Abstract
Previous studies suggested that natural killer (NK) cells are involved in the regulation of the growth and differentiation of pluripotent hematopoietic stem cells. To establish whether the effector cells responsible for the in vivo resistance to bone marrow (BM) transplants and the in vitro inhibition of colony-forming units (CFU) may represent identical or overlapping populations, we used a rat system for syngeneic BM transplantation, with and without the transfer of large numbers of peripheral blood large granular lymphocytes (LGLs). BM reconstitution was measured by the in vivo formation of syngeneic CFU in the spleen (CFU-s). Because of the very low frequency of CFU-s in normal rat BM, we fractionated BM cells in Percoll density gradients, which provided a 2- to 5-fold enrichment in CFU-s in the lower-density fractions. Although these fractions contained less than 10% of the total cells, they contained greater than 75% of the CFU-s and allowed for the transfer of significantly fewer donor cells. At the time of BM transplantation, radiation-resistant asialoganglioside GM1-positive LGLs, with high NK activity, accounted for a significant percentage of the lymphoid cells in the irradiated recipient. The in vivo regulatory role of these cells on engraftment was demonstrated by their depletion (by i.v. injection of small amounts of anti-asialo-GM1 antiserum before BM transplantation), which resulted in a significant increase in the number of CFU-s. Conversely, a 50% inhibition in CFU-s was found when CFU-s-enriched BM fractions were preincubated in vitro with LGLs. Additional experiments, involving selective in vivo depletion of NK cells followed by LGL repopulation, directly demonstrated the involvement of LGLs in the regulation and growth of syngeneic pluripotent hematopoietic stem cells. Our results further support the hypothesis that LGLs are involved directly or via humoral factors in the homeostasis and regulation of hematopoietic stem cell growth and differentiation.
Full text
PDF

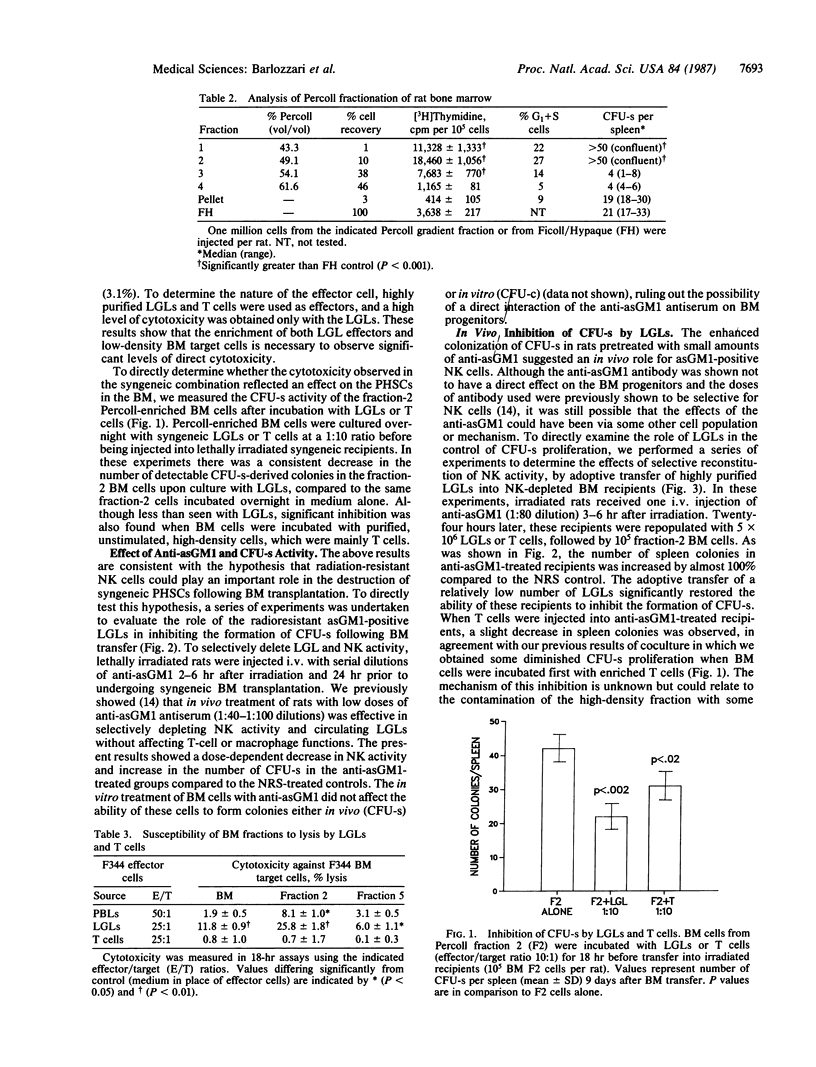
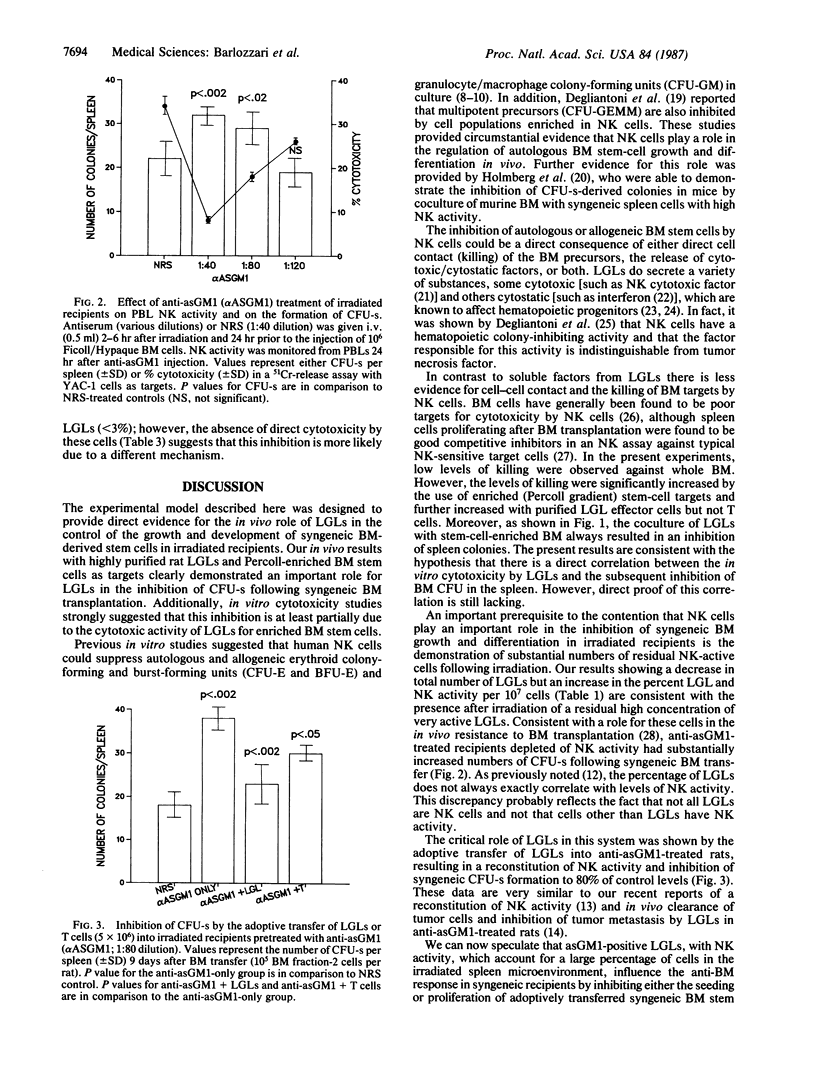

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alarcón B., Fresno M. Specific effect of anti-transferrin antibodies on natural killer cells directed against tumor cells. Evidence for the transferrin receptor being one of the target structures recognized by NK cells. J Immunol. 1985 Feb;134(2):1286–1291. [PubMed] [Google Scholar]
