In this study the authors address the mechanistic basis of the therapeutic benefit afforded by treatment with the naturally occurring small molecule sulforaphane in the context of a keratin-based skin blistering disease, epidermolysis bullosa simplex.
Abstract
Treatment with the natural chemical sulforaphane (SF) ameliorates skin blistering in keratin 14 (K14)-deficient mice, correlating with the induction of K16 and K17 in the basal layer of epidermis (Kerns et al., PNAS 104:14460, 2007). Here we