 10.3109/13550280009013158. [DOI] [PubMed] [Google Scholar]
- Jayasuriya H, Herath KB, Ondeyka JG, et al. Isolation and structure of antagonists of chemokine receptor (CCR5) J Nat Prod. 2004;67:1036–1038. doi: 10.1021/np049974l. [DOI] [PubMed] [Google Scholar]
- Kaul M, Garden GA, Lipton SA. Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature. 2001;410:988–994. doi: 10.1038/35073667. [DOI] [PubMed] [Google Scholar]
- Kelder W, McArthur JC, Nance-Sproson T, McClernon D, Griffin DE. Beta-chemokines MCP-1 and RANTES are selectively increased in cerebrospinal fluid of patients with human immunodeficiency virus-associated dementia. Annals of Neurology. 1998;44:831–835. doi: 10.1002/ana.410440521. [DOI] [PubMed] [Google Scholar]
- Khurdayan VK, Buch S, El-Hage N, et al. Preferential vulnerability of astroglia and glial precursors to combined opioid and HIV-1 Tat exposure in vitro. Eur J Neurosci. 2004;19:3171–3182. doi: 10.1111/j.0953-816X.2004.03461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilroy GE, Foster SJ, Wu X, et al. Cytokine profile of human adipose-derived stem cells: expression of angiogenic, hematopoietic, and pro-inflammatory factors. J Cell Physiol. 2007;212:702–709. doi: 10.1002/jcp.21068. [DOI] [PubMed] [Google Scholar]
- Komitova M, Eriksson PS. Sox-2 is expressed by neural progenitors and astroglia in the adult rat brain. Neurosci Lett. 2004;369:24–27. doi: 10.1016/j.neulet.2004.07.035. [DOI] [PubMed] [Google Scholar]
- Kong LY, McMillian MK, Hudson PM, Jin L, Hong JS. Inhibition of lipopolysaccharide-induced nitric oxide and cytokine production by ultralow concentrations of dynorphins in mixed glia cultures. Journal of Pharmacology and Experimental Therapeutics. 1997;280:61–66. [PubMed] [Google Scholar]
- Kong LY, Wilson BC, McMillian MK, Bing G, Hudson PM, Hong JS. The effects of the HIV-1 envelope protein gp120 on the production of nitric oxide and proinflammatory cytokines in mixed glial cell cultures. Cell Immunol. 1996;172:77–83. doi: 10.1006/cimm.1996.0217. [DOI] [PubMed] [Google Scholar]
- Kramer-Hammerle S, Hahn A, Brack-Werner R, Werner T. Elucidating effects of long-term expression of HIV-1 Nef on astrocytes by microarray, promoter, and literature analyses. Gene. 2005a;358:31–38. doi: 10.1016/j.gene.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Kramer-Hammerle S, Rothenaigner I, Wolff H, Bell JE, Brack-Werner R. Cells of the central nervous system as targets and reservoirs of the human immunodeficiency virus. Virus Res. 2005b;111:194–213. doi: 10.1016/j.virusres.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Kutsch O, Oh J, Nath A, Benveniste EN. Induction of the chemokines interleukin-8 and IP-10 by human immunodeficiency virus type 1 tat in astrocytes. Journal of Virology. 2000;74:9214–9221. doi: 10.1128/jvi.74.19.9214-9221.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence DM, Durham LC, Schwartz L, Seth P, Maric D, Major EO. Human immunodeficiency virus type 1 infection of human brain-derived progenitor cells. Journal of Virology. 2004;78:7319–7328. doi: 10.1128/JVI.78.14.7319-7328.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B, Sharron M, Blanpain C, et al. Epitope mapping of CCR5 reveals multiple conformational states and distinct but overlapping structures involved in chemokine and coreceptor function. J Biol Chem. 1999;274:9617–9626. doi: 10.1074/jbc.274.14.9617. [DOI] [PubMed] [Google Scholar]
- Lehmann MH, Masanetz S, Kramer S, Erfle V. HIV-1 Nef upregulates CCL2/MCP-1 expression in astrocytes in a myristoylation- and calmodulin-dependent manner. J Cell Sci. 2006;119:4520–4530. doi: 10.1242/jcs.03231. [DOI] [PubMed] [Google Scholar]
