Abstract
After publication of the updated World Health Organization (WHO) classification of tumors of hematopoietic and lymphoid tissues in 2008, the Pathology Working Group of the International Lymphoma Epidemiology Consortium (InterLymph) now presents an update of the hierarchical classification of lymphoid neoplasms for epidemiologic research based on the 2001 WHO classification, which we published in 2007. The updated hierarchical classification incorporates all of the major and provisional entities in the 2008 WHO classification, including newly defined entities based on age, site, certain infections, and molecular characteristics, as well as borderline categories, early and “in situ” lesions, disorders with limited capacity for clinical progression, lesions without current International Classification of Diseases for Oncology, 3rd Edition codes, and immunodeficiency-associated lymphoproliferative disorders. WHO subtypes are defined in hierarchical groupings, with newly defined groups for small B-cell lymphomas with plasmacytic differentiation and for primary cutaneous T-cell lymphomas. We suggest approaches for applying the hierarchical classification in various epidemiologic settings, including strategies for dealing with multiple coexisting lymphoma subtypes in one patient, and cases with incomplete pathologic information. The pathology materials useful for state-of-the-art epidemiology studies are also discussed. We encourage epidemiologists to adopt the updated InterLymph hierarchical classification, which incorporates the most recent WHO entities while demonstrating their relationship to older classifications.
Introduction
In 2007, a hierarchical scheme for the classification of lymphoid neoplasms for epidemiology was proposed by members of the International Lymphoma Epidemiology Consortium (InterLymph) to further the aims of etiologic research.1 The hierarchical classification scheme was based on the 2001 World Health Organization (WHO) classification of lymphoid neoplasms2 and the International Classification of Diseases for Oncology, 3rd Edition (ICD-O-3)3 and encouraged the adoption of the WHO classification of lymphoid disease entities by epidemiologic studies. The InterLymph scheme defined standardized groupings of lymphoid neoplasms at a range of hierarchical levels and provided a method for using cases coded under previous classification schemes (eg, Working Formulation, REAL, and ICD-O-2) in pooled studies. In epidemiologic studies, this hierarchical classification has facilitated subtype-specific analyses to the most detailed extent possible, based on the sample size and level of pathology information available, which is critical for comparing subtype-specific data among studies, achieving homogeneity among cases diagnosed using different systems within a single study, and for combining data from multiple studies for pooled analyses. The InterLymph classification scheme has been adopted by individual epidemiologic studies,4 pooled analyses,5–10 descriptive epidemiologic studies using cancer registry data,11 and by the 2009 Surveillance Epidemiology and End Results (SEER) Cancer Statistics Review.12
The WHO classification of lymphoid neoplasms was updated in 2008,13 with several key changes from the 2001 classification, including the introduction of several new and provisional entities defined on the basis of age at diagnosis, site, molecular characteristics, and association with inflammation and viral infections. The 2008 WHO classification also refined the criteria for diagnosing certain entities, such as chronic lymphocytic leukemia (CLL) and plasma cell neoplasms, provided categories for lesions with borderline features, and more comprehensively addressed early and “in situ” counterparts of lymphoma entities. New codes were proposed for new entities without ICD-O-3 codes; these were expected to be incorporated in the 4th edition of ICD-O, but remained subject to changes.
Herein, we propose an updated InterLymph hierarchical classification for future epidemiologic studies of lymphoid neoplasms that take into account the 2008 revisions to the WHO classification. Categories not included in the previous InterLymph hierarchical classification, including immunodeficiency-associated lymphoproliferative disorders, early and “in situ” lesions, and multiple coexisting lymphoma subtypes diagnosed in a patient, are accounted for in the updated classification. Although several of the new 2008 WHO entities have no ICD-O-3 codes, they are now being reported by pathologists along with their 2008 WHO (proposed ICD-O-4) codes. We therefore believe it is important to include these entities in the updated classification, so that their epidemiology may be studied and to enable cancer registries to recognize where they should be placed in the classification of lymphoid neoplasms. As with the previous classification, we recommend methodologies for incorporating the 2008 WHO classification in future studies and for converting cases classified using previous classification schemes, which is essential for long-term studies, such as cohort studies, completed or ongoing studies, and calculation of time trends.
Methods
Development of an updated InterLymph nested classification according to the 2008 WHO classification
A nested hierarchical classification, based on the 2008 WHO classification, was developed by the InterLymph Pathology Working Group (http://epi.grants.cancer.gov/InterLymph). InterLymph is an organization of international investigators conducting epidemiologic research on lymphoid neoplasms. The Pathology Working Group within InterLymph is composed of hematopathologists, epidemiologists, and other investigators with an interest in integrating pathology concepts into epidemiologic studies. The resultant nested hierarchical classification system represents the consensus view of the Working Group members after discussion of the various issues in a series of teleconferences and e-mails in 2009-2010 and at the InterLymph annual meeting in April 2010. The hierarchical groupings within the previous and the updated classification are defined by numerous parameters, including morphology, immunophenotype, genotype, stage of differentiation, and clinical features. Some of the new WHO subtypes are defined by anatomic site or age at diagnosis (eg, subtypes of diffuse large B-cell lymphoma) or the occurrence of a particular infection (eg, lymphomas associated with Epstein-Barr virus infection). These characteristics may be shared across different subtypes, which precludes nesting them in the classification structure.
All of the major WHO 2008 entities and provisional entities are included in the updated classification, except for some proposed divisions within subtypes that would be impracticable to incorporate into epidemiologic studies, such as the morphologic variants of mantle cell lymphoma and peripheral T-cell lymphoma, not otherwise specified. Readers are referred to the 2008 WHO publication13 for criteria for the different lymphoid neoplasm subtypes. Very rare subtypes are accounted for, so they can be analyzed in large studies with sufficient case numbers or pooled studies. Provisional WHO borderline categories between diffuse large B-cell lymphoma (DLBCL), usually the primary mediastinal type, and classical Hodgkin lymphoma, and between DLBCL and Burkitt lymphoma, are included. Categories are also provided for lymphomas of B-cell, T-cell, and unknown lineage that are not otherwise specified (NOS). Lymphomas of more than one histologic type found in one patient at any site and approximately the same time are listed as “multiple coexisting lymphoma subtypes.”