- Barlozzari T., Leonhardt J., Wiltrout R. H., Herberman R. B., Reynolds C. W. Direct evidence for the role of LGL in the inhibition of experimental tumor metastases. J Immunol. 1985 Apr;134(4):2783–2789. [PubMed] [Google Scholar]
- Barlozzari T., Reynolds C. W., Herberman R. B. In vivo role of natural killer cells: involvement of large granular lymphocytes in the clearance of tumor cells in anti-asialo GM1-treated rats. J Immunol. 1983 Aug;131(2):1024–1027. [PubMed] [Google Scholar]
- Daley J. P., Nakamura I. Natural resistance of lethally irradiated F1 hybrid mice to parental marrow grafts is a function of H-2/Hh-restricted effectors. J Exp Med. 1984 Apr 1;159(4):1132–1148. doi: 10.1084/jem.159.4.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degliantoni G., Murphy M., Kobayashi M., Francis M. K., Perussia B., Trinchieri G. Natural killer (NK) cell-derived hematopoietic colony-inhibiting activity and NK cytotoxic factor. Relationship with tumor necrosis factor and synergism with immune interferon. J Exp Med. 1985 Nov 1;162(5):1512–1530. doi: 10.1084/jem.162.5.1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degliantoni G., Perussia B., Mangoni L., Trinchieri G. Inhibition of bone marrow colony formation by human natural killer cells and by natural killer cell-derived colony-inhibiting activity. J Exp Med. 1985 May 1;161(5):1152–1168. doi: 10.1084/jem.161.5.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goss G. D., Wittwer M. A., Bezwoda W. R., Herman J., Rabson A., Seymour L., Derman D. P., Mendelow B. Effect of natural killer cells on syngeneic bone marrow: in vitro and in vivo studies demonstrating graft failure due to NK cells in an identical twin treated by bone marrow transplantation. Blood. 1985 Nov;66(5):1043–1046. [PubMed] [Google Scholar]
- Hansson M., Beran M., Andersson B., Kiessling R. Inhibition of in vitro granulopoiesis by autologous allogeneic human NK cells. J Immunol. 1982 Jul;129(1):126–132. [PubMed] [Google Scholar]
- Hansson M., Kiessling R., Andersson B. Human fetal thymus and bone marrow contain target cells for natural killer cells. Eur J Immunol. 1981 Jan;11(1):8–12. doi: 10.1002/eji.1830110103. [DOI] [PubMed] [Google Scholar]
- Hansson M., Kiessling R., Andersson B., Kärre K., Roder J. NK cell-sensitive T-cell subpopulation in thymus: inverse correlation to host NK activity. Nature. 1979 Mar 8;278(5700):174–176. doi: 10.1038/278174a0. [DOI] [PubMed] [Google Scholar]
