Abstract
Although the regulation of smooth muscle cell (SMC) gene expression by cGMP-dependent protein kinase (PKG) is now recognized, the mechanisms underlying these effects are not fully understood. In this study, we report that PKG-I stimulates myocardin/serum response factor (SRF)-dependent gene expression in vascular SMCs. The expression of PKG in PKG-deficient cells enhanced myocardin-induced SM22 promoter activity in a concentration-dependent fashion. However, neither SRF nor myocardin expression was affected. To investigate alternative mechanisms, we examined whether PKG affects the phosphorylation of E26-like protein-1 (Elk-1), a SRF/myocardin transcription antagonist. The activation of PKG caused an increase in a higher molecular mass form of phospho-Elk-1 that was determined to be small ubiquitin-related modifier (sumo)ylated Elk-1. PKG increased Elk-1 sumoylation twofold compared with the PKG-deficient cells, and Elk-1 sumoylation was reduced using dominant-negative sumo-conjugating enzyme, DN-Ubc9, confirming PKG-dependent sumoylation of phospho-Elk-1 in vascular SMCs. In addition, PKG stimulated Elk-1 sumoylation in COS-7 cells overexpressing Elk-1, sumo-1, and PKG-I. The increased expression of PKG in vascular SMCs inhibited Elk-1 binding to SMC-specific promoters, SM22 and smooth muscle myosin heavy chain, as measured by EMSA and chromatin immunoprecipitation assay, and PKG suppressed the Elk-1 inhibition of SM22 reporter gene expression. Taken together, these data suggest that PKG-I decreases Elk-1 activity by sumo modification of Elk-1, thereby increasing myocardin-SRF activity on SMC-specific gene expression.
Keywords: guanosine 3′,5′-cyclic monophosphate-dependent protein kinase; small ubiquitin-like modifier; myocardin; E26-like protein-1
vascular smooth muscle cells (SMCs) undergo dramatic phenotypic changes in both culture and in vivo (8, 37). It has been shown that in response to arterial injury in vivo or exposure to growth conditions in vitro, SMCs become more proliferative and express lower levels of contractile proteins and other signaling molecules associated with the more contractile “differentiated” phenotype (7, 9, 38, 43). These changes in gene expression profiles are believed to underlie SMC phenotypic modulation, a phenomenon that is associated with several vascular disorders such as atherosclerosis and vascular fibrosis. Understanding the mechanisms that control SMC-specific gene expression is therefore an important aspect for understanding vascular disease.
Over the last several years, a significant amount of progress has been made toward understanding the mechanisms regulating SMC-specific gene expression, especially at the level of promoter regulation and chromatin remodeling. It is now widely accepted that serum response factor (SRF) is an important transcription factor that increases the expression of a wide variety of SMC-specific genes such as smooth muscle-specific myosin heavy chain (SM-MHC), SM22α, telokin, and α-actin (32, 34). The specificity for SRF to increase SMC promoter activity is conferred by members of the myocardin-related factor family that act as cotranscriptional regulators with SRF (10, 19). Myocardin itself has been shown to increase SMC-specific gene expression in many non-SMCs, thus illustrating the importance of this protein in phenotypic properties of SMCs (29).
Like many transcription factors, SRF activity is regulated by numerous proteins, including other transcription factors and chromatin binding proteins. One such transcription factor is a member of the ets family of proteins, E26-like protein-1 (Elk-1) (51, 57). Elk-1, like SRF, is widely expressed in cells. When phosphorylated by mitogen-activated protein (MAP) kinases in response to growth signals, Elk-1 binds to SRF and displaces myocardin binding, which in turn reduces SMC-specific gene expression (51). Elk-1 and other transcription factors are also regulated by a posttranslational small ubiquitin-related modifier (sumo)ylation, which affects various activities of these proteins including nuclear uptake, phosphorylation, and binding to chromatin DNA (36). In the case of Elk-1, it has been proposed that sumoylation represses its transcriptional activity and possibly inhibits its interaction with SRF (54).
The various signaling pathways that ultimately regulate SRF, Elk-1, and other transcription factors in SMCs are not well understood. One pathway that may regulate the proliferative and/or differentiation state of vascular SMCs is the nitric oxide (NO)/cGMP pathway. NO donors and cGMP analogs have been shown to suppress SMC proliferation (15, 20). A number of years ago, our laboratory reported that rat aortic SMCs, when cultured in vitro, have a decreased expression of the major cGMP downstream mediator, cGMP-dependent protein kinase (PKG) (14, 16). When introduced into PKG-deficient rat aortic SMCs by transfection or adenoviral transduction, PKG increases the expression of SMC-specific gene products such as SM-MHC, α-actin, and calponin, suggesting an important role for PKG in regulating SMC-specific gene expression (1, 3, 31). In turn, the downregulation of PKG expression in SMCs, such as occurs with multiple passaging (14) or in the presence of inflammatory mediators such as interleukin-1 (4), is associated with dedifferentiation or modulation to the more proliferative phenotype. Therefore, the cGMP/PKG pathway appears to represent an important regulatory pathway for a phenotypic modulation and SMC-specific gene regulation.
In this study, we explore one possible mechanism of action of cGMP/PKG in the regulation of SMC-specific gene expression. The results reported here suggest that cGMP/PKG suppresses the activity of Elk-1 on myocardin-SRF transcription through the sumoylation of Elk-1.
EXPERIMENTAL PROCEDURES
DNA constructs.
The PKG-Iα construct was cloned into pcDNA3.1 (Invitrogen). The pCMV5-Elk-1 construct tagged with FLAG and His was provided by Dr. Andrew Sharrocks (Univ. of Manchester, UK) and recloned into pcDNA3.1. The expression plasmid for mouse myocardin isoform B was provided by Dr. Eric Olson (Univ. of Texas, Dallas, TX), whereas hemagglutinin (HA) -tagged sumo-1 was provided by Dr. Edward Yeh (Univ. of Texas, MDACC, TX). The dominant negative (DN)-Ubc9 cDNA was provided by Dr. Ronald Hay (Univ. of St. Andrews, St. Andrews, UK). Mouse SM22α-luciferase (−447 ± 62 bp) construct was provided by Dr. Joseph Miano (Univ. of Rochester Medical School, Rochester, NY).
Cell culture, transfection, and reporter gene assays.
Animal procedures were reviewed and approved by the Institutional Animal Care and Use Committee of the University of Alabama. SMCs were isolated from the thoracic and abdominal aortas of Sprague-Dawley rats (200 to 250 g) as described previously (1, 14). A7r5 and COS-7 cells were cultured in DMEM (Cellgro)-10% FBS (Invitrogen) and passaged routinely. Transient and stable plasmid transfection of cells was performed by using Lipofectamine 2000 (Invitrogen) or FuGene6 (Roche Diagnostics, Indianapolis, IN). To acquire stable PKG-transfected rat aortic SMCs, empty or PKG-Iα-expressing plasmids were used for transfection and selected under 500 μg/ml of G418 antibiotics (Sigma) as described previously (1, 18). Luciferase assays were carried out using dual-luciferase reporter assay system (Promega). Cells were seeded in 24-well plates at 3 to 4 × 104 per well, and transfections were performed using Fugene 6 transfecting reagent (Roche). The total amount of DNA per well was kept constant by adding the corresponding amount of expression vector, pcDNA3.1, without a cDNA insert. The promoter activity was expressed as relative light units by the measurement of the firefly luciferase activity relative to the internal control Renilla luciferase or β-galactosidase activity using a manual luminometer (TD 20/20 Luminometer Turner Design). A minimum of three independent transfections was performed at least in duplicate. Results are expressed as means ± SD.
Adenovirus transduction.
Adenovirus carrying the coding sequence of PKG-Iα or green fluorescent protein was produced and used for the infection of rat aortic SMCs according to the manufacturer's protocol (Vector BioLabs). To express PKG-Iα in rat aortic SMCs, the cells were plated at a density of 800,000 cells per 100-mm plate and grown overnight. On the next day, either control adenovirus or recombinant PKG-Iα adenovirus was added directly to the culture medium at a multiplicity of infection of 20 after changing to the fresh complete medium. After 1 day of incubation, the culture medium was replaced with serum-deprived DMEM including 1 mg/ml of BSA and cultured for 2 to 3 days. The level of PKG-Iα expression was constantly monitored by Western blot analysis.
Preparation of nuclear and cytoplasmic extracts.