The immunodeficiency-associated lymphoproliferative disorders, certain early and “in situ” lesions related to WHO subtypes, and subtypes with a limited capacity for clinical progression are also included. New to this version of the InterLymph hierarchical classification, we have included entities without current ICD-O-3 codes, in anticipation that codes will be developed in the future. When a new entity has been given a new WHO (ie, proposed ICD-O-4) code in the 2008 WHO monograph, this code is listed alongside the ICD-O-3 code used under the assumption that the new codes will be incorporated in the upcoming 4th edition of the ICD-O (ICD-O-4). When this occurs, the former ICD-O-3 codes will become obsolete. Those entities without malignant ICD-O-3 codes, such as early and “in situ” lesions, lymphomatoid granulomatosis, and lymphomatoid papulosis, are designated as lymphoproliferative disorder (LPD). LPD is also used as a general term for lymphoproliferative disorders associated with nontransplant immune disorders.
Results
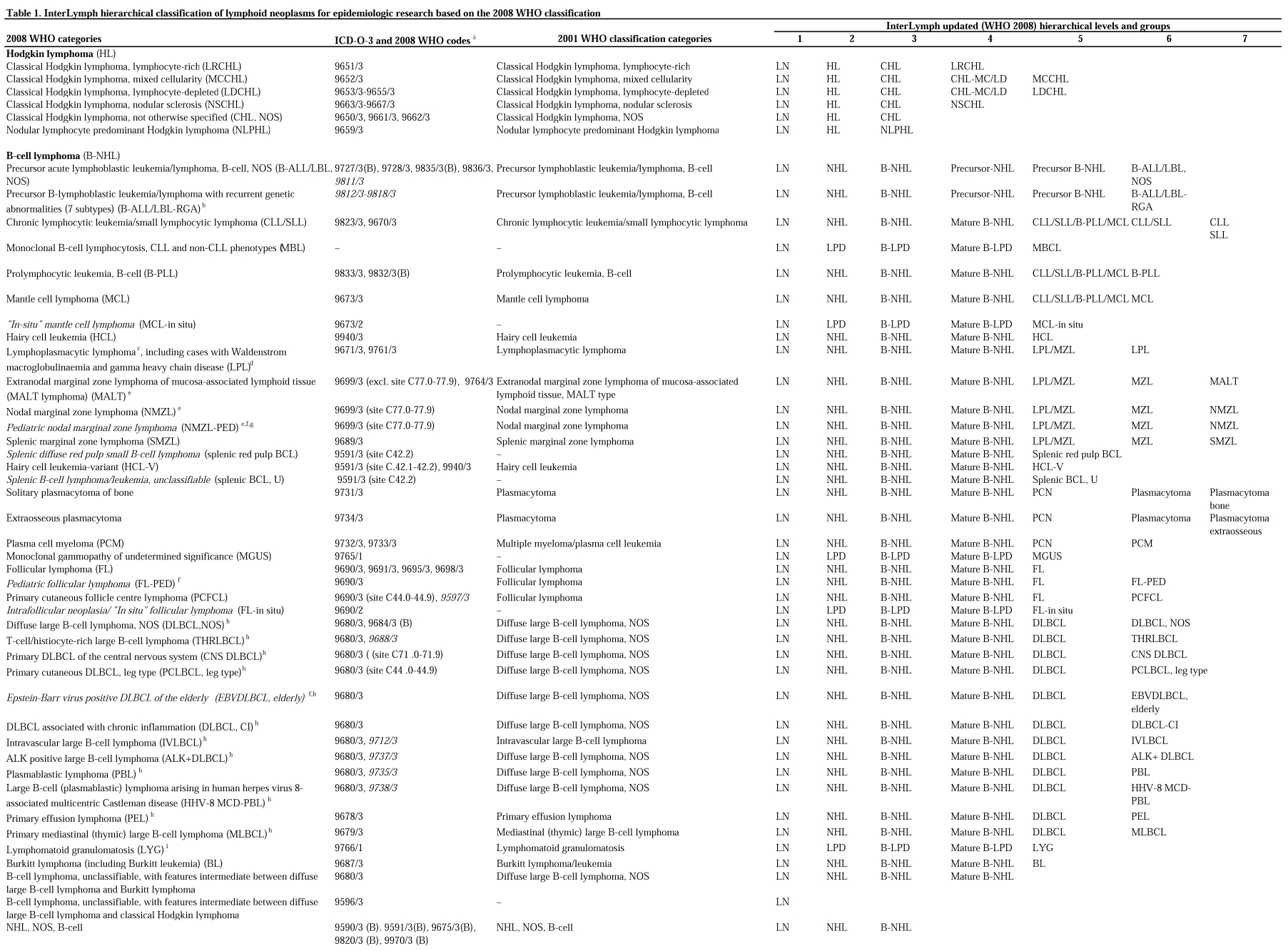
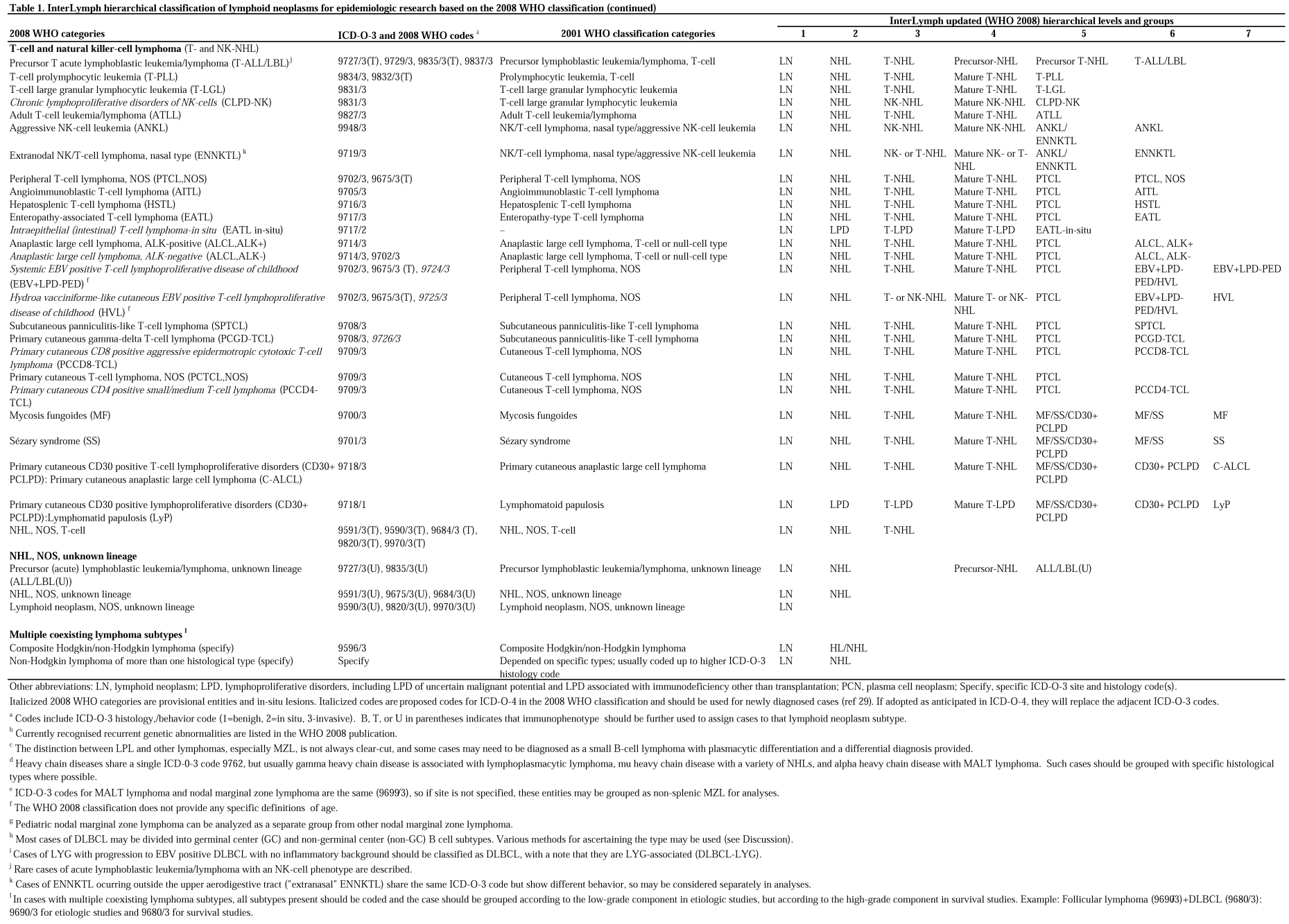
Tables 1 and 2 follow the same format as the prior InterLymph nested classification.1 The first 2 columns provide a list of the 2008 WHO categories and provisional categories, and the corresponding existing ICD-O-3 codes and 2008 WHO (proposed ICD-O-4) codes. The third column includes the previous 2001 WHO classification categories for historical comparison. The subsequent columns show the updated InterLymph (ie, WHO 2008-based) hierarchical levels, 1-7, with hierarchical groups of lymphoid neoplasms listed beneath each level. Level 1 includes all lymphoid neoplasms (LNs) but distinguishes the immunodeficiency-associated lymphoid neoplasms (LN-IDs). Level 2 separates Hodgkin lymphoma (HL) from non-Hodgkin lymphoma (NHL) and other LPDs.
