Abstract
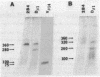
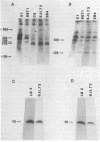
We used pulsed-field gel electrophoresis to determine the organization of the beta-chain gene of the T-cell receptor for antigen in normal and mutant inbred strains of mice. In normal mice, the variable (V)- and constant (C)- region elements of this locus span 700-800 kilobases of chromosomal DNA. All but one of the V beta gene segments analyzed lie 5' of the J beta C beta locus (J beta represents the joining region), with the closest being 280-360 kilobases away. The mutant mouse strain SJL has an internal V beta-region gene deletion that compacts the V beta region by 100-200 kilobases. Taken together with other data, these results indicate that the beta-chain locus can use either a looping-out/deletion or an inversion mechanism to appose V beta to DJ beta gene segments (D is the diversity region) and can accomplish the former (at least) over very large distances.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alt F. W., Baltimore D. Joining of immunoglobulin heavy chain gene segments: implications from a chromosome with evidence of three D-JH fusions. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4118–4122. doi: 10.1073/pnas.79.13.4118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behlke M. A., Chou H. S., Huppi K., Loh D. Y. Murine T-cell receptor mutants with deletions of beta-chain variable region genes. Proc Natl Acad Sci U S A. 1986 Feb;83(3):767–771. doi: 10.1073/pnas.83.3.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernards A., Kooter J. M., Michels P. A., Moberts R. M., Borst P. Pulsed field gradient electrophoresis of DNA digested in agarose allows the sizing of the large duplication unit of a surface antigen gene in trypanosomes. Gene. 1986;42(3):313–322. doi: 10.1016/0378-1119(86)90235-0. [DOI] [PubMed] [Google Scholar]
- Bird A. P. CpG-rich islands and the function of DNA methylation. Nature. 1986 May 15;321(6067):209–213. doi: 10.1038/321209a0. [DOI] [PubMed] [Google Scholar]
- Brenner M. B., McLean J., Dialynas D. P., Strominger J. L., Smith J. A., Owen F. L., Seidman J. G., Ip S., Rosen F., Krangel M. S. Identification of a putative second T-cell receptor. Nature. 1986 Jul 10;322(6075):145–149. doi: 10.1038/322145a0. [DOI] [PubMed] [Google Scholar]
- Carle G. F., Olson M. V. Separation of chromosomal DNA molecules from yeast by orthogonal-field-alternation gel electrophoresis. Nucleic Acids Res. 1984 Jul 25;12(14):5647–5664. doi: 10.1093/nar/12.14.5647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien Y. H., Gascoigne N. R., Kavaler J., Lee N. E., Davis M. M. Somatic recombination in a murine T-cell receptor gene. Nature. 1984 May 24;309(5966):322–326. doi: 10.1038/309322a0. [DOI] [PubMed] [Google Scholar]
- Chou H. S., Anderson S. J., Louie M. C., Godambe S. A., Pozzi M. R., Behlke M. A., Huppi K., Loh D. Y. Tandem linkage and unusual RNA splicing of the T-cell receptor beta-chain variable-region genes. Proc Natl Acad Sci U S A. 1987 Apr;84(7):1992–1996. doi: 10.1073/pnas.84.7.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou H. S., Behlke M. A., Godambe S. A., Russell J. H., Brooks C. G., Loh D. Y. T cell receptor genes in an alloreactive CTL clone: implications for rearrangement and germline diversity of variable gene segments. EMBO J. 1986 Sep;5(9):2149–2155. doi: 10.1002/j.1460-2075.1986.tb04478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fujimoto S., Yamagishi H. Isolation of an excision product of T-cell receptor alpha-chain gene rearrangements. Nature. 1987 May 21;327(6119):242–243. doi: 10.1038/327242a0. [DOI] [PubMed] [Google Scholar]
- Gascoigne N. R., Waters S., Elliott J. F., Victor-Kobrin C., Goodnow C., Davis M. M., Bona C. A. Expression of T cell receptor genes in an antigen-specific hybridoma and radiation-induced variants. J Exp Med. 1986 Jul 1;164(1):113–130. doi: 10.1084/jem.164.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HSU T. C., SOMERS C. E. Properties of L cells resistant to 5-bromodeoxyuridine. Exp Cell Res. 1962 Mar;26:404–410. doi: 10.1016/0014-4827(62)90192-1. [DOI] [PubMed] [Google Scholar]
- Hardy D. A., Bell J. I., Long E. O., Lindsten T., McDevitt H. O. Mapping of the class II region of the human major histocompatibility complex by pulsed-field gel electrophoresis. Nature. 1986 Oct 2;323(6087):453–455. doi: 10.1038/323453a0. [DOI] [PubMed] [Google Scholar]
- Hedrick S. M., Nielsen E. A., Kavaler J., Cohen D. I., Davis M. M. Sequence relationships between putative T-cell receptor polypeptides and immunoglobulins. Nature. 1984 Mar 8;308(5955):153–158. doi: 10.1038/308153a0. [DOI] [PubMed] [Google Scholar]
- Hieter P. A., Hollis G. F., Korsmeyer S. J., Waldmann T. A., Leder P. Clustered arrangement of immunoglobulin lambda constant region genes in man. Nature. 1981 Dec 10;294(5841):536–540. doi: 10.1038/294536a0. [DOI] [PubMed] [Google Scholar]