Nuclear and cytoplasmic fractionation was performed using nuclear and cytoplasmic extraction reagent (Pierce) supplemented with 1× protease inhibitors and 1× phosphatase inhibitors (Pierce) according to the manufacturer's protocol. In brief, treated cells were placed on ice, washed three times with cold PBS, and harvested directly or snap frozen until use. The cells were harvested by scraping off the dish in an appropriate volume of cytoplasmic extraction reagent I containing protease inhibitors, phosphatase inhibitors, and 20 μM of N-ethylmaleimide (NEM; Sigma) and incubated on ice for 10 min. After cytoplasmic extraction reagent II was added with an additional incubation on ice for 1 min, the nuclei were collected by centrifugation at 16,000 g for 5 min and the supernatants were collected for cytoplasmic extracts. The nuclei were resuspended by adding nuclear extraction reagent and incubated on ice for a total of 50 min with vortexing for 15 s every 10 min. The nuclear extracts were collected as the supernatant by centrifugation at 16,000 g for 10 min. All extracts were used directly or snap frozen and kept at −80°C until use.
SDS-PAGE and immunoblot analysis.
Cells (SMCs or COS-7) were lysed in a denaturing lysis buffer consisting of 20 mM Tris·HCl (pH 7.4), 150 mM NaCl, and 1% SDS, containing 1× protease inhibitor cocktail (Pierce), 1× phophatase inhibitor cocktail (Pierce), and 20 μM of NEM (Sigma) or in SDS sample buffer and were briefly sonicated (Vir Sonic Ultrasonic Cell Disrupter 100) to reduce sample viscosity. After being heated to a boil, the samples were applied to 8% or ∼8–17.5% gradient SDS-PAGE and transferred to nitrocellulose membranes for the molecules of interest using the following primary antibodies: anti-PKG-I (Stressgen), anti-SM-MHC (provided by Dr. Primal deLanorelle, Univ. of Illinois, Chicago), anti-SRF, anti-Elk-1, anti-myocardin (Santa Cruz), anti-sumo-1, anti-phospho-Elk-1, anti-phospho-vasodilator-stimulated phosphoprotein (Ser239), anti-phospho-Erk, anti-FLAG (cell signaling), anti-β actin (Sigma). Protein bands were visualized using the enhanced chemiluminescence system (Pierce).
Immunoprecipitation.
For immunoprecipitation, the total volume of nuclear extracts (∼50–100 μg) were made to 0.5 ml with radioimmunoprecipitation assay buffer consisting of 50 mM Tris·HCl (pH 7.5), 300 mM NaCl, 0.2% SDS, 2% Nonidet P-40, and 2% sodium deoxycholate, supplemented with protease inhibitor cocktail and phosphatase inhibitors. To this lysate, 2 μg of normal rabbit IgG (Santa Cruz) were added to preclear the lysate. After incubation at 4°C for 30 min, 40 μl of protein A/G agarose (Santa Cruz) were added and incubated for an additional 30 min and centrifuged at 1,000 g for 5 min. The resulting supernatant was incubated with primary antibody (10 μl of anti-sumo-1 antibody, Cell Signaling) at 4°C for 1 h following overnight incubation after adding 20 μl of protein A/G agarose. The agarose beads were collected by centrifugation at 1,000 g for 5 min, and the pellet was resuspended in cold radioimmunoprecipitation assay buffer and washed four times with the same buffer by centrifuging at 1,000 g for 5 min before boiling in the SDS sample buffer. Immunoblotting following 8% SDS-PAGE was conducted to detect the sumoylated Elk-1.
Denaturing purification and analysis of in vivo sumo-Elk conjugates.
Control or stably PKG-I expressing COS-7 cells were transfected with plasmids expressing HA-sumo-1 and His-Elk-1. Two days later, the cells were harvested in 1 ml of lysis buffer consisting of 6 M guanadine-HCl, 100 mM NaH2PO4, 0.3 M NaCl, 10 mM Tris·Cl (pH 8.0), 20 mM β-mercaptoethanol, and 20 mM imidazole, containing 1× protease inhibitor cocktail, calyculin A, and 20 μM of NEM. One-twentieth amounts of cell lysates were precipitated with 10% trichloroacetic acid for total protein. His-tagged Elk-1 was purified by overnight incubation with 30 μl of nickel-nitrilotriacetic acid agarose (Qiagen) prewashed with lysis buffer. Collected agarose-bound proteins were washed with serial wash buffer containing 8 M urea, 100 mM NaH2PO4, 0.3 M NaCl, 10 mM Tris·Cl, and 0.2% Triton X-100 at pH 8.0, pH 6.3, and pH 5.9 and then wash buffer at pH 4.5. All wash buffers were made to 20 mM β-mercaptoethanol and 20 mM imidazole before use, except for the final wash buffer. The final wash buffer at pH 4.5 was made to 720 mM β-mercaptoethanol and 200 mM imidazole to elute the proteins. After heat denaturation with SDS sample buffer, the proteins were separated by SDS-PAGE, followed by immunoblotting against HA antibody to detect sumo-modified Elk-1.
Electrophoretic mobility shift assay.
Nuclear extracts were prepared, incubated with double-stranded oligonucleotide probe corresponding to the SM22α promoter for 20 min at room temperature, and analyzed by 4.5% nondenaturing PAGE. The sequences of the SM22-promoter probe contain the CC (A/T) GG (CArG) box (underlined) and the wild- or mutant-type of ternary complex factor (TCF) site (bold) and are shown below: wild-type, AGCTGTTTCAGGGTCCTGCCCATAAAAGGTTTTTCCCGGCCGCC; and the TCF mutant, AGCTGTTTCAGGGTCCTGCCCATAAAAGGTTTTTAACGGCCGCC (51). In the antibody supershift assays or competitive EMSA, nuclear extracts were preincubated with 0.6 μg of the indicated antibodies or 50-fold excess of the unlabeled probe for 20 min on ice, respectively, before the addition of the labeled probe. After electrophoresis, the gels were dried and imaged by autoradiography.
Chromatin immunoprecipitation assay.
The chromatin immunoprecipitation (ChIP) assay kit and protocol were from Upstate Biotechnology and used with minor modification. In brief, protein and chromatin from SMCs were cross-linked by adding formaldehyde directly to culture medium at a final concentration of 1% and incubated for 10 min at room temperature. To terminate the cross-linking process, glycine was added to a final concentration of 125 mM. The cells were washed three times with ice-cold PBS containing 1× protease inhibitor cocktail, calyculin A, and NEM. The cells were harvested in cell lysis buffer containing 5 mM PIPES-KOH (pH 8.0), 85 mM KCl, and 0.5% Nonidet P-40, including the same supplements. After incubation on ice for 20 min, the lysates were centrifuged at 5,000 rpm for 5 min. Nuclear pellets were resuspended in 200 μl of SDS lysis buffer containing 1% SDS, 10 mM EDTA, and 50 mM Tris·HCl (pH 8.1), including the same supplements per two million cells. After incubation for 10 min on ice, four cycles of sonication for 15 s at a setting of 4 using VirSonic Ultrasonic Cell Disrupter 100 were conducted to obtain about 500 bp of chromatin fragments. The sonicated DNA protein complexes were then centrifuged at 4°C for 10 min at 16,000 g to remove insoluble material. The supernatant was diluted 1:10 with dilution buffer containing 0.01% SDS, 1.1% Triton X-100, 1.2 mM EDTA, 16.7 mM Tris·Cl (pH 8.1), and 167 mM NaCl, including the same supplements. The diluted supernatant was precleared by incubation with 80 μl of protein A agarose/salmon sperm DNA (PAA/SSD) at 4°C for 1 h. After centrifugation at 4,000 g for 1 min, 2.5% of the supernatant was saved to serve as an input control and the remaining supernatant was subsequently incubated with 8 μg of Elk-1 antibody (Santa Cruz) at 4°C overnight. As a negative control, 60 μl of precleared PAA/SSA were added to diluted supernatant without antibody and incubated at 4°C for 1 h and subsequently washed with washing buffer as described below. Unbound supernatant resulting from control incubation was used for the immunoprecipitation with SRF antibody (4 μg, Santa Cruz) at 4°C overnight. On the next day, the antibody-agarose complex was obtained by incubation with precleared PAA/SSD at 4°C for 1 h. After being washed with four different washing buffers [low-salt wash buffer contained 0.1% SDS, 1% Triton X-100, 2 mM EDTA, 20 mM Tris·Cl (pH 8.1), and 150 mM NaCl; high-salt wash buffer contained 0.1% SDS, 1% Triton X-100, 2 mM EDTA, 20 mM Tris·Cl (pH 8.1), and 500 mM NaCl; LiCl wash buffer contained 0.25 M LiCl, 1% IGEPAL-CA630, 1% deoxycholic acid, 1 mM EDTA, 10 mM Tris·Cl (pH 8.1); TE buffer two times], the DNA protein complex was eluted with 100 μl of fresh elution buffer, containing 1% SDS and 0.1 M NaHCO3, from agarose by shaking at room temperature for 15 min. After the supernatant was collected by centrifugation at 4,000 g for 1 min, another elution was performed, and the eluates were combined. The combined eluates along with input control were reverse cross-linked to release the DNA from the protein by incubating tubes in a 65°C water bath for 4 h to overnight. After RNase A and Proteinase K treatment, the remaining DNA was purified using PCR purification kit (Qiagen). The amounts of purified DNA fragments bound to immunoprecipitated proteins of interest were determined by PCR using primers specific for the promoter sequence of interest. Primer sequences for ChIP assays were as follows: SM22α promoter region, GGTCCTGCCCATAAAAGGTTT and TGCCCATGGAAGTCTGCTTGG; the exon 5 region, AAGCCCAGGAGCATAAGAGGGACT and GAAGGACAGTGGGCTGGCCATCAG (51); SM-MHC promoter region, CTGGAGCTCTTATTAGTACTGGGGTCCC and ACTCAGGCCATAAAAGGAAGTCGAGGCAGAGTTGG; SM-MHC promoter region lacking CArG, ATGTCAGATGTCCTCTCACTGCTTTATTCC and AGCAAACAGCTTTAAATACGTATTGGCTTC (32); and c-fos CArG region, CATTGAATCAGGTGCGAATGTTCGC and GCCGTGGAAACCTGCTGACGCA (57). The PCR products were separated on a 3% agarose gel.
