Abstract
The aim of the study was to investigate the influence of severe hyperthyroidism on plasma high-density lipoprotein cholesterol (HDL-C). Recently, it was shown in mice that increasing doses of triiodothyronine (T3) upregulate hepatic expression of scavenger receptor-BI (SR-BI), resulting in increased clearance of plasma HDL-C. Here we show that severe hyperthyroidism in mice did not affect hepatic expression of SR-BI, but reduced hepatic expression of ATP-binding cassette transporter 1 (ABCA1), accompanied by a 40%-reduction of HDL-C. Sterol content of bile, liver and feces was markedly increased, accompanied by upregulation of hepatic CYP7A1, and ATP-binding cassette half-transporter ABCG5, which is known to promote biliary sterol secretion upon dimerization with ABCG8. Both control and hyperthyroid mice exerted identical plasma clearance of intravenously injected [3H] HDL-C, supporting the view that severe hyperthyroidism does not affect HDL-C clearance, but rather its formation via hepatic ABCA1.
Thyroid disorders influence plasma levels of low-density lipoprotein cholesterol (LDL-C) and HDL-C. Changes in LDL-C have been extensively studied and were attributed to changes in hepatic expression of LDL receptor (LDLr) (1). However, little is known about the causes of HDL-C decrease under hyperthyroid conditions.
Recently, Parini and coworkers showed that intraperitoneal treatment of mice with increasing doses of T3 resulted in upregulation of hepatic SR-BI (2), which was accompanied by a reduction of plasma HDL-C. SR-BI, a CD36 family member, mediates high affinity binding of HDL and the selective uptake of HDL-derived lipids into liver and represents a physiologically relevant receptor for HDL-C metabolism (3-8). A further protein expressed in liver which was shown to influence HDL-C metabolism is ABCA1 (9). Hepatic ABCA1 is the major transporter that facilitates the efflux of cholesterol to poorly lipidated apoA-I to form nascent or preβ HDL; in fact, ABCA1-KO mice are characterized by virtually undetectable plasma HDL-C (10-12).
In the current study we describe that induction of severe hyperthyroidism in mice results in a dramatic decrease of plasma HDL-C levels. Our data suggest that in this model of hyperthyroidism lowering of plasma HDL-C is due to diminished lipidation via ABCA1.
Materials and Methods
Reagents
3,3′,5′-Triiodo-L-thyronine was purchased from Sigma, St. Louis, MO.
Animals
Male Balb/c mice were obtained from Charles River Laboratories, Kisslegg, Germany, and housed under protocols approved by the Austrian Animal Care and Use Committee. All procedures and care of animals were approved by the Austrian Animal Care and Use Committee. Mice of 20g were divided into two groups and intraperitoneally injected with 5 μg T3 in PBS or with PBS alone as control, respectively (13). Both, control and T3-treated mice were under a 12-h light, 12-h dark schedule (lights off at 7 p.m.), and were injected at 8 a.m. After 14 days of daily treatment, animals were fasted for 5 h after the last injection and anesthetized. Blood samples were taken, mice sacrificed by cervical dislocation, and organ biopsies were snap-frozen.
Bile and liver cholesterol analysis
The abdominal cavity was opened through a ventral incision. After ligation of the bile ductus and transection of ligamentum falciparum, the gall bladder was removed in toto for exposure. Gall bladder volume was calculated using the formula for ellipsoids: 4/3 × Π × a × b2. Subsequently, bile was aspirated and stored at 4°C. Biliary cholesterol of pooled bile was measured within 14 days using ABX Diagnostics commercial kits (ABX Diagnostics, Montpellier, France). Liver was subdiveded into 4 parts, weighed and snap-frozen. Liver total cholesterol was extracted and measured as described (14).
Hepatic cholesterol synthesis
Hepatic cholesterol synthesis was determined according to a previously described protocol (15). In brief, mice of 20g were daily injected with T3 for 2 weeks as described above. After a 18 h fasting period, animals received a final T3 injection with at 8 a.m. of day 15. After another 2 h, [1(2)-14C]acetate (100 kBq / animal) was intraperitoneally injected. 2 h later (4 h after T3 administration), animals were sacrificed and exsanguinated. The abdominal cavity was subsequently opened and liver specimens taken, weighed and snap-frozen. Liver cholesterol was extracted as described (15), and [14C]cholesterol measured by liquid scintillation counting.
Lipoprotein parameters
Total cholesterol and triglycerides (TG) were measured in whole plasma of each animal employing ABX Diagnostics commercial kits (ABX Diagnostics, Montpellier, France). Additionally, plasma samples of six animals of each group were combined and subjected to FPLC fractionation analysis with two tandem Superose 6 columns (GE Healthcare, Austria) as described previously (16). Apolipoprotein (apo) A-I measurements were performed by an immunonephelometric assay as described (17).