- Lendahl U, Zimmerman LB, McKay RD. CNS stem cells express a new class of intermediate filament protein. Cell. 1990;60:585–595. doi: 10.1016/0092-8674(90)90662-x. [DOI] [PubMed] [Google Scholar]
- Letendre SL, Lanier ER, McCutchan JA. Cerebrospinal fluid beta chemokine concentrations in neurocognitively impaired individuals infected with human immunodeficiency virus type 1. Journal of Infectious Disease. 1999;180:310–319. doi: 10.1086/314866. [DOI] [PubMed] [Google Scholar]
- Levesque S, Wilson B, Gregoria V, Thorpe LB, Dallas S, Polikov VS, Hong JS, Block ML. Reactive microgliosis: extracellular {micro}-calpain and microglia-mediated dopaminergic neurotoxicity. Brain. 2010;133:808–821. doi: 10.1093/brain/awp333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligon KL, Fancy SP, Franklin RJ, Rowitch DH. Olig gene function in CNS development and disease. Glia. 2006;54:1–10. doi: 10.1002/glia.20273. [DOI] [PubMed] [Google Scholar]
- Liu CH, Hwang SM. Cytokine interactions in mesenchymal stem cells from cord blood. Cytokine. 2005;32:270–279. doi: 10.1016/j.cyto.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Llado J, Haenggeli C, Maragakis NJ, Snyder EY, Rothstein JD. Neural stem cells protect against glutamate-induced excitotoxicity and promote survival of injured motor neurons through the secretion of neurotrophic factors. Mol Cell Neurosci. 2004;27:322–331. doi: 10.1016/j.mcn.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Lobato MN, Caldwell MB, Ng P, Oxtoby MJ. Encephalopathy in children with perinatally acquired human immunodeficiency virus infection. Pediatric Spectrum of Disease Clinical Consortium. J Pediatr. 1995;126:710–715. doi: 10.1016/s0022-3476(95)70397-7. [DOI] [PubMed] [Google Scholar]
- Luo Y, Berman MA, Abromson-Leeman SR, Dorf ME. Tumor necrosis factor is required for RANTES-induced astrocyte monocyte chemoattractant protein-1 production. Glia. 2003;43:119–127. doi: 10.1002/glia.10231. [DOI] [PubMed] [Google Scholar]
- Madrigal JL, Leza JC, Polak P, Kalinin S, Feinstein DL. Astrocyte-derived MCP-1 mediates neuroprotective effects of noradrenaline. J Neurosci. 2009;29:263–267. doi: 10.1523/JNEUROSCI.4926-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahajan SD, Aalinkeel R, Reynolds JL, Nair BB, Fernandez SF, Schwartz SA, Nair MP. Morphine exacerbates HIV-1 viral protein gp120 induced modulation of chemokine gene expression in U373 astrocytoma cells. Curr HIV Res. 2005;3:277–288. doi: 10.2174/1570162054368048. [DOI] [PubMed] [Google Scholar]
- McManus CM, Weidenheim K, Woodman SE, Nunez J, Hesselgesser J, Nath A, Berman JW. Chemokine and chemokine-receptor expression in human glial elements: induction by the HIV protein, Tat, and chemokine autoregulation. American Journal of Pathology. 2000;156:1441–1453. doi: 10.1016/S0002-9440(10)65013-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menten P, Wuyts A, Van Damme J. Macrophage inflammatory protein-1. Cytokine Growth Factor Rev. 2002;13:455–481. doi: 10.1016/s1359-6101(02)00045-x. [DOI] [PubMed] [Google Scholar]
- Molloy AP, Martin FT, Dwyer RM, Griffin TP, Murphy M, Barry FP, O'Brien T, Kerin MJ. Mesenchymal stem cell secretion of chemokines during differentiation into osteoblasts, and their potential role in mediating interactions with breast cancer cells. Int J Cancer. 2009;124:326–332. doi: 10.1002/ijc.23939. [DOI] [PubMed] [Google Scholar]
- Nath A, Conant K, Chen P, Scott C, Major EO. Transient exposure to HIV-1 Tat protein results in cytokine production in macrophages and astrocytes : A hit and run phenomenon. Journal of Biological Chemistry. 1999;274:17098–17102. doi: 10.1074/jbc.274.24.17098. [DOI] [PubMed] [Google Scholar]
