Abstract
Mcl-1 inhibits apoptosis in well-differentiated cells by sequestering BAD, BID, and BAX and other apoptotic molecules. pAKT blocks apoptotsis by facilitating the interaction of BAD with BCL-XL. Expression of pAKT and Mcl-1 have been described in colon cancer, however, the relationship between pAKT and Mcl-1 has not. Mcl-1 and pAKT immunohistochemistry was performed using colorectal cancer tissue microarray (TMA). The Holm step-down method was used to adjust for multiple testing. Mcl-1 and pAKT scores, stage, and grade were compared using Spearman's correlation coefficient. Metastasis and no metastasis groups were compared using the Wilcoxon rank sum test. Mcl-1 and pAKT scores were compared for normal colorectal mucosa (NR), adenoma (AD), and colorectal cancer (CRC) cohorts. The mean (SD) pAKT expression in NR (14) was 2.0 (1.4), in AD (8) was 3.0 (1.7), and in CRC (101) was 5.6 (2.4). These differences were statistically significant. For Mcl-1 the mean (SD) expression was 4.1 (1.7) in NR, 3.2 (1.2) in AD, and 3.3 (2.6) in CRC. Mcl-1 and pAKT scores were directly correlated during various stages of colon car-cinogenesis (p = 0.04). Mcl-1 showed direct correlation with tumor grade (p = 0.001) and tumor stage (p = 0.02) and with presence of metastasis (p = 0.008). We report the correlation of Mcl-1 protein expression with higher grade and stage in colorectal cancer. Mcl-1 correlated also with pAKT expression. We also report the up regulation of pAKT during the transition from NR to CRC.
Keywords: Mcl-1, pAKT, colon adenocarcinoma, normal colon, immunohistochemistry
Introduction
Colorectal adenocarcinoma is the most common form of colonic cancer affecting approximately 112,000 new patients every year. Colo-rectal cancer accounted for approximately 19% of all cancer-related deaths in the United States in 2007 [1]. Colorectal adenocarcinoma affects patients usually older then 40 years, except in individuals with genetic predisposition to this form of cancer [2]. The patients with high stage tumors are those that frequently develop metas-tases and succumb to the cancer [3].
Sporadic colorectal cancer usually develops following the accumulation of multiple sequential genetic changes within a cell. While somatic mutations of the APC tumor suppressor gene are the first step toward carcinogenesis, the accumulation of other sequential genetic or epigenetic events activate oncogenes (Ras, c-Src), or inactivate tumor suppressor genes (DCC, DPC-4, P53, and others) [4-9]. These genetic changes are thought to be translated in functional alterations that eventually provide the tumor cell with new malignant attributes such as increase mobility, capability of invading the surrounding stroma, of evading the immune system, and of metastasizing. Recently, the “serrated pathway” was identified as another pathway responsible for colon carcinogenesis. This pathway involves errors in mismatch repair genes and involvement of cyclin B, Braf, TGFBR2, and others [10]
It has been shown that inhibition of apoptosis is critical to colorectal Tumorigenesis [11]. For example, it has been proposed that overexpression of Bcl-XL in cancer may suppress the activity of the proapoptotic molecules Bax and Bak, contributing to cancer progression [12, 13]. It seems that, also in CRC, the dissociation of Bax and Bcl-XL promotes Bax multimerization and mitochondrial translocation, triggering apop-tosis [14].
Mcl-1 (myeloid cell leukemia-1) is a Bcl-2 family protein that interferes with mitochondrial activation to inhibit apoptosis. Altered expression pattern of Mcl-1, as well as of Bax and Bcl-XL, has been described during colorectal cancer progression [11-13, 16]. Backus et al. have described the interesting co-localization of Bax, Mcl-1 and Bcl-XL reactivity to the apical areas of the normal intestinal mucosa, as opposed to the diffuse cytoplasmic staining in the tumor cells [11].
