Abstract
Matrix metalloproteinases (MMP) have been implicated in increased invasive and metastatic potential of tumors, possibly via interactions with the extracellular matrix and angiogenesis. This study investigates the relationship between MMP-2 immunoexpression and angiogenesis in a series of lung carcinomas metastatic to the central nervous system (CNS). Twenty eight metastatic carcinoma cases with adequate brain-tumor interface were identified from the archives at the Moffitt Cancer Center. MMP-2 expression was determined by immunohistochemistry using an antibody directed against pro and active forms (NeoMarkers). Similarly, microvessels were identified on parallel sections with anti-CD34 antibody (Biogenix). Angiogenesis profiles within the tumor and at the CNS/tumor interface were morphometrically assessed by the Image Pro Plus image analysis system. Briefly, CD34 positive vessels were quantitated and correlated with presence or absence of MMP-2 expression in the tumor. Mean microvessel area (MMVA) and mean microvessel number (MMVN) were assessed within areas of brain-tumor interface and within the tumor and expressed as a ratio relative to the tumor. Sixteen (57.14%) metastatic tumors were strongly immunoreac-tive for MMP-2, while 12 (42.86%) were negative. MMP-2 positive tumors had a higher MMVA and MMVN ratio at the CNS/tumor interface in comparison to MMP-2 negative neoplasms. MMP-2 expression thus appears to confer enhanced vascular proliferation particularly at the brain-tumor interface which would support the contention of enhanced capability of growth and invasion within the CNS, possibly modulated by MMP2. The relationship between MMP-2 expression and angiogenesis has been previously reported and its biological and therapeutic implications remain the focus of investigations.
Keywords: Matrix metalloproteinases (MMP), angiogenesis, lung cancer, CNS metastasis
Introduction
The ability of cancer cells to proliferate locally as well as their ability to metastasize is by far the most important and clinically significant contributors to prognosis. Interactions within the tumor microenvironment and extracellular matrix (ECM) including, but not limited to, proteolytic enzymes, their inhibitors and a wide range of cytokines remain fundamental players in this process. Matrix metalloproteinases (MMP) are a family of glycoproteins that utilize zinc at the catalytic site. They are secreted as zymogens and are activated by proteinases within conserved and terminal region [1]. MMP play an important functional role in many normal physiological activities such as tissue remodeling, reproduction, and organogenesis [2]. Over recent years a significant amount of evidence has accumulated that directly implicates members of the matrix metalloproteinases family in tumor invasion and metastasis [3-4]. Studies have demonstrated a positive correlation between the expression of MMP and the invasive and metastatic potential of various malignant tumors including lung [5], head, and neck [6], breast [7], prostate [8], ovarian [9], pancreatic [10-11], colorectal [12] and gastric carcinoma [13-14]. The expression of MMP2 and MMP3 is closely related to lymphoid metastasis and vascular invasion in squamous cell carcinoma of the esophagus [15]. Similarly MMP2 expression correlates with poor prognosis in cervical squamous carcinoma and the expression of MMP2 serves as a marker for malignant transformation of cervical epithelial cells [16-17]. The specifics of MMP interactions with the matrix and particularly endothelial cells continue to be the focus of ongoing studies.
Materials and methods
Immunohistochemistry
Paraffin-embedded blocks of 28 metastatic lung adenocarcinoma to brain were identified from the surgical pathology archives. Tumors were not further defined in terms of histologic variants, size of tumor or degree of necrosis, except to ensure that all sections examined showed viable tumor and brain interface. Tissue sections were cut at 5 microns and were applied to “Plus” slides (Fisher Scientific). They were depar-affinized in xylene and hydrated through graded alcohols. Endogenous peroxidase was quenched with 3% hydrogen peroxide in methanol. Following several rinses in phosphate buffered saline (PBS, pH 7.4, 0.3% Triton X-100), slides were incubated for 20 minutes in primary antibody. MMP-2 expression was determined using a mouse monoclonal antibody directed against both pro and active forms (NeoMarkers, clone A-gel VC2), with overnight incubation at 4°C at a dilution of 1:75. After multiple PBS rinses, the slides were exposed to secondary antibody, biotinylated goat anti-mouse antibody and then to peroxidase conjugated strepavidin. Finally sections were incubated with the chromogen very intense purple (VIP), counterstained with hematoxylin, dehydrated through alcohols and xylene and cover slipped with a synthetic permanent mounting medium. Human placental tissue served as a positive control, and negative controls included elimination of the primary antibody or use of non-specific IgG.