Statistics.
Data are presented as means ± SD. Statistical analyses were done using a Student's t-test or with a one-way analysis of variance with post hoc (Newman multiple comparison test) when multiple groups were compared. A P value of <0.05 was taken as statistically significant.
RESULTS
PKG-I enhanced myocardin-induced SM22 promoter activity.
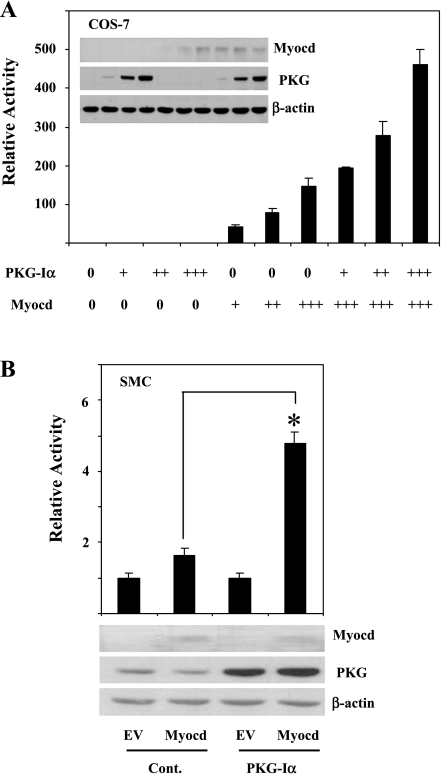
Previous work by our laboratory (3) and that of Zhang et al. (56) demonstrated that the expression of PKG-I in passaged SMCs, deficient in endogenous PKG-I, induced SMC-specific gene expression. Because myocardin is a key transcriptional regulator of the expression of SMC-specific gene expression, we tested whether PKG-I expression affected myocardin-induced SMC gene promoter activity in COS-7 cells. COS-7 cells are useful to examine the specific effects of PKG-I because in addition to not expressing myocardin, the cells express no PKG-I mRNA or protein. As shown in Fig. 1A, myocardin significantly increased SM22 promoter activity some 100- to 300-fold. PKG-I expression alone had no effect on the SM22 promoter activity in the cells. When PKG-I was cotransfected with myocardin, SM22 promoter activity was increased two- to threefold over myocardin alone (Fig. 1A). We also examined whether PKG-I increased myocardin-induced SM22 promoter activity in rat aortic SMCs stably transfected with PKG-Iα vector. Unlike COS-7 cells, rat aortic SMCs express myocardin, although more highly passaged cells express lower levels than lower passaged cells (10). As shown in Fig. 1B, SMCs stably transfected with PKG-I had no detectable effect on the level of endogenous myocardin expression but had higher levels of myocardin-induced SM22 promoter activity (∼3-fold) compared with control-transfected SMCs.
Fig. 1.
PKG enhanced myocardin (Myocd)-induced SM22 promoter activity. A: COS-7 cells were transiently transfected with Myocd (6, 12.5, or 25 ng)- with or without PKG-Iα (10, 50, or 100 ng)-expressing plasmids and SM22 promoter-driven firefly luciferase reporter plasmid. Inset: lysates for essays were immunoblotted to confirm that cotransfection of PKG and Myocd does not affect the expression of Myocd protein. B: Myocd-expressing plasmids (0.2 μg) were transiently cotransfected with SM22 promoter-driven reporter plasmid in control rat aortic smooth muscle cells [SMCs; control (Cont)] or stably PKG-expressing rat aortic SMCs (PKG-Iα). EV, empty vector. A similar expression of myocardin protein in the control and PKG-Iα cell lines was shown by immunoblot. SM22 promoter-driven firefly luciferase activities were normalized with the cotransfected renilla luciferase activity (A) or β-galactosidase activity (B). Relative luciferase activity is expressed as fold activation above the level of expression of the reporter gene alone. *P < 0.01 indicated myocardin-induced promoter activity in PKG-expressing cells vs. the control group. Data are representative of at least 3 independent experiments performed in triplicate.
PKG-I increases the unusual size of phospho-Elk-1 species in rat aortic SMCs.
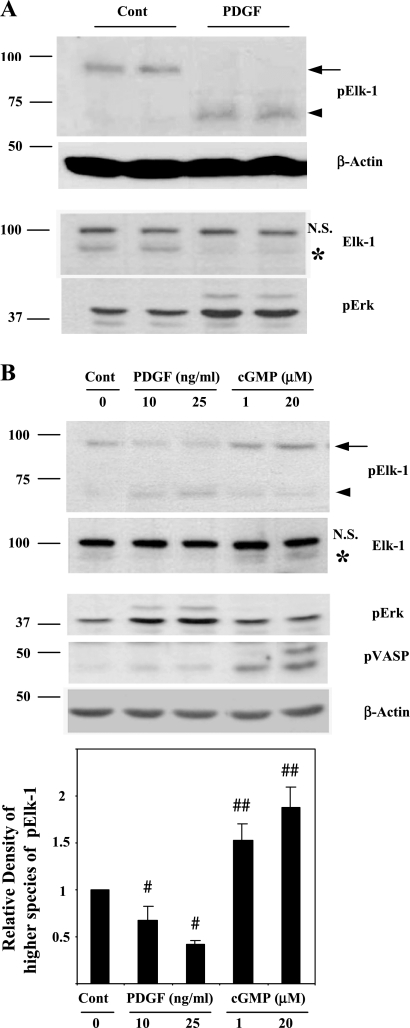
Olson's laboratory proposed that the ets-binding protein, Elk-1, when phosphorylated by MAP kinases competes with myocardin binding to SRF (51). According to this model, Elk-1 phosphorylation suppresses SMC-specific gene expression by dislodging myocardin from SRF, thus reducing transcription. To increase myocardin-SRF specific gene expression, our initial hypothesis was that PKG may decrease Elk-1 phosphorylation, possibly by inhibiting MAP kinases or stimulating protein phosphatases (46, 52, 60). Thus we examined the level of phospho-Elk-1 (pElk-1) in rat aortic SMCs in response to PDGF by immunoblotting using a specific pElk-1 antibody. In primary cultures of rat aortic SMCs, PDGF induced the phosphorylation of Elk-1 as expected (arrowhead in Fig. 2A). However, in nontreated SMCs (Cont), there was an unexpected higher molecular mass species (molecular mass, ∼90 kDa) that reacted with the pElk-1 antibody (arrow, Fig. 2A), which was decreased by PDGF treatment. Immunoblot using Elk-1 antibody was performed to show that this higher molecular mass species was Elk-1. As shown in the Elk-1 blot of Fig. 2A, anti-Elk-1 (as opposed to anti-pElk-1) also reacted with the higher molecular mass protein that showed a similar decrease in response to PDGF (asterisks). That this higher molecular mass species was authentic, Elk-1 was supported by other observations: 1) preincubation of anti-pElk-1 antibody with blocking peptide (pElk-1 peptide, Santa Cruz) completely abolished the pElk-1 signals, and 2) PDGF specifically decreased the presence of the higher molecular mass band while increasing the level of the 65 kDa protein, suggesting that this protein was not an unrelated, nonspecific protein.
Fig. 2.