Measurement of PLTP plasma activity
Plasma activity of PLTP was performed as described (16).
[3H]HDL turnover studies
Murine HDL was prepared by ultracentrifugation in the density range of 1.063-1.21 g/ml (18) and radiolabeled with [3H]cholesteryl oleoyl ether (PerkinElmer, Boston, MA) as described (16). 25 μg [3H] HDL were injected into the tail vein of control and hyperthyroid mice, respectively and blood samples were drawn at 0.08, 5, 15, and 25 h from the retrobulbar plexus. Plasma samples were analyzed by liquid scintillation counting. The radioactivity of 0.08 h post-injection is defined as 100% of injected radioactivity.
Fecal sterol analysis
50 mg of dried feces were boiled in 1 ml alkaline methanol (1M NaOH / Methanol, 1:3 v/v) at 80°C for 2 h after addition of 50 nmoles 5α-Cholestane as internal standard for neutral sterol analysis. After cooling down to room temperature, neutral sterols were extracted using three times 3 ml of petroleum ether, boiling range 60-80°C. The residual sample was diluted 1: 9 with distilled water. 100 μl of the solution was subjected to a enzymatic total bile acid measurement (19). The extracted neutral sterols were converted to trimethylsilyl derivatives. Neutral sterol composition of prepared feces samples was determined by capillary gas chromatography on an Agilent gas chromatograph (HP 6890) equipped with a 25 m × 0.25 mm CP-Sil-19 fused silica column (Varian, Middelburg, The Netherlands) and a Flame Ionization Detector. The working conditions were the following: Injector temperature 280°C; pressure 16.0 psi; column flow constant at 0.8 ml/min; oven temperature program: 240°C (4 min), 10°C/min to 280°C ( 27 min); detector temperature 300°C.
Plant sterol measurement in plasma
Sitosterol and campesterol were extracted from 10 μl plasma from each animal in duplicate samples. Samples were derivatized with trimethylsilane reagent (pyridin:hexametyl disilan:trimetylchlorosilane 3:2:1, v/v/v) prior to gas-chromatography-mass spectrometry analysis (20). D5-campesterol/sitosterol was used as internal standard. The levels of sitosterol and campesterol in plasma reflect cholesterol absorption (21).
Protein extraction and Western blot analysis
Preparation of hepatic proteins and Western blot analysis were performed as described (16). Immunodetection of SR-BI was carried out by the use of a rabbit antibody against SR-BI (NB 400-104, Novus Biologicals, Littleton, CO), detection of ABCA1 was performed with a polyclonal rabbit anti-ABCA1 antibody (NB 400-105, Novus Biologicals, Littleton, CO). The chemoluminescent reaction was performed using Super Signal West Dura Reagent (Pierce, Rockford, IL, USA) and blots were visualized by Fluor-S-Imager using Quantity One V4.1 software (BioRad, Hercules, CA, USA).
RNA isolation, reverse transcription, and quantitative real-time PCR
Total RNA was extracted using RNAbee according to the manufacturer's protocol (Tel-test Inc., Friendswood, Texas, USA) and reverse transcribed with Omniscript-RT Kit (Qiagen, Hilden, Germany). Primers and probes for murine ABCA1, ABCG5, ABCG8, CYP7A1 were described previously (22), primers and probes for murine NPC1L1 elsewhere (23). GUSB was used as reference (Applied Biosystems, Foster City, CA, USA). Taqman real-time PCR reactions were performed on a Mx4000® Multiplex Quantitative PCR System (Stratagene, Amsterdam, The Netherlands).
Other measurements
Free T3 (fT3) and free 3,5,3′,5′-tetraiodothyronine (fT4) plasma levels were measured using an immunoassay kit (Roche Diagnostics, Mannheim, Germany). TSH could not be measured because no reliable assay existed at the time these studies were performed.
Statistical analysis
Results are presented as means ± s.e.m. The statistical significance of the differences between the means of the experimental groups was tested by the Student's t-test for unpaired data. A difference was considered statistically significant when P < 0.05.