Table 1.
InterLymph hierarchical classification of lymphoid neoplasms for epidemiologic research based on the 2008 WHO classification
Table 2.
InterLymph hierarchical classification of immunodeficiency-associated lymphoproliferative disorders for epidemiologic research based on the 2008 WHO classification*
Level 3 separates classical HL (CHL) from nodular lymphocyte predominant Hodgkin lymphoma (NLPHL). Nodular sclerosis CHL and lymphocyte-rich CHL are separated in level 4 from the remaining categories of CHL (ie, mixed cellularity and lymphocyte-depleted CHL), which are grouped in level 4 and separated in level 5.
For NHL and the LPDs, level 3 categories are distinguished by cell lineage. Subtypes are then grouped in level 4 by stage of differentiation into precursor cell lymphoma and mature NHL and LPD. Finally, the B-, T-, and natural killer (NK)–cell NHLs and LPDs are further characterized in levels 5-7, based on similarities in morphologic, phenotypic, genotypic, and clinical features into major NHL and LN-ID groups and, finally, more specific WHO subtypes.
The LN-IDs are grouped in level 5 of Table 2 as transplantation-associated (PTLD), or cases associated with primary immunodeficiency (PID-LPD), HIV infection (HIV-LPD), or iatrogenic immunosuppression (IAT-LPD), and are further separated in level 6. LN-ID cases that fall into distinct WHO lymphoma categories should, in addition, be classified and coded in their respective WHO categories in level 6.
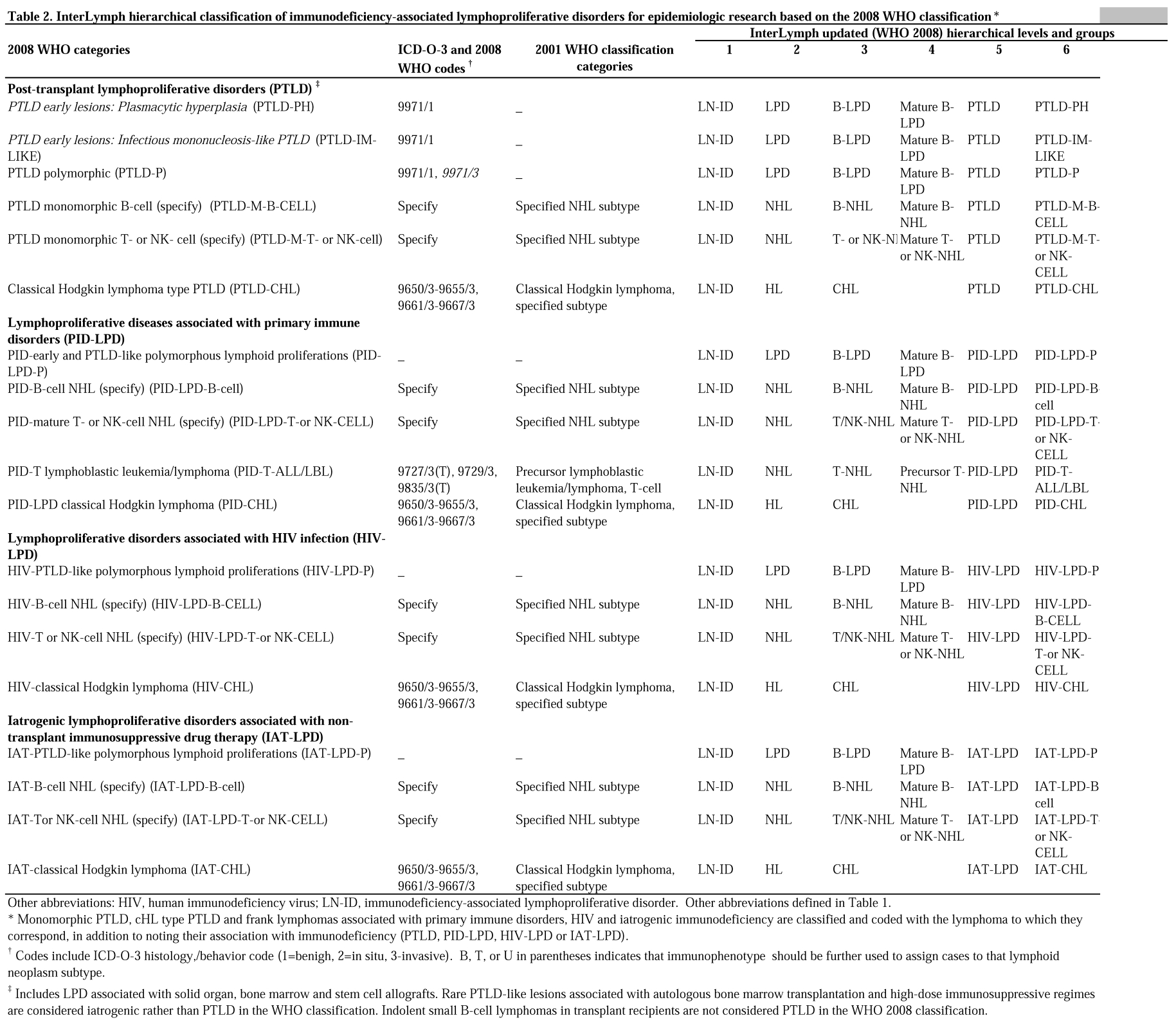
Table 3 describes pathology materials that may be used for epidemiologic studies of lymphoid neoplasms, to the extent that such techniques are available.
