Research highlights
▸ Adolescents show increased amygdala activity when encoding positive stimuli compared to adults. ▸ No group differences in brain activity were present during encoding of negative stimuli. ▸ Results have implications for the increased reward and risk taking behavior during adolescence.
Keywords: Adolescents, Adults, Encoding, Positive stimuli, Amygdala, Hippocampus
Abstract
While studies among adults implicate the amygdala and interconnecting brain regions in encoding emotional stimuli, few studies have examined whether developmental changes occur within this emotional-memory network during adolescence. The present study examined whether adolescents and adults differentially engaged the amygdala and hippocampus during successful encoding of emotional pictures, with either positive or negative valence. Eighteen adults and twelve adolescents underwent event-related fMRI while encoding emotional pictures. Approximately 30 min later, outside the scanner, subjects were asked to recall the pictures seen during the scan. Age group differences in brain activity in the amygdala and hippocampus during encoding of the pictures that were later successfully and unsuccessfully recalled were separately compared for the positive and negative pictures. Adolescents, relative to adults, demonstrated enhanced activity in the right amygdala during encoding of positive pictures that were later recalled compared to not recalled. There were no age group differences in amygdala or hippocampal activity during successful encoding of negative pictures. The findings of preferential activity within the adolescent right amygdala during successful encoding of positive pictures may have implications for the increased reward and novelty seeking behavior, as well as elevated rates of psychopathology, observed during this distinct developmental period.
1. Introduction
Animal and adult human data indicate that the amygdala plays a critical role in the formation of implicit and explicit emotional memories. In adult humans, implicit memory studies using fear conditioning paradigms report increased amygdala activity during fear acquisition even when the stimuli are presented outside of awareness (Büchel et al., 1998, Davis and Whalen, 2001, LaBar et al., 1998, Maren, 2001). Studies of explicit memory show enhanced amygdala activity during successful encoding of highly arousing stimuli, such as negative pictures (Dolcos et al., 2004, Hamann et al., 1999, Kensinger and Schacter, 2006), words (Kensinger and Schacter, 2006, Strange and Dolan, 2004), film clips (Cahill et al., 1996, Kilpatrick and Cahill, 2003) and scenes (Canli et al., 2000). Enhanced amygdala activity is also present during encoding of positive stimuli, thereby delineating a broader role for this region in encoding arousing stimuli (Hamann et al., 1999, Kensinger and Schacter, 2006).
The amygdala modulates explicit long term memory for emotionally arousing material primarily through its interactions with other brain structures, notably the hippocampus (Cahill et al., 1995, Cahill et al., 1996, Cahill and McGaugh, 1998, Hamann et al., 1999, McGaugh, 1992, McGaugh, 2004, Packard and Teather, 1998, Phelps, 2004, Richardson et al., 2004). Animal data reveal strong reciprocal connections between the amygdala and hippocampus (e.g., McGaugh, 2000, McGaugh et al., 1996, McGaugh et al., 2002, Pikkarainen et al., 1999, Pitkänen et al., 2000) that enable the amygdala to strengthen synapses within the hippocampus to facilitate learning (Akirav and Richter-Levin, 1999). Human studies data also suggest that coupled activation of the amygdala and hippocampus is necessary for successful encoding of emotional stimuli (Hamann et al., 1999, Kensinger et al., 2002, Richardson et al., 2004).
The emotion processing literature indicates that the adult amygdala responds to a wide variety of positive and negative stimuli (see reviews by Phan et al., 2002 and Sergerie et al., 2008), whereas studies of amygdala responses in youth have predominantly focused on examining responses to faces with different emotional valences (e.g., Baird et al., 1999). Responses to facial expressions may vary across childhood due to changes in brain structure and function. Adolescence in particular is characterized by significant brain reorganization, heightened emotionality, reward seeking, and risk taking, as well as a dramatic increase in rates of psychiatric disorders such as depression, anxiety, and substance abuse (Angold et al., 1998, Ernst and Fudge, 2009, Lewinsohn et al., 1993). These changes may contribute to distinct responses to emotional stimuli. Several neurobiological models of adolescent brain development have been proposed to explain the metamorphosis that occurs during this period. The primary hypothesis across these models is that adolescence is characterized by an imbalance between regulatory systems involving the prefrontal cortex and emotional systems involving subcortical structures such as the amygdala (Casey et al., 2010, Ernst et al., 2005b, Ernst and Fudge, 2009). Such an imbalance is speculated to arise from slower maturation of the prefrontal cortex relative to subcortical structures resulting in less control over emotional behavior, and may give rise to distinct responses to affective stimuli, such as enhanced sensitivity to reward.
In humans, amygdala responses to fearful stimuli change from childhood to adulthood. For example, adolescents exhibit stronger amygdala responses to fearful faces and weaker functional connectivity between the amygdala and hippocampus compared to adults (Guyer et al., 2008, McClure et al., 2004, Monk et al., 2003). Another study showed enhanced amygdala responses to fearful faces in adults and neutral faces in adolescents, which may have been perceived as ambiguous (Thomas et al., 2001). Differential lateralization of amygdala responses to fearful faces is also present, with bilateral activity present in children and adults but only right-sided activity in adolescents (Killgore and Yurgelun-Todd, 2004). There are no developmental differences in the response to happy faces (Guyer et al., 2008, Monk et al., 2003). However, more research is needed in this area as amygdala responses may vary for different types of stimuli as well as cognitive processes.
Only one study has examined developmental differences in the neural circuits mediating successful memory for emotional stimuli (Nelson et al., 2003). This study used fMRI and a subsequent memory paradigm that presented facial expressions with different emotions during encoding and then administered a postscan recognition task 20–40 min later. The fMRI data were subsequently sorted according to whether pictures were correctly recalled (successful encoding) or not recalled. Results showed age group differences in neural activity during encoding depending on the face emotion type; none of these group differences arose in the amygdala, however.
Similar to Nelson et al. (2003), the current study also uses fMRI and a subsequent memory paradigm but with some differences in the methods. The stimuli are non-facial pictures, instead of facial expressions, with positive and negative valence, and the postscan memory test is a recall as opposed to recognition task. Adolescent data on the neural responses to non-facial emotive stimuli are sparse but much needed, as pictures represent events and stimuli that individuals encounter on a daily basis. Moreover, neural responses to evocative faces and pictures may vary across development. The study also used a recall task since it would be more likely to ensure stronger representations of the successfully encoded pictures. Finally, the study focused on examining encoding differences in the amygdala and hippocampus because of their coordinated role in the encoding of emotional stimuli. The findings from this study are important for understanding the cognitive and neural factors that contribute to emotional memory across development, and for pursuing studies of dysfunctional emotional memory circuitry in psychiatric disorders.
Our hypotheses regarding age group differences in encoding of the emotional pictures diverged based on data from the emotion processing literature. For the negative pictures, we expected that adolescents, relative to adults, would exhibit greater amygdala and hippocampal activity during successful encoding based on preliminary data indicating stronger amygdala responses to fearful stimuli in adolescents relative to adults (Guyer et al., 2008, Killgore and Yurgelun-Todd, 2004, Monk et al., 2003). During encoding of successfully recalled positive pictures, no age group differences in amygdala-hippocampal responses were expected given the absence of this finding in previous studies.
2. Methods
2.1. Subjects
Thirty-three subjects (19 adults and 14 adolescents) were enrolled in the study. Subjects were recruited from the Washington, D.C. area through flyers and other advertisements seeking healthy volunteers. Exclusion criteria were assessed during in-person, face-to-face structured interviews performed by experienced clinicians. These were performed prior to enrollment and screened for the presence of psychiatric and medical illness, substance abuse, head trauma, and neurological disorders. Written consent was obtained from all participants. For subjects younger than 18 years, written consent was obtained from the parent or guardian. As part of the consent process, parents were allowed to inspect all material used in this study to ensure that it was considered tolerable for their adolescents. Subjects were financially compensated for their participation following the National Institutes of Health (NIH) guidelines. All experimental protocols were approved by the institutional review board at the NIMH.
Only subjects without any psychopathology were included in the study. All subjects were screened for psychopathology, using the Kiddie-Schedule for Affective Disorders and Schizophrenia (K-SADS; Kaufman et al., 2000) for adolescents and the Structured Clinical Interview for DSM Disorders (SCID; Spitzer et al., 1992) for adults. All subjects had an IQ greater than 85, as determined by the Wechsler Abbreviated Scale of Intelligence (WASI; Psychological Corporation, 1999).
Three subjects were excluded from the imaging analyses. One adolescent subject did not recall any pictures (positive or negative) in the memory task and two additional subjects (1 adult and 1 adolescent) exhibited excessive motion in the scanner. As these were the only exclusions that applied to imaging analyses, the final sample for fMRI data consisted of 30 subjects, 18 adults [mean age = 30.22 years (SD = 6.0, range = 22.0–44.6 years); mean IQ = 114.56 (SD = 8.94, range = 105–136); 7 females; 78.6% right handed] and 12 adolescents [mean age = 14.95 years (SD = 1.6, range = 12.0–17.4 years); mean IQ = 113.63 (SD = 13.29, range = 95–136); 5 females; 87.5% right handed].
Additionally, certain subjects were excluded from select analyses of the behavioral but not imaging data. One adolescent did not recall any positive pictures. This subject was excluded only from the analyses of recall data for the positive pictures, resulting in a sample size of 18 adults and 11 adolescents for this data category. Since this subject still contributed to memory recall data for negative pictures, the sample size for analyses of the recalled negative pictures remained 18 adults and 12 adolescents. For two adults and one adolescent, subjective valence and arousal ratings were not recorded during scanning due to technical malfunction; these subjects were excluded only from analyses of the subjective emotional ratings, but not the fMRI and memory recall analyses. Thus for subjective rating analyses alone, the final sample consisted of 16 adults and 11 adolescents.