Microvessels were similarly identified on parallel sections with anti-human CD34 antibody (Biogenix, clone QBend/10). Briefly, tissue sections were deparaffinized, hydrated and endogenous peroxidase activity quenched with hydrogen peroxide in methanol. For antigen retrieval, slides were microwaved in citrate buffer for 15 mins at low power. Primary antibody was added at 1:10 dilution and incubated overnight at 4°C. Biogenex multilink system was utilized for secondary antibody detection and VIP chromogen was used for detection. Epithelial cell immunoreactivity with MMP2 was arbitrarily identified as positive (focal or diffuse, involving more than 30% of the tumor- although most tumors were either significantly positive or only minimally reactive) or negative, if less than 30% immunoreactive cells. Only epithelial cell immunoreactivity as opposed to stromal staining was recorded as positive as mesenchymal elements, including blood vessels occasional stained positive without epithelial reactivity.
Morphometry
CD34 immunoreactive, angiogenesis profiles within the tumor and at the CNS/tumor interface were morphometrically assessed by segmentation thresholding using the Image Pro Plus image analysis software system. Briefly, images were captured at 200x magnification from 3 fields/case, which were selected based on areas with maximal CD34 staining. The stained area of CD34 positive vessels was highlighted on images from the interface and the tumor and measured in pixels. Final results (MMVA) are expressed as the ratio of these numbers. In an analogous manner, individual, distinctly separate vessels were also manually tagged and counted and expressed as a ratio of number of vessels at the interface and number of vessels within the tumor, providing the MMVN. This quantitation was correlated with MMP2 expression, the latter being expressed as positive (greater than 30% of tumor cells showing moderate intensity of staining) or negative (less than 30% of tumor cells staining). Although the selection of the 30% staining cut-off remains an arbitrary number, the distinction between 30% and as much as 60% of tumor cells showing MMP2 expression was minimal. The reporting of data as a ratio relative to the tumor was felt to be more representative than just reporting numbers within normal brain, the tumor and the interface individually. Comparison of raw data e.g. number of blood vessels in the tumor and at the interface was equally representative of the findings, but did not take into consideration the inherent vascularity of the tumor itself.
Statistical analysis
Statistical analysis was performed using Graph-Pad Prism software (Version 5.03). The comparison between mean values was performed using non-parametric 2-tailed t-test (Mann-Whitney test). Statistical significance was defined as a p-value of less than 0.05.
Results
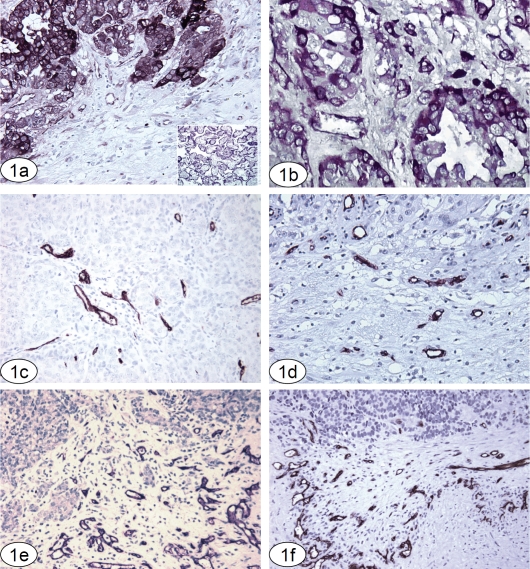
MMP2 immunoreactivity was readily identified in 57.14% (16/28) of metastatic tumors, with most cases demonstrating unequivocal expression. 42.86% of cases (12/28) showed no staining, or either weak focal epithelial or focal stromal reaction, both of which were considered negative. MMP2-positive tumor cells were intensely stained and easily identified, particularly at the interface with adjacent normal or reactive brain tissue (Figure 1a and b). Vessels at the interface and within the tumor often expressed some MMP2 immunoreactivity. CD34 immunoreactivity was generally strong and specific, restricted to the walls of small vessels and the intraluminal aspect of larger vessels. Vessels within the tumor and at the CNS-tumor interface were evaluated in MMP2 negative and positive tumors (Figure 1c-f).