Table 3.
Pathology materials useful for epidemiologic studies of lymphoid neoplasms
| Applications | Material needed | Preferred specimen(s) | Collection media | Method(s) |
|---|---|---|---|---|
| Tumor cell phenotyping | Protein | Tumor tissue, blood lymphocytes, bone marrow aspirates, malignant effusions | FFPE tissue, frozen tissue, fresh cells in tissue culture media, or glass tubes with anticoagulant (EDTA or heparin) | Flow cytometry or immunohistochemistry |
| Chromosomal abnormalities, such as translocations, gains, deletions, gene amplifications | DNA | Tumor tissue | FFPE tissue, fresh tissue, blood cells, bone marrow aspirates | FISH, genomic SNP/CN arrays, real-time PCR, LDI-PCR, genome sequencing |
| Detection of tumor oncogene products, receptors for growth factors, cytokines | Protein | Tumor tissue, tissue microarrays | FFPE tissue, fresh or frozen tissue | Immunohistochemistry, Western blot, flow cytometry, real-time PCR |
| Quantitative gene expression | mRNA, cDNA | Tumor tissue | FFPE tissue, fresh or frozen tissue or cells; use of RNA preservative, such as RNA trizol | Real-time PCR, cDNA microarrays, other hybridization platforms, transcriptome sequencing |
| Detect single-stranded RNAs (typically 21-23 nucleotides long) that regulate gene expression | Micro-RNAs | Tumor tissue | FFPE tissue, fresh or frozen tissue or cells | Real-time PCR, quantitative PCR, Northern blot, miRNA-profiling |
| Detection of gene polymorphisms; single-nucleotide polymorphisms (SNPs); DNA sequencing | DNA | Blood leukocytes, buccal smears | Blood clots, buffy coats, buccal cell samples | Multiple genotyping and sequencing platforms |
| Viral antibody detection | Serum or plasma | Clotted blood or serum | Blood-collection tubes with EDTA or ACD | ELISA, real-time PCR, immunofluorescence |
| Serum markers, such as cytokines, growth factors, vitamin levels, chemicals, etc. | Serum or plasma | Peripheral blood | Blood-collection tube, glass with EDTA or ACD | ELISA, mass spectroscopy |
| Proteomic biomarkers | Serum or plasma | Peripheral blood | Blood-collection tube, glass with EDTA or ACD | Mass spectroscopy |
Abbreviations: FFPE, formalin-fixed paraffin embedded; FISH, fluorescence in situ hybridization; SNP, single-nucleotide polymorphism; CN, copy number; PCR, polymerase chain reaction; LDI-PCR, long-distance-inverse PCR; miRNA, micro-RNA; ACD, acid citrate dextrose; EDTA, ethylenediamine tetra-acetic acid; ELISA, enzyme-linked immunosorbent assay.
Discussion
To facilitate the epidemiologic study of lymphoid neoplasms, an updated InterLymph hierarchical classification for epidemiology has been developed based on the 2008 WHO classification. The classification incorporates new 2008 WHO entities and new hierarchical groups. We recommend classifying each case to the highest hierarchical level possible, thus allowing for analysis by WHO subtype, when such detail is available. However, when there are limitations in the pathology information or sample size, hierarchical groups can be used. By using these groups, it is also possible to explore etiologic similarities and differences among lymphoma subtypes. The groups are intended to be flexible and may be modified by individual studies to shed the greatest possible light on the hypotheses under evaluation. The use of groups also allows the pooling of epidemiologic data from diverse geographic areas, including studies carried out in less economically developed countries in which full WHO classification may not yet be in widespread use but might include populations of great interest because of exposure or genetic differences. The hierarchical groups also provide for inclusion in epidemiologic studies of study participants whose lymphoid neoplasms cannot be classified to a particular subtype, and thus remain classified as lymphoma, NOS, or are incompletely specified for various reasons, including an inadequate specimen, inadequate or unavailable ancillary investigations, or a genuinely difficult case. Lymphoma, NOS has represented 5%-15% of SEER-based cases in recent years1 and can potentially lead to an underestimation of subtype-specific distributions and possible bias of subtype-specific studies and analyses. Educational programs directed at pathologists and pathology residents in training may help to reduce this significant problem.
The use of specific hierarchical groupings depends primarily on the classification system and sample size. Current and future studies that include cases diagnosed after the introduction of the 2001 WHO classification should ideally use hierarchical level 3 or higher groups for HL analyses. Level 5 or higher should be used whenever possible for B-, T-, and NK-cell lymphoma, LPD, and LN-ID, particularly for mature B-cell NHL, which represents more than 90% of NHL worldwide. Higher levels allow the assessment of the more frequent subtypes. In studies using older classifications, including the Working Formulation (WF) and REAL classification, and ICD-O-1, ICO-O-2, ICD-9, and ICD-10 codes, it should be possible to directly compare lymphoma entities and groups where diagnostic reliability has been sufficiently high over time for statistical analysis in epidemiologic studies with large case numbers. For example, in the original InterLymph classification paper,1 we presented evidence from 4 studies that the reliability of classification of cases as DLBCL was 88.2% for WF category G, and for follicular lymphoma reliability was 88.9% for WF categories B-D. For lymphoma subtypes not yet defined in the classification used in a particular study, it would be necessary to analyze at lower hierarchical levels using the conversion rules presented in the original InterLymph WHO nested classification.1 For example, under these rules, cases from studies classified using the WF cannot be included in subtype analyses of marginal zone lymphoma (MZL), mantle-cell lymphoma, and most T-cell lymphomas, because these cases (albeit in small numbers) were, at that time, unrecognized within various WF categories. Historical comparisons are important in long-running studies, such as cohort studies, in descriptive studies of time trends, and comparing studies from different eras. This includes studies examining the impact on survival time trends of new therapeutic interventions, such as the introduction of rituximab for treatment of DLBCL and follicular lymphoma (FL), new treatment options in multiple myeloma, and the introduction of other novel therapies.