2.2. Emotional memory task
The task included a total of 160 stimulus trials that were divided into four 4.5 min runs with 32 pictures (positive, negative and neutral valence) and eight baseline fixation cross trials presented in each run; 10 to 11 pictures within each emotion category were presented in each run. The 32 pictures for each subject were randomly selected from a larger bank of 98 pictures. The stimuli were color photographs taken from the International Affective Picture System (IAPS; Lang et al., 1997), an established stimulus set with normative data on arousal and valence ratings (Bradley et al., 1992, Greenwald et al., 1989). The pictures were the size of the entire field of view for the Avotec Vision Goggles in the fMRI scanner. Selection of the emotional and neutral pictures was based on McManis et al. (2001). Sample pictures in the emotional and neutral categories were as follows: positive (attractive animals, food), negative (scary animals, weapons), neutral (household objects, appliances). Appendix A lists the specific IAPS picture numbers that were included in the task.
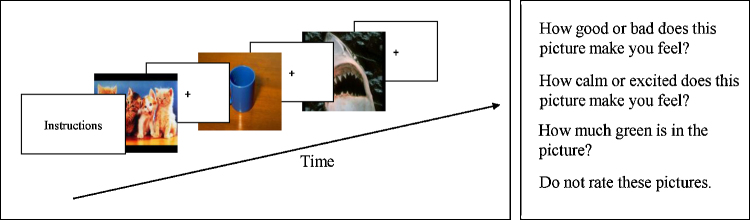
We considered various techniques for constraining attention during encoding based on prior research using the subsequent memory paradigm in children and adolescents (e.g., Nelson et al., 2003, Pine et al., 2004, Roberson-Nay et al., 2006). This prior work suggested that the advantages of having subjects rate each stimulus on different features at each viewing outweighed the disadvantages of not knowing precisely when each recalled stimulus was encoded (see discussion in Roberson-Nay et al., 2006). With this paradigm, subjects viewed each of the 32 pictures a total of four times, once in each of the four runs. Each picture was viewed under a different viewing instruction: 1) passive viewing of the picture, 2) rate the valence (“How good or bad does the picture make you feel?”), 3) rate the arousal (“How calm or excited does the picture make you feel?”), 4) rate the greenness level (“How much green is in the picture?”). This type of design directed subjects’ attention to different aspects of the pictures across the different viewing conditions, thereby facilitating encoding success by reducing boredom associated with repeated viewing of the same pictures and maximizing memory recall. Subjects made their ratings using the Self Assessment Manikin (SAM), an established rating scale for assessing valence and arousal (Lang, 1980). The SAM displays a row of five manikins with increasing expressions of pleasure (1 = very bad, 5 = very good) or arousal (1 = very calm, 5 = very excited). The scale was modified to measure increasing amounts of green (1 = no green to 5 = very much green). Ratings were made using a five-key response box.
The order of viewing conditions and the presentation of the 32 emotional and neutral pictures were randomized in each run for each subject. Additionally, 8 fixation trials of the same duration as memory-encoding trials were randomly interspersed in each run. This design, whereby fixation events appear randomly embedded within a task, has been used frequently in other studies of development (Simmonds et al., 2007, Suskauer et al., 2008a, Suskauer et al., 2008b), including studies using the subsequent-memory paradigm (Guyer et al., 2008, Nelson et al., 2003). This design ensures that picture viewing occurs at a randomly varying time points across the dynamic range of hemodynamic response functions (HRF). This design feature enables successful deconvolution of the overlapping HRFs during the encoding phase for distinct classes of picture events (e.g., successfully or unsuccessfully remembered pictures of one or another valence). This design has come to be known as the “rapid event-related design,” as originally developed by Friston et al. (1999).
Trials were presented in attention blocks. Each attention block started with the presentation of a rating instruction screen for 3 s followed by 5 trials. Four of the five trials in each block were picture trials (one from each emotion category and one randomly selected) and one was a fixation cross or null event. Each trial consisted of a 3 s display of an image followed by a 3 s fixation cross. While viewing the picture, subjects were required to think about their rating and told to perform their rating during the fixation cross that followed each picture. The same instruction screen was presented twice consecutively and each time was followed by five trials. A different instruction screen would then appear and was presented for two consecutive blocks of five trials. This pattern was repeated until 40 stimuli in each run were presented (see Fig. 1).
Fig. 1.
fMRI task design and stimuli. One of the four rating conditions listed to the right was presented on the instruction screen (3 s) after which 5 trials that consisted of either picture trials or a fixation cross were presented (3 s each). Each picture was followed by a fixation cross (3 s) at which time the subject made his or her rating.
Approximately 20–40 min after the fMRI task, subjects were administered a surprise free recall task outside the scanner. Subjects were not told there would be a memory task after the scan in order to minimize the possibility that each subject might adopt different mnemonic strategies during the task. The reliance on implicit rather than explicit encoding strategies also created a situation more similar to the encoding of affective stimuli in everyday life. During the delay period between the functional scan and the surprise memory test, subjects received a structural brain scan and completed research questionnaires pertaining to the current as well as other studies within the lab. Procedures during the delay period were standardized across all subjects in order to minimize delay variability. When the research questionnaires were completed, subjects were spontaneously told that within the next few seconds, they would be asked to verbally recall as many pictures as possible that were seen in the scanner. Subject responses during the free recall task were hand recorded by the experimenter. Subjects were given 90 s for the recall task but most subjects stopped generating items before the procedure was over. This free recall procedure was based on the findings of Pine et al. (2002), which showed developmental differences in memory using a timed recall task and fMRI.
The memory recall data were used to segregate fMRI scanning data according to whether stimuli viewed in each emotion category were correctly recalled or not recalled. This analytic strategy allowed us to identify brain regions that were more active when viewing the most salient stimuli. Accordingly, six regressors were modeled that reflected brain activity during scanning according to recall status: recalled positive pictures, not recalled positive pictures, recalled negative pictures, not recalled negative pictures, recalled neutral pictures, and not recalled neutral pictures.
As noted above, the current task design was adapted from subsequent memory paradigms used in prior behavioral and fMRI studies that have reported age-related differences in memory for emotional faces in healthy and clinically depressed adolescents (Nelson et al., 2003, Pine et al., 2004, Roberson-Nay et al., 2006). Adult studies have also used similar paradigms to examine encoding processes for words (Alkire et al., 1998, Buckner et al., 2001, Wagner et al., 1998) as well as emotional and non-emotional scenes (Brewer et al., 1998, Canli et al., 2000).
2.3. Image acquisition
Functional magnetic resonance images were acquired on a General Electric Signa 3Tesla magnet at NIMH. Head movement was restricted by the use of foam padding, and subjects viewed visual images through Avotec Silent Vision Glasses (Stuart, FL) located directly above their eyes.
Gradient echo planar images (EPI) were acquired using echoplanar single shot gradient echo T2* weighting after sagittal localization and a manual shim procedure. EPI images were acquired contiguously in twenty-three 5 mm axial slices per brain volume, positioned parallel to the AC-PC line with the following scan parameters: matrix = 64 mm × 64 mm; TR = 2000 ms; TE = 40 ms; FOV (field of view) = 240 mm; voxels were 3.75 mm × 3.75 mm × 5 mm. After EPI images were acquired, a high resolution T1 weighted anatomical image was acquired to facilitate spatial normalization. Scan parameters for the anatomic scan were: 180 one mm sagittal slices; FOV = 256; NEX = 1; TR = 11.4 ms; TE = 4.4 ms; matrix = 256 × 256; TI = 300 ms; bandwidth = 130 Hz/pixel; 33 kHz/256 pixels.
2.4. Data analysis
2.4.1. Behavioral data
The picture recall data and the subjective rating (valence, arousal) data were examined with repeated measures ANOVA. To examine age-related differences in the picture recall data, a 2 (age group: adolescents, adults) × 3 (picture valence: negative, positive, neutral) ANOVA was performed. Since the number of pictures for each picture valence was either 10 or 11, the proportion of recalled stimuli for each picture valence was calculated and entered into the ANOVA. Age group differences in the subjective valence and arousal ratings were each analyzed separately using a 2 (age group) × 3 (picture valence) × 2 (recall status: recalled, not recalled) ANOVA. Significant main effects and interactions were followed up with paired t-tests. Cohen's d values were calculated to determine the effect size of the comparisons.
2.4.2. Neuroimaging data
Functional MRI preprocessing and data analysis were carried out with SPM5 (Wellcome Department of Imaging Neuroscience, 2005). Functional data were corrected for slice timing, motion corrected by realignment to the first volume for each subject, and coregistered with the anatomical data. Subjects with greater than 3 mm of motion in any direction were excluded. Normalization was performed through the unified segmentation routine in SPM5 (Ashburner and Friston, 2005) using the subject's anatomical image, after which the segmentation parameters were applied to the EPI images. This step was followed by smoothing the images with an 8-mm FWHM Gaussian kernel.