IGF1-dependent activation of AKT effects proliferation, transformation, resistance to apoptosis, and metastatic potential of colon cancer cells [17]. The indication that AKT activation has a pivotal role in colorectal carcinogenesis also derives from the observation that mice lacking the catalytic subunit of PI3 kinase gamma develop spontaneous intestinal adenocarcinomas [18]. It is known that loss of PTEN protein activates phosphoinositol (PI)-3 kinase, with generation of PI 3,4,5-triphosphate and recruite-ment and activation of AKT to the plasma membrane [19]. It has become evident that AKT activation facilitates cell transformation and tumori-genesis affecting multiple pathways regulating not only apoptosis [20], but also the cell cycle [21], cell motility [22], and angiogenesis [23].
Studies on clinical samples have show that AKT activation is increased in 46% of colorectal carcinomas, and its association with Ki-67 proliferation index and inversely associated with the presence of apoptosis [24]. Others have shown that AKT activation increases also during the transition from benign polyps to carcinoma, and that such activation is inversely correlated to PTEN expression, a tumor suppressor protein known to inhibit the activation of PI3K/AKT pathway in colon cancer [25]. Despite the similar antiapoptotic function of Mcl-1 and pAKT, we found no reports in the current English literature on their correlation in colorectal cancer.
In this study, using stage oriented human colorectal cancer tissue microarrays; we evaluated changes in Akt activity and in Mcl-1 expression during the progression from normal colonic mucosa to adenoma, to invasive colorectal adeno-carcinoma, using immunohistochemistry. The results were correlated with clinical pathologic findings.
Materials and methods
Selection of cases
Using stage oriented human colorectal cancer tissue microarrays (prepared in the Histology laboratory of the Moffitt Cancer Center Tissue Core Facility), 123 tissue samples (101 CRC, 8 adenomas, and 14 samples of normal colonic mucosa) were analyzed for Mcl-1 expression and pAKT activation by immunohistochemistry. All of the tumors used for the tissue array construction were CRC identified from the Moffitt Cancer Center, Anatomic Pathology Department's database, CoPath®, and representing surgical resection specimens obtained between 1990 and 2002. All of the specimens were preserved in 10% buffered formalin, prior to embedding in paraffin, for at least 12 hours. The tumors were staged according to the TNM system, following the recommendations of the American Joint Committee on Cancer, 1988. The stage of the invasive tumors was as follows: 12 patients had stage I, 37 stage II, 43 stage III, and 9 stage IV disease. All tumors occurred in the absence of genetic cancer syndromes such as human non polyposis colon cancer syndrome (HNPCC), familial adenomatous polyposis syndrome (FAP), etc.; also cancers arising in the background of ulcerative colitis or Crohn's disease were excluded from the study. The NR samples were taken near the resected colorectal margin, away from the tumor site, from patients that underwent colon resection for CRC.
Immunohistochemistry
The usage of Tissue Microarray in our study allowed the entire cohort (NR, AD and CRC) to be analyzed in one batch on only 4 slides (2 test slides, in addition to positive and negative controls). Thus reagent concentration, incubation times, temperature, humidity, wash conditions, and antigen retrieval were identical for each case. The tissues were stained with mouse monoclonal antibodies toward pAKT (clone 587F11, 1:100 dilution, Cell Signaling Tech., Beverly, MA) and Mcl-1 (clone RC13, 1:400 dilution, GeneTex Inc.). The slides were dewaxed by heating at 55° C for 30 minutes and by three washes, five minutes each, with xylene. Tissues were rehydrated by a series of five-minute washes in 100%, 95%, and 80% ethanol, and distilled water. Endogenous peroxidase activity was blocked with 3% hydrogen peroxide for 20 minutes. After blocking with universal blocking serum (Ventana Medical Systems, Inc., Tucson, Arizona OMNI MAP) for 30 minutes, the samples were incubated with the primary antibodies at 4° C overnight. The samples were then incubated with biotin-labeled secondary antibody and streptavidin-horseradish peroxidase for 30 minutes each (Ventana Medical Systems). The slides were developed with 3,3'-diaminobenzidine tetrahydrochloride substrate (Ventana Medical Systems Inc. Tucson, Arizona) and counterstained with hematoxylin (Ventana Medical Systems Inc. Tucson, Arizona). The tissue samples were dehydrated and coversli-ped. Standard cell conditioning (following the Ventana proprietarian recommendations) was used for antigen retrieval. The specificity of the anti-Mcl-1 and anti-pAKT monoclonal antibodies was confirmed by immunostains of colon cancer cell lines constitutively expressing Mcl-1 and pAKT antibodies, and colon cancer cell lines KO for Mcl-1 and pAKT [12, 13]. Negative control was included by using non immune mouse sera and omitting the primary antibodies during the primary antibody incubation step.