- Persidsky Y, Gendelman HE. Mononuclear phagocyte immunity and the neuropathogenesis of HIV-1 infection. Journal of Leukocyte Biology. 2003;74:691–701. doi: 10.1189/jlb.0503205. [DOI] [PubMed] [Google Scholar]
- Polazzi E, Contestabile A. Reciprocal interactions between microglia and neurons: from survival to neuropathology. Rev Neurosci. 2002;13:221–242. doi: 10.1515/revneuro.2002.13.3.221. [DOI] [PubMed] [Google Scholar]
- Rafei M, Hsieh J, Fortier S, et al. Mesenchymal stromal cell-derived CCL2 suppresses plasma cell immunoglobulin production via STAT3 inactivation and PAX5 induction. Blood. 2008;112:4991–4998. doi: 10.1182/blood-2008-07-166892. [DOI] [PubMed] [Google Scholar]
- Rappaport J, Joseph J, Croul S, Alexander G, Del Valle L, Amini S, Khalili K. Molecular pathway involved in HIV-1-induced CNS pathology: role of viral regulatory protein, Tat. Journal of Leukocyte Biology. 1999;65:458–465. doi: 10.1002/jlb.65.4.458. [DOI] [PubMed] [Google Scholar]
- Ronaldson PT, Bendayan R. HIV-1 viral envelope glycoprotein gp120 triggers an inflammatory response in cultured rat astrocytes and regulates the functional expression of P-glycoprotein. Mol Pharmacol. 2006;70:1087–1098. doi: 10.1124/mol.106.025973. [DOI] [PubMed] [Google Scholar]
- Rothenaigner I, Kramer S, Ziegler M, Wolff H, Kleinschmidt A, Brack-Werner R. Long-term HIV-1 infection of neural progenitor populations. AIDS. 2007;21:2271–2281. doi: 10.1097/QAD.0b013e3282f12f27. [DOI] [PubMed] [Google Scholar]
- Schall TJ, Bacon K, Toy KJ, Goeddel DV. Selective attraction of monocytes and T lymphocytes of the memory phenotype by cytokine RANTES. Nature. 1990;347:669–671. doi: 10.1038/347669a0. [DOI] [PubMed] [Google Scholar]
- Schinkothe T, Bloch W, Schmidt A. In vitro secreting profile of human mesenchymal stem cells. Stem Cells Dev. 2008;17:199–206. doi: 10.1089/scd.2007.0175. [DOI] [PubMed] [Google Scholar]
- Schmidtmayerova H, Nottet HS, Nuovo G, et al. Human immunodeficiency virus type 1 infection alters chemokine beta peptide expression in human monocytes: implications for recruitment of leukocytes into brain and lymph nodes. Proc Natl Acad Sci U S A. 1996;93:700–704. doi: 10.1073/pnas.93.2.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz L, Major EO. Neural progenitors and HIV-1-associated central nervous system disease in adults and children. Curr HIV Res. 2006;4:319–327. doi: 10.2174/157016206777709438. [DOI] [PubMed] [Google Scholar]
- Sheng WS, Hu S, Ni HT, Rowen TN, Lokensgard JR, Peterson PK. TNF-alpha-induced chemokine production and apoptosis in human neural precursor cells. J Leukoc Biol. 2005;78:1233–1241. doi: 10.1189/jlb.0405221. [DOI] [PubMed] [Google Scholar]
- Shingo T, Sorokan ST, Shimazaki T, Weiss S. Erythropoietin regulates the in vitro and in vivo production of neuronal progenitors by mammalian forebrain neural stem cells. J Neurosci. 2001;21:9733–9743. doi: 10.1523/JNEUROSCI.21-24-09733.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simard AR, Soulet D, Gowing G, Julien JP, Rivest S. Bone marrow-derived microglia play a critical role in restricting senile plaque formation in Alzheimer's disease. Neuron. 2006;49:489–502. doi: 10.1016/j.neuron.2006.01.022. [DOI] [PubMed] [Google Scholar]
- Sommer I, Schachner M. Cells that are O4 antigen-positive and O1 antigen-negative differentiate into O1 antigen-positive oligodendrocytes. Neuroscience Letters. 1982;29:183–188. doi: 10.1016/0304-3940(82)90351-2. [DOI] [PubMed] [Google Scholar]
- Tanaka S, Kamachi Y, Tanouchi A, Hamada H, Jing N, Kondoh H. Interplay of SOX and POU factors in regulation of the Nestin gene in neural primordial cells. Mol Cell Biol. 2004;24:8834–8846. doi: 10.1128/MCB.24.20.8834-8846.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taupin P, Ray J, Fischer WH, Suhr ST, Hakansson K, Grubb A, Gage FH. FGF-2-responsive neural stem cell proliferation requires CCg, a novel autocrine/paracrine cofactor. Neuron. 2000;28:385–397. doi: 10.1016/s0896-6273(00)00119-7. [DOI] [PubMed] [Google Scholar]