At the individual subject level, event-related response amplitudes were estimated using the General Linear Model (GLM) for four regressors of interest: 1) recalled positive pictures, 2) not recalled positive pictures, 3) recalled negative pictures, and 4) not recalled negative pictures. The neutral pictures were modeled as a single regressor of no interest because five subjects (3 adults, 2 adolescents) recalled no neutral pictures, which compromised power to study memory for the neutral pictures. Since the focus of this study was memory and not attention, the four regressors of interest as well as the neutral regressor were created by collapsing events for each regressor across the four different viewing conditions. Two additional regressors of no interest were modeled, the instruction screens and the button press during the behavioral response. The fMRI response to each event type was modeled as a rectangular pulse (of 3 s duration) convolved with the hemodynamic response function.
Since we entered the study with a priori hypotheses regarding the amygdala and hippocampus, we conducted a region of interest (ROI) analysis using the MarsBar ROI toolbox for SPM (http://marsbar.sourceforge.net). MarsBar was used to the extract mean contrast value (i.e., the mean difference in beta values) across all voxels within a particular ROI. Brain activity during encoding was examined within four ROIs: the right and left amygdalae, and the right and left hippocampi. These regions were defined using standard anatomical criteria on a single MNI template and applied to normalized brains at the group level (Nelson et al., 2003, Szeszko et al., 1999, Szeszko et al., 2002). Given prior data on laterality of amygdala functions, we examined age group differences separately in the right and left amygdala and hippocampus. For each subject, response was contrasted between viewing of recalled pictures and baseline fixation trials, and between viewing of not recalled stimuli and baseline fixation trials, within each ROI. These contrasts were performed for the positive and negative pictures separately. Mean contrast values were entered into SPSS and subjected to repeated measures analysis of variance (ANOVA).
Based on our valence-specific hypotheses, age group differences in brain activity during encoding of the positive and negative pictures were examined separately rather than comparing encoding of the negative versus positive pictures to each other. For each picture valence, we performed a 2 (age group) × 2 (recall, not recall) repeated measures ANOVA on the mean contrast values for each of the four ROIs. As such, we performed a total of 8 ANOVAs, four examining age group differences in ROI activity during successful encoding of negative stimuli and the other four examining age group differences in ROI activity during successful encoding of the positive stimuli. These ANOVAs allowed us to identify the brain regions in which age group differences in activity for recalled and not recalled stimuli were present for each valence category.
Due to the relatively low number of recalled pictures, our primary analyses used a threshold of two or more pictures recalled in each valence category (positive recalled pictures: 14 adults, 11 adolescents; negative pictures: 16 adults, 12 adolescents). If group differences in ROI activity were present for either the positive or negative recalled pictures, we repeated our analyses using more stringent recall criteria, i.e., a cutoff of three or more recalled stimuli (positive: 10 adults, 9 adolescents; negative: 15 adults, 11 adolescents), and report those data after reporting the results of the primary analyses.
According to our hypotheses, we expected a significant group × recall interaction for the mean contrast values in each of the four ROIs during encoding of the negative stimuli; this interaction would result from adolescents demonstrating greater mean contrast values during successful encoding of negative pictures compared to adults in each of the ROIs. For the positive pictures, no group × recall status interactions were expected in any of the ROIs. Consistent with our age group predictions, significant interactions in any ROI were probed with independent t-tests comparing between-group differences in the mean contrast values for recalled and not recalled stimuli separately for the positive and negative pictures. If a significant interaction could not be accounted for by any age group differences, no further t-tests were performed. Cohen's d was calculated to determine the effect size of the comparisons.
As a secondary analysis, we examined whether subjective arousal for the positive and negative pictures correlated with amygdala activity. These analyses may provide preliminary insights on brain behavior relationships pertaining to responses to emotionally evocative pictures.
For all analyses, an alpha level of 0.05 was considered statistically significant. It is important to note that the mean contrast values, which represent the average BOLD signal across each ROI, were employed in these analyses. As a result, these analyses are not based on multi-voxel tests. A Bonferroni correction was not applied to the ANOVAs due to the exploratory nature of the study.
3. Results
3.1. Behavioral data
3.1.1. Memory recall performance
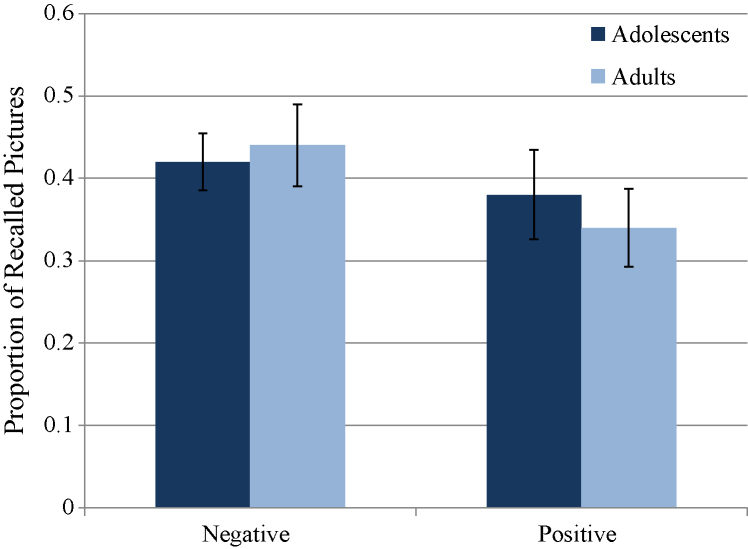
The recall data are presented in Fig. 2. A 2 (age group) × 2 (picture valence) ANOVA was performed to examine group differences in the proportion of recalled pictures for each picture valence (positive, negative, neutral). Results showed no main effects or interactions involving the factor of age. There was a main effect of picture valence [F(2,56) = 15.20, p < 0.001], with paired t-tests showing that subjects recalled more negative pictures than positive [t(29) = 2.65, p = 0.013, d = 0.38] or neutral [t(29) = 5.43, p < 0.001, d = 1.30] pictures. Significantly more positive pictures were recalled than neutral pictures [t(29) = 3.06, p = 0.005, d = 0.84]. These data indicate that any potential developmental differences in the fMRI data were not the result of differences in behavioral performance.
Fig. 2.
Proportion of pictures recalled 20–40 min after encoding during the fMRI scan*.
*For the positive and negative pictures, the data represent the proportion of stimuli recalled out of 11 pictures in each category. The neutral recall data represent the proportion of stimuli recalled out of 10 neutral pictures. Error bars represent the standard error.
3.1.2. Subjective emotional ratings
The mean subjective valence and arousal ratings of the pictures made during encoding are presented in Table 1. A 2 (age group) × 2 (picture valence) × 2 (recall status) ANOVA on the subjective valence ratings indicated no main effects or interactions involving the age group or recall status factors. There was a main effect of picture valence [F(2,36) = 90.71, p < 0.001] indicating that subjects provided higher subjective valence ratings for positive than negative [t(26) = 14.06, p < 0.001, d = 3.05] and neutral [t(26) = 14.34, p < 0.001, d = 1.69] pictures. Neutral pictures were rated significantly higher than negative pictures [t(26) = 4.89, p < 0.001, d = 1.49].
Table 1.
Mean subjective valence and arousal ratingsa of the pictures made by adolescents (n = 11) and adults (n = 16) during fMRI scanning.
| Positive |
Negative |
Neutral |
||||
|---|---|---|---|---|---|---|
| Recalled | Not recalled | Recalled | Not recalled | Recalled | Not recalled | |
| Valence | ||||||
| Adolescent | 4.28 (1.00) | 3.99 (0.55) | 1.83 (0.76) | 1.92 (0.55) | 2.79 (0.40) | 2.56 (0.67) |
| Adult | 3.88 (0.68) | 4.02 (0.30) | 1.58 (0.47) | 1.89 (0.61) | 2.60 (0.79) | 2.50 (0.41) |
| Arousal | ||||||
| Adolescent | 2.44 (1.16) | 2.70 (0.98) | 2.48 (1.20) | 2.64 (1.07) | 1.25 (0.46) | 1.32 (0.49) |
| Adult | 3.10 (0.93) | 2.99 (0.90) | 3.64 (1.13) | 3.33 (1.02) | 1.35 (0.50) | 1.56 (0.46) |
Ratings ranged from 1 to 5 for valence (1 = very bad to 5 = very good) and arousal (1 = very calm to 5 = very excited).
We performed a similar age group × picture valence × recall status ANOVA on the subjective arousal ratings and also found no main effects or interactions involving the age group or recall status factors. A main effect of picture valence [F(2,36) = 16.53, p < 0.001] was present with t-tests showing significantly higher arousal ratings for the negative [t(24) = 4.63, p < 0.001, d = 1.92] and positive [t(24) = 7.04, p < 0.001, d = 1.80] pictures compared to neutral pictures. Positive and negative picture arousal ratings were not significantly different (p = 0.70).
3.2. Neuroimaging data
A total of eight 2 (age group) × 2 (recall, not recall) repeated measure ANOVAs were performed, four examining group × recall status interactions for the negative stimuli in each of the four ROIs and the other four examining group × recall status interactions for the positive pictures in each ROI.
3.2.1. Group × recall status interactions for the negative stimuli
There were no significant age group × recall status interactions in the right amygdala [F(1, 26) = 0.14, p = 0.72], left amygdala [F (1, 26) = 1.02, p = 0.32], right hippocampus [F(1, 26) = 0.40, p = 0.53], or left hippocampus [F(1, 26) = 0.52, p = 0.48]. These data indicate that contrary to our hypotheses, adolescents and adults did not differ in the degree of amygdala and hippocampal activity engaged during successful encoding of negative pictures.