Immunohistochemical data analysis
The Mcl-1 and pAKT stained tissue cores were examined by two independent observers (EA, DC); and a consensus score was reached for each specimen. The positive reaction for both antibodies was scored into four grades, according to the intensity of the staining: 0, 1+, 2+, and 3+. The percentages of Mcl-1 or pAKT positive cells were also scored into four categories: 0 (0%), 1 (1-33%), 2 (34-66%), and 3 (67-100%). The product of the intensity by percentage scores was used as the final score. The final scores were classified as: 0 negative; 1-3, weak; 4-6, moderate; and 7-9, strong. The specimens were also classified by the types of tissue staining positive: normal colonic mucosa, adenoma, and adenocarcinoma.
Statistical analysis
Descriptive statistics for the scores were generated and reported for each tissue group. Mcl-1 and pAKT scores were compared for normal (N), adenoma (AD), and adenocarcinoma (CRC) cohorts. The Holm step-down method was used to adjust for multiple testing. Mcl-1 and pAKT score, stage, and grade were compared using Spearman's correlation coefficient. Mcl-1 and pAKT expression scores for metastasis and no metastasis groups were compared using the Wilcoxon rank sum test. The tests were called significant at the significance level of 0.05.
Results
Clinical pathologic findings
The clinical pathological findings are summarized in Table 1. The patients with cancer had an average age of 64 years (range, 24-92). Sixty -six were male and 35 were female. All the tumors occurred in the absence of familial adeno-matous polyposis or HNPCC. The tumors ranged in size between 1.4 cm. and 14.5 cm (average 5.2 cm), mostly polypoid and ulcerated. Twenty tumors involved the cecum, 25 the ascending colon, 4 the transverse colon, 9 the descending colon, 24 the sigmoid colon, 10 the rectosigmoid junction, and 9 the rectum. Thirteen tumors were well differentiated, 74 were moderately differentiated, and 14 were poorly differentiated. Of the invasive tumors, 12 were Duke's stage A, 37 Duke's stage B, 43 Duke's stage C, and 9 Duke's stage D. Only 2 patients, both with rectal cancer, received preoperative radiation, to reduce the size of their tumors.
Table 1.
Clinical pathological findings
| Age | 24-92 (average 64) |
|---|---|
| Sex | Female: N= 35 |
| Male: N= 66 | |
| Tumor Size | 1.4 - 14.5 cm (average 5.2 cm) |
| Tumor Location | Cecum: 20 |
| Ascending colon: 25 | |
| Transverse colon: 4 | |
| Descending colon: 9 | |
| Sigmoid: 24 | |
| Rectosigmoid: 10 | |
| Rectum: 9 | |
| Tumor Grade | Well differentiated: 13 |
| Moderately differentiated: 74 | |
| Poorly differentiated: 14 | |
| Tumor Stage | Stage I: 12 |
| Stage II: 37 | |
| Stage III: 43 | |
| Stage IV: 9 |
Immunohistochemical results
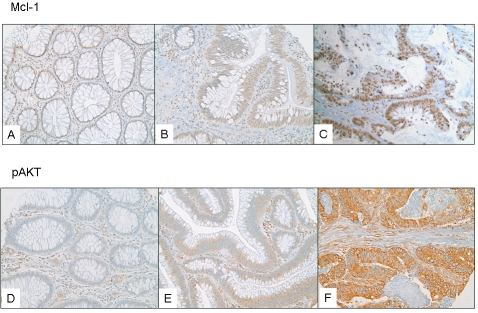
All of the Mcl-1 positively stained cases had nuclear staining. The pAKT stain was predominantly cytoplasmic, however in some cases nuclear and cytoplasmic stain was observed. The cytoplasmic staining was diffusely granular with variation in intensity seen within the same lesion of some cases. Cases with variable staining were graded based on the predominant staining intensity and the percentage of positive stain was determined based on the amount of the lesion demonstrating the predominant intensity. For Mcl-1 the mean (SD) expression was 4.1 (1.7) in NR, 3.2 (1.2) in AD, and 3.3 (2.6) in CRC (Figure 1A, B, C). For pAKT the mean (SD) pAKT expression in NR (14) was 2.0 (1.4), in AD (8) was 3.0 (1.7), and in CRC (101) was 5.6 (2.4) (Figure 1D, E, F). Despite the fact that a decrease in Mcl-1 staining was observed between NR vs. AD and CRC, the presence of staining within CRC cases correlated with grade and stage.