Detection of higher molecular species of phospho-E26-like protein-1 (pElk-1) in SMCs. A: low-passaged rat aortic SMCs were cultured in 10% FBS in DMEM until grown to confluence, incubated in serum-deprived medium (1 mg/ml of BSA in DMEM) for 3 days, and then stimulated with or without PDGF-BB (10 ng/ml) for 30 min. B, top: rat aortic SMCs were treated with PDGF-BB or 8-(p-chlorophenylthio)-cGMP (1 or 20 μM) for 30 min after culturing in serum-deprived medium for 3 days. B, bottom: pooled data from 3 experiments quantifying higher molecular mass species of pElk-1 (arrow). #P < 0.001, PDGF treated vs. control; ##P < 0.01, cGMP treated vs. control. Total protein extracts were prepared and immunoblotted with indicated antibodies. Arrows indicate high molecular mass pElk-1, and arrowheads indicate PDGF-induced pElk-1 (pElk-1 blots of A and B). *Corresponding high molecular mass Elk-1 signals on Elk-1 blots of A and B. NS, nonspecific bands. pErk and phospho-vasodilator-stimulated phosphoprotein (pVASP)-239 were detected to show activations of Erk-Elk-1 and PKG, respectively.
When primary cultured rat aortic SMCs were treated with the cGMP analog 8-(p-chlorophenylthio)-cGMP together with treatment with PDGF for comparison, there was an increase in the higher molecular mass species in response to 8-(p-chlorophenylthio)-cGMP (arrow in pElk-1 blot of Fig. 2B). The Elk-1 blot was again performed to show the similar effects of PDGF or the cGMP analog and to confirm that it reacted with Elk-1 antibody (asterisk in Elk-1 blot of Fig. 2B).
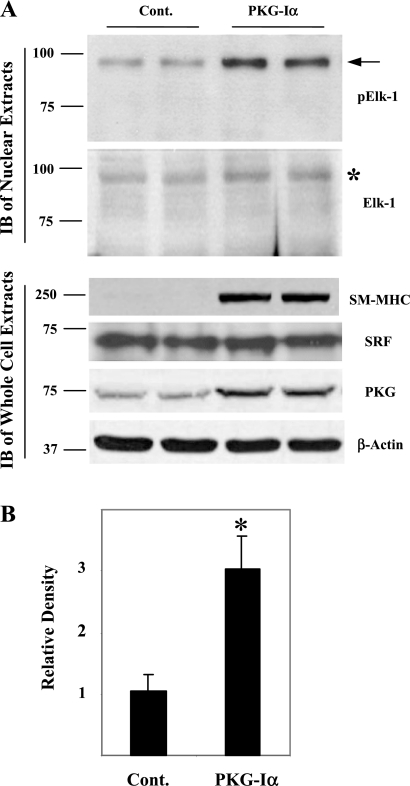
To confirm that PKG increased the higher molecular mass form of pElk-1 in vascular SMCs, we examined the effects of overexpression of PKG-I on the abundance of the higher molecular mass pElk-1 species in more highly passaged rat aortic SMCs. In cells stably overexpressing PKG-I, there was a two- to threefold increase in the higher molecular mass pElk-1 that also reacted with anti-Elk-1 antibody (asterisk in Elk-1 blot), compared with control transfected SMCs (Fig. 3A, top, and B), confirming the effect of cGMP/PKG to increase the levels of this species. Also shown is the effect of PKG-I to increase the expression of the SMC-specific phenotypic marker, SM-MHC, without changing the levels of SRF protein in whole cell extracts (Fig. 3A, bottom). These results demonstrate two species of pElk-1 and show that PDGF reduced the higher molecular mass form. The mechanism of this effect of PDGF is not clear. The results also suggest that cGMP/PKG antagonized the effect of PDGF not by inhibiting Elk-1 phosphorylation but by shifting pElk-1 to the unusual higher molecular mass form. These observations led us to define the characteristics of these species.
Fig. 3.
Effect of PKG-Iα expression on high molecular mass pElk-1 in rat aortic SMCs. Rat aortic SMCs at passage 3 to 5 were transfected with control pcDNA3.1 vector (Cont) or PKG-Iα-pcDNA3.1 (PKG-Iα) using Lipofectamine 2000. Stably transfected cells were selected using 500 μg/ml of G418. Cells grown to confluence were serum deprived in DMEM containing 1 mg/ml of BSA for 3 days. A: nuclear extracts or whole cell extracts were prepared and immunoblotted (IB) with indicated antibodies. Arrow on pElk-1 blot indicates high molecular mass pElk-1 *Corresponding high molecular mass Elk-1 signal on Elk-1 blot. Data are a representative of 3 experiments. SM-MHC, smooth muscle-myosin heavy chain; SRF, serum response factor. B: quantitative analysis of 3 pElk-1 blots was performed using ImageJ software. Results are expressed as means ± SD. *P < 0.01, PKG-expressing cells (PKG-Iα) vs. the control group (Cont).
The higher molecular mass species is sumoylated Elk-1.
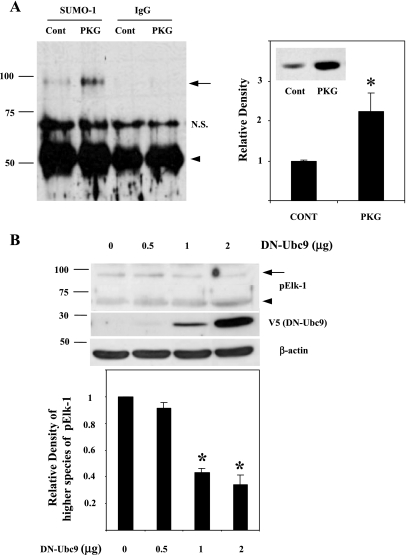
Previous work has shown that Elk-1 undergoes posttranslational modification by sumoylation (54), raising the possibility that the higher molecular mass form of pElk-1 may have been a posttranslationally sumo-modified form of pElk-1. Our results regarding the unusual size of pELK-1 species thus led us to explore the possibility that PKG expression stimulates sumo-modification of pElk-1 in SMCs. As shown in Fig. 4A, left, immunoprecipitation of nuclear extracts using anti-sumo-1 antibody followed by immunoblotting with pElk-1 antibody demonstrated the higher molecular mass species of Elk-1 was indeed sumoylated pElk-1. Furthermore, adenoviral-induced PKG-Iα expression in rat aortic SMCs increased sumoylated pElk-1 by approximately twofold compared with control rat aortic SMCs (Fig. 4A, right). Of note, sumo proteins have molecular masses of ∼15 kDa. However, sumo-modified proteins generally migrate anomalously on SDS-PAGE as proteins of 20 kDa larger than the unmodified protein. Hence, the migration of pElk-1 in the 90-kDa range suggested a monosumoylated species, as has been reported previously (17, 28, 44).
Fig. 4.
Sumoylation of a higher molecular mass pElk-1 in the PKG-I overexpressing rat aortic SMCs. Immunoprecipitation was performed with nuclear extracts from adenoviral-mediated green fluorescent protein (Cont) or PKG-Iα (PKG)-expressing rat aortic SMCs using anti-small ubiquitin-related modifier-1 (sumo-1) antibody or normal IgG (IgG) followed by immunoblotting with anti-pElk-1 antibody. A, left: increased levels of sumoylated Elk-1 in the PKG-overexpressing cells was shown and indicated with arrow. Arrowhead indicates IgG heavy chain. A, right: results are expressed as means ± SD and typical of 3 independent experiments. *P < 0.05, PKG-Iα-expressing cells vs. Cont. Inset: lysates from control- and PKG-transfected cells were immunoblotted for PKG levels. B, top: rat aortic SMCs were transfected with dominant negative (DN)-Ubc9 expressing plasmids (0, 0.5, 1, or 2 μg). After 2 days, whole cell extracts were prepared with 1× denaturing lysis buffer and subjected to ∼8–17.5% of gradient SDS-PAGE and immunoblotting. Arrow indicates high molecular mass pElk-1, and arrowhead indicates DN-UbC9-mediated pElk-1. B, bottom: pooled data from 3 separate experiments quantifying higher molecular mass species of pElk-1 (arrow). *P < 0.01, DN-Ubc9 vs. control.
To further study the effect of PKG expression on Elk-1 sumoylation, we transfected SMCs with a dominant-negative form of the E2 sumo ligase, DN-Ubc9, which is unable to transfer sumo proteins to the target proteins (48). As shown in Fig. 4B, a similar decrease in higher molecular mass pElk-1 (arrow) and an increase in lower molecular mass pElk-1 (arrowhead) were observed in accordance with an increased expression of DN-Ubc9, confirming that the higher molecular form of pElk-1 was PKG-induced sumoylated Elk-1.