3.2.2. Group × recall status interactions for the positive stimuli
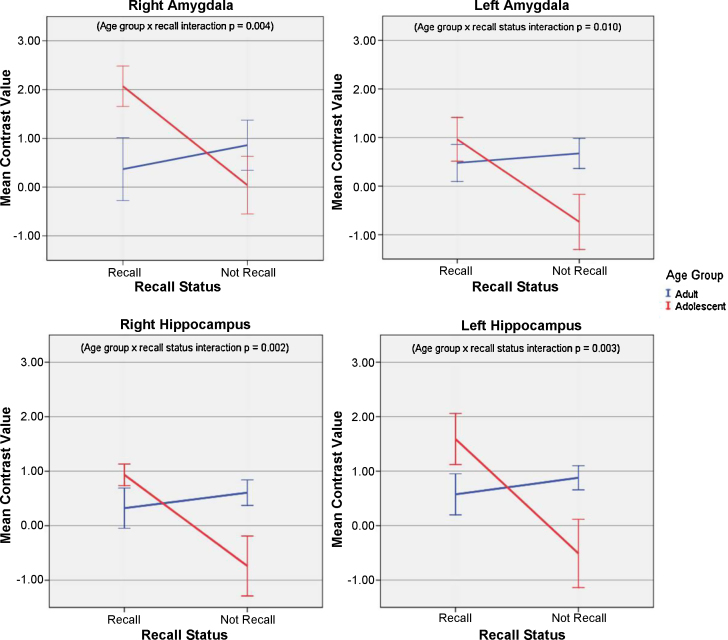
Fig. 3 displays the mean contrast values in each of the four ROIs for the positive recalled and not recalled stimuli for each age group. Significant age group × recall interactions were present in all four ROIs: right amygdala [F(1, 23) = 10.17, p = 0.004], left amygdala [F(1, 23) = 7.86, p = 0.01], right hippocampus [F(1, 23) = 12.21, p = 0.002], and left hippocampus [F(1, 23) = 10.96, p = 0.003].
Fig. 3.
Results of the age group × recall status repeated measures ANOVA on the mean contrast values* in the right and left amygdala and hippocampus during encoding of the positive pictures.
*Mean contrast values represent the average regional activity during viewing of successfully recalled and not recalled positive pictures. Error bars represent the standard error. Significant interactions were present in all four regions of interest.
Table 2 presents the results of the follow-up independent t-tests comparing the mean contrast values for the recalled positive stimuli for adolescents and adults. Results showed that the mean contrast value for positive recalled stimuli was significantly greater in adolescents compared to adults in the right amygdala [t(23) = 2.36, p = 0.027, d = 0.98]. In the left amygdala, right hippocampus, and left hippocampus, there were no significant differences in the mean contrast value for recalled positive stimuli between adolescents and adults, although the mean contrast values were higher for recalled positive stimuli than not recalled positive stimuli in each of these regions. These findings contradict our initial hypotheses. Whereas we originally predicted no age group differences in brain activity during successful encoding of positive stimuli, our findings indicate that adolescents demonstrate enhanced activity, relative to adults, within the right amygdala during successful encoding of positive stimuli.
Table 2.
Significant results from the independent t-tests comparing age group differences in ROI activity during encoding of successfully recalled positive pictures.
| t-value | p-value | Cohen's d | |
|---|---|---|---|
| Right amygdalaa | 2.36 | 0.027 | 0.98 |
| Left amygdala | 0.72 | 0.48 | 0.29 |
| Right hippocampus | 1.49 | 0.15 | 0.62 |
| Left hippocampus | 1.38 | 0.18 | 0.55 |
Adolescents exhibited greater right amygdala activity during encoding of successfully recalled positive pictures compared to adults.
Significant age group × recall interactions for the positive pictures were also present in the right amygdala [F(1, 17) = 7.95, p = 0.012] when the 3+ recall cutoff criteria were applied. Follow-up independent t-tests for the 3+ cutoff values revealed the same findings as for the primary analyses, i.e., adolescents exhibited greater mean contrast values compared to adults during encoding of the recalled positive stimuli in the right amygdala [t(17) = 2.30, p = 0.034, d = 1.07].
3.2.3. Secondary analyses
Bivariate correlations were performed to examine whether subjective arousal ratings correlated with amygdala activity for each picture type. For this analysis, amygdala activity was calculated as the average contrast value within the region across the four viewing conditions. Significant positive correlations were observed in the left amygdala for the negative stimuli, i.e., greater subjective arousal for all negative pictures was associated with greater amygdala activity in the left amygdala in adults (r = 0.62; p = 0.04,) and adolescents (r = 0 .75; p = 0 .02). Adolescents also showed a positive correlation between subjective arousal for recalled negative pictures and left amygdala activity (r = 0.68, p = 0.04). Significant correlations were not present for the positive pictures.
4. Discussion
This is the first study to report enhanced right amygdala activity in adolescents during successful encoding of positively valenced pictures compared to adults. Conversely, no group differences in brain activity emerged during successful encoding of negative pictures. These data indicate that one possible change in the transition from adolescence to adulthood involves valence-specific changes in encoding activity within the right amygdala. Only one previous study has compared age group differences in implicit encoding of recalled stimuli between adolescents and adults (Nelson et al., 2003); findings showed several valence-specific age group differences in brain regions mediating encoding, but none involving the amygdala. Methodological differences may contribute to the discrepant findings between Nelson et al. (2003) and our study, such as stimulus salience (color vs. black and white photographs) and memory retrieval methods (recall vs. recognition).
Several mechanisms may account for the adolescents’ enhanced right amygdala activity during encoding of positive pictures. Positive pictures may elicit relatively greater arousal in adolescents versus adults, which can affect various stages of memory formation (LaBar and Phelps, 1998, Sharot and Phelps, 2004). Enhanced arousal can lead to more focused attention on the arousing aspects of the stimulus (Easterbrook, 1959, Lockhart, 2002) and more efficient stimulus processing (Hansen and Hansen, 1988). Both processes are influenced by ongoing retrieval processes that facilitate greater elaboration and encoding of the positive stimuli (Ochsner, 2000). Although not significant, the enhanced bilateral hippocampal activity in adolescents may reflect such elaborate encoding processes, and could reach significance with more power. This enhanced arousal hypothesis, however, is unsupported by our behavioral data since the age group × picture valence interactions for the subjective arousal ratings were not significant. Furthermore, we were unable to precisely correlate subjective arousal with amygdala activity since arousal could have occurred during any one of the four picture viewings and not necessarily when arousal was subjectively measured. Finally, underdevelopment of prefrontal-amygdala connections may have resulted in less regulation of amygdala activity, hence requiring adolescents to expend greater effort to encode the recalled stimuli (Casey et al., 2000, Casey et al., 2005, Luna et al., 2004), although this is unlikely given that the findings were specific to the positive stimuli rather than generalized to encoding of both positive and recalled negative stimuli.
The finding of enhanced right amygdala activity during encoding of positive stimuli in adolescents has developmental and clinical implications. Enhanced neural activity during the encoding and recall of emotional stimuli can enhance survival by promoting defensive strategies and reproductive success (Adolphs and Damasio, 2000). A positive encoding bias may be evolutionarily necessary for adolescents to achieve developmental goals, notably autonomy and individuation. The downside, however, is that this bias may lead to adverse outcomes such as drug and alcohol abuse (Chambers et al., 2003, Koob and Le Moal, 1997, Reyna and Farley, 2006, Spear, 2000, Steinberg, 2008). Encoding processes within the amygdala are modulated by interconnected regions other than the hippocampus, including the ventral striatum, a key dopaminergic structure that is integral to reward processing (Camara et al., 2009, Cardinal et al., 2002, Everitt et al., 1999, Groenewegen et al., 1999, Voorn et al., 1986). The dopaminergic system undergoes significant changes during adolescence (Ernst et al., 2005a, Galvan et al., 2006, May et al., 2004) that may facilitate enhanced neural activity during encoding of positive stimuli and contribute to increased reward seeking and risk taking behavior (Bjork et al., 2004, Chambers et al., 2003, Ernst et al., 2005b). Further research is needed to understand the interrelationships amongst encoding processes, behavioral outcomes, and amygdala-based circuitry activity in this age group.
Our findings were restricted to the right amygdala only. Currently, there are no established models positing dynamic shifts in left and right amygdala functions across development. Several meta-analytic reviews of adult emotion processing with a wide range of methodologies indicate that the left amygdala more frequently activates than the right amygdala, particularly to negative stimuli (Baas et al., 2004, Fusar-Poli et al., 2009, Wager et al., 2003). Other studies have examined whether laterality differences are reflected by the types of emotional stimuli, e.g., unconscious versus conscious, innate versus conditioned, novel versus familiar stimuli, and learned versus experiential fear (see review by Zald, 2003). These data regarding laterality of amygdala functions remain inconclusive, however. From a developmental perspective, it is possible that brain changes during adolescence result in differential programming, such that the right amygdala preferentially subserves encoding of positive stimuli. The current methods can be used to examine the timing of lateralization during development and whether gender by laterality interactions are present in adolescents as they are in adults.
There were no group differences in neural activity during successful encoding of the negative pictures, suggesting that adolescents exhibit mature levels of processing for the negative stimuli whereas neural differences only exist for the positive stimuli. Alternatively, our 90 s recall time limit may have possibly constrained further recall of negative pictures, thereby increasing power to detect group differences. Some evidence from emotion processing studies suggests that adolescents display heightened amygdala responses to fearful stimuli compared to adults and that these responses may be lateralized to the right amygdala (Guyer et al., 2008, Killgore and Yurgelun-Todd, 2004, McClure et al., 2004, Monk et al., 2003). Further studies of emotional memory in adolescents are needed to understand these inconsistencies.