Figure 1.
Lung carcinoma metastatic to the CNS, with strong immunoreactivity for MMP2. MMP2 expression was well defined in the neoplastic elements. Occasional mild staining is seen within the adjacent neuropil and some blood vessels. MMP2 immunoreaction, original magnification 200×. Inset: Placental tissue, positive control. (b) MMP2 positive adenocarcinoma, with strong cytoplasmic and focal cytoplasmic membrane positivity. MMP2 immunoreaction, original magnification 400×. (c, d, e and f) CD34 immunoreactivity was specific and confined to blood vessels. Sections illustrating angiogenesis within a representative tumor (1c); at the tumor/brain interface in an MMP2 negative tumor (1d) and at the interface in 2 different MMP2 positive tumors (1e and f). CD34 immunoreaction, original magnification 200×. All sections are paraffin embedded, formalin fixed tissue, using a VIP (purple) chromogen, counter-stained with hematoxylin.
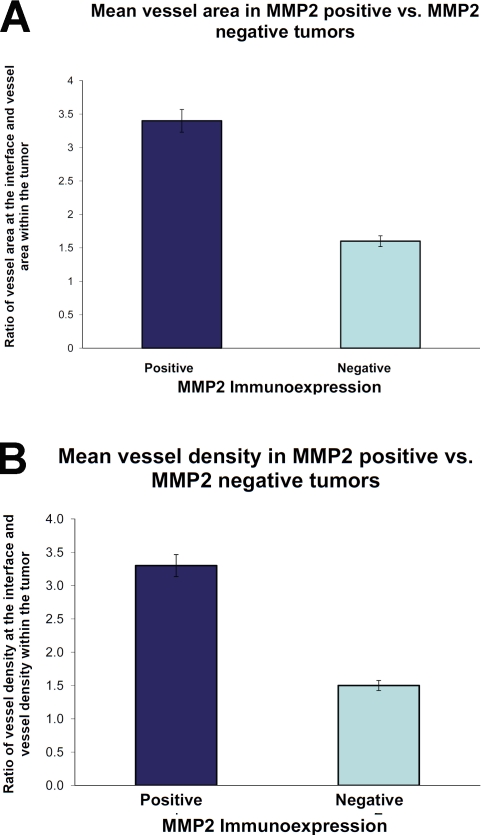
MMP2 positive tumors had a 1.7 fold higher MMVA ratio and 1.9 fold higher MMVN ratio at the CNS/tumor interface in comparison to MMP-2 negative neoplasms. The mean microvessel area (MMVA) was significantly higher in MMP2 positive tumors (3.14, range 1 - 12.4) in comparison to MMP2 negative tumors (1.8, range 0.1 - 7.8) (p = 0.0015) Figure 2a. In all cases examined, a higher vessel number was apparent at the tumor brain interface or the invasive edge of the tumor in MMP2-positive cases (mean 3.2, range 1.0-7.1) versus MMP-negative cases (mean 1.69, range 0.3-4.4), which was highly significant (p=<0.0001) (Figure 2b).
Figure 2.
The ratio of vascularity between “interface” and CNS metastasis expressed as mean microvessel area (a); and mean microvessel number in MMP2 positive and MMP2 negative metastatic lung adeno-carcinoma (b).