Sumoylation of Elk-1 by PKG-I in COS-7 cells.
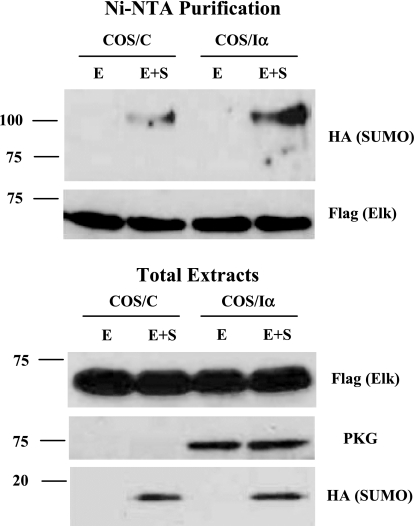
To further explore the effects of PKG-I on Elk-1 sumoylation in intact cells, we examined the effect of PKG-I expression on Elk-1 sumoylation in COS-7 cells, where the direct effect of the kinase could be more easily studied. COS-7 cells were transfected with plasmids encoding His-tagged Elk-1 plus HA-tagged sumo-1 in control (COS/C) or PKG-Iα (COS/Iα) stably transfected cells. Following nickel affinity chromatography and immunoblotting using anti-HA antibody, we observed that there was a large increase in the level of sumoylated Elk-1 in PKG-I expressing COS-7 cells when both Elk-1 (E) and sumo-1 (S) were expressed compared with control cells (Fig. 5, top, lanes 2 and 4).
Fig. 5.
PKG-I increases Elk-1 sumoylation in vivo. His-Flag-Elk-1 (E) and hemagglutinin (HA) -SUMO-1 (S) expression vectors were transfected into control COS-7 cells (COS/C) or stably PKG-1α expressing COS-7 cells (COS/Iα). After 2 days, cells were lysed in 1 ml of 6 M guanethidine HCl, 0.1 M NaH2PO4, 0.01 M Tris·HCl (pH 8.0), and 0.3 M NaCl plus 20 mM imidazole, 20 mM β-mercaptoethanol, 15 mM N-ethylmaleimide (NEM), 5 nM calyculin A, and 1× proteinase cocktail. The lysates were mixed with 30 μl of Ni2+-nitrilotriacetic acid (Ni-NTA) agarose beads prewashed with lysis buffer and incubated overnight at 4°C. The beads were washed twice with lysis buffer and the following wash buffers: 8 M urea, 0.1 M NaH2PO4, 0.01 M Tris·HCl (pH 8.0, pH 6.3, and pH 5.9). All wash buffers contain 20 mM imidazole and 20 mM β-mercaptoethanol. After the final wash, the beads were eluted with 8 M urea, 0.1 M NaH2PO4, and 0.01 M Tris·HCl (pH 4.5) containing 200 mM imidazole and 720 mM β-mercaptoethanol. The eluates and total extracts were subjected to SDS-PAGE and immunoblotting with indicated antibodies.
PKG-I modulates Elk-1 inhibition on the myocardin-induced SM22 promoter activity.
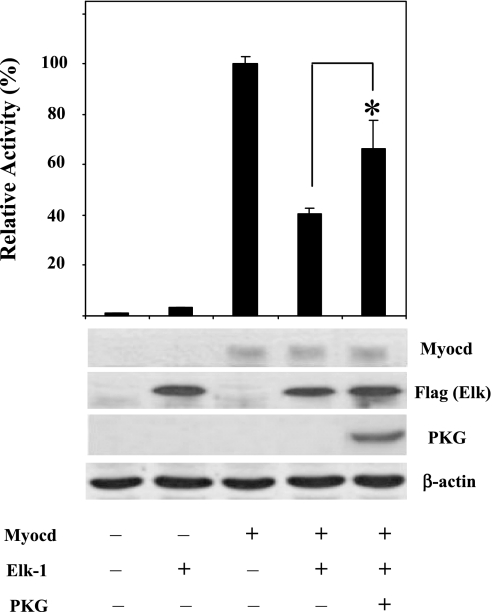
It is well established that Elk-1 regulates cell proliferation, increases growth-related gene expression, and suppresses SMC-specific gene expression (5, 51, 57). To test whether PKG-I inhibits the effects of Elk-1 on myocardin-induced SM22 promoter activity, COS-7 cells were cotransfected with a combination of expression plasmids for myocardin, Elk-1, and PKG-I together with the SM22 promoter-reporter construct. Consistent with previous studies and our results (Fig. 1), myocardin increased SM22 promoter activity (Fig. 6, lane 3) and this effect was reduced by Elk-1 cotransfection (Fig. 6, lane 4). Elk-1 repression of myocardin-induced SM22 promoter activity was reversed in the presence of PKG-I expression (Fig. 6, lane 5). These results show that PKG-I reduced the Elk-1 suppression of myocardin-induced SM22 promoter activity, suggesting a more direct role of PKG in SMC-specific gene regulation through the inhibition of Elk-1 activity.
Fig. 6.
PKG-I modified Elk-1 inhibition of myocardin-induced SM22 promoter activity. COS-7 cells were transiently transfected with Flag-Elk-1 (25 ng), myocardin (25 ng), or both with or without PKG-Iα (50 ng)-expressing plasmids. SM22 promoter-driven firefly luciferase activities were normalized with the cotransfected renilla luciferase activity. Relative luciferase activity is expressed as fold activation above the level of expression of the reporter gene alone. Relative levels of protein expression were shown by immunoblots. Experiment was repeated 3 times in triplicate, and the data presented are from a representative experiment. *P < 0.05 compared with Elk-suppressed Myocd-induced SM22 promoter activity.
Elk-1 binding to SMC-specific gene promoters is decreased by PKG-I in rat aortic SMCs.
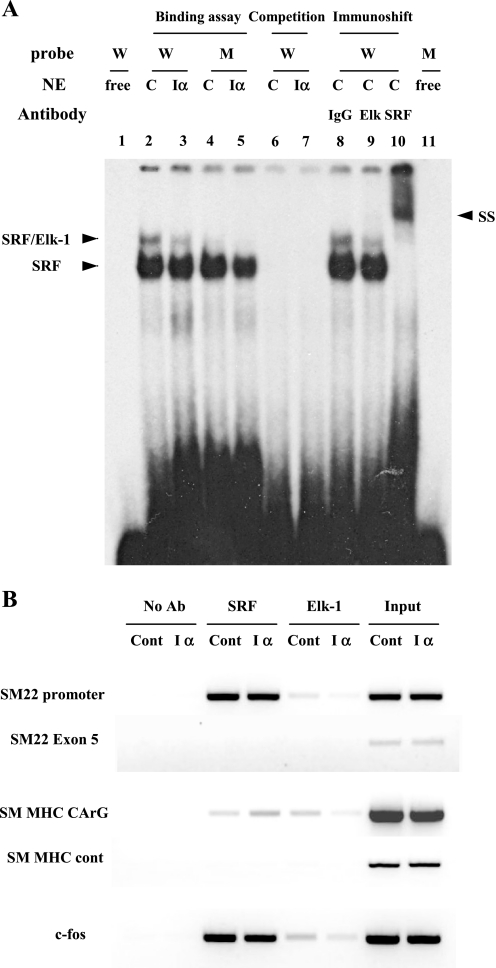
The inhibitory effect of Elk-1 on SMC-specific gene promoter activity and its reversal by PKG led us to examine the role of PKG in modulating SRF and Elk-1 binding to SMC-specific gene promoters using EMSA (in vitro) and ChIP assay (in vivo). In EMSA analysis, the incubation of nuclear extracts from PKG-Iα-expressing rat aortic SMCs (Iα) with a radiolabeled probe corresponding to the SM22 promoter region that contains a consensus CArG box and the TCF site revealed a decreased SRF-Elk-1 complex (SRF/Elk-1) compared with the incubation with control extracts (C) (compare lanes 2 and 3 in Fig. 7A). These complexes, not the SRF-alone complexes, were completely erased in the binding reaction with mutant oligos containing a TCF-site mutation, thus confirming the PKG-I effect on the reduced Elk-1 binding (Fig. 7, lanes 4 and 5). Nuclear extracts from control rat aortic SMCs (C) were used in the EMSA immunoshift assay to confirm that PKG-mediated reduction of the complex was SRF-Elk-1 complex. As shown in Fig. 7A, lane 9, and previously (51), Elk-1 antibody reduced only the higher band intensity, whereas SRF antibody erased both bands completely and induced a supershift (Fig. 7, lane 10), suggesting that PKG-I expression was responsible for the reduced binding of Elk-1 to the smooth muscle-specific SM22 promoter.