Figure 1.
(A) a case of NR showing weak and diffuse Mcl-1 nuclear staining; (B) a case of AD showing weak nuclear staining; (C) a case showing strongly and diffusely Mcl-1 positive CRC; (D) The pAKT stain was predominantly cytoplasmic and the IHC score increased progressively from NR; (E) The pAKT stain was predominantly cytoplasmic and the IHC score increased progressively from AD; (F) The pAKT stain was predominantly cytoplasmic and the IHC score increased progressively from CRC;
Statistical analysis
The statistical analysis results are summarized in Table 2. A Wilcoxon rank sum test revealed a statistically significant difference in pAKT staining score between NR and CRC (p-value<0.0001), and between AD and CRC (p-value = 0.006), but not between AD and NR (p-value = 0.61). The same test did not detect significant correlation in Mcl-1 staining scores between NR and CRC (p-value = 0.61), AD and CRC (p = 0.76), and between AD and NR (p-value = 0.76). When considering the CRC cohort, we observed a correlation between Mcl-1 expression and AKT activation (Spearman's cor-relation coefficient 0.23, p-value = 0.04). Mcl-1 showed direct correlation with tumor grade (Spearman correlation coefficient = 0.36; p = 0.001) and tumor stage (Spearman correlation coefficient = 0.26; p = 0.02). Also, the Wilcoxon rank sum test showed a significant correlation of the Mcl-1 staining scores with presence of metastasis (p = 0.008), however, only 9 cases with metastases were available for evaluation. This finding needs to be re-evaluated using a larger cohort. pAKT staining score did not correlate with grade, stage, or presence of metasta-
Table 2.
Immunostains results and statistics
| pAKT | Mean(sd) | Median | Minimum | Maximum |
|---|---|---|---|---|
| Normal (N) | 2.0 (1.4) | 2.5 | 0 | 4 |
| Adenoma (AD) | 3.0 (1.7) | 3 | 0 | 6 |
| Carcinoma (CA) | 5.6 (2.4) | 6 | 0 | 9 |
| *Wilcoxon rank sum test: CA vs. N, p-value <0.0001; AD vs. CA, p-value = 0.006; AD vs. N, p-value = 0.61 | ||||
| Mcl-1 | Mean(sd) | Median | Minimum | Maximum |
| Normal (N) | 4.1 (1.7) | 3 | 2 | 6 |
| Adenoma (AD) | 3.2 (1.2) | 3 | 2 | 6 |
| Carcinoma (CA) | 3.3 (2.6) | 3 | 0 | 9 |
| * Wilcoxon rank sum test: CA vs. N, p-value = 0.61; AD vs. CA, p-value = 0.76; AD vs. N, p-value = 0.76 | ||||
| Other Statistics: | ||||
| Presence of Metastasis | pAKT, p-value = 0.26; Mcl-1, p-value = 0.008 | |||
| Grade | pAKT, p-value = 0.72; Mcl-1, p-value = 0.001 | |||
| Stage | pAKT, p-value = 0.49; Mcl-1, p-value = 0.02 | |||
Discussion
Programmed cell death (PCD) is defined as a physiological process that plays a critical role in normal development, cellular differentiation, and tissue homeostasis of multicellular organisms [26]. Dysregulation of this physiological cell death process contributes to the patho-genesis of human diseases including cancer [26,27].