Results
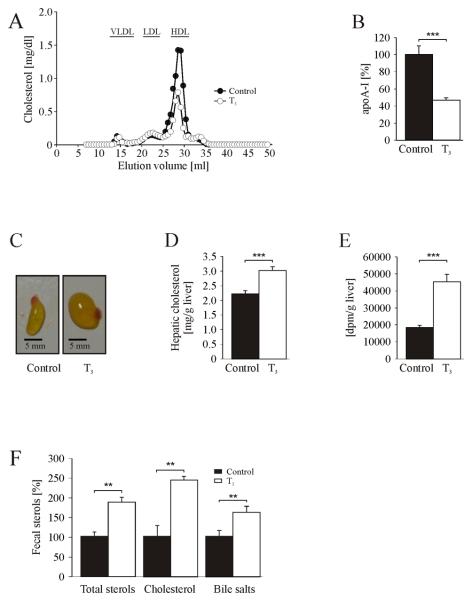
To induce severe hyperthyroidism in mice, we employed a dosage of T3 described in a previously published study (13). After 14 days of treatment, hyperthyroid mice did not show alterations of body weight, when compared to vehicle-treated animals (Table 1). Circulating free thyroid hormone (fT3) was increased ~5-fold, and free tetraiodothyronine (fT4) was not detectable in hyperthyroid animals, indicating a negative feedback inhibition of T3/T4 production in the thyroid caused by exogenously administered T3. Plasma total cholesterol in T3-treated mice was decreased by 40%, whereas no significant changes of plasma triglycerides were observed (Table 1). Hyperthyroid animals showed a 40% decrease of HDL-C (FIG. 1A) with a concomitant decrease of its major apolipoprotein apoA-I (−50%) (FIG. 1B), and no significant changes of apoB-containing lipoproteins (FIG. 1A).
Table 1.
Body weight and plasma parameters of hyperthyroid mice. Chow-fed Balb/c mice were treated with T3 or PBS for 14 days. (n=6-12 per group, unless otherwise stated). fT3, free triiodothyronine; fT4, free tetraiodothyronine.
| Control | T3 | P-value | |
|---|---|---|---|
| Body weight (g) | 19.6 ± 1.5 | 20.7 ± 2.3 | 0.34 |
| Plasma fT3 (pmol/l) | 1.8 ± 0.0 | 8.8 ± 0.3 | < 0.001 |
| Plasma fT4 (pmol/l) | 17.4 ± 4.4 | non detectable | < 0.001 |
| Plasma cholesterol (mg/dl) | 89.8 ± 19.2 | 54.2 ± 5.3 | < 0.001 |
| Plasma triglycerides (mg/dl) | 112.0 ± 23.7 | 123.3 ± 50.3 | 0.63 |
| Biliary cholesterol (μg/μl) | 2.8 | 5.7 | (Pooled bile) |
FIG.1.
Hyperthyroidism lowers plasma HDL-C and promotes excretion of hepatic cholesterol. Chow-fed Balb/c mice were treated with T3 or PBS for 14 days. (A) FPLC analysis of pooled plasma from control and T3-treated mice (n=6 per group). (B) Immunonephelometric measurement of plasma apoA-I concentration (n=5-6 per group). (C) Representative picture of gall bladders from control and T3-treated animals. (D) Enzymatic analysis of hepatic cholesterol concentration (n=6 per group). (E) Hepatic cholesterol de novo synthesis. [14C]cholesterol [dpm] is normalized to g liver (n=6 per group). (F) Feces were collected for 48 hours and analyzed by capillary gas chromatography (n=5-6 per group). *P < 0.05, **P < 0.01, ***P < 0.001 versus corresponding controls; ns, non significant; data presented in % are normalized to the respective controls.
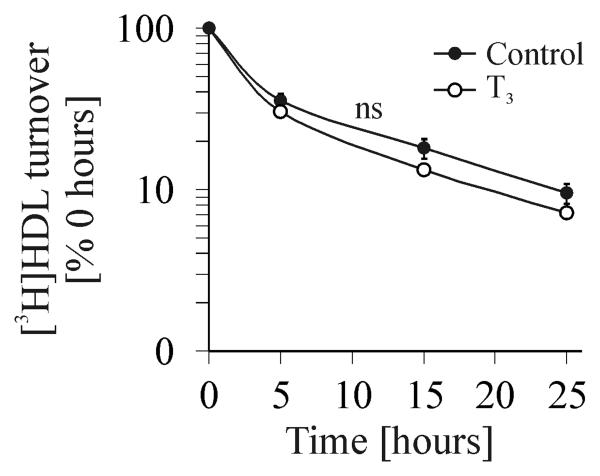
Hyperthyroid animals had a 2.7-fold larger gall bladder volume, when compared to controls (19.2 ± 2.2 μl and 52.5 ± 1.9 μl, P<0.001, n=3) (FIG. 1C), and the cholesterol concentration in gall bladder bile was increased 2.6-fold (Table 1). In addition, these animals showed a 40% increase in hepatic cholesterol (FIG. 1D). To determine the source of increased hepatic cholesterol, we measured cholesterol de novo synthesis, and indeed found a 2.5-fold amount of newly synthesized cholesterol in livers of T3-treated mice (FIG. 1E). Analysis of fecal sterol excretion in hyperthyroid mice revealed markedly increased levels of cholesterol as well as of bile acid mass (FIG. 1F). Both control and hyperthyroid mice showed identical plasma decay of intravenously injected [3H]HDL cholesteryl oleoyl ether (FIG. 2), thus excluding a different HDL-C clearance. HDL-C concentrations might also be influenced by plasma activity of phospholipid transfer protein (PLTP), the major lipid transfer protein in the mouse (24). However, we did not observe any differences in PLTP activity between control and hyperthyroid animals (data not shown).