The updated InterLymph hierarchical classification for epidemiology includes several notable changes from the original InterLymph classification scheme. Nodular lymphocyte-rich classical HL is separated from mixed cellularity and lymphocyte-depleted classical HL, based on differences in clinical behavior, morphology, and association with Epstein-Barr virus (EBV). EBV-related HL appears to differ etiologically from non–EBV-related HL, and epidemiologic study of HL cases according to EBV status may be considered as an alternative to the study of traditional WHO HL subtypes.14,15
Newly defined groupings within the InterLymph NHL categories include lymphoplasmacytic lymphoma/marginal zone lymphoma (LPL/MZL), reflecting the observation in the 2008 WHO publication that the distinction between LPL and other subtypes of small-cell lymphoma with plasmacytic differentiation, especially some MZLs, is not always clear cut, and “some cases may need to be diagnosed as a small B-cell lymphoma with plasmacytic differentiation and a differential diagnosis provided.”16 This misclassification is especially likely in epidemiologic studies for which full clinical information is not always available. Because the ICD-O-3 code for nodal marginal zone lymphoma (NMZL) and extranodal marginal zone lymphoma (MALT) is the same, these cases may need to be grouped for analyses as nonsplenic MZL, if the site is not known. The increasing evidence that etiologic factors may vary by site of extranodal MZL8,17,18 suggests that these differences could be considered in future WHO and ICD-O classifications.
In level 5 of the mature T- and NK-cell lymphomas, we continue to separate a group of entities that present with leukemic/disseminated disease, including T-cell prolymphocytic leukemia, T-cell large granular lymphocytic leukemia, chronic lymphoproliferative disease of NK-cells, and adult T-cell leukemia/lymphoma with its strong association with human T-lymphotropic virus 1. The group of extranodal NK/T-cell lymphoma and aggressive NK-cell leukemia is also separated in level 5 because of the NK/T- and NK-cell phenotypes, respectively, and the strong association of each with EBV. Extranodal NK/T-cell lymphoma with an extranasal presentation shares the same ICD-O-3 code with the nasal type and is included in the same group. However, major clinical differences in these entities have been noted,19 and so, we suggest that extranasal cases may be considered separately in some analyses. From the increasing number of subtypes of T-cell lymphoma presenting in the skin, a new group has been created in level 5, comprising mycosis fungoides, Sézary syndrome, and primary cutaneous CD30-positive T-cell lymphoproliferative disorders, which may be amenable to epidemiologic study because of overlap in phenotypic, genotypic, and clinical features.20 The remaining mature T-cell lymphomas are grouped as “peripheral T-cell lymphoma” in level 5, because few epidemiologic studies would have adequate numbers of the T-cell entities in levels 6 and 7 for meaningful analysis. To date, there have been few epidemiologic studies of T-, NK-, and NK/T-cell lymphoma21 or of mycosis fungoides or other peripheral T-cell lymphomas,22 and these studies have included small numbers of cases.
A key new feature of the updated InterLymph classification is the inclusion of early and “in situ” lesions and other disorders that may have a limited capacity for progression, as defined in the 2008 WHO classification. These lesions are not considered reportable to most cancer registries as invasive cancers and, thus, are mostly relevant to specialized clinical and epidemiologic studies. They are included because of the insight they provide into early lymphomagenesis. Some of these lesions are identified by clinical features combined with flow cytometry (monoclonal B-cell lymphocytosis; MBL) or serum-protein electrophoresis (monoclonal gammopathy of uncertain significance; MGUS). Etiologic investigations have only been undertaken recently, but promising new leads have been suggested for MGUS.23,24 Also included are “in situ” counterparts of WHO entities, as well as lymphomatoid granulomatosis, lymphomatoid papulosis, and some of the immunodeficiency-associated lymphoproliferative disorders. It should be noted that in the current WHO classification, definitions of MBL versus CLL and MGUS versus other plasma-cell neoplasms have changed, and polymorphous posttransplantation lymphoproliferative disorder has been given a malignant 2008 WHO (proposed ICD-O-4) code. With the exception of lymphomatoid papulosis, these early and “in situ” lesions have not been placed in hierarchical groups, but this can be done in studies that identify significant numbers of these cases.
In the previous nested classification scheme, we did not address the immunodeficiency-associated lymphoproliferative disorders defined in the 2001 and 2008 WHO classifications because of their complex relationship with other lymphoid neoplasms, the coding difficulties mentioned above, and the exclusion of these cases from most population-based epidemiologic studies. It is acknowledged that the reporting of these conditions will be incomplete and, in the iatrogenic immunodeficiency group, there are difficulties in distinguishing an immunosuppressive therapy effect from an underlying disease effect.25 However, population-based epidemiologic studies have shown that these conditions have distinct lymphoma profiles,26 and they have provided important insights into lymphomagenesis because of their association with oncogenic viral infections.27 We recommend that these conditions be noted as LN-ID (PTLD, PID-LPD, HIV-LPD, or IAT-LPD), if reported to registries, and also assigned 2008 WHO subtypes and ICD-O-3 codes, where available. It will be helpful if ICD-O-4 codes are assigned to all of these disorders in the future.