Mcl-1, also known as myeloid cell leukemia-1, is a 37-kd protein with significant homology with Bcl-2. Mcl-1 was isolated from a human myeloid leukemia cell line undergoing differentiation, and in these cells Mcl-1 protein was only induced transiently [15]. Mcl-1 interferes with the activation of the mitochondrial pathway of apop-tosis and contributes to the resistance of neo-plastic cells towards apoptosis. This is demon-strated by the fact that Mcl-1 is capable of abrogating c-Myc induced apoptosis in Chinese hamster ovarian cells [28]. It has been postulated that in the absence of Bcl-2, Mcl-1 may interact with Bax to induce its anti-apoptotic effect [29].
Several investigators have studied the expression of Mcl-1 protein in human tissues. In one study, Mcl-1 protein was found in well differentiated complex epithelia, such as epidermis, intestine, colon, prostate, nasopharynx, and upper airway, usually localized to the upper portion of the cells [13]. This localization is in antithesis with that of Bcl-2 protein, usually found in the cytoplasm of cells bordering the basement membrane and in less differentiated cells. This may reflect different functions of Bcl-2 and Mcl-1 in the in vivo regulation of apoptosis. While Bcl-2 has been shown to inhibit PCD to support the long term survival of epithelia related stem cells; ultimately regulating their self renewal [30-32]. Other studies have reported Mcl-1 protein to be localized in either the mitochondrial membrane [33], or in the nucleus [15, 34]. Furthermore, Liu H. etal. observed Mcl-1 predominant localization in mitochondria if the cells were tested within 24 hours of seeding, and in the nucleus and/or cytoplasm if tested after long culture time [35].
In our study, using the monoclonal antibody MCL1 (RC13), the Mcl-1 protein stain was consistently nuclear, as expected according to Gene Tex proprietarian instructions. We found an overall decrease in Mcl-1 expression during the progression from NR to AD and/or CRC. However, this was due to the presence of a subset of NR with high Mcl-1 scores. When considering the CRC cohort alone most of the tumors had moderate or strong Mcl-1 expression and correlated well with pAKT expression. Our data are in contrast with the finding of Krajewska et al. reporting the increase in Mcl-1 during the transition from N to AD, but the downregulation of Mcl-1 protein in the invasive tumors. The authors explained this finding as a selection bias as they studied tumors that were mostly poorly differentiated.
In this study we found a statistically significant correlation of Mcl-1 with tumor grade and stage. However, these findings must be confirmed on larger patient cohorts. In fact, the tumors we studied included a preponderant percentage of moderately differentiated CRCs, and only 9 cancers were metastatic.
We also report the increased expression of pAKT during the progression from N to AD to CA. This is an expected finding following our previous observation that in colon cancer the expression of insulin-like growth factor receptor 1 (IGF1-R) increases with the progression of the disease and that the tumorigenic and anti-apoptotic functions of this receptor are modulated via activation of AKT [17, 36].
Itoh et al. have described pAKT in the nucleus and cytoplasm of epithelial cells of normal colorectal mucosa. pAKT was localized especially on the surface as opposed to the deep layer of the mucosa [24]. In the same study, 46% of the colorectal carcinoma samples, showed high level of pAKT. The authors reported a close association between pAKT and Ki-67 proliferative activity, and between pAKT and number of apoptotic bodies [24]. Others have shown pAKT activity to be significantly higher in colorectal cancers than polyps [25]. In our study we observed a similar finding with up-regulation of pAKT during the transition from normal colorectal mucosa to carcinoma, supporting a role for pAKT in colon carcinogenesis.
When considering the CRC cohort alone, we observed a direct correlation between Mcl-1 and pAKT, reflecting the common anti-apoptotic function of these proteins. These proteins likely favor the progression of colorectal carcinomas by promoting cell growth and by rescuing tumor cells from apoptosis. We also observed the progressive AKT activation during the transition from NR to AD to CRC. These changes were statistically significant. However, pAKT did not correlate with tumor grade and/or stage in this size limited study.