FIG.2.
[3H]HDL turnover study. After 14 days of treatment with T3 or PBS, 25 μg [3H] HDL cholesteryl oleoyl ether were injected into the tail vein of control and hyperthyroid mice, respectively and blood samples were drawn from the retrobulbar plexus at the indicated time points. Plasma samples were analyzed by liquid scintillation counting. The radioactivity of 0.08 h post-injection is defined as 100% of injected radioactivity. ns, non significant.
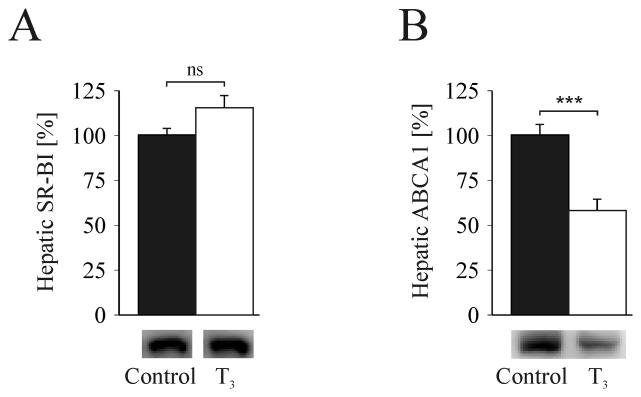
Subsequently, we performed Western blot analysis of the two major hepatic proteins involved in HDL metabolism, SR-BI and ABCA1 (3, 25-28). In agreement with [3H]HDL turnover studies, no significant changes in the hepatic expression of the HDL-receptor SR-BI were observed (FIG. 3A). However, ABCA1 was decreased by 40% in livers of hyperthyroid mice (FIG. 3B). Timmins et al. have previously shown that targeted inactivation of hepatic ABCA1 in mice results in an 80% reduction of HDL-C (29). Thus, ABCA1 was suggested to be responsible for the vast majority of initial lipidation of lipid-poor apoA-I (30).
FIG.3.
Hepatic expression of the major proteins known to influence HDL-C metabolism. Western blot analysis of (A) SR-BI and (B) ABCA1 expression (n=6 per group). Results are representative of 3 independent studies. T3, treated with T3 for 14 days; ***P < 0.001 versus corresponding controls; ns, non significant; data presented in % are normalized to the respective controls.
Real-time PCR measurements of hyperthyroid liver specimens revealed a transcriptional downregulation of ABCA1, increased mRNA levels of CYP7A1, the rate-limiting enzyme for conversion of cholesterol into bile acids, and an increased expression of hepatic ATP-binding cassette half-transporter ABCG5, which is known to promote biliary sterol secretion upon dimerization with ABCG8 (ABCG5/G8) (31) (FIG. 4A). Yu et al. previously showed overexpression of ABCG5/G8 to enhance biliary cholesterol secretion, to increase neutral sterol loss via the feces, and to strongly reduce the absorption of dietary sterols (31). Accordingly, induction of hepatic ABCG5/G8 in our hyperthyroid mice was associated with increased biliary cholesterol levels, increased fecal neutral sterol loss and reduced plasma phytosterol levels (FIG. 4B).
FIG.4.
Influence of hyperthyroidism on enterohepatic sterol metabolism. (A) Taqman real-time PCR analysis of hepatic genes involved in cholesterol metabolism (ABCA1, ABCG5, ABCG8) and bile acid synthesis (CYP7A1) (n=4-10 per group). (B) Gas-chromatography-mass spectrometry analysis of diet-derived phytosterols in plasma, normalized to cholesterol. Plasma phytosterol levels reflect intestinal absorption of cholesterol (n=5 per group). T3, treated with T3 for 14 days; **P < 0.01 versus corresponding controls; data are normalized to the respective controls.