Discussion
The steps involved in establishment of metastasis include clonal selection of a metastatic sub-population, detachment of these cells from the primary tumor by a process of local invasion, tumor migration and penetration of blood and/ or lymphatic vessels to enter into the circulation. These cells are arrested at a distant site and must now extravasate from the circulation to penetrate the target tissue. Having passed through the barriers imposed by the circulatory system, the tumor cell now permeates through the extracellular matrix establishing itself as a colony where it must continue to proliferate [18]. A critical step in the establishment of metastasis at any site is the elaboration of a blood supply, typically derived from the host site. Tumor host interactions significantly modulate the process of metastasis, particularly by release and activation of extracellular matrix degrading enzymes and promotion of angiogenesis.
While a considerable body of evidence implicates MMPs and tissue inhibitors of matrix metalloproteinases (TIMPs) in cancer spread, there are a number of areas that remain unclear. MMP2 knockout mice have been shown to develop normally, displaying no apparent anatomic or vascular abnormalities; however, they exhibited reduced tumor growth and metastasis in a Lewis lung carcinoma and melanoma cell line [19]. Tumor induced angiogenesis in these animals was shown to be partly suppressed. These studies suggest a very interesting distinction in that the interactions and properties of MMPs in modulating angiogenesis in tumors may be quite different from their role during development.
The development and establishment of vascular supply is a key element of growth, maturation as well as maintenance of tissue under physiological conditions [20]. It appears reasonable that dissemination of tumor cells from a primary site may occur fairly early in the malignant process. However, the establishment of metastasis only appears to become biologically significant when the peripherally implanted cell colony establishes its own blood supply, derived from the host site [21]. A number of factors that stimulate vascular proliferation and vessel formation have been identified including acidic and basic fibroblast growth factor, transforming growth factor and TNF alpha [22]. Although tissue remodeling is a well recognized function of MMPs, the latter also interact with and are modulated by a network of cytokines and growth factors. Cytokines and growth factors bound to proteoglycans abound within the ECM and basement membrane. Enzymatic degradation of the ECM thus results in the release and diffusion of cytokines and growth factors as well as activation of ECM molecules important in tissue pathology [23]. The interactions of MMPs and the wide range of cytokines and growth factors present within the tumor microenvironment remain complex and are not fully understood. This study addresses just one of these intricate relationships.
The evidence that MMPs might have a role in angiogenesis emerged from observations that TIMPS as well as synthetic MMP inhibitors can greatly reduce the extent of endothelial cell tube formation in vitro [24-25]. Several MMPs like MMP1, MMP13, MMP3, MMP2, MMP9 and MT1-MMP have been shown to be produced by endothelial cells grown in vitro [25-27]. MMPs have been shown to promote endothelial cell migration and may also function to trigger the angiogenic switch. MMP9 brings about the release of VEGF from the matrix and it has been suggested that MMP2 activity is necessary for the switch to an angiogenic phenotype [28-29]. Tumor cells may act in a paracrine manner, providing the necessary cues required for endothelial cells to infiltrate the tumor, or vice versa. The activated endothelial cell secretes proteinases like MMPs to break down the basement membrane in order to embark upon its migration into the neoplasm. As recently reviewed [30], the new vessels derived from the processes of angiogenesis and vasculogenesis within the tumor are poorly formed, compromising their functional integrity. These structural and functional deficiencies facilitate transendo-thelial migration of tumor cells. This is manifest most significantly at the invasive margin or the tumor -stroma interface, predisposing to enhanced tumor kinetics [31]. Identification of the topographical distribution of functional activity of MMPs has been greatly enhanced by an array of high resolution imaging techniques. Elegant studies [32] using such methods have localized active MMP activity primarily at the tumorstroma interface in mouse xenograft models. Similarly, using “proteolytic beacons” Scherer et al [33] have shown enhanced MMP activity at the tumor edge in colon and pancreatic carcinoma models. Other studies have also utilized advanced fluorescence protocols to describe increased activity of MMP2, -9 and -14 at the tumor edge [34].