- Herberman R. B., Nunn M. E., Lavrin D. H. Natural cytotoxic reactivity of mouse lymphoid cells against syngeneic acid allogeneic tumors. I. Distribution of reactivity and specificity. Int J Cancer. 1975 Aug 15;16(2):216–229. doi: 10.1002/ijc.2910160204. [DOI] [PubMed] [Google Scholar]
- Hiserodt J. C., Laybourn K. A., Varani J. Expression of a laminin-like substance on the surface of murine natural killer (NK) lymphocytes and its role in NK recognition of tumor target cells. J Immunol. 1985 Aug;135(2):1484–1487. [PubMed] [Google Scholar]
- Hochman P. S., Cudkowicz G., Dausset J. Decline of natural killer cell activity in sublethally irradiated mice. J Natl Cancer Inst. 1978 Jul;61(1):265–268. doi: 10.1093/jnci/61.1.265. [DOI] [PubMed] [Google Scholar]
- Holmberg L. A., Miller B. A., Ault K. A. The effect of natural killer cells on the development of syngeneic hematopoietic progenitors. J Immunol. 1984 Dec;133(6):2933–2939. [PubMed] [Google Scholar]
- Kiessling R., Hochman P. S., Haller O., Shearer G. M., Wigzell H., Cudkowicz G. Evidence for a similar or common mechanism for natural killer cell activity and resistance to hemopoietic grafts. Eur J Immunol. 1977 Sep;7(9):655–663. doi: 10.1002/eji.1830070915. [DOI] [PubMed] [Google Scholar]
- Klimpel G. R., Fleischmann W. R., Jr, Klimpel K. D. Gamma interferon (IFN gamma) and IFN alpha/beta suppress murine myeloid colony formation (CFU-C)N: magnitude of suppression is dependent upon level of colony-stimulating factor (CSF). J Immunol. 1982 Jul;129(1):76–80. [PubMed] [Google Scholar]
- Mangan K. F., Hartnett M. E., Matis S. A., Winkelstein A., Abo T. Natural killer cells suppress human erythroid stem cell proliferation in vitro. Blood. 1984 Feb;63(2):260–269. [PubMed] [Google Scholar]
- Nunn M. E., Herberman R. B., Holden H. T. Natural cell-mediated cytotoxicity in mice against non-lymphoid tumor cells and some normal cells. Int J Cancer. 1977 Sep 15;20(3):381–387. doi: 10.1002/ijc.2910200309. [DOI] [PubMed] [Google Scholar]
- O'Brien T., Kendra J., Stephens H., Knight R., Barrett A. J. Recognition and regulation of progenitor marrow elements by NK cells in the mouse. Immunology. 1983 Aug;49(4):717–725. [PMC free article] [PubMed] [Google Scholar]
- Pistoia V., Ghio R., Nocera A., Leprini A., Perata A., Ferrarini M. Large granular lymphocytes have a promoting activity on human peripheral blood erythroid burst-forming units. Blood. 1985 Feb;65(2):464–472. [PubMed] [Google Scholar]