Another point to note is that the current study as well as prior studies of emotion processing included adolescents with a wide age range, i.e., 12–17 years. Physical, cognitive and neural development across adolescence varies considerably. For example, early adolescence is characterized by pubertal changes and poor affective regulation, whereas late adolescence is associated with maturation of the frontal lobes and regulatory competence (Steinberg, 2005). Grouping these different stages of adolescence may therefore contribute to the heterogeneity of findings across emotion processing studies and emphasizes the need for examining age group interactions between behavior, affective and neural processes within this period.
In adults, enhanced amygdala activity during encoding predicts memory for both positive and negative stimuli (Cahill et al., 1996, Canli et al., 1999, Canli et al., 2000, Canli et al., 2002b, Hamann, 2001, Hamann et al., 1999, Tabert et al., 2001). Although these studies vary widely in their stimuli and retrieval methods, the majority correlated encoding activity with long term memory, assessed several weeks after scanning. Moreover, the most consistent evidence implicating the amygdala in emotional memory emerges for highly aversive stimuli (e.g., Cahill et al., 1996, Canli et al., 2002a). In terms of positive pictures, erotic images are known to elicit the greatest arousal in adult studies (Bradley and Lang, 2007, Lang et al., 1997, Ribeiro et al., 2007). We considered using such stimuli in our study, but the Institutional Review Board (IRB) review indicated that these stimuli were not suitable for use with minors. The stimuli selected for our study were therefore clearly less arousing than in these prior studies of emotional memory in adults. As a result, our data provide evidence for right amygdala involvement in encoding relatively mild emotional stimuli after a shorter delay of 20–40 min. When combined with prior data in adults for more arousing stimuli, our findings suggest that the threshold for engaging an amygdala-based memory system is lower in adolescents than adults.
Consistent with prior literature, our recall data revealed a memory bias for emotional compared to neutral stimuli (Bradley et al., 1992, Christianson and Fallman, 1990, Heuer and Reisberg, 1990, Kleinsmith and Kaplan, 1963, LaBar and Phelps, 1998). Adolescents, however, did not exhibit superior memory for positive pictures despite enhanced brain activity during successful encoding. Nelson et al. (2003) also found no age group differences in emotional memory performance. The absence of an emotion-enhancing effect on memory performance in adolescents may relate to age group differences in storage and retrieval processes, which are each mediated by a distinctly different set of brain circuits from those involved in encoding (Gabrieli et al., 1997, Kavcic et al., 2003, Sowell et al., 2001). Retrieval processes may be underdeveloped in adolescents, leading to lower than expected memory performance (Ofen et al., 2007). Also, the use of the recall method may have resulted in a smaller number of stimuli recalled in each group, making it harder to appreciate group differences in recall that may exist. Finally, enhanced neural activity for the positive stimuli in adolescents may result from arousal-mediated memory consolidation mechanisms that occur over time, beyond the short delay period in this study (Kleinsmith and Kaplan, 1963, McGaugh, 1992).
Emotional memory as well as amygdala-hippocampal dysfunction are present in various adult psychiatric disorders such as depression (Drevets et al., 2008, Fu et al., 2004, Sheline et al., 2001), bipolar disorder (Drevets et al., 2008, Kauer-Sant’Anna et al., 2008), substance abuse (Robbins et al., 2008), PTSD (Bremner, 2007) and schizophrenia (Kosaka et al., 2002, Phillips et al., 1999, Weniger et al., 2006, Exner et al., 2004). Abnormal encoding processes are also present in adolescent psychiatric disorders (Roberson-Nay et al., 2006, Dickstein et al., 2007). These data indicate that relationships between emotional memory and brain function in adolescents merit further investigation.
There are several limitations of our study. The small sample size and number of replicates for each contrast was small, thereby increasing the chance of type II errors, particularly in the adult group. While more power may have enabled us to detect additional group differences, it is important to note that our findings revealing an effect of age group on activation for positive recalled versus positive not recalled stimuli emerged despite relatively limited power. Moreover, this difference was not driven by group differences in the number of recalled items. The relatively low number of trials should therefore not call into question the validity of the group differences that were detected. Second, we have no proof that the recalled positive pictures elicited relatively greater arousal in adolescents relative to adults. Using physiological measures in future studies will help establish links between arousal and amygdala activity. Additionally, we could not precisely determine whether encoding was condition-specific or the result of repeated viewings because each picture was presented four times. We attempted to correlate subjective arousal with amygdala activity but these results are very preliminary. In future studies, it will be important for investigators to capture encoding more directly, as well as examine brain behavior relationships amongst subjective arousal, memory performance, and brain activity in response to evocative pictures. Next, transient mood effects experienced during scanning or recall could have influenced encoding processes and therefore it will be necessary in subsequent research to include a mood assessment prior to scanning and recall. Third, the not recalled stimuli could have been encoded and therefore future studies should consider including a recognition memory test. Fourth, the stimuli used in our study of adolescents and young adults were based on IAPS ratings in children (ages 7–14 years, McManis et al., 2001) and therefore do not account for potential age-related shifts in the perception of the emotional stimuli. This issue of applying emotional ratings from one age group to another is an ongoing issue in developmental studies of emotional processing and can be addressed in future studies by obtaining emotional ratings of the stimuli or considering the use of different stimulus sets for each age group.
Another limitation is that the picture and fixation trials were randomized in order to disentangle the HRFs for each trial during encoding. This design has the advantage of reducing the overall time of the imaging task, which was necessary in the current study to increase tolerability of these procedures among youth. However, the design does not easily generate data on the HRF within a trial, which can be more easily estimated using alternative designs that generate longer tasks. The relatively short task duration also reduced the number of events that could be included in the analysis. Both of these design features reduce power. As a result, any negative findings in the current study, such as the relative dearth of activation in the adult sub-sample, should be interpreted cautiously; more powerful methods may further increase our ability to detect between-group differences in future studies. Nevertheless, the key finding in the current study concerned the between-group differences in activation that were detected. Neither design limitation can account for this significant, positive finding, as both of these limitations would have the effect of reducing our ability to detect differences. Finally, we were unable to examine whether the amygdala has a role in the encoding of non-emotional stimuli because too few neutral stimuli were recalled.
In summary, this study found enhanced right amygdala activity during encoding of positive stimuli between adolescents and adults. This finding has significant implications for understanding the etiology of adolescent behaviors, such as novelty seeking and risk taking behavior, as well as specific types of psychiatric disorders that emerge during this developmental period. Our results, however, are preliminary given the small size. Therefore, replication with larger sample sizes, as well as different tasks and stimuli, is needed before drawing firm conclusions regarding the nature of adolescent encoding processes.
Appendix A. IAPS picture numbers presented in the behavioral task.
Positive: 1463, 1610, 1710, 1722, 1750, 1920, 2070, 2216, 2260, 2310, 2345, 2360, 2791, 5480, 5621, 5910, 7330, 7390, 7400, 7410, 7502, 8021, 8031, 8162, 8420, 8461, 8490, 8496, 8501, 8502, 8510, 8531, 8540.
Negative: 1050, 1052, 1111, 1120, 1201, 1220, 1300, 1302, 1321, 1930, 1931, 2722, 2800, 3022, 3230, 3280, 6010, 6190, 6200, 6230, 6300, 6370, 6510, 6830, 6836, 6840, 7360, 7380, 9050, 9250, 9440, 9480, 9910.
Neutral: 5130, 5532, 5740, 7000, 7002, 7004, 7006, 7009, 7010, 7020, 7025, 7030, 7031, 7040, 7050, 7060, 7080, 7090, 7100, 7130, 7140, 7150, 7170, 7175, 7211, 7217, 7224, 7235, 7500, 7595, 7705, 7950.