The current study seeks to further define the relationship between angiogenic profiles and metastatic carcinoma, particularly at the tumor's interface with normal brain. Our data shows that tumors expressing MMP2 have more proliferating vasculature at the tumor-brain interface than MMP2-negative tumors, implicating a role for MMP2 in stimulation of angiogenesis. The vasculature as defined by CD34 immunore-activity and by morphology ranges from single layer to multiple layered vessels, supported by smooth muscle/pericytes in some areas. As the tumor advances to invade the adjacent brain and elaborates new vasculature to sustain itself, it appears likely that MMP2 expression may be a key player in this process, enhancing both invasion and vascularization. While immunoexpression by itself may not define causality, the findings do support the higher likelihood of such a mechanism. In the context of the functions previously elucidated for matrix metallo-proteinases, our data corroborates the role of MMP2 in these events. It further substantiates that even in the CNS, where the microenvironment is unique in terms of the presence of the blood brain barrier and the absence of collagen in the matrix, this function of MMP2 likely holds true. The CNS remains a unique site for metastasis by virtue of its limited space and devastating clinical outcome. Understanding the biologic aspects of brain metastasis remains crucial for the development of novel therapeutic interventions that will eventually allow a better quality of life and reduced morbidity for affected patients.
References
- 1.Fanjul-Fernandez M, Folgueras AR, Cabrera S, Lopez-Otin C. Matrix metalloproteinases: Evolution, gene regulation and functional analysis in mouse models. Biochim Biophys Acta. 1803:3–19. doi: 10.1016/j.bbamcr.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 2.Page-McCaw A, Ewald AJ, Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol. 2007;8:221–233. doi: 10.1038/nrm2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shapiro SD. Matrix metalloproteinase degradation of extracellular matrix: biological consequences. Curr Opin Cell Biol. 1998;10:602–608. doi: 10.1016/s0955-0674(98)80035-5. [DOI] [PubMed] [Google Scholar]
- 4.Kahari VM, Saarialho-Kere U. Matrix metalloproteinases and their inhibitors in tumour growth and invasion. Ann Med. 1999;31:34–45. doi: 10.3109/07853899909019260. [DOI] [PubMed] [Google Scholar]
- 5.Kodate M, Kasai T, Hashimoto H, Yasumoto K, Iwata Y, Manabe H. Expression of matrix metalloproteinase (gelatinase) in T1 adenocarcinoma of the lung. Pathol Int. 1997;47:461–469. doi: 10.1111/j.1440-1827.1997.tb04525.x. [DOI] [PubMed] [Google Scholar]
- 6.Franchi A, Santucci M, Masini E, Sardi I, Paglierani M, Gallo O. Expression of matrix metalloproteinase 1, matrix metalloproteinase 2, and matrix metalloproteinase 9 in carcinoma of the head and neck. Cancer. 2002;95:1902–1910. doi: 10.1002/cncr.10916. [DOI] [PubMed] [Google Scholar]