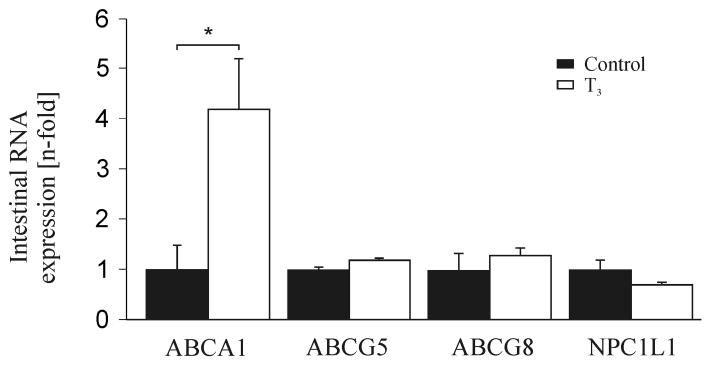
Finally, we investigated the expression of cholesterol transporters within the small intestine of hyperthyroid mice. No significant changes of intestinal ABCG5/G8 and Niemann-Pick C1 Like 1 protein (NPC1L1) (32, 33) were found (FIG. 5). Interestingly, intestinal expression of ABCA1 was increased 4-fold, which might have attenuated the fall in plasma HDL-C (34) (FIG. 5).
FIG.5.
Taqman real-time PCR analysis of intestinal cholesterol transporters (n=4-10 per group). T3, treated with T3 for 14 days; *P < 0.05 versus corresponding controls; data are normalized to the respective controls.
Discussion
Recent work by Brewer Jr. and colleagues indicates that hepatic expression of ABCA1 is crucial for whole body cholesterol homeostasis and, more precisely, for plasma HDL-C levels (9, 26). Our data suggest that severe hyperthyroidism reduces HDL formation by downregulating hepatic ABCA1, as targeted knockdown of hepatic ABCA1 expression has been demonstrated to reduce HDL-C (28, 29). In line with the mentioned studies, we also found a significant decrease of apoA-I plasma levels. However, we cannot rule out that other enzymes involved in lipoprotein metabolism might play a role in the hyperthyroid scenario, f.i. LCAT, endothelial lipase, hepatic lipase or lipid transfer proteins. In this study we analyzed PLTP, the major transfer protein in mice, and found no effect of severe hyperthyroidism on its plasma activity. T3 treatment has previously been shown to be associated with increased hepatic SR-BI expression (2). Surprisingly, in our experiments, severe hyperthyroidism did not influence SR-BI protein expression in liver. However, this finding is in good agreement with data by Johansson et al. analyzing both T3 and a liver selective TH analogue in mice (2).
As severe hyperthyroidism stimulated hepatic de novo cholesterol synthesis (FIG. 1E), which is known to be conferred by the induction of HMG CoA reductase (1), the question arised where this excess of cholesterol would be directed to. In our experiments, hepatic as well as biliary cholesterol were increased by T3-treatment. Hyperthyroidism also increased fecal cholesterol as well as bile acid mass, and induced hepatic expression of ABCG5 and CYP7A1. These data suggest that increased hepatic production of cholesterol may be counterbalanced by diverting cholesterol from the plasma to the bile and ultimately to the feces. Increased levels of neutral sterols from the bile, in turn, may compete with cholesterol and plant sterols of dietary origin, thus resulting in decreased intestinal absorption of dietary sterols (35).
The presented study is based exclusively on experiments in mice. Since mice transport the majority of plasma cholesterol as HDL, our data may not be fully applicable to the situation in humans. In the human system, a significant portion of HDL-cholesterol is transferred to LDL-particles via cholesteryl ester transfer protein (CETP). LDL cholesterol, in turn, is cleared by the hepatic LDL receptor. It is known that patients with subclinical hyperthyroidism, or patients treated with TSH-suppressive LT4 doses exhibit a significant reduction in plasma HDL-C levels (1). Accordingly, high doses of thyroid hormone might indeed lead to an overall impaired reverse cholesterol transport. Corresponding experiments in a CETP-expressing animal, f.i. in rabbits, might help to further understand the role of T3 in lipoprotein metabolism.
From our results in mice we conclude that severe hyperthyroidism may reduce the formation of nascent HDL particles by a marked downregulation of hepatic ABCA1. Our results suggest newly synthesized cholesterol to be retained in the liver and to be actively converted into bile salts for biliary excretion and/or directly transported into bile, thus increasing the sterol content in feces of hyperthyroid mice.