Cases diagnosed with more than one subtype of lymphoma presenting in the same or different sites at the same time, as well as over time, are of interest in epidemiologic studies. The different lymphoma subtypes may represent clonal evolution or transformation, as in the not uncommon simultaneous or sequential occurrence of FL and DLBCL in the same patient, or the lymphomas may be composed of unrelated clones. There is increasing awareness of the plasticity and transdifferentiation of lymphoid cells, for example, the development of clonally related histiocytic/dendritic cell sarcomas in patients with mature B-cell lymphomas,28 and there are numerous possible combinations of categories of NHL, HL, and other hematopoietic neoplasms in a patient. In etiologic studies, the low-grade NHL should be used in analyses when 2 codes are identified, as recommended in the previous InterLymph classification paper.1 However, in survival studies, we suggest that the high-grade lymphoma is likely more relevant because it determines both the natural history and treatment approach. The importance of separately reporting the simultaneous occurrence of low- and high-grade lymphoma, especially DLBCL with FL, is emphasized in the 2008 WHO classification. However, this situation is not, at present, addressed effectively in cancer registries, leading to a nonstandardized approach in different epidemiologic studies. Because of complex SEER rules regarding the assignment of multiple primary tumors versus one primary tumor, and the fact that only the higher numerical ICD-O histology coded will usually be recorded in cases regarded as having a single primary,29 cases with more than one subtype of lymphoma may not necessarily be identifiable in cancer registries and studies in which detailed pathology reports are not available. At present, only composite HL and NHL occurring together in one node or multiple lymph nodes in one lymph node region are recognized by a single “composite lymphoma” ICD-O-3 code (9596/3). Furthermore, the coexistence of multiple non-Hodgkin components is not accounted for under SEER rules if they occur concurrently, unless they are in entirely separate organs. The term “composite lymphoma” has been used broadly and variably in the pathology literature for various combinations of lymphoma subtypes, including more than one NHL type,30 and definitions have continued to evolve over time. To avoid confusion, we have instead used the term “multiple coexisting lymphoma subtypes,” and we recommend that all subtypes of lymphoma present in a patient should be included in the designation of the case within a cancer registry and for epidemiologic studies.
Future epidemiologic studies should be designed to incorporate the WHO 2008 and updated InterLymph classification systems. This requires morphologic assessment and immunophenotyping (ie, flow cytometry and/or immunohistochemistry) in nearly all cases, with cytogenetics, fluorescence in situ hybridization, and gene-rearrangement studies needed to confirm some diagnoses. Clinical information is also important for classification, including age at diagnosis, sites of disease, geographic origin, and known etiologic factors, such as autoimmune disorders, immunodeficiency, and infections. We also recommend the collection of tumor tissue for epidemiologic studies, as this will ensure the highest quality pathology classification by central review. Collection of peripheral blood for DNA, plasma, and serum will further expand the range of studies to address etiologic and prognostic questions. As disease classification evolves, these materials will be critical for updating studies. For example, the molecular subclassification of DLBCL into germinal center B-cell (GCB) and non-GCB/activated B-cell subtypes was originally done by gene expression profiling (GEP) on fresh frozen tissue.31,32 Although this is not mandatory in the 2008 WHO classification because GEP is not in routine use in many laboratories, immunohistochemical surrogates have been developed for this purpose,33,34 along with novel techniques for GEP on paraffin tissues.35,36 In addition, cytogenetic techniques could be used to refine GEP findings. For example, comparative genomic hybridization has been used to improve GEP survival prediction in DLBCL.37 It is also possible to examine cytogenetic changes shared by more than one histologic subtype, such as the use of fluorescence in situ hybridization and other techniques to study the t(14;18) translocation.38,39 These techniques can easily be incorporated in epidemiologic studies using stored biologic specimens. Recommendations for types of specimens to consider collecting are outlined in Table 3.
Epidemiologic studies investigating survival will also require clinical information, such as extent of disease,40 prognostic factors, such as the International Prognostic Index,41 Follicular Lymphoma International Prognostic Index,42 and Mantle Cell Lymphoma International Prognostic Index,43 as well as therapy and survival data. Furthermore, several therapeutic trials, which include central pathology review and survival information, are now also gathering epidemiologic information.
The 2008 WHO classification and the updated InterLymph hierarchical classification for lymphoid neoplasms still have limitations for epidemiologic studies. The 2008 WHO classification recognizes the importance of early and “in situ” lymphoid disorders. However, the static nature of this classification, used primarily for clinical decision making, in conjunction with a lack of reporting of early disorders to cancer registries, presents difficulties for identifying predictors for malignant progression, lack of progression, or regression of these disorders. A related issue is the changing, and somewhat arbitrary, nature of the demarcation between early or predisposing lesions and subsequent early malignant lesions, such as the distinction between monoclonal B-cell lymphocytosis and CLL. Modification, over time, of the minimal diagnostic criteria for a diagnosis of CLL attempts to clarify the early lesion from frank malignancy, but does not provide major new insights for optimal timing of treatment, predictors for progression, or strategies for prevention. The WHO 2008 classification has incorporated greater recognition of borderline categories with overlapping morphologic and immunophenotypic characteristics, such as mediastinal nodular sclerosis classical HL and primary mediastinal large B-cell lymphoma. More understanding is needed to resolve uncertainties about overlapping entities, and epidemiologic studies may contribute insights on risk-factor associations that may clarify further possible distinctions versus true overlap of such disorders. Similarly, problematic entities, such as lymphomas that include more than one subtype in a patient, and lymphoma subtypes, such as DLBCL and FL, that have been subdivided in the 2008 WHO classification because they are heterogeneous in nature, require further investigation that may include epidemiologic study as a key element in clarifying the biological significance of the current classification. New paradigms in classification may be needed to allow for detailed, systematic characterization of lymphoproliferative disorders at multiple points in time. This approach could provide key etiologic and clinical insights into predictors for progression, nonprogression, or regression, allow for flexibility in setting boundaries between potentially overlapping entities, improve understanding and contribute to the development of optimal treatments for lymphomas composed of more than one histology, and enhance etiologic and clinical decision making about how to consider potentially heterogeneous or homogeneous lymphoid neoplasms that are currently designated (possibly inappropriately) as single or multiple entities, respectively. Thus far, we have limited the InterLymph classification for epidemiology to lymphoid neoplasms, because that has been the focus of InterLymph. However, we recognize that tumors of myeloid cells and histiocytic and dendritic cells are related hematologic neoplasms that require much more epidemiologic research, and could be considered in future updates of this classification for epidemiology studies.
In conclusion, on behalf of the Pathology Working Group of the InterLymph Consortium, we have proposed an updated, nested classification of lymphoid neoplasms for epidemiologic research, based on the 2008 WHO classification. The classification is intended to provide epidemiologists with a practical approach to using the updated WHO classification. Although many of the new entities in the classification cannot be identified in historical series and cancer registries, current epidemiologic studies and registries should now make every effort to incorporate the 2008 WHO classification into their databases and analyses. InterLymph is committed to a periodic review of the lymphoma classification used in epidemiologic studies as our understanding of the biology of the various lymphoma subtypes continues to evolve.