- 7.Talvensaari-Mattila A, Paakko P, Hoyhtya M, Blanco-Sequeiros G, Turpeenniemi-Hujanen T. Matrix metalloproteinase-2 immunoreactive protea marker of aggressiveness in breast carcinoma. Cancer. 1998;83:1153–1162. doi: 10.1002/(sici)1097-0142(19980915)83:6<1153::aid-cncr14>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 8.Still K, Robson CN, Autzen P, Robinson MC, Hamdy FC. Localization and quantification of mRNA for matrix metalloproteinase-2 (MMP-2) and tissue inhibitor of matrix metalloproteinase2 (TIMP-2) in human benign and malignant prostatic tissue. Prostate. 2000;42:18–25. doi: 10.1002/(sici)1097-0045(20000101)42:1<18::aid-pros3>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 9.Westerlund A, Apaja-Sarkkinen M, Hoyhtya M, Puistola U, Turpeenniemi-Hujanen T. Gelatinase A-immunoreactive protein in ovarian lesions- prognostic value in epithelial ovarian cancer. Gynecol Oncol. 1999;75:91–98. doi: 10.1006/gyno.1999.5533. [DOI] [PubMed] [Google Scholar]
- 10.Bramhall SR, Stamp GW, Dunn J, Lemoine NR, Neoptolemos JP. Expression of collagenase (MMP2), stromelysin (MMP3) and tissue inhibitor of the metalloproteinases (TIMP1) in pancreatic and ampullary disease. Br J Cancer. 1996;73:972–978. doi: 10.1038/bjc.1996.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koshiba T, Hosotani R, Wada M, Miyamoto Y, Fujimoto K, Lee JU, Doi R, Arii S, Imamura M. Involvement of matrix metalloproteinase-2 activity in invasion and metastasis of pancreatic carcinoma. Cancer. 1998;82:642–650. doi: 10.1002/(sici)1097-0142(19980215)82:4<642::aid-cncr5>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 12.Liabakk NB, Talbot I, Smith RA, Wilkinson K, Balkwill F. Matrix metalloprotease 2 (MMP-2) and matrix metalloprotease 9 (MMP-9) type IV collagenases in colorectal cancer. Cancer Res. 1996;56:190–196. [PubMed] [Google Scholar]
- 13.Nomura H, Fujimoto N, Seiki M, Mai M, Okada Y. Enhanced production of matrix metalloproteinases and activation of matrix metalloproteinase 2 (gelatinase A) in human gastric carcinomas. Int J Cancer. 1996;69:9–16. doi: 10.1002/(SICI)1097-0215(19960220)69:1<9::AID-IJC3>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 14.Sier CF, Kubben FJ, Ganesh S, Heerding MM, Griffioen G, Hanemaaijer R, van Krieken JH, Lamers CB, Verspaget HW. Tissue levels of matrix metalloproteinases MMP-2 and MMP-9 are related to the overall survival of patients with gastric carcinoma. Br J Cancer. 1996;74:413. doi: 10.1038/bjc.1996.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shima I, Sasaguri Y, Kusukawa J, Yamana H, Fujita H, Kakegawa T, Morimatsu M. Production of matrix metalloproteinase-2 and metalloproteinase-3 related to malignant behavior of esophageal carcinoma. A clinicopathologic study. Cancer. 1992;70:2747–2753. doi: 10.1002/1097-0142(19921215)70:12<2747::aid-cncr2820701204>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 16.Davidson B, Goldberg I, Kopolovic J, Lerner-Geva L, Gotlieb WH, Ben-Baruch G, Reich R. MMP2 and TIMP-2 expression correlates with poor prognosis in cervical carcinoma–a clinicopathologic study using immunohistochemistry and mRNA in situ hybridization. Gynecol Oncol. 1999;73:372–382. doi: 10.1006/gyno.1999.5381. [DOI] [PubMed] [Google Scholar]
- 17.Talvensaari-Mattila A, Paakko P, Turpeenniemi-Hujanen T. MMP-2 positivity and age less than 40 years increases the risk for recurrence in premenopausal patients with node-positive breast carcinoma. Breast Cancer Res Treat. 1999;58:287–293. doi: 10.1023/a:1006326513176. [DOI] [PubMed] [Google Scholar]
- 18.Stetler-Stevenson WG, Aznavoorian S, Liotta LA. Tumor cell interactions with the extracellular matrix during invasion and metastasis. Annu Rev Cell Biol. 1993;9:541–573. doi: 10.1146/annurev.cb.09.110193.002545. [DOI] [PubMed] [Google Scholar]