Acknowledgements
This work was supported by the Hans & Blanca Moser Stiftung (No. 61-1994/95 to I.T.), by the Medizinische Forschungsfoerderung Innsbruck (MFI No. 4316 to I.T.), by the Jubiläumsfond der Oesterreichischen Nationalbank (OENB, No. 12156 to I.T. and A.R.) and by the Fonds zur Foerderung der wissenschaftlichen Forschung (FWF, P19999-B05 to A.R.).
Abbreviations
- T3
3,3′,5′-Triiodo-L-thyronine
- T4
3,5,3′,5′-tetraiodothyronine
- SR-BI
scavenger receptor class B, type I
- ABCA1
ATP-binding cassette transporter 1
- ABCG5
ATP-binding cassette transporter 5, ABCG8, ATP-binding cassette transporter 8
- CYP7A1
Cholesterol 7α-Hydroxylase
- NPC1L1
Niemann-Pick C1 Like 1 protein
- apoA-I
apolipoprotein A-I
- apoB
apolipoprotein B
- HDL-C
high density lipoprotein cholesterol
- LDL-C
low density lipoprotein cholesterol
- VLDL-C
very low density lipoprotein colesterol
References
- 1.Duntas LH. Thyroid disease and lipids. Thyroid. 2002;12:287–93. doi: 10.1089/10507250252949405. [DOI] [PubMed] [Google Scholar]
- 2.Johansson L, Rudling M, Scanlan TS, Lundasen T, Webb P, Baxter J, Angelin B, Parini P. Selective thyroid receptor modulation by GC-1 reduces serum lipids and stimulates steps of reverse cholesterol transport in euthyroid mice. Proc Natl Acad Sci U S A. 2005;102:10297–302. doi: 10.1073/pnas.0504379102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kozarsky KF, Donahee MH, Rigotti A, Iqbal SN, Edelman ER, Krieger M. Overexpression of the HDL receptor SR-BI alters plasma HDL and bile cholesterol levels. Nature. 1997;387:414–7. doi: 10.1038/387414a0. [DOI] [PubMed] [Google Scholar]
- 4.Krieger M. Scavenger receptor class B type I is a multiligand HDL receptor that influences diverse physiologic systems. J Clin Invest. 2001;108:793–7. doi: 10.1172/JCI14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Temel RE, Trigatti B, DeMattos RB, Azhar S, Krieger M, Williams DL. Scavenger receptor class B, type I (SR-BI) is the major route for the delivery of high density lipoprotein cholesterol to the steroidogenic pathway in cultured mouse adrenocortical cells. Proc Natl Acad Sci U S A. 1997;94:13600–5. doi: 10.1073/pnas.94.25.13600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ueda Y, Royer L, Gong E, Zhang J, Cooper PN, Francone O, Rubin EM. Lower plasma levels and accelerated clearance of high density lipoprotein (HDL) and non-HDL cholesterol in scavenger receptor class B type I transgenic mice. J Biol Chem. 1999;274:7165–71. doi: 10.1074/jbc.274.11.7165. [DOI] [PubMed] [Google Scholar]
- 7.Arai T, Rinninger F, Varban L, Fairchild-Huntress V, Liang CP, Chen W, Seo T, Deckelbaum R, Huszar D, Tall AR. Decreased selective uptake of high density lipoprotein cholesteryl esters in apolipoprotein E knock-out mice. Proc Natl Acad Sci U S A. 1999;96:12050–5. doi: 10.1073/pnas.96.21.12050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sehayek E, Ono JG, Shefer S, Nguyen LB, Wang N, Batta AK, Salen G, Smith JD, Tall AR, Breslow JL. Biliary cholesterol excretion: a novel mechanism that regulates dietary cholesterol absorption. Proc Natl Acad Sci U S A. 1998;95:10194–9. doi: 10.1073/pnas.95.17.10194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brewer HB, Jr., Remaley AT, Neufeld EB, Basso F, Joyce C. Regulation of plasma high-density lipoprotein levels by the ABCA1 transporter and the emerging role of high-density lipoprotein in the treatment of cardiovascular disease. Arterioscler Thromb Vasc Biol. 2004;24:1755–60. doi: 10.1161/01.ATV.0000142804.27420.5b. [DOI] [PubMed] [Google Scholar]