Fig. 7.
Effect of PKG-I on Elk-1 and SRF binding to SMC-specific promoters. A: EMSA analysis demonstrating a PKG inhibition of Elk-1 binding to SM22 promoter. Nuclear extracts (NEs) from control rat aortic SMCs (C) or stably PKG-Iα-expressing rat aortic SMCs (Iα) were incubated with wild (W) or mutant (M) forms of radiolabeled oligonucleotide probes for SM22 promoter as described in experimental procedures. Protein DNA complexes were separated by 4.5% of nondenaturated polyacrylamide gel electrophoresis. Competition, preincubation of NEs with 50-fold excess of the unlabeled probe; immunoshift, preincubation of extracts with indicated antibodies before adding the wild form of labeled probe; SS, supershift. B: chromatin immunoprecipitation assays demonstrate that PKG-I inhibits Elk-1 binding to endogenous SMC-specific promoters. Control (Cont) or PKG-Iα-expressing rat aortic SMCs (Iα) were cultured until subconfluent. Cross-linked protein DNA complexes were immunoprecipitated using anti-Elk-1. Extracts from an equal number of cells were incubated without antibody and used as a negative control for each immunoprecipitation (No Ab). Supernatant from a negative control was collected and incubated with anti-SRF (SRF). The coprecipitated DNA was purified and amplified by PCR using primers for SM22 promoter region or exon 5 region, SM-MHC CC (A/T) GG (CArG) region or CArG-lacking control region, and c-fos CArG region. Data are representative of 3 independent experiments.
To further test a possible role of PKG-I in Elk-1 binding to the SMC-specific gene promoters in vivo, we performed ChIP assays. In stably transfected PKG-I-expressing rat aortic SMCs, less Elk-1 binding to its specific binding sites on both the SM22 and SM-MHC promoters was observed compared with control stably transfected cells (Fig. 7B, lanes 5 and 6). Furthermore, there was also decreased binding of Elk-1 to the c-fos promoter in PKG-expressing SMCs, suggesting an overall effect of PKG-I to reduce Elk-1 availability for gene expression. It was also noted that SRF binding was increased, particularly to the SM-MHC promoter, in PKG-I-expressing SMCs compared with control cells. Taken together, these results suggest that the suppressive effect of PKG-Iα on Elk-1 binding to CArG regions in SMC gene promoters mediates the increased myocardin-SRF activity on SMC-specific gene expression.
DISCUSSION
The unique characteristic of vascular SMCs known as phenotypic plasticity plays a critical role in development as well as in phenotype modulation between differentiated SMCs and proliferative, synthetic SMCs (38). The differentiated phenotype is typically distinguished from the synthetic phenotype by the expression of high levels of various contractile proteins including SM-MHC, calponin, and smooth muscle α-actin. Knowledge of the cellular pathways that control SMC-specific gene expression and differentiation is important for understanding this phenotypic switch. In this study, we investigated mechanisms relating to the cGMP/PKG pathway in SMC-specific gene expression because studies in our laboratory (3) and others (56, 58) have shown that this pathway stimulates vascular SMC differentiation.
Our studies were prompted by progress in defining the molecular mechanisms of SMC-specific gene expression at the nuclear transcription level. SRF is the key transcription factor that is required for transcription of SMC-specific genes (34). In turn, SRF is regulated by numerous cotranscriptional and regulatory proteins that include myocardin and related proteins (19, 29), GATA proteins (35), and Elk-1 (5). Because SRF is a ubiquitous transcription factor that regulates gene expression in a wide variety of cell types, the specificity for SRF to regulate SMC-specific gene expression depends on myocardin (19, 29). However, SRF is also under the control of transcription factors such as Elk-1 that inhibit SMC-specific gene expression (51, 57). The activation of SMCs by growth factors that downregulate SMC-specific gene expression and promote vascular SMC phenotypic modulation to the fibroproliferative “synthetic” phenotype is associated with the activation of the MAP kinase pathway and the phosphorylation of Elk-1. Phosphorylated Elk-1 inhibits myocardin association with SRF on SMC promoters (51). Thus there has been much interest in defining the cytosolic signaling pathways that control both SRF and Elk-1 in SMCs.
The NO/cGMP/PKG signaling pathway has been shown to contribute to numerous SMC functions including relaxation, cell proliferation, and gene expression (26, 30, 31, 41). Previous studies from our laboratory (3) and that of Zhang et al. (56) and Zhou et al. (58) have shown that the cGMP/PKG pathway stimulates SRF-dependent SMC-specific gene expression and promotes a more “contractile” (differentiated) phenotype for SMCs. In this study, we examined the hypothesis that PKG decreases Elk-1 suppressive activity on SMC-specific gene expression. Specifically, we studied the posttranslational modification of Elk-1 by the cGMP/PKG pathway.
One posttranslational signaling pathway that has been shown to regulate the activity of a number of transcription factors including Elk-1 is sumoylation (21, 50). Like ubiquitination, protein sumoylation involves the covalent binding of sumo proteins to ϵ-lysine residues of target proteins. Unlike ubiquitination, however, sumoylation rarely targets proteins for proteasome degradation. Rather, sumoylation promotes structural changes in target proteins that allow the proteins to be directed to specific cellular organelles and/or to be changed in their activities (23, 33). It has been reported that a sumo-modification of Elk-1 suppresses its binding to nuclear targets (45). In this report, we show evidence that PKG increases sumo-modification of Elk-1 using immunoprecipitation, intact cell sumoylation, and dominant-negative approaches. These data suggest that increased SMC-specific gene expression is due in part to PKG-activated sumo-modification of Elk-1. Our data demonstrate that an overexpression in COS-7 cells of sumo-1, Elk-1, and PKG-I were all required to increase the sumoylation of Elk-1 and Elk-1 inhibition of myocardin-dependent SM22 promoter activity (Figs. 5 and 6). Reporter gene, EMSA, and ChIP assays demonstrate that PKG suppresses the inhibitory effect of Elk-1 on SRF/myocardin-induced SMC-specific gene expression (Figs. 6 and 7). These results provide a possible molecular mechanism for PKG in the upregulation of smooth muscle gene expression and modulation to the contractile phenotype.
The precise mechanism by which PKG stimulates Elk-1 sumoylation is not yet known. In vitro sumoylation carried out with purified PKG and Elk-1 using the sumoylation kit (Biomol) did not stimulate Elk-1 sumoylation (data not shown), suggesting that perhaps PKG increases the sumoylation of Elk-1 through a more specific sumo E3 ligase pathway in the cell. For example, the nucleoporin RAN binding protein 2 (RanBP2) has been shown to strongly enhance the sumoylation of SP100 with the ability to confer substrate specificity, (40) and, of interest, human RanBP2 contains a PKG consensus sequence (RRIT) at the COOH-terminal region (55), suggesting RanBP2 as a possible target of PKG action in Elk-1 sumoylation. More work needs to be directed toward identifying the specific target of PKG action.
It was initially surprising that two posttranslational regulation pathways, sumoylation (repressive) and phosphorylation (active), operated simultaneously with Elk-1. The phosphorylation of Elk-1 by the MAP kinase pathway is transient (5) and leads to a decreased sumo conjugation, resulting in Elk-1 regaining transcriptional activity (54). Perhaps this is the mechanism underlying the effect of PDGF to decrease the amount of higher molecular mass Elk-1 (Fig. 2). However, the coordinated regulation of transcription factors by two different modifications (phosphorylation and sumoylation) has been reported in a number of cases including heat shock factors, myocyte enhancer factor 2D (MEF2D), and estrogen-related receptor-α and -γ (22, 24, 25, 49). In the case of MEF2D, the serine residue adjacent to the sumoylation motif was required for the sumoylation of MEF2D, and the transcriptional activity of MEF2D was inhibited by sumoylation that was increased by the phosphorylation. Likewise, it has been demonstrated that sumoylation suppresses a Ser383 phosphorylation-independent transcriptional capacity of Elk-1 (54), suggesting a possible coexistence of two modifications on Elk-1.