Acknowledgments
This work was supported, in part, by the Intramural Research Program of the National Cancer Institute, National Institutes of Health, Department of Health and Human Services, Bethesda, MD, National Cancer Institute grants R01 CA92153 and R01 CA97274 (to J.R.C.), and National Institutes of Health grant P20RR018757 (M.E.K.).
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: All authors helped develop the updated classification and contributed to the final version of this report. J.J.T. drafted and revised the report; L.M.M., J.J.T., J.R.C., M.S.L., C.A.C., M.M., and D.D.W., with others, developed the original architecture of the InterLymph nested classification published in 2007; and M.E.K. designed Table 3.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jennifer J. Turner, Department of Histopathology, Douglass Hanly Moir Pathology, 14 Giffnock Ave, Macquarie Park, NSW 2113, Australia; e-mail: jtur8838@bigpond.net.au.
References
- 1.Morton LM, Turner JJ, Cerhan JR, et al. Proposed classification of lymphoid neoplasms for epidemiologic research from the Pathology Working Group of the International Lymphoma Epidemiology Consortium (InterLymph). Blood. 2007;110(2):695–708. doi: 10.1182/blood-2006-11-051672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jaffe ES, Harris NL, Stein H, Vardiman JW. Lyon, France: IARC Press; 2001. World Health Organization Classification of Tumours: Pathology and Genetics, Tumours of Hematopoietic and Lymphoid Tissues. [Google Scholar]
- 3.Percy C, Fritz A, Ries L. 3d ed. Cancer Statistics Branch, DCCPS, SEER Program, National Cancer Institute; 2001. Conversion of neoplasms by topography and morphology from the International Classification of Diseases for Oncology, second edition, to International Classification of Diseases for Oncology. [Google Scholar]
- 4.Morton LM, Wang SS, Cozen W, et al. Etiologic heterogeneity among non-Hodgkin lymphoma subtypes. Blood. 2008;112(13):5150–5160. doi: 10.1182/blood-2008-01-133587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang SS, Slager SL, Brennan P, et al. Family history of hematopoietic malignancies and risk of non-Hodgkin lymphoma (NHL): a pooled analysis of 10 211 cases and 11 905 controls from the International Lymphoma Epidemiology Consortium (InterLymph). Blood. 2007;109(8):3479–3488. doi: 10.1182/blood-2006-06-031948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kricker A, Armstrong BK, Hughes AM, et al. Personal sun exposure and risk of non-Hodgkin lymphoma: a pooled analysis from the Interlymph Consortium. Int J Cancer. 2008;122(1):144–154. doi: 10.1002/ijc.23003. [DOI] [PubMed] [Google Scholar]
- 7.Willett EV, Morton LM, Hartge P, et al. Non-Hodgkin lymphoma and obesity: a pooled analysis from the InterLymph Consortium. Int J Cancer. 2008;122(9):2062–2070. doi: 10.1002/ijc.23344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ekstrom Smedby K, Vajdic CM, Falster M, et al. Autoimmune disorders and risk of non-Hodgkin lymphoma subtypes: a pooled analysis within the InterLymph Consortium. Blood. 2008;111(8):4029–4038. doi: 10.1182/blood-2007-10-119974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Sanjose S, Benavente Y, Vajdic CM, et al. Hepatitis C and non-Hodgkin lymphoma among 4784 cases and 6269 controls from the International Lymphoma Epidemiology Consortium. Clin Gastroenterol Hepatol. 2008;6(4):451–458. doi: 10.1016/j.cgh.2008.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vajdic CM, Falster MO, de Sanjose S, et al. Atopic disease and risk of non-Hodgkin lymphoma: an InterLymph pooled analysis. Cancer Res. 2009;69(16):6482–6489. doi: 10.1158/0008-5472.CAN-08-4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sant M, Allemani C, De Angelis R, et al. Influence of morphology on survival for non-Hodgkin lymphoma in Europe and the United States. Eur J Cancer. 2008;44(4):579–587. doi: 10.1016/j.ejca.2007.12.016. [DOI] [PubMed] [Google Scholar]
- 12.Horner MJ, Ries LAG, Krapcho M, et al. Bethesda, MD: [Accessed April 30, 2010]. SEER Cancer Statistics Review, 1975-2006, National Cancer Institute. http://seer.cancer.gov/csr/1975_2006/, based on November 2008 SEER data submission, posted to the SEER website, 2009. [Google Scholar]
- 13.Swerdlow S, Campo E, Harris N. Lyon, France: IARC Press; 2008. World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues. [Google Scholar]
- 14.Jarrett RF. Risk factors for Hodgkin's lymphoma by EBV status and significance of detection of EBV genomes in serum of patients with EBV-associated Hodgkin's lymphoma. Leuk Lymphoma. 2003;44(suppl 3):S27–S32. doi: 10.1080/10428190310001623801. [DOI] [PubMed] [Google Scholar]
- 15.Hjalgrim H, Frisch M, Ekbom A, Kyvik KO, Melbye M, Green A. Cancer and diabetes—a follow-up study of two population-based cohorts of diabetic patients. J Intern Med. 1997;241(6):471–475. doi: 10.1111/j.1365-2796.1997.tb00004.x. [DOI] [PubMed] [Google Scholar]
- 16.Swerdlow SH, Berger F, Pileri SA, Harris NL, Jaffe ES, Stein H. Lymphoplasmacytic lymphoma. In: Swerdlow SH, Campo E, Harris NL, et al., editors. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th ed. Lyon, France: IARC; 2008. pp. 194–195. [Google Scholar]
- 17.Engels EA, Cerhan JR, Linet MS, et al. Immune-related conditions and immune-modulating medications as risk factors for non-Hodgkin's lymphoma: a case-control study. Am J Epidemiol. 2005;162(12):1153–1161. doi: 10.1093/aje/kwi341. [DOI] [PubMed] [Google Scholar]
- 18.Mbulaiteye SM, Hisada M, El-Omar EM. Helicobacter pylori–associated global gastric cancer burden. Front Biosci. 2009;14:1490–1504. doi: 10.2741/3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Au WY, Weisenburger DD, Intragumtornchai T, et al. Clinical differences between nasal and extranasal natural killer/T-cell lymphoma: a study of 136 cases from the International Peripheral T-Cell Lymphoma Project. Blood. 2009;113(17):3931–3937. doi: 10.1182/blood-2008-10-185256. [DOI] [PubMed] [Google Scholar]