- Torres-Munoz J, Stockton P, Tacoronte N, Roberts B, Maronpot RR, Petito CK. Detection of HIV-1 gene sequences in hippocampal neurons isolated from postmortem AIDS brains by laser capture microdissection. J Neuropathol Exp Neurol. 2001;60:885–892. doi: 10.1093/jnen/60.9.885. [DOI] [PubMed] [Google Scholar]
- Tran PB, Ren D, Miller RJ. The HIV-1 coat protein gp120 regulates CXCR4-mediated signaling in neural progenitor cells. J Neuroimmunol. 2005;160:68–76. doi: 10.1016/j.jneuroim.2004.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trillo-Pazos G, Diamanturos A, Rislove L, et al. Detection of HIV-1 DNA in microglia/macrophages, astrocytes and neurons isolated from brain tissue with HIV-1 encephalitis by laser capture microdissection. Brain Pathol. 2003;13:144–154. doi: 10.1111/j.1750-3639.2003.tb00014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Rie A, Harrington PR, Dow A, Robertson K. Neurologic and neurodevelopmental manifestations of pediatric HIV/AIDS: a global perspective. Eur J Paediatr Neurol. 2007;11:1–9. doi: 10.1016/j.ejpn.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Vangelista L, Secchi M, Lusso P. Rational design of novel HIV-1 entry inhibitors by RANTES engineering. Vaccine. 2008;26:3008–3015. doi: 10.1016/j.vaccine.2007.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner W, Roderburg C, Wein F, Diehlmann A, Frankhauser M, Schubert R, Eckstein V, Ho AD. Molecular and secretory profiles of human mesenchymal stromal cells and their abilities to maintain primitive hematopoietic progenitors. Stem Cells. 2007;25:2638–2647. doi: 10.1634/stemcells.2007-0280. [DOI] [PubMed] [Google Scholar]
- Walton NM, Sutter BM, Laywell ED, Levkoff LH, Kearns SM, Marshall GP, 2nd, Scheffler B, Steindler DA. Microglia instruct subventricular zone neurogenesis. Glia. 2006;54:815–825. doi: 10.1002/glia.20419. [DOI] [PubMed] [Google Scholar]
- Wang Z, Trillo-Pazos G, Kim SY, et al. Effects of human immunodeficiency virus type 1 on astrocyte gene expression and function: Potential role in neuropathogenesis. Journal of Neurovirology. 2004;10:25–32. doi: 10.1080/753312749. [DOI] [PubMed] [Google Scholar]
- Whitney NP, Eidem TM, Peng H, Huang Y, Zheng JC. Inflammation mediates varying effects in neurogenesis: relevance to the pathogenesis of brain injury and neurodegenerative disorders. J Neurochem. 2009;108:1343–1359. doi: 10.1111/j.1471-4159.2009.05886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff RH, Tekki-Kessaris N, Stiles CD, Rowitch DH, Richardson WD. Oligodendrocyte development in the spinal cord and telencephalon: common themes and new perspectives. Int J Dev Neurosci. 2001;19:379–385. doi: 10.1016/s0736-5748(00)00083-6. [DOI] [PubMed] [Google Scholar]
- Yao H, Peng F, Dhillon N, Callen S, Bokhari S, Stehno-Bittel L, Ahmad SO, Wang JQ, Buch S. Involvement of TRPC channels in CCL2-mediated neuroprotection against tat toxicity. J Neurosci. 2009;29:1657–1669. doi: 10.1523/JNEUROSCI.2781-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung MC, Pulliam L, Lau AS. The HIV envelope protein gp120 is toxic to human brain-cell cultures through the induction of interleukin-6 and tumor necrosis factor-alpha. AIDS. 1995;9:137–143. [PubMed] [Google Scholar]
- Ziv Y, Ron N, Butovsky O, Landa G, Sudai E, Greenberg N, Cohen H, Kipnis J, Schwartz M. Immune cells contribute to the maintenance of neurogenesis and spatial learning abilities in adulthood. Nat Neurosci. 2006;9:268–275. doi: 10.1038/nn1629. [DOI] [PubMed] [Google Scholar]