In the numerous reports describing the effects of posttranslational modification of proteins by sumoylation, an interesting regulatory mechanism was recently described in which oxidative stress induced or decreased reversible sumoylation of cellular substrates depending on the concentration of reactive oxygen species (ROS) (2, 59). ROS have also been recognized to mediate vascular SMC differentiation, and recently, the NAD(P)H oxidase, Nox4, was found to be required for differentiation marker gene expression in vascular SMCs (12, 53). Of interest is that the NO/cGMP/PKG pathway is known to be regulated by oxidative stress, and H2O2 oxidizes PKG-Iα to directly activate the kinase itself (6). Based on these reports, it is possible that PKG-mediated Elk-1 sumoylation might be regulated by ROS, resulting in the SMC differentiation. Recently, Zhou et al. (58) reported an inhibitory role for PKG-I on Elk-1 activity in a model of hypoxia-induced pulmonary SMC phenotypic modulation. In this model, PKG under normoxic conditions contributes to a high level of myocardin expression and less Elk-1 binding to SRF, resulting in the activation of SMC-specific gene expression. While this is consistent with our results regarding the inhibitory role of PKG on Elk-1 activity, in our experiments PKG overexpression did not change the levels of either Elk-1 or myocardin, as determined by RT-PCR/immunoblot (data not shown).
SRF/myocardin-dependent expression of smooth muscle-specific genes is regulated and coordinated by multiple mechanisms in addition to the Elk-1 suppression. And Elk-1 activity can be regulated by many posttranslational modifications including phosphorylation and sumoylation events (5, 54). This may explain why PKG induces only a partial reversal of Elk-1 suppression of SM22 promoter activity (Fig. 6), and thus it is our hypothesis that PKG affects primarily the Elk-1 suppression of SRF/myocardin gene expression through sumoylation. It would be misleading to conclude that sumoylation is the only mechanism by which PKG stimulates SMC-specific gene expression. Pilz and coworkers (56) reported that PKG catalyzes the phosphorylation of cysteine-rich LIM-only protein (CRP4) in SMCs, resulting in the association of CRP4 with SRF and GATA6 (56). The CRP4 complex was found to increase SRF binding and SMC-specific gene expression. Thus there are likely multiple mechanisms by which cGMP/PKG pathway might stimulate SRF/myocardin-dependent gene expression in addition to the sumoylation of Elk-1 described here.
PKG-I has now been shown to stimulate SMC-specific gene expression by increasing SRF/myocardin-dependent transcription. We propose that the cGMP/PKG pathway exists in adult, differentiated SMCs as a mechanism to relay signals derived from endothelial (NO) and plasma sources (natriuretic peptides) to maintain the contractile phenotype of the cell in the vessel wall. However, since PKG-I knockout animals develop normal SMCs (39), it is likely that other pathways than the cGMP/PKG pathway stimulate and maintain SRF/myocardin-dependent gene expression during embryogenesis. We surmise that both embryonic pathway(s) and the adult cGMP/PKG pathway ultimately act to stimulate the same or similar SRF/myocardin nuclear events.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-053426 and HL-066164.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1.Boerth NJ, Dey NB, Cornwell TL, Lincoln TM. Cyclic GMP-dependent protein kinase regulates vascular smooth muscle cell phenotype. J Vasc Res 34: 245–259, 1997 [DOI] [PubMed] [Google Scholar]
- 2.Bossis G, Melchior F. Regulation of SUMOylation by reversible oxidation of SUMO conjugating enzymes. Mol Cell 21: 349–357, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Brophy CM, Woodrum DA, Pollock J, Dickinson M, Komalavilas P, Cornwell TL, Lincoln TM. cGMP-dependent protein kinase expression restores contractile function in cultured vascular smooth muscle cells. J Vasc Res 39: 95–103, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Browner NC, Sellak H, Lincoln TM. Downregulation of cGMP-dependent protein kinase expression by inflammatory cytokines in vascular smooth muscle cells. Am J Physiol Cell Physiol 287: C88–C96, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Buchwalter G, Gross C, Wasylyk B. Ets ternary complex transcription factors. Gene 7: 1–14, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Burgoyne JR, Madhani M, Cuello F, Charles RL, Brennan JP, Schröder E, Browning DD, Eaton P. Cysteine redox sensor in PKGIa enables oxidant-induced activation. Science 317: 1393–1397, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Campbell JH, Campbell GR. Smooth muscle phenotypic changes in arterial wall homeostasis: implications for the pathogenesis of atherosclerosis. Exp Mol Pathol 42: 136–162, 1985 [DOI] [PubMed] [Google Scholar]
- 8.Chamley-Campbell JH, Campbell GR, Ross R. The smooth muscle cell in culture. Physiol Rev 59: 1–61, 1979 [DOI] [PubMed] [Google Scholar]
- 9.Chamley-Campbell JH, Campbell GR, Ross R. Phenotype-dependent response of cultured aortic smooth muscle to serum mitogens. J Cell Biol 89: 379–383, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen J, Kitchen DM, Streb JW, Miano JM. Myocardin: a component of a molecular switch for smooth muscle differentiation. J Mol Cell Cardiol 34: 1345–1356, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Christensen EN, Mendelsohn ME. Cyclic GMP-dependent protein kinase Ialpha inhibits thrombin receptor-mediated calcium mobilization in vascular smooth muscle cells. J Biol Chem 281: 8409–8416, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Clempus RE, Sorescu D, Dikalova AE, Pounkova L, Jo P, Sorescu GP, Schmidt HH, Lassègue B, Griendling KK. Nox4 is required for maintenance of the differentiated vascular smooth muscle cell phenotype. Arterioscler Thromb Vasc Biol 27: 42–48, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Corbin JD, Doskeland SO. Studies of two different intrachain cGMP-binding sites of cGMP-dependent protein kinase. J Biol Chem 258: 11391–11397, 1983 [PubMed] [Google Scholar]
- 14.Cornwell TL, Lincoln TM. Regulation of intracellular Ca2+ levels in cultured vascular smooth muscle cells. Reduction of Ca2+ by atriopeptin and 8-bromo-cyclic GMP is mediated by cyclic GMP-dependent protein kinase. J Biol Chem 264: 1146–1155, 1989 [PubMed] [Google Scholar]
- 15.Cornwell TL, Arnold E, Boerth NJ, Lincoln TM. Inhibition of smooth muscle cell growth by nitric oxide and activation of cAMP-dependent protein kinase by cGMP. Am J Physiol Cell Physiol 267: C1405–C1413, 1994 [DOI] [PubMed] [Google Scholar]
- 16.Cornwell TL, Soff GA, Traynor AE, Lincoln TM. Regulation of the expression of cyclic GMP-dependent protein kinase by cell density in vascular smooth muscle cells. J Vasc Res 31: 330–337, 1994 [DOI] [PubMed] [Google Scholar]
- 17.Degerny C, Monte D, Beaudoin C, Jaffray E, Portois L, Hay RT, de Launoit Y, Baert JL. SUMO modification of the Ets-related transcription factor ERM inhibits its transcriptional activity. J Biol Chem 280: 24330–24338, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Dey NB, Boerth NJ, Murphy-Ullrich JE, Chang PL, Prince CW, Lincoln TM. Cyclic GMP-dependent protein kinase inhibits osteopontin and thrombospondin production in rat aortic smooth muscle cells. Circ Res 82: 139–146, 1998 [DOI] [PubMed] [Google Scholar]
- 19.Du KL, Ip HS, Li J, Chen M, Dandre F, Yu W, Lu MM, Owens GK, Parmacek MS. Myocardin is a critical serum response factor cofactor in the transcriptional program regulating smooth muscle cell differentiation. Mol Cell Biol 23: 2425–2437, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garg UC, Hassid A. Nitric oxide-generating vasodilators and 8-bromo-cyclic guanosine monophosphate inhibit mitogenesis and proliferation of cultured rat vascular smooth muscle cells. J Clin Invest 83: 1774–1777, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Girdwood DW, Tatham MH, Hay RT. SUMO and transcriptional regulation. Semin Cell Dev Biol 15: 201–210, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Grégoire S, Tremblay AM, Xiao L, Yang Q, Ma K, Nie J, Mao Z, Wu Z, Giguère V, Yang XJ. Control of MEF2 transcriptional activity by coordinated phosphorylation and sumoylation. J Biol Chem 281: 4423–4433, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Hay RT. SUMO: a history of modification. Mol Cell 18: 1–12, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Hietakangas V, Ahlskog JK, Jakobsson AM, Hellesuo M, Sahlberg NM, Holmberg CL, Mikhailov A, Palvimo JJ, Pirkkala L, Sistonen L. Phosphorylation of serine 303 is a prerequisite for the stress-inducible SUMO modification of heat shock factor 1. Mol Cell Biol 23: 2953–2968, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hietakangas V, Anckar J, Blomster HA, Fujimoto M, Palvimo JJ, Nakai A, Sistonen L. PDSM, a motif for phosphorylation-dependent SUMO modification. Proc Natl Acad Sci USA 103: 45–50, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hofmann F, Ammendola A, Schlossmann J. Rising behind NO: cGMP-dependent protein kinases. J Cell Sci 113: 1671–1676, 2000 [DOI] [PubMed] [Google Scholar]