- 20.Kadin ME. Pathobiology of CD30+ cutaneous T-cell lymphomas. J Cutan Pathol. 2006;33(1 suppl):10–17. doi: 10.1111/j.0303-6987.2006.00507.x. [DOI] [PubMed] [Google Scholar]
- 21.Xu JX, Hoshida Y, Yang WI, et al. Life-style and environmental factors in the development of nasal NK/T-cell lymphoma: a case-control study in East Asia. Int J Cancer. 2007;120(2):406–410. doi: 10.1002/ijc.22313. [DOI] [PubMed] [Google Scholar]
- 22.Morales-Suarez-Varela MM, Olsen J, Johansen P, et al. Occupational risk factors for mycosis fungoides: a European multicenter case-control study. J Occup Environ Med. 2004;46(3):205–211. doi: 10.1097/01.jom.0000116819.01813.8c. [DOI] [PubMed] [Google Scholar]
- 23.Brown LM, Gridley G, Check D, Landgren O. Risk of multiple myeloma and monoclonal gammopathy of undetermined significance among white and black male United States veterans with prior autoimmune, infectious, inflammatory, and allergic disorders. Blood. 2008;111(7):3388–3394. doi: 10.1182/blood-2007-10-121285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Landgren O, Kyle RA, Hoppin JA, et al. Pesticide exposure and risk of monoclonal gammopathy of undetermined significance in the Agricultural Health Study. Blood. 2009;113(25):6386–6391. doi: 10.1182/blood-2009-02-203471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baecklund E, Backlin C, Iliadou A, et al. Characteristics of diffuse large B cell lymphomas in rheumatoid arthritis. Arthritis Rheum. 2006;54(12):3774–3781. doi: 10.1002/art.22277. [DOI] [PubMed] [Google Scholar]
- 26.Vajdic CM, Van Leeuwen MT, Turner JJ, et al. No excess risk of follicular lymphoma in kidney transplant and HIV-related immune deficiency. Int J Cancer. 2010;127(11):2732–2735. doi: 10.1002/ijc.25272. [DOI] [PubMed] [Google Scholar]
- 27.Bouvard V, Baan R, Straif K, et al. A review of human carcinogens—Part B: biological agents. Lancet Oncol. 2009;10(4):321–322. doi: 10.1016/s1470-2045(09)70096-8. [DOI] [PubMed] [Google Scholar]
- 28.Feldman AL, Arber DA, Pittaluga S, et al. Clonally related follicular lymphomas and histiocytic/dendritic cell sarcomas: evidence for transdifferentiation of the follicular lymphoma clone. Blood. 2008;111(12):5433–5439. doi: 10.1182/blood-2007-11-124792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnson CH, Adamo M, Peace S, Percy-Laurry A. Hematopoietic and Lymphoid Neoplasm Case Reportability and Coding Manual. Bethesda, MD: National Cancer Institute; 2010. [Accessed April 30, 2010]. 20892-8316. http://seer.cancer.gov/tools/heme/index.html. [Google Scholar]
- 30.Kim H, Hendrickson R, Dorfman RF. Composite lymphoma. Cancer. 1977;40(3):959–976. doi: 10.1002/1097-0142(197709)40:3<959::aid-cncr2820400302>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 31.Alizadeh AA, Eisen MB, Davis RE, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000;403(6769):503–511. doi: 10.1038/35000501. [DOI] [PubMed] [Google Scholar]
- 32.Rosenwald A, Wright G, Chan WC, et al. The use of molecular profiling to predict survival after chemotherapy for diffuse large-B-cell lymphoma. N Engl J Med. 2002;346(25):1937–1947. doi: 10.1056/NEJMoa012914. [DOI] [PubMed] [Google Scholar]
- 33.Hans CP, Weisenburger DD, Greiner TC, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103(1):275–282. doi: 10.1182/blood-2003-05-1545. [DOI] [PubMed] [Google Scholar]
- 34.Choi WW, Weisenburger DD, Greiner TC, et al. A new immunostain algorithm classifies diffuse large B-cell lymphoma into molecular subtypes with high accuracy. Clin Cancer Res. 2009;15(17):5494–5502. doi: 10.1158/1078-0432.CCR-09-0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roberts RA, Sabalos CM, LeBlanc ML, et al. Quantitative nuclease protection assay in paraffin-embedded tissue replicates prognostic microarray gene expression in diffuse large-B-cell lymphoma. Lab Invest. 2007;87(10):979–997. doi: 10.1038/labinvest.3700665. [DOI] [PubMed] [Google Scholar]
- 36.Rimsza LM, Leblanc ML, Unger JM, et al. Gene expression predicts overall survival in paraffin-embedded tissues of diffuse large B-cell lymphoma treated with R-CHOP. Blood. 2008;112(8):3425–3433. doi: 10.1182/blood-2008-02-137372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bea S, Zettl A, Wright G, et al. Diffuse large B-cell lymphoma subgroups have distinct genetic profiles that influence tumor biology and improve gene-expression–based survival prediction. Blood. 2005;106(9):3183–3190. doi: 10.1182/blood-2005-04-1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chiu BC, Lan Q, Dave BJ, Blair A, Zahm SH, Weisenburger DD. The utility of t(14;18) in understanding risk factors for non-Hodgkin lymphoma. J Natl Cancer Inst Monogr. 2008;(39):69–73. doi: 10.1093/jncimonographs/lgn018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leich E, Salaverria I, Bea S, et al. Follicular lymphomas with and without translocation t(14;18) differ in gene expression profiles and genetic alterations. Blood. 2009;114(4):826–834. doi: 10.1182/blood-2009-01-198580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Edge SE, Byrd DR, Carducci MA, Compton CA, editors. AJCC Cancer Staging Manual. 7th ed. New York, NY: Springer; 2010. [Google Scholar]
- 41.The International Non-Hodgkin's Lymphoma Prognostic Factors Project. A predictive model for aggressive non-Hodgkin's lymphoma. N Engl J Med. 1993;329(14):987–994. doi: 10.1056/NEJM199309303291402. [DOI] [PubMed] [Google Scholar]
- 42.Solal-Celigny P, Roy P, Colombat P, et al. Follicular lymphoma international prognostic index. Blood. 2004;104(5):1258–1265. doi: 10.1182/blood-2003-12-4434. [DOI] [PubMed] [Google Scholar]
- 43.Hoster E, Dreyling M, Klapper W, et al. A new prognostic index (MIPI) for patients with advanced-stage mantle cell lymphoma. Blood. 2008;111(2):558–565. doi: 10.1182/blood-2007-06-095331. [DOI] [PubMed] [Google Scholar]