Conclusion
This study describes, for the first time, the direct correlation between Mcl-1 protein expression and AKT activation in colorectal cancer. In this study we found that Mcl-1 expression is significantly correlated with tumor grade and stage, and that AKT activation level increases during the progression from NR to AD to CRC.
Acknowledgments
We thank the Histology Section of the Tissue Core at the Moffitt Cancer Center and Research Institute for the support in performing the im-muno-histochemical stains. We also thank Andrea Dattilo for her help with preparation of the manuscript. The authors have no conflict of interests.
References
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer Statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Nishisho I, Nakamura Y, Miyoshi Y, Miki Y, Ando H, Horii A, Koyama K, Utsunomiya J, Baba S, Hedge P. Mutations of chromosome 5q21 genes in FAP and colorectal cancer patients. Science. 1991;253(5020):665–9. doi: 10.1126/science.1651563. [DOI] [PubMed] [Google Scholar]
- 3.Astler VB, Coller FA. The prognostic significance of direct extension of carcinoma of the colon and rectum. Ann Surg. 1954;139:846–851. doi: 10.1097/00000658-195406000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Winawer SJ, Fletcher RH, Miller L, Godlee F, Stolar MH, Mulrow CD, Woolf SH, Glick SN, Ganiats TG, Bond JH, Rosen L, Zapka JG, Olsen SJ, Giardiello FM, Sisk JE, Van Antwerp R, Brown-Davis C, Marciniak DA, Mayer RJ. Colorectal cancer screening: clinical guidelines and rationale. Gastroenterology. 1997;112:594–642. doi: 10.1053/gast.1997.v112.agast970594. [DOI] [PubMed] [Google Scholar]
- 5.Nakamura Y, Nishisho I, Kinzler KW, Vogelstein B, Miyoshi Y, Miki Y, Ando H, Horii A, Nagase H. Mutations of the adenomatous polyposis coli gene in familial polyposis coli patients and sporadic colorectal tumors. Princess Takamatsu Symp. 1991;22:285–92. [PubMed] [Google Scholar]
- 6.Cho KR, Oliner JD, Simons JW, Hedrick L, Fearon ER, Preisinger AC, Hedge P, Silverman GA, Vogelstein B. The DCC gene: structural analysis and mutations in colorectal carcinomas. Genomics. 1994;19:525–31. doi: 10.1006/geno.1994.1102. [DOI] [PubMed] [Google Scholar]
- 7.Thiagalingam S, Lengauer C, Leach FS, Schutte M, Hahn SA, Overhauser J, Willson JK, Markowitz S, Hamilton SR, Kern SE, Kinzler KW, Vogelstein B. Evaluation of candidate tumor suppressor genes on chromosome 18 in colorectal cancers. Nat Genet. 1996;13:343–6. doi: 10.1038/ng0796-343. [DOI] [PubMed] [Google Scholar]
- 8.Polyak K, Xia Y, Zweier JL, Kinzler KW, Vogelstein B. A model for p53-induced apoptosis. Nature. 1997;389(6648):300–5. doi: 10.1038/38525. [DOI] [PubMed] [Google Scholar]
- 9.Irby RB, Mao W, Coppola D, Kang J, Loubeau JM, Trudeau W, Karl R, Fujita DJ, Jove R, Yeatman TJ. Activating SRC mutation in a subset of advanced human colon cancers. Nat Genet. 1999;21(2):187–90. doi: 10.1038/5971. [DOI] [PubMed] [Google Scholar]
- 10.Kelsen DP, Daly JM, Kern SE, Levin B, Tepper JE, Van Cutsem E. 2nd ed. Philadelphia: Lippincott, Williams and Wilkins; 2008. Principles and Practice Gastrointestinal Oncology. [Google Scholar]