References
- Adolphs R., Damasio A.R. The interaction of affect and cognition. In: Forgas J.P., editor. Handbook of Affect and Social Cognition. Erlbaum; Mahwah, NJ: 2000. pp. 27–49. [Google Scholar]
- Akirav I., Richter-Levin G. Biphasic modulation of hippocampal plasticity by behavioral stress and basolateral amygdala stimulation in the rat. J. Neurosci. 1999;19:10530–10535. doi: 10.1523/JNEUROSCI.19-23-10530.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkire M.T., Haier R.J., Fallon J.H., Cahill L. Hippocampal, but not amygdala, activity at encoding correlates with long-term, free recall of nonemotional information. Proc. Natl. Acad. Sci. U.S.A. 1998;95:14506–14510. doi: 10.1073/pnas.95.24.14506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angold A., Costello E.J., Worthman C.M. Puberty and depression: the roles of age, pubertal status, and pubertal timing. Psychol. Med. 1998;28:51–61. doi: 10.1017/s003329179700593x. [DOI] [PubMed] [Google Scholar]
- Ashburner J., Friston K.J. Unified segmentation. NeuroImage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Baas D., Aleman A., Kahn R.S. Lateralization of amygdala activation: a systematic review of functional neuroimaging studies. Brain Res. Rev. 2004;45:96–103. doi: 10.1016/j.brainresrev.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Baird A.A., Gruber S.A., Fein D.A., Maas L.C., Steingard R.J., Renshaw P.F. Functional magnetic resonance imaging of facial affect recognition in children and adolescents. J. Am. Acad. Child Adolesc. Psychiatry. 1999;38:195–199. doi: 10.1097/00004583-199902000-00019. [DOI] [PubMed] [Google Scholar]
- Bjork J.M., Knutson B., Fong G.W., Caggiano D.M., Bennett S.M., Hommer D.W. Incentive-elicited brain activation in adolescents: similarities and differences from young adults. J. Neurosci. 2004;24:1793–1802. doi: 10.1523/JNEUROSCI.4862-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley M.M., Greenwald M.K., Petry M.C., Lang P.J. Remembering pictures: pleasure and arousal in memory. J. Exp. Psychol. Learn. Mem. Cogn. 1992;18:379–390. doi: 10.1037//0278-7393.18.2.379. [DOI] [PubMed] [Google Scholar]
- Bradley M.M., Lang P.J. The International Affective Picture System (IAPS) in the study of emotion and attention. In: Coan J.A., Allen J.B.J., editors. Handbook of Emotion Elicitation and Assessment. Oxford University Press; New York: 2007. pp. 29–46. [Google Scholar]
- Bremner J.D. Functional neuroimaging in post-traumatic stress disorder. Expert Rev. Neurother. 2007;7:393–405. doi: 10.1586/14737175.7.4.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer J.B., Zhao Z., Desmond J.E., Glover G.H., Gabrieli J.D.E. Making memories: brain activity that predicts how well visual experience will be remembered. Science. 1998;281:1185–1187. doi: 10.1126/science.281.5380.1185. [DOI] [PubMed] [Google Scholar]
- Büchel C., Morris J., Dolan R.J., Friston K.J. Brain systems mediating aversive conditioning: an event-related fMRI study. Neuron. 1998;20:947–957. doi: 10.1016/s0896-6273(00)80476-6. [DOI] [PubMed] [Google Scholar]
- Buckner R.L., Wheeler M.E., Sheridan M.A. Encoding processes during retrieval tasks. J. Cogn. Neurosci. 2001;13:406–415. doi: 10.1162/08989290151137430. [DOI] [PubMed] [Google Scholar]
- Cahill L., Babinsky R., Markowitsch H.J., McGaugh J.L. The amygdala and emotional memory. Nature. 1995;377:295–296. doi: 10.1038/377295a0. [DOI] [PubMed] [Google Scholar]
- Cahill L., Haier R.J., Fallon J., Alkire M.T., Tang C. Amygdala activity at encoding correlated with long-term, free recall of emotional information. Proc. Acad. Sci. 1996;93:8016–8021. doi: 10.1073/pnas.93.15.8016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill L., McGaugh J.L. Mechanisms of emotional arousal and lasting declarative memory. Trends Neurosci. 1998;21:294–299. doi: 10.1016/s0166-2236(97)01214-9. [DOI] [PubMed] [Google Scholar]
- Camara E., Rodriguez-Fornells A., Münte T.F. Functional connectivity of reward processing in the brain. Front. Hum. Neurosci. 2009;2:1–14. doi: 10.3389/neuro.09.019.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canli T., Desmond J.E., Zhao Z., Gabrieli J.D. Sex differences in the neural basis of emotional memories. Proc. Natl. Acad. Sci. U.S.A. 2002;99:10789–10794. doi: 10.1073/pnas.162356599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canli T., Sivers H., Whitfield S.L., Gotlib I.H., Gabrieli J.D. Amygdala response to happy faces as a function of extraversion. Science. 2002;296:2191. doi: 10.1126/science.1068749. [DOI] [PubMed] [Google Scholar]
- Canli T., Zhao Z., Brewer J., Gabrieli J.D.E., Cahill L. Event-related activation in the human amygdala associates with later memory for individual emotional experience. J. Neurosci. 2000;20:1–5. doi: 10.1523/JNEUROSCI.20-19-j0004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canli T., Zhao Z., Desmond J.E., Glover G., Gabrieli J.D.E. fMRI identifies a network of structures correlated with retention of positive and negative emotional memory. Psychobiology. 1999;27:441–452. [Google Scholar]
- Cardinal R.N., Parkinson J.A., Hall J., Everitt B.J. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci. Biobehav. Rev. 2002;26:321–352. doi: 10.1016/s0149-7634(02)00007-6. [DOI] [PubMed] [Google Scholar]
- Casey B.J., Giedd J.N., Thomas K.M. Structural and functional brain development and its relation to cognitive development. Biol. Psychol. 2000;54:241–257. doi: 10.1016/s0301-0511(00)00058-2. [DOI] [PubMed] [Google Scholar]
- Casey B.J., Jones R.M., Levita L., Libby V., Pattwell S.S., Ruberry E.J. The storm and stress of adolescence: insights from human imaging and mouse genetics. Dev. Psychobiol. 2010;52:225–235. doi: 10.1002/dev.20447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey B.J., Tottenham N., Liston C., Durston S. Imaging the developing brain: what have we learned about cognitive development? Trends Cogn. Sci. 2005;9:104–110. doi: 10.1016/j.tics.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Chambers R.A., Taylor J.R., Potenza M.N. Developmental neurocircuitry of motivation in adolescence: a critical period of addiction vulnerability. Am. J. Psychiatry. 2003;160:1041–1052. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson S.A., Fallman L. The role of age on reactivity and memory for emotional pictures. Scand. J. Psychol. 1990;31:291–301. doi: 10.1111/j.1467-9450.1990.tb00841.x. [DOI] [PubMed] [Google Scholar]
- Davis M., Whalen P.J. The amygdala: vigilance and emotion. Mol. Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- Dickstein D.P., Rich B.A., Roberson-Nay R., Berghorst L., Vinton D. Neural activation during encoding of emotional faces in pediatric bipolar disorder. Bipolar Disord. 2007;9:679–692. doi: 10.1111/j.1399-5618.2007.00418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolcos F., LaBar K.S., Cabeza R. Interaction between the amygdala and the medial temporal lobe memory system predicts better memory for emotional events. Neuron. 2004;42:855–863. doi: 10.1016/s0896-6273(04)00289-2. [DOI] [PubMed] [Google Scholar]
- Drevets W.C., Price J.L., Furey M.L. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct. Funct. 2008;213:93–118. doi: 10.1007/s00429-008-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easterbrook J.A. The effect of emotion on cue utilization and the organization of behavior. Psychol. Rev. 1959;66:183–201. doi: 10.1037/h0047707. [DOI] [PubMed] [Google Scholar]
- Ernst M., Fudge J.L. A developmental neurobiological model of motivated behavior: anatomy, connectivity and ontogeny of the triadic nodes. Neurosci. Biobehav. Rev. 2009;33:367–382. doi: 10.1016/j.neubiorev.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M., Nelson E.E., Jazbec S., McClure E.B., Monk C.S., Leibenluft E. Amygdala and nucleus accumbens in responses to receipt and omission of gains in adults and adolescents. NeuroImage. 2005;25:1279–1291. doi: 10.1016/j.neuroimage.2004.12.038. [DOI] [PubMed] [Google Scholar]
- Ernst M., Pine D.S., Hardin M. Triadic model of the neurobiology of motivated behavior in adolescence. Psychol. Med. 2005;35:1–14. doi: 10.1017/S0033291705005891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt B.J., Parkinson J.A., Olmstead M.C., Arroyo M., Robledo P., Robbins T.W. Associative processes in addiction and reward: the role of amygdala-ventral striatal subsystems. Ann. N.Y. Acad. Sci. 1999;877:412–438. doi: 10.1111/j.1749-6632.1999.tb09280.x. [DOI] [PubMed] [Google Scholar]
- Exner C., Boucsein K., Degner D., Irle E., Weniger G. Impaired emotional learning and reduced hippocampal size in schizophrenia: a 3-month follow-up. Schizophr. Res. 2004;71:493–503. doi: 10.1016/j.schres.2004.02.023. [DOI] [PubMed] [Google Scholar]
- Friston K.J., Zarahn E., Josephs O., Henson R.N.A., Dale A.M. Scholastic designs in event-related fMRI. Neuroimage. 1999;10:607–619. doi: 10.1006/nimg.1999.0498. [DOI] [PubMed] [Google Scholar]