- 27.Jarchau T, Hausler C, Markert T, Pohler D, Vanderkerchhove J, De Jonge HR, Lohmann SM, Walter U. Cloning, expression, and in situ localization of rat intestinal cGMP-dependent protein kinase II. Proc Natl Acad Sci USA 91: 9426–9430, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnson ES. Protein modification by SUMO. Annu Rev Biochem 73: 355–382, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Li S, Wang DZ, Wang Z, Richardson JA, Olson EN. The serum response factor coactivator myocardin is required for vascular smooth muscle development. Proc Natl Acad Sci USA 100: 9366–9370, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lincoln TM, Dey N, Sellak H. Invited review: cGMP-dependent protein kinase signaling mechanisms in smooth muscle: from the regulation of tone to gene expression. J Appl Physiol 91: 1421–1430, 2001 [DOI] [PubMed] [Google Scholar]
- 31.Lincoln TM, Wu X, Sellak H, Dey N, Choi CS. Regulation of vascular smooth muscle cell phenotype by cyclic GMP and cyclic GMP-dependent protein kinase. Front Biosci 11: 356–367, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Manabe I, Owens GK. CArG elements control smooth muscle subtype-specific expression of smooth muscle myosin in vivo. J Clin Invest 107: 823–834, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Melchior F, Schergaut M, Pichler A. SUMO: ligases, isopeptidases and nuclear pores. Trends Biochem Sci 28: 612–618, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Miano JM. Serum response factor: toggling between disparate programs of gene expression. J Mol Cell Cardiol 35: 577–593, 2003 [DOI] [PubMed] [Google Scholar]
- 35.Nishida W, Nakamura M, Mori S, Takahashi M, Ohkawa Y, Tadokoro S, Yoshida K, Hiwada K, Hayashi K, Sobue K. A triad of serum response factor and the GATA and NK families governs the transcription of smooth and cardiac muscle genes. J Biol Chem 277: 7308–7317, 2002 [DOI] [PubMed] [Google Scholar]
- 36.Ouyang J, Valin A, Gill G. Regulation of transcription factor activity by SUMO modification. Methods Mol Biol 497: 141–152, 2009 [DOI] [PubMed] [Google Scholar]
- 37.Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev 84: 767–801, 2004 [DOI] [PubMed] [Google Scholar]
- 38.Owens GK. Regulation of differentiation of vascular smooth muscle cells. Physiol Rev 75: 487–517, 1995 [DOI] [PubMed] [Google Scholar]
- 39.Pfeifer A, Klatt P, Massberg S, Ny L, Sausbier M, Hirneiss C, Wang GX, Korthm M, Aszodi A, Andersson KE, Krombach F, Mayerhofer A, Ruth P, Fassler R, Hofmann F. Defective smooth muscle regulation in cGMP kinase I-deficient mice. EMBO J 17: 3045–3051, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pichler A, Gast A, Seeler JS, Dejean A, Melchior F. The nucleoporin RanBP2 has SUMO1 E3 ligase activity. Cell 108: 109–120, 2002 [DOI] [PubMed] [Google Scholar]
- 41.Pilz RB, Casteel DE. Regulation of gene expression by cyclic GMP. Circ Res 93: 1034–1052, 2003 [DOI] [PubMed] [Google Scholar]
- 42.Richie-Jannetta R, Busch JL, Higgins KA, Corbin JD, Francis SH. Isolated regulatory domains of cGMP-dependent protein kinase Ialpha and Ibeta retain dimerization and native cGMP-binding properties and undergo isoform-specific conformational changes. J Biol Chem 281: 6977–6984, 2006 [DOI] [PubMed] [Google Scholar]
- 43.Rovner AS, Murphy RA, Owens GK. Expression of smooth muscle and nonmuscle myosin heavy chains in cultured vascular smooth muscle cells. J Biol Chem 261: 14740–14745, 1989 [PubMed] [Google Scholar]
- 44.Sacher M, Pfander B, Jentsch S. Identification of SUMO-protein conjugates. Method Enzymol 399: 392–404, 2005 [DOI] [PubMed] [Google Scholar]
- 45.Salinas S, Briançon-Marjollet A, Bossis G, Lopez MA, Piechaczyk M, Jariel-Encontre I, Debant A, Hipskind RA. SUMOylation regulates nucleo-cytoplasmic shuttling of Elk-1. J Cell Biol 165: 767–773, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Surks HK, Mochizuki N, Kasai Y, Georgescu SP, Tang KM, Ito M, Lincoln TM, Mendelsohn ME. Regulation of myosin phosphatase by a specific interaction with cGMP- dependent protein kinase Ialpha. Science 286: 1583–1587, 1999 [DOI] [PubMed] [Google Scholar]
- 47.Tamura N, Itoh H, Ogawa Y, Nakagawa O, Harada M, Chun TH, Suga S, Yoshimasa T, Nakao K. cDNA cloning and gene expression of human type Ialpha cGMP-dependent protein kinase. Hypertension 27: 552–557, 1996 [DOI] [PubMed] [Google Scholar]
- 48.Tashiro K, Pando MP, Kanegae Y, Wamsley PM, Inoue S, Verma IM. Direct involvement of the ubiquitin-conjugating enzyme Ubc9/Hus5 in the degradation of IkappaBalpha. Proc Natl Acad Sci USA 22: 7862–7867, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tremblay AM, Wilson BJ, Yang XJ, Giguère V. Phosphorylation-dependent sumoylation regulates estrogen-related receptor-alpha and -gamma transcriptional activity through a synergy control motif. Mol Endocrinol 22: 570–584, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Verger A, Perdomo J, Crossley M. Modification with SUMO. A role in transcriptional regulation. EMBO Rep 4: 137–142, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang Z, Wang DZ, Hockemeyer D, McAnally J, Nordheim A, Olson EN. Myocardin and ternary complex factors compete for SRF to control smooth muscle gene expression. Nature 428: 185–189, 2004 [DOI] [PubMed] [Google Scholar]
- 52.Wooldridge AA, MacDonald JA, Erdodi F, Ma C, Borman MA, Hartshorne DJ, Haystead TA. Smooth muscle phosphatase is regulated in vivo by exclusion of phosphorylation of threonine 696 of MYPT1 by phosphorylation of Serine 695 in response to cyclic nucleotides. J Biol Chem 279: 34496–34504, 2004 [DOI] [PubMed] [Google Scholar]
- 53.Xiao Q, Luo Z, Pepe AE, Margariti A, Zeng L, Xu Q. Embryonic stem cell differentiation into smooth muscle cells is mediated by Nox4-produced H2O2. Am J Physiol Cell Physiol 296: C711–C723, 2009 [DOI] [PubMed] [Google Scholar]
- 54.Yang SH, Jaffray E, Hay RT, Sharrocks AD. Dynamic interplay of the SUMO and ERK pathways in regulating Elk-1 transcriptional activity. Mol Cell 12: 63–74, 2003 [DOI] [PubMed] [Google Scholar]
- 55.Yokoyama N, Hayashi N, Seki T, Pante N, Ohba T, Nishii K, Kuma K, Hayashida T, Miyata T, Aebi U, Fukui M, Nishimoto T. A giant nucleopore protein that binds Ran/TC4. Nature 376: 184–188, 1995 [DOI] [PubMed] [Google Scholar]
- 56.Zhang T, Zhuang S, Casteel DE, Looney DJ, Boss GR, Pilz RB. A cysteine-rich LIM-only protein mediates regulation of smooth muscle-specific gene expression by cGMP-dependent protein kinase. J Biol Chem 282: 33367–33380, 2007 [DOI] [PubMed] [Google Scholar]
- 57.Zhou J, Hu G, Herring BP. Smooth muscle-specific genes are differentially sensitive to inhibition by Elk-1. Mol Cell Biol 25: 9874–9885, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhou W, Negash S, Liu J, Raj JU. Modulation of pulmonary vascular smooth muscle cell phenotype in hypoxia: role of cGMP-dependent protein kinase and myocardin. Am J Physiol Lung Cell Mol Physiol 296: L780–L789, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhou W, Ryan JJ, Zhou H. Global analyses of sumoylated proteins in Saccharomyces cerevisiae. Induction of protein sumoylation by cellular stresses. J Biol Chem 279: 32262–32268, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhou XB, Ruth P, Schlossmann J, Hofmann F, Korth M. Protein phosphatase 2A is essential for the activation of Ca2+-activated K+ currents by cGMP-dependent protein kinase in tracheal smooth muscle and Chinese hamster ovary cells. J Biol Chem 271: 19760–19767, 1996 [DOI] [PubMed] [Google Scholar]