- Hinds K. R., Litman G. W. Major reorganization of immunoglobulin VH segmental elements during vertebrate evolution. Nature. 1986 Apr 10;320(6062):546–549. doi: 10.1038/320546a0. [DOI] [PubMed] [Google Scholar]
- Kronenberg M., Siu G., Hood L. E., Shastri N. The molecular genetics of the T-cell antigen receptor and T-cell antigen recognition. Annu Rev Immunol. 1986;4:529–591. doi: 10.1146/annurev.iy.04.040186.002525. [DOI] [PubMed] [Google Scholar]
- Lai E., Barth R. K., Hood L. Genomic organization of the mouse T-cell receptor beta-chain gene family. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3846–3850. doi: 10.1073/pnas.84.11.3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis S., Rosenberg N., Alt F., Baltimore D. Continuing kappa-gene rearrangement in a cell line transformed by Abelson murine leukemia virus. Cell. 1982 Oct;30(3):807–816. doi: 10.1016/0092-8674(82)90285-9. [DOI] [PubMed] [Google Scholar]
- Lindsten T., Fowlkes B. J., Samelson L. E., Davis M. M., Chien Y. H. Transient rearrangements of the T cell antigen receptor alpha locus in early thymocytes. J Exp Med. 1987 Sep 1;166(3):761–775. doi: 10.1084/jem.166.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malissen M., McCoy C., Blanc D., Trucy J., Devaux C., Schmitt-Verhulst A. M., Fitch F., Hood L., Malissen B. Direct evidence for chromosomal inversion during T-cell receptor beta-gene rearrangements. Nature. 1986 Jan 2;319(6048):28–33. doi: 10.1038/319028a0. [DOI] [PubMed] [Google Scholar]
- Okazaki K., Davis D. D., Sakano H. T cell receptor beta gene sequences in the circular DNA of thymocyte nuclei: direct evidence for intramolecular DNA deletion in V-D-J joining. Cell. 1987 May 22;49(4):477–485. doi: 10.1016/0092-8674(87)90450-8. [DOI] [PubMed] [Google Scholar]
- Pardoll D. M., Fowlkes B. J., Lechler R. I., Germain R. N., Schwartz R. H. Early genetic events in T cell development analyzed by in situ hybridization. J Exp Med. 1987 Jun 1;165(6):1624–1638. doi: 10.1084/jem.165.6.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patten P., Yokota T., Rothbard J., Chien Y., Arai K., Davis M. M. Structure, expression and divergence of T-cell receptor beta-chain variable regions. Nature. 1984 Nov 1;312(5989):40–46. doi: 10.1038/312040a0. [DOI] [PubMed] [Google Scholar]
- Pohlenz H. D., Straubinger B., Thiebe R., Pech M., Zimmer F. J., Zachau H. G. The human V kappa locus. Characterization of extended immunoglobulin gene regions by cosmid cloning. J Mol Biol. 1987 Jan 20;193(2):241–253. doi: 10.1016/0022-2836(87)90216-6. [DOI] [PubMed] [Google Scholar]
- Reynaud C. A., Anquez V., Dahan A., Weill J. C. A single rearrangement event generates most of the chicken immunoglobulin light chain diversity. Cell. 1985 Feb;40(2):283–291. doi: 10.1016/0092-8674(85)90142-4. [DOI] [PubMed] [Google Scholar]
- Russell G. J., Walker P. M., Elton R. A., Subak-Sharpe J. H. Doublet frequency analysis of fractionated vertebrate nuclear DNA. J Mol Biol. 1976 Nov;108(1):1–23. doi: 10.1016/s0022-2836(76)80090-3. [DOI] [PubMed] [Google Scholar]
- SWARTZ M. N., TRAUTNER T. A., KORNBERG A. Enzymatic synthesis of deoxyribonucleic acid. XI. Further studies on nearest neighbor base sequences in deoxyribonucleic acids. J Biol Chem. 1962 Jun;237:1961–1967. [PubMed] [Google Scholar]
- Schwartz D. C., Cantor C. R. Separation of yeast chromosome-sized DNAs by pulsed field gradient gel electrophoresis. Cell. 1984 May;37(1):67–75. doi: 10.1016/0092-8674(84)90301-5. [DOI] [PubMed] [Google Scholar]
- Shulman M., Wilde C. D., Köhler G. A better cell line for making hybridomas secreting specific antibodies. Nature. 1978 Nov 16;276(5685):269–270. doi: 10.1038/276269a0. [DOI] [PubMed] [Google Scholar]
- Siu G., Kronenberg M., Strauss E., Haars R., Mak T. W., Hood L. The structure, rearrangement and expression of D beta gene segments of the murine T-cell antigen receptor. 1984 Sep 27-Oct 3Nature. 311(5984):344–350. doi: 10.1038/311344a0. [DOI] [PubMed] [Google Scholar]
- Steinmetz M., Minard K., Horvath S., McNicholas J., Srelinger J., Wake C., Long E., Mach B., Hood L. A molecular map of the immune response region from the major histocompatibility complex of the mouse. Nature. 1982 Nov 4;300(5887):35–42. doi: 10.1038/300035a0. [DOI] [PubMed] [Google Scholar]
- Tonegawa S. Somatic generation of antibody diversity. Nature. 1983 Apr 14;302(5909):575–581. doi: 10.1038/302575a0. [DOI] [PubMed] [Google Scholar]
- Van der Ploeg L. H., Schwartz D. C., Cantor C. R., Borst P. Antigenic variation in Trypanosoma brucei analyzed by electrophoretic separation of chromosome-sized DNA molecules. Cell. 1984 May;37(1):77–84. doi: 10.1016/0092-8674(84)90302-7. [DOI] [PubMed] [Google Scholar]
- Yancopoulos G. D., Alt F. W. Regulation of the assembly and expression of variable-region genes. Annu Rev Immunol. 1986;4:339–368. doi: 10.1146/annurev.iy.04.040186.002011. [DOI] [PubMed] [Google Scholar]