- 19.Itoh T, Tanioka M, Yoshida H, Yoshioka T, Nishimoto H, Itohara S. Reduced angiogenesis and tumor progression in gelatinase A-deficient mice. Cancer Res. 1998;58:1048–1051. [PubMed] [Google Scholar]
- 20.Folkman J, Klagsbrun M. Angiogenic factors. Science. 1987;235:442–447. doi: 10.1126/science.2432664. [DOI] [PubMed] [Google Scholar]
- 21.Winlaw DS. Angiogenesis in the pathobiology and treatment of vascular and malignant diseases. Ann Thorac Surg. 1997;64:1204–1211. doi: 10.1016/s0003-4975(97)00716-9. [DOI] [PubMed] [Google Scholar]
- 22.Noel A, Jost M, Maquoi E. Matrix metalloproteinases at cancer tumor-host interface. Semin Cell Dev Biol. 2008;19:52–60. doi: 10.1016/j.semcdb.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 23.McCawley LJ, Matrisian LM. Matrix metalloproteinases: they're not just for matrix anymore! Curr Opin Cell Biol. 2001;13:534–540. doi: 10.1016/s0955-0674(00)00248-9. [DOI] [PubMed] [Google Scholar]
- 24.Montesano R, Orci L. Tumor-promoting phorbol esters induce angiogenesis in vitro. Cell. 1985;42:469–477. doi: 10.1016/0092-8674(85)90104-7. [DOI] [PubMed] [Google Scholar]
- 25.Schnaper HW, Kleinman HK. Regulation of cell function by extracellular matrix. Pediatr Nephrol. 1993;7:96–104. doi: 10.1007/BF00861587. [DOI] [PubMed] [Google Scholar]
- 26.Cornelius LA, Nehring LC, Roby JD, Parks WC, Welgus HG. Human dermal microvascular endothelial cells produce matrix metalloproteinases in response to angiogenic factors and migration. J Invest Dermatol. 1995;105:170–176. doi: 10.1111/1523-1747.ep12317080. [DOI] [PubMed] [Google Scholar]
- 27.Lewalle JM, Munaut C, Pichot B, Cataldo D, Baramova E, Foidart JM. Plasma membrane-dependent activation of gelatinase A in human vascular endothelial cells. J Cell Physiol. 1995;165:475–483. doi: 10.1002/jcp.1041650305. [DOI] [PubMed] [Google Scholar]
- 28.Bergers G, Brekken R, McMahon G, Vu TH, Itoh T, Tamaki K, Tanzawa K, Thorpe P, Itohara S, Werb Z, Hanahan D. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol. 2000;2:737–744. doi: 10.1038/35036374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fang J, Shing Y, Wiederschain D, Yan L, Butterfield C, Jackson G, Harper J, Tamvakopoulos G, Moses MA. Matrix metalloproteinase-2 is required for the switch to the angiogenic phenotype in a tumor model. Proc Natl Acad Sci U S A. 2000;97:3884–3889. doi: 10.1073/pnas.97.8.3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141:52–67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Padera TP, Kadambi A, di Tomaso E, Carreira CM, Brown EB, Boucher Y, Choi NC, Mathisen D, Wain J, Mark EJ, Munn LL, Jain RK. Lymphatic metastasis in the absence of functional intratumor lymphatics. Science. 2002;296:1883–1886. doi: 10.1126/science.1071420. [DOI] [PubMed] [Google Scholar]
- 32.Olson ES, Aguilera TA, Jiang T, Ellies LG, Nguyen QT, Wong EH, Gross LA, Tsien RY. In vivo characterization of activatable cell penetrating peptides for targeting protease activity in cancer. Integr Biol (Camb) 2009;1:382–393. doi: 10.1039/b904890a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scherer RL, McIntyre JO, Matrisian LM. Imaging matrix metalloproteinases in cancer. Cancer Metastasis Rev. 2008;27:679–690. doi: 10.1007/s10555-008-9152-9. [DOI] [PubMed] [Google Scholar]
- 34.Packard BZ, Artym VV, Komoriya A, Yamada KM. Direct visualization of protease activity on cells migrating in three-dimensions. Matrix Biol. 2009;28:3–10. doi: 10.1016/j.matbio.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]