- 10.McNeish J, Aiello RJ, Guyot D, Turi T, Gabel C, Aldinger C, Hoppe KL, Roach ML, Royer LJ, de Wet J, Broccardo C, Chimini G, Francone OL. High density lipoprotein deficiency and foam cell accumulation in mice with targeted disruption of ATP-binding cassette transporter-1. Proc Natl Acad Sci U S A. 2000;97:4245–50. doi: 10.1073/pnas.97.8.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Christiansen-Weber TA, Voland JR, Wu Y, Ngo K, Roland BL, Nguyen S, Peterson PA, Fung-Leung WP. Functional loss of ABCA1 in mice causes severe placental malformation, aberrant lipid distribution, and kidney glomerulonephritis as well as high-density lipoprotein cholesterol deficiency. Am J Pathol. 2000;157:1017–29. doi: 10.1016/S0002-9440(10)64614-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Orso E, Broccardo C, Kaminski WE, Bottcher A, Liebisch G, Drobnik W, Gotz A, Chambenoit O, Diederich W, Langmann T, Spruss T, Luciani MF, Rothe G, Lackner KJ, Chimini G, Schmitz G. Transport of lipids from golgi to plasma membrane is defective in tangier disease patients and Abc1-deficient mice. Nat Genet. 2000;24:192–6. doi: 10.1038/72869. [DOI] [PubMed] [Google Scholar]
- 13.Drover VA, Agellon LB. Regulation of the human cholesterol 7alpha-hydroxylase gene (CYP7A1) by thyroid hormone in transgenic mice. Endocrinology. 2004;145:574–81. doi: 10.1210/en.2003-0993. [DOI] [PubMed] [Google Scholar]
- 14.Loison C, Mendy F, Serougne C, Lutton C. Dietary myristic acid modifies the HDL-cholesterol concentration and liver scavenger receptor BI expression in the hamster. Br J Nutr. 2002;87:199–210. doi: 10.1079/BJNBJN2002521. [DOI] [PubMed] [Google Scholar]
- 15.Bischoff H, Angerbauer R, Bender J, Bischoff E, Faggiotto A, Petzinna D, Pfitzner J, Porter MC, Schmidt D, Thomas G. Cerivastatin: pharmacology of a novel synthetic and highly active HMG-CoA reductase inhibitor. Atherosclerosis. 1997;135:119–30. doi: 10.1016/s0021-9150(97)00188-3. [DOI] [PubMed] [Google Scholar]
- 16.Tancevski I, Frank S, Massoner P, Stanzl U, Schgoer W, Wehinger A, Fievet C, Eller P, Patsch JR, Ritsch A. Increased plasma levels of LDL cholesterol in rabbits after adenoviral overexpression of human scavenger receptor class B type I. J Mol Med. 2005;83:927–32. doi: 10.1007/s00109-005-0695-8. [DOI] [PubMed] [Google Scholar]
- 17.Singaraja RR, Fievet C, Castro G, James ER, Hennuyer N, Clee SM, Bissada N, Choy JC, Fruchart JC, McManus BM, Staels B, Hayden MR. Increased ABCA1 activity protects against atherosclerosis. J Clin Invest. 2002;110:35–42. doi: 10.1172/JCI15748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ji Y, Wang N, Ramakrishnan R, Sehayek E, Huszar D, Breslow JL, Tall AR. Hepatic scavenger receptor BI promotes rapid clearance of high density lipoprotein free cholesterol and its transport into bile. J Biol Chem. 1999;274:33398–402. doi: 10.1074/jbc.274.47.33398. [DOI] [PubMed] [Google Scholar]
- 19.Mashige F, Imai K, Osuga T. A simple and sensitive assay of total serum bile acids. Clin Chim Acta. 1976;70:79–86. doi: 10.1016/0009-8981(76)90007-3. [DOI] [PubMed] [Google Scholar]
- 20.Lutjohann D, Bjorkhem I, Beil UF, von Bergmann K. Sterol absorption and sterol balance in phytosterolemia evaluated by deuterium-labeled sterols: effect of sitostanol treatment. J Lipid Res. 1995;36:1763–73. [PubMed] [Google Scholar]
- 21.Miettinen TA, Tilvis RS, Kesaniemi YA. Serum plant sterols and cholesterol precursors reflect cholesterol absorption and synthesis in volunteers of a randomly selected male population. Am J Epidemiol. 1990;131:20–31. doi: 10.1093/oxfordjournals.aje.a115479. [DOI] [PubMed] [Google Scholar]
- 22.Naik SU, Wang X, Da Silva JS, Jaye M, Macphee CH, Reilly MP, Billheimer JT, Rothblat GH, Rader DJ. Pharmacological activation of liver X receptors promotes reverse cholesterol transport in vivo. Circulation. 2006;113:90–7. doi: 10.1161/CIRCULATIONAHA.105.560177. [DOI] [PubMed] [Google Scholar]