- 11.Backus HHJ, Van Groeningen CJ, Vos W, Dukers DF, Bloemena E, Wouters D, Pinedo HM, Peters GJ. Differential expression of cell cycle and apoptosis related proteins in colorectal mucosa, primary colon tumours, and liver metastases. J Clin Pathol. 2002;55:206–211. doi: 10.1136/jcp.55.3.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krajewska M, Moss S, Krajewski S, Song K, Holt PR, Reed JC. Elevated expression of Bcl-X and reduced Bak in primary colorectal adenocarcinomas. Cancer Res. 1996;56:2422–2427. [PubMed] [Google Scholar]
- 13.Krajewski S, Bodrug S, Krajewska M, Shabaik A, Gascoyne R, Berean K, Reed JC. Immunohis-tochemical analysis of Mcl-1 protein in human tissues. Am J Pathol. 1995;146:1309–1319. [PMC free article] [PubMed] [Google Scholar]
- 14.Ming L, Wang P, Bank A, Yu J, Zhang L. Puma dissociates Bax and Bcl-XLto induce apoptosis in colon cancer cells. JBC. 2006;281(23):16034–16042. doi: 10.1074/jbc.M513587200. [DOI] [PubMed] [Google Scholar]
- 15.Kozopas KM, Yang T, Buchan HL, Zhou P, Craig RW. MCL1, a gene expressed in program myeloid cell differentiation, has sequence similarity to BCL2. Pro. Natl. Acad. Sci. USA. 1993;90:3516–3520. doi: 10.1073/pnas.90.8.3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schulze-Bergkamen H, Ehrenberg R, Hickmann L, Vick B, Urbanik T, Schimanski CC, Berger MR, Schad A, Weber A, Heeger S, Galle PR, Moehler M. Bcl-XL and Myeloid cell leukaemia-1 contribute to apoptosis resistance of colorectal cancer cells. World J Gastroenterol. 2008;14:3829–3840. doi: 10.3748/wjg.14.3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sekharam M, Zhao H, Sun M, Fang Q, Zhang Q, Yuan Z, Dan HC, Boulware D, Cheng JQ, Coppola D. Insulin-like growth factor 1 receptor enhances invasion and induces resistance to apoptosis of colon cancer cells through the Akt/Bcl-x(L) pathway. Cancer Res. 2003;63:7708–16. [PubMed] [Google Scholar]
- 18.Sasaki T, Irie-Sasaki J, Horie Y, Bachmaier K, Fata JE, Li M, Suzuki A, Bouchard D, Ho A, Redston M, Gallinger S, Khokha R, Mak TW, Hawkins PT, Stephens L, Scherer SW, Tsao M, Penninger JM. Colorectal carcinoma in mice lacking catalytic subunit of PI(3)K gamma. Nature. 2000;406(6798):897–902. doi: 10.1038/35022585. [DOI] [PubMed] [Google Scholar]
- 19.Cantley LC, Neel BG. New insights into tumor suppression: PTEN suppresses tumor formation by restraining the phophoinositide 3-kinase/AKT pathway. Proc Natl Acad Sci USA. 1999;96(8):4240–4245. doi: 10.1073/pnas.96.8.4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khwaja A. Akt is more than just a Bad kinase. Nature. 1999;401(6748):33–34. doi: 10.1038/43354. [DOI] [PubMed] [Google Scholar]
- 21.El-Deiry WS. Akt takes centre stage in cell-cycle deregulation. Nat Cell Biol. 2001;3(3):E71–73. doi: 10.1038/35060148. [DOI] [PubMed] [Google Scholar]
- 22.Sheng H, Shao J, Washington MK, DuBois RN. Prostaglandin E2 increases growth and motility of colorectal carcinoma cells. J Biol Chem. 2001;276(21):18075–18081. doi: 10.1074/jbc.M009689200. [DOI] [PubMed] [Google Scholar]
- 23.Kureishi Y, Luo Z, Shiojima I, Bialik A, Fulton D, Lefer DJ, Sessa WC, Walsh K. The HMG-CoA reductase inhibitor simvastatin activates the protein kinase Akt and promotes angiogenesis in normocholesterolemic animals. Nat Med. 2000;6(9):1004–1010. doi: 10.1038/79510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Itoh N, Semba S, Ito M, Takeda H, Kawata S, Yamakawa M. Phosphorylation of Akt/PKB is required for suppression of cancer cell apoptosis and tumor progression in human colorectal carcinoma. Cancer. 2002;94(12):127–134. doi: 10.1002/cncr.10591. [DOI] [PubMed] [Google Scholar]