- Reynolds C. W., Timonen T., Herberman R. B. Natural killer (NK) cell activity in the rat. I. Isolation and characterization of the effector cells. J Immunol. 1981 Jul;127(1):282–287. [PubMed] [Google Scholar]
- Riccardi C., Santoni A., Barlozzari T., Herberman R. B. In vivo reactivity of mouse natural killer (NK) cells against normal bone marrow cells. Cell Immunol. 1981 May 1;60(1):136–143. doi: 10.1016/0008-8749(81)90254-9. [DOI] [PubMed] [Google Scholar]
- Rolstad B., Ford W. L. The rapid elimination of allogeneic lymphocytes: relationship to established mechanisms of immunity and to lymphocyte traffic. Immunol Rev. 1983;73:87–113. doi: 10.1111/j.1600-065x.1983.tb01080.x. [DOI] [PubMed] [Google Scholar]
- SIMINOVITCH L., MCCULLOCH E. A., TILL J. E. THE DISTRIBUTION OF COLONY-FORMING CELLS AMONG SPLEEN COLONIES. J Cell Physiol. 1963 Dec;62:327–336. doi: 10.1002/jcp.1030620313. [DOI] [PubMed] [Google Scholar]
- Stern P., Gidlund M., Orn A., Wigzell H. Natural killer cells mediate lysis of embryonal carcinoma cells lacking MHC. Nature. 1980 May 29;285(5763):341–342. doi: 10.1038/285341a0. [DOI] [PubMed] [Google Scholar]
- Timonen T., Ortaldo J. R., Herberman R. B. Characteristics of human large granular lymphocytes and relationship to natural killer and K cells. J Exp Med. 1981 Mar 1;153(3):569–582. doi: 10.1084/jem.153.3.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner J. F., Dennert G. Effects of a cloned cell line with NK activity on bone marrow transplants, tumour development and metastasis in vivo. Nature. 1982 Nov 4;300(5887):31–34. doi: 10.1038/300031a0. [DOI] [PubMed] [Google Scholar]
- Wiltrout R. H., Taramelli D., Holden H. T. Measurement of macrophage-mediated cytotoxicity against adherent and non-adherent target cells by release of 11 indium-oxine. J Immunol Methods. 1981;43(3):319–331. doi: 10.1016/0022-1759(81)90180-0. [DOI] [PubMed] [Google Scholar]
- Wright S. C., Bonavida B. Studies on the mechanism of natural killer (NK) cell-mediated cytotoxicity (CMC). I. Release of cytotoxic factors specific for NK-sensitive target cells (NKCF) during co-culture of NK effector cells with NK target cells. J Immunol. 1982 Jul;129(1):433–439. [PubMed] [Google Scholar]
- Zoumbos N. C., Djeu J. Y., Young N. S. Interferon is the suppressor of hematopoiesis generated by stimulated lymphocytes in vitro. J Immunol. 1984 Aug;133(2):769–774. [PubMed] [Google Scholar]