- 23.van der Veen JN, Kruit JK, Havinga R, Baller JF, Chimini G, Lestavel S, Staels B, Groot PH, Groen AK, Kuipers F. Reduced cholesterol absorption upon PPARdelta activation coincides with decreased intestinal expression of NPC1L1. J Lipid Res. 2005;46:526–34. doi: 10.1194/jlr.M400400-JLR200. [DOI] [PubMed] [Google Scholar]
- 24.Jiang XC, Bruce C, Mar J, Lin M, Ji Y, Francone OL, Tall AR. Targeted mutation of plasma phospholipid transfer protein gene markedly reduces high-density lipoprotein levels. J Clin Invest. 1999;103:907–14. doi: 10.1172/JCI5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krieger M. Charting the fate of the "good cholesterol": identification and characterization of the high-density lipoprotein receptor SR-BI. Annu Rev Biochem. 1999;68:523–58. doi: 10.1146/annurev.biochem.68.1.523. [DOI] [PubMed] [Google Scholar]
- 26.Basso F, Freeman L, Knapper CL, Remaley A, Stonik J, Neufeld EB, Tansey T, Amar MJ, Fruchart-Najib J, Duverger N, Santamarina-Fojo S, Brewer HB., Jr Role of the hepatic ABCA1 transporter in modulating intrahepatic cholesterol and plasma HDL cholesterol concentrations. J Lipid Res. 2003;44:296–302. doi: 10.1194/jlr.M200414-JLR200. [DOI] [PubMed] [Google Scholar]
- 27.Joyce C, Freeman L, Brewer HB, Jr., Santamarina-Fojo S. Study of ABCA1 function in transgenic mice. Arterioscler Thromb Vasc Biol. 2003;23:965–71. doi: 10.1161/01.ATV.0000055194.85073.FF. [DOI] [PubMed] [Google Scholar]
- 28.Ragozin S, Niemeier A, Laatsch A, Loeffler B, Merkel M, Beisiegel U, Heeren J. Knockdown of hepatic ABCA1 by RNA interference decreases plasma HDL cholesterol levels and influences postprandial lipemia in mice. Arterioscler Thromb Vasc Biol. 2005;25:1433–8. doi: 10.1161/01.ATV.0000166616.86723.d0. [DOI] [PubMed] [Google Scholar]
- 29.Timmins JM, Lee JY, Boudyguina E, Kluckman KD, Brunham LR, Mulya A, Gebre AK, Coutinho JM, Colvin PL, Smith TL, Hayden MR, Maeda N, Parks JS. Targeted inactivation of hepatic Abca1 causes profound hypoalphalipoproteinemia and kidney hypercatabolism of apoA-I. J Clin Invest. 2005;115:1333–42. doi: 10.1172/JCI23915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rader DJ. Molecular regulation of HDL metabolism and function: implications for novel therapies. J Clin Invest. 2006;116:3090–100. doi: 10.1172/JCI30163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu L, Li-Hawkins J, Hammer RE, Berge KE, Horton JD, Cohen JC, Hobbs HH. Overexpression of ABCG5 and ABCG8 promotes biliary cholesterol secretion and reduces fractional absorption of dietary cholesterol. J Clin Invest. 2002;110:671–80. doi: 10.1172/JCI16001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Altmann SW, Davis HR, Jr., Zhu LJ, Yao X, Hoos LM, Tetzloff G, Iyer SP, Maguire M, Golovko A, Zeng M, Wang L, Murgolo N, Graziano MP. Niemann-Pick C1 Like 1 protein is critical for intestinal cholesterol absorption. Science. 2004;303:1201–4. doi: 10.1126/science.1093131. [DOI] [PubMed] [Google Scholar]
- 33.Kruit JK, Groen AK, van Berkel TJ, Kuipers F. Emerging roles of the intestine in control of cholesterol metabolism. World J Gastroenterol. 2006;12:6429–39. doi: 10.3748/wjg.v12.i40.6429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brunham LR, Kruit JK, Iqbal J, Fievet C, Timmins JM, Pape TD, Coburn BA, Bissada N, Staels B, Groen AK, Hussain MM, Parks JS, Kuipers F, Hayden MR. Intestinal ABCA1 directly contributes to HDL biogenesis in vivo. J Clin Invest. 2006;116:1052–62. doi: 10.1172/JCI27352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu L, Hammer RE, Li-Hawkins J, Von Bergmann K, Lutjohann D, Cohen JC, Hobbs HH. Disruption of Abcg5 and Abcg8 in mice reveals their crucial role in biliary cholesterol secretion. Proc Natl Acad Sci U S A. 2002;99:16237–42. doi: 10.1073/pnas.252582399. [DOI] [PMC free article] [PubMed] [Google Scholar]