- 25.Colakoglu T, Yildirim S, Kayaselcuk F, Nursal TZ, Ezer A, Noyan T, Karakayali H, Haberal M. Clinicopathological significance of PTEN loss and the phosphoinositide 3-kinase/Akt pathway in sporadic colorectal neoplasms: is PTEN loss predictor of local recurrence? Am J Surg. 2008;195:719–725. doi: 10.1016/j.amjsurg.2007.05.061. [DOI] [PubMed] [Google Scholar]
- 26.Cotran RS, Kumar V, Collins T. Robbins Pathologic basis of disease. 7th ed. Philadelphia: Elsevier Saunders; 2004. Cellular injury and cell death. [Google Scholar]
- 27.Maddika S, Ande SR, Panigrahi S, Weglarczyk K, Zuse A, Eshraghi M, Manda KD, Wiechec E, Los M. Cell survival, cell death and cell cycle pathways are interconnected: implications for cancer therapy. Drug Resistance Updates. 2007;10:13–29. doi: 10.1016/j.drup.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 28.Reynolds JE, Yang T, Qian L, Jenkinson JD, Zhou P, Eastman A, Craig RW. Mcl-1, a member of the Bcl-2 family, delays apoptosis induced by c-Myc overexpression in Chinese hamster ovary cells. Cancer Res. 1994;54:6348–6352. [PubMed] [Google Scholar]
- 29.Krajewski S, Krajewska M, Shabaik A, Miyashita T, Wang HG, Reed JC. Immunohistochemi-cal determination of in vivo distribution of Bax, a dominant inhibitor of Bcl-2. Am J Pathol. 1994;145:1323–1333. [PMC free article] [PubMed] [Google Scholar]
- 30.Adams JM, Kelly PN, Dakic A, Carotta S, Nutt SL, Strasser A. Role of “cancer stem cells” and cell survival in tumor development and maintenance. Cold Spring Harb Symp Quant Biol. 2008;73:451–9. doi: 10.1101/sqb.2008.73.004. [DOI] [PubMed] [Google Scholar]
- 31.LeBrun DP, Warnke RA, Cleary ML. Expression of bcl-2 in fetal tissues suggests a role in morphogenesis. Am J Pathol. 1993;142:743–753. [PMC free article] [PubMed] [Google Scholar]
- 32.Kamada S, Shimono A, Shinto Y, Tsujimura T, Takahashi T, Noda T, Kitamura Y, Kondoh H, Tsujimoto Y. Bcl-2 deficiency in mice leads to pleiotropic abnormalities: accelerated lymphoid cell death in thymus and spleen, polycystic kidney, distorted intestine and hair hypopigmentation. Cancer Res. 1994;55:354–359. [PubMed] [Google Scholar]
- 33.Yang T, Kozopas KM, Craig RW. The intracellular distribution and pattern of expression of Mel -1 overlap with, but are not identical to those of Bcl-2. J Cell Biol. 1995;128:1173–1184. doi: 10.1083/jcb.128.6.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang D, Li F, Weidner D, Mnjoyan ZH, Fujise K. Physical and functional interaction between myeloid cell leukemia 1 protein (MCL1) and fortilthe potential role of MCL1 as a fortilin chaperone. J Biol Chem. 2002;7:37430–37438. doi: 10.1074/jbc.M207413200. [DOI] [PubMed] [Google Scholar]
- 35.Liu H, Peng HW, Cheng YS, Yuan HS, Yang-Yen HF. Stabilization and Enhancement of the An-tiapoptotic Activity of Mcl-1 by TCTP. Mol Cell Biol. 2005;25(8):3117–3126. doi: 10.1128/MCB.25.8.3117-3126.2005. 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hakam A, Yeatman TJ, Lu L, Mora L, Marcet G, Nicosia SV, Karl RC, Coppola D. Expression of insulin-like growth factor - 1 receptor in human colorectal cancer. Hum Pathol. 1999;30(10):1128–33. doi: 10.1016/s0046-8177(99)90027-8. [DOI] [PubMed] [Google Scholar]