- Fu C.H., Williams S., Cleare A., Brammer M., Walsh K.J. Attenuation of the neural response to sad faces in major depression by antidepressant treatment: a prospective, event-related functional magnetic resonance imaging study. Arch. Gen. Psychiatry. 2004;61:877–889. doi: 10.1001/archpsyc.61.9.877. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P., Placentino A., Carletti F., Allen P., Landi P. Laterality effect on emotional faces processing: ALE meta-analysis of evidence. Neurosci. Lett. 2009;452:262–267. doi: 10.1016/j.neulet.2009.01.065. [DOI] [PubMed] [Google Scholar]
- Gabrieli J.D.E., Brewer J.B., Desmond J.E., Glover G.H. Separate neural bases of two fundamental memory processes in the human medial temporal lobe. Science. 1997;11:264–266. doi: 10.1126/science.276.5310.264. [DOI] [PubMed] [Google Scholar]
- Galvan A., Hare T.A., Parra C.E., Penn J., Voss H., Glover G. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. J. Neurosci. 2006;26:6885–6892. doi: 10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwald M.K., Cook E.W., Lang P.J. Affective judgment and psychophysiological response: dimensional covariation in the evaluation of pictorial stimuli. J. Psychophysiol. 1989;3:51–64. [Google Scholar]
- Groenewegen H.J., Wright C.I., Beijer A.V., Voorn P. Convergence and segregation of ventral striatal inputs and outputs. Ann. N.Y. Acad. Sci. 1999;877:49–63. doi: 10.1111/j.1749-6632.1999.tb09260.x. [DOI] [PubMed] [Google Scholar]
- Guyer A.E., Monk C.S., McClure-Tone E.B., Nelson E.E., Roberson-Nay R. Developmental examination of amygdala response to facial expressions. J. Cogn. Neurosci. 2008;20:1–18. doi: 10.1162/jocn.2008.20114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann S. Cognitive and neural mechanisms of emotional memory. Trends Cogn. Sci. 2001;5:394–400. doi: 10.1016/s1364-6613(00)01707-1. [DOI] [PubMed] [Google Scholar]
- Hamann S.B., Ely T.D., Grafton S.T., Kilts C.D. Amygdala activity related to enhanced memory for pleasant and aversive stimuli. Nat. Neurosci. 1999;2:289–293. doi: 10.1038/6404. [DOI] [PubMed] [Google Scholar]
- Hansen C.H., Hansen R.D. Finding the face in the crowd: an anger superiority effect. J. Pers. Soc. Psychol. 1988;54:917–924. doi: 10.1037//0022-3514.54.6.917. [DOI] [PubMed] [Google Scholar]
- Heuer F., Reisberg D. Vivid memories of emotional events: the accuracy of remembered minutiae. Mem. Cognit. 1990;18:496–506. doi: 10.3758/bf03198482. [DOI] [PubMed] [Google Scholar]
- Kauer-Sant’Anna M., Yatham N.L., Tramontina J., Weyne F., Cereser K.M. Emotional memory in bipolar disorder. Br. J. Psychiatry. 2008;192:458–463. doi: 10.1192/bjp.bp.107.040295. [DOI] [PubMed] [Google Scholar]
- Kaufman J., Birmaher B., Brent D.A., Ryan N.D., Rao U. K-SADS-Pl. J. Am. Acad. Child Adolesc. Psychiatry. 2000;39:1208. doi: 10.1097/00004583-200010000-00002. [DOI] [PubMed] [Google Scholar]
- Kavcic V., Zhong J., Yoshiura T., Doty R.W. Frontal cortex, laterality, and memory: encoding versus retrieval. Acta Neurobiol. Exp. 2003;63:337–350. doi: 10.55782/ane-2003-1473. [DOI] [PubMed] [Google Scholar]
- Kensinger E.A., Brierley B., Medford N., Growdon J.H., Corkin S. Effects of normal aging and Alzheimer's disease on emotional memory. Emotion. 2002;2:118–134. doi: 10.1037/1528-3542.2.2.118. [DOI] [PubMed] [Google Scholar]
- Kensinger E.A., Schacter D.L. Amygdala activity is associated with the successful encoding of item, but not source, information for positive and negative stimuli. J. Neurosci. 2006;26:2564–2570. doi: 10.1523/JNEUROSCI.5241-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killgore W.D., Yurgelun-Todd D.A. Sex-related developmental differences in lateralized activation of the prefrontal cortex and amygdala during perception of facial affect. Percept. Mot. Skills. 2004;99:371–391. doi: 10.2466/pms.99.2.371-391. [DOI] [PubMed] [Google Scholar]
- Kilpatrick L., Cahill L. Amygdala modulation of parahippocampal and frontal regions during emotionally influenced memory storage. NeuroImage. 2003;20:2091–2099. doi: 10.1016/j.neuroimage.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Kleinsmith L.J., Kaplan S. Paired-associate learning as a function of arousal and interpolated interval. J. Exp. Psychol. 1963;65:190–193. doi: 10.1037/h0040288. [DOI] [PubMed] [Google Scholar]
- Koob G.F., Le Moal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278:52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- Kosaka H., Omori M., Murata T., Iidaka T., Yamada H. Differential amygdala response during facial recognition in patients with schizophrenia: an fMRI study. Schizophr. Res. 2002;57:87–95. doi: 10.1016/s0920-9964(01)00324-3. [DOI] [PubMed] [Google Scholar]
- LaBar K.S., Gatenby J.C., Gore J.C., LeDoux J.E., Phelps E.A. Human amygdala activation during conditioned fear acquisition and extinction: a mixed-trial fMRI study. Neuron. 1998;20:937–945. doi: 10.1016/s0896-6273(00)80475-4. [DOI] [PubMed] [Google Scholar]
- LaBar K.S., Phelps E.A. Arousal-mediated memory consolidation: role of the medial temporal lobe in humans. Psychol. Sci. 1998;9:490–493. [Google Scholar]
- Lang P.J. Behavioral treatment and bio-behavioral assessment: computer applications. In: Sidowski J.B., Johnson J.H., Williams T.A., editors. Technology in Mental Health Care Delivery Systems. Ablex; Norwood, NJ: 1980. pp. 119–137. [Google Scholar]
- Lang P.J., Bradley M.M., Cuthbert B.N. NIMH Center for the Study of Emotion and Attention; Gainesville, FL: 1997. International Affective Picture System. [Google Scholar]
- Lewinsohn P.M., Hops H., Roberts R.E., Seeley J.R., Andrews J.A. Adolescent psychopathology: I. Prevalence and incidence of depression and other DSM-III-R disorders in high school students. J. Abnorm. Psychol. 1993;1:133–144. doi: 10.1037//0021-843x.102.1.133. [DOI] [PubMed] [Google Scholar]
- Lockhart R.S. Levels of processing, transfer-appropriate processing, and the concept of robust encoding. Memory. 2002;10:397–403. doi: 10.1080/09658210244000225. [DOI] [PubMed] [Google Scholar]
- Luna B., Garver K.E., Urban T.A., Lazar N.A., Sweeney J.A. Maturation of cognitive processes from late childhood to adulthood. Child Dev. 2004;75:1357–1372. doi: 10.1111/j.1467-8624.2004.00745.x. [DOI] [PubMed] [Google Scholar]
- Maren S. Neurobiology of Pavlovian fear conditioning. Annu. Rev. Neurosci. 2001;24:897–931. doi: 10.1146/annurev.neuro.24.1.897. [DOI] [PubMed] [Google Scholar]
- May J.C., Delgado M.R., Dahl R.E., Stenger V.A., Ryan N.D. Event-related functional magnetic resonance imaging of reward-related brain circuitry in children and adolescents. Biol. Psychiatry. 2004;55:359–366. doi: 10.1016/j.biopsych.2003.11.008. [DOI] [PubMed] [Google Scholar]
- McClure E.B., Monk C.S., Nelson E.E., Zarahn E., Leibenluft E. A developmental examination of gender differences in brain engagement during evaluation of threat. Biol. Psychiatry. 2004;55:1047–1055. doi: 10.1016/j.biopsych.2004.02.013. [DOI] [PubMed] [Google Scholar]
- McGaugh J.L. Affect, neuromodulatory systems and memory storage. In: Christianson S., editor. The Handbook of Emotion and Memory: Research and Theory. Erlbaum; Hillsdale, NJ: 1992. pp. 269–288. [Google Scholar]
- McGaugh J.L. Memory: a century of consolidation. Science. 2000;14:248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- McGaugh J.L. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu. Rev. Neurosci. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- McGaugh J.L., Cahill L., Roozendaal B. Involvement of the amygdala in memory storage: interaction with other brain systems. Proc. Natl. Acad. Sci. U.S.A. 1996;93(24):13508–13514. doi: 10.1073/pnas.93.24.13508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaugh J.L., McIntyre C.K., Power A.E. Amygdala modulation of memory consolidation: interaction with other brain systems. Neurobiol. Learn. Mem. 2002;78(3):539–552. doi: 10.1006/nlme.2002.4082. [DOI] [PubMed] [Google Scholar]
- McManis M.H., Bradley M.M., Berg W.K., Cuthbert B.N., Lang P.J. Emotional reactions in children: verbal, physiological, and behavioral responses to affective pictures. Psychophysiology. 2001;38:222–231. [PubMed] [Google Scholar]
- Monk C.S., McClure E.B., Nelson E.E., Zarahn E., Bilder R.M. Adolescent immaturity in attention-related brain engagement to emotional facial expressions. NeuroImage. 2003;20:420–428. doi: 10.1016/s1053-8119(03)00355-0. [DOI] [PubMed] [Google Scholar]
- Nelson E.E., McClure E.B., Monk C.S., Zarahn E., Leibenluft E. Developmental differences in neuronal engagement during implicit encoding of emotional faces: an event-related fMRI study. J. Child Psychol. Psychiatry. 2003;44:1015–1024. doi: 10.1111/1469-7610.00186. [DOI] [PubMed] [Google Scholar]
- Ochsner K.N. Are affective events richly recollected or simply familiar? The experience and process of recognizing feelings past. J. Exp. Psychol. Gen. 2000;129:242–261. doi: 10.1037//0096-3445.129.2.242. [DOI] [PubMed] [Google Scholar]
- Ofen N., Kao Y.C., Sokol-Hessner P., Kim H., Whitfield-Gabrieli S. Development of the declarative memory system in the human brain. Nat. Neurosci. 2007;10:1198–1205. doi: 10.1038/nn1950. [DOI] [PubMed] [Google Scholar]
- Packard M.G., Teather L.A. Amygdala modulation of multiple memory systems: hippocampus and caudate-putamen. Neurobiol. Learn. Mem. 1998;69:163–203. doi: 10.1006/nlme.1997.3815. [DOI] [PubMed] [Google Scholar]
- Phan K.L., Wager T., Taylor S.F., Liberzon I. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. NeuroImage. 2002;16:331–348. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- Phelps E.A. Human emotion and memory: interactions of the amygdala and hippocampal complex. Curr. Opin. Neurobiol. 2004;14:198–202. doi: 10.1016/j.conb.2004.03.015. [DOI] [PubMed] [Google Scholar]
- Phillips M.L., Williams L., Senior C., Bullmore E.T., Brammer M.J. A differential neural response to threatening and non-threatening negative facial expressions in paranoid and non-paranoid schizophrenics. Psychiatry Res. 1999;92:11–31. doi: 10.1016/s0925-4927(99)00031-1. [DOI] [PubMed] [Google Scholar]
- Pikkarainen M., Ronko S., Savander V., Insausti R., Pitkänen A. Projections from the lateral, basal, and accessory basal nuclei of the amygdala to the hippocampal formation in rat. J. Comp. Neurol. 1999;403:229–260. [PubMed] [Google Scholar]
- Pine D.S., Grun J., Maguire E.A., Burgess N., Zarahn E., Koda V. Neurodevelopmental aspects of spatial navigation: a virtual reality fMRI study. NeuroImage. 2002;15:396–406. doi: 10.1006/nimg.2001.0988. [DOI] [PubMed] [Google Scholar]
- Pine D.S., Lissek S., Klein R.G., Mannuzza S., Moulton J.L., 3rd Face-memory and emotion: associations with major depression in children and adolescents. J. Child Psychol. Psychiatry. 2004;45:1199–1208. doi: 10.1111/j.1469-7610.2004.00311.x. [DOI] [PubMed] [Google Scholar]
- Pitkänen A., Pikkarainen M., Nurminen N., Ylinen A. Reciprocal connections between the amygdala and hippocampal formation, perirhinal cortex, and postrhinal cortex in rat. A review. Ann. N.Y. Acad. Sci. 2000;911:369–391. doi: 10.1111/j.1749-6632.2000.tb06738.x. [DOI] [PubMed] [Google Scholar]
- Psychological Corporation . Harcort Brace and Company; San Antonio: 1999. The Wechsler Abbreviated Scale of Intelligence. [Google Scholar]
- Reyna V.F., Farley F. Risk and rationality in adolescent decision making: implications for theory, practice, and public policy. Psychol. Sci. Public Interest. 2006;7:1–44. doi: 10.1111/j.1529-1006.2006.00026.x. [DOI] [PubMed] [Google Scholar]
- Ribeiro R.L., Teixeira-Silva F., Pompéia S., Bueno O.F. IAPS includes photographs that elicit low-arousal physiological responses in healthy volunteers. Physiol. Behav. 2007;91:671–675. doi: 10.1016/j.physbeh.2007.03.031. [DOI] [PubMed] [Google Scholar]
- Richardson M.P., Strange B.A., Dolan R.J. Encoding of emotional memories depends on amygdala and hippocampus and their interactions. Nat. Neurosci. 2004;7:278–285. doi: 10.1038/nn1190. [DOI] [PubMed] [Google Scholar]
- Robbins T.W., Ersche K.D., Everitt B.J. Drug addiction and the memory systems of the brain. Ann. N.Y. Acad. Sci. 2008;1141:1–21. doi: 10.1196/annals.1441.020. [DOI] [PubMed] [Google Scholar]
- Roberson-Nay R., McClure E.B., Monk C.S., Nelson E.E., Guyer A.E. Increased amygdala activity during successful memory encoding in adolescent major depressive disorder: an FMRI study. Biol. Psychiatry. 2006;60:966–973. doi: 10.1016/j.biopsych.2006.02.018. [DOI] [PubMed] [Google Scholar]
- Sergerie K., Chochol C., Armony J.L. The role of the amygdala in emotional processing: a quantitative meta-analysis of functional neuroimaging studies. Neurosci. Biobehav. Rev. 2008;32:811–830. doi: 10.1016/j.neubiorev.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Sharot T., Phelps E.A. How arousal modulates memory: disentangling the effects of attention and retention. Cogn. Affect. Behav. Neurosci. 2004;4:294–306. doi: 10.3758/cabn.4.3.294. [DOI] [PubMed] [Google Scholar]
- Sheline Y.I., Barch D., Donnelly J., Ollinger J., Snyder A., Mintun M. Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: an fMRI study. Biol. Psychiatry. 2001;50:651–658. doi: 10.1016/s0006-3223(01)01263-x. [DOI] [PubMed] [Google Scholar]
- Simmonds D.J., Fotedar S.G., Suskauer S.J., Pekar J.J., Denckla M.B., Mostofsky S.H. Functional brain correlates of response time variability in children. Neuropsychologia. 2007;45:2147–2157. doi: 10.1016/j.neuropsychologia.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Sowell E.R., Delis D., Stiles J., Jernigan T.L. Improved memory functioning and frontal lobe maturation between childhood and adolescence: a structural MRI study. J. Int. Neuropsychol. Soc. 2001;7:312–322. doi: 10.1017/s135561770173305x. [DOI] [PubMed] [Google Scholar]
- Spear L.P. The adolescent brain and age-related behavioral manifestations. Neurosci. Biobehav. Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spitzer R.L., Williams J.B., Gibbon M., First M.B. The Structured Clinical Interview for DSM-IIIR (SCID). I: history, rationale, and description. Arch. Gen. Psychiatry. 1992;49:624–629. doi: 10.1001/archpsyc.1992.01820080032005. [DOI] [PubMed] [Google Scholar]
- Steinberg L. Cognitive and affective development during adolescence. Trends Cogn. Sci. 2005;9(2):69–74. doi: 10.1016/j.tics.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Steinberg L. A social neuroscience perspective on adolescent risk-taking. Dev. Rev. 2008;28:78–106. doi: 10.1016/j.dr.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange B.A., Dolan R.J. Beta-adrenergic modulation of emotional memory-evoked human amygdala and hippocampal responses. Proc. Natl. Acad. Sci. U.S.A. 2004;101:11454–11458. doi: 10.1073/pnas.0404282101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suskauer S.J., Simmonds D.J., Caffo B.S., Denckla M.B., Pekar J.J., Mostofsky S.H. fMRI of intrasubject variability in ADHD: anomalous premotor activity with prefrontal compensation. J. Am. Acad. Child Adolesc. Psychiatry. 2008;47(10):1141–1150. doi: 10.1097/CHI.0b013e3181825b1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suskauer S.J., Simmonds D.J., Fotedar S., Blankner J.G., Pekar J.J., Denckla M.B. Functional magnetic resonance imaging evidence for abnormalities in response selection in attention deficit hyperactivity disorder: differences in activation associated with response inhibition but not habitual motor response. J. Cogn. Neurosci. 2008;20:478–493. doi: 10.1162/jocn.2008.20032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szeszko P.R., Robinson D., Alvir J.M., Bilder R.M., Lencz T. Orbital frontal and amygdala volume reductions in obsessive-compulsive disorder. Arch. Gen. Psychiatry. 1999;56:913–919. doi: 10.1001/archpsyc.56.10.913. [DOI] [PubMed] [Google Scholar]
- Szeszko P.R., Strous R.D., Goldman R.S., Ashtari M., Knuth K.H. Neuropsychological correlates of hippocampal volume in patients experiencing a first episode of schizophrenia. Am. J. Psychiatry. 2002;159:217–226. doi: 10.1176/appi.ajp.159.2.217. [DOI] [PubMed] [Google Scholar]
- Tabert M.H., Borod J.C., Tang C.Y., Lange G., Wei T.C. Differential amygdala activation during emotional decision and recognition memory tasks using unpleasant words: an fMRI study. Neuropsychologia. 2001;39:556–573. doi: 10.1016/s0028-3932(00)00157-3. [DOI] [PubMed] [Google Scholar]
- Thomas K.M., Drevets W.C., Whalen P.J., Eccard C.H., Dahl R.E. Amygdala response to facial expressions in children and adults. Biol. Psychiatry. 2001;49:309–316. doi: 10.1016/s0006-3223(00)01066-0. [DOI] [PubMed] [Google Scholar]
- Voorn P., Jorritsma-Byham B., Van Dijk C., Buijs R.M. The dopaminergic innervation of the ventral striatum in the rat: a light- and electron-microscopical study with antibodies against dopamine. J. Comp. Neurol. 1986;251:84–99. doi: 10.1002/cne.902510106. [DOI] [PubMed] [Google Scholar]
- Wager T.D., Phan K.L., Liberzon I., Taylor S.F. Valence, gender, and lateralization of functional brain anatomy in emotion: a meta-analysis of findings from neuroimaging. NeuroImage. 2003;19:513–531. doi: 10.1016/s1053-8119(03)00078-8. [DOI] [PubMed] [Google Scholar]
- Wagner A.D., Poldrack R.A., Eldridge L.L., Desmond J.E., Glover G.H., Gabrieli J.D. Material-specific lateralization of prefrontal activation during episodic encoding and retrieval. Neuroreport. 1998;9:3711–3717. doi: 10.1097/00001756-199811160-00026. [DOI] [PubMed] [Google Scholar]
- Weniger G., Lange C., Irle E. Abnormal size of the amygdala predicts impaired emotional memory in major depressive disorder. J. Affect. Disord. 2006;94:219–229. doi: 10.1016/j.jad.2006.04.017. [DOI] [PubMed] [Google Scholar]
- Zald D.H. The human amygdala and the emotional evaluation of sensory stimuli. Brain Res. Brain Res. Rev. 2003;41:88–123. doi: 10.1016/s0165-0173(02)00248-5. [DOI] [PubMed] [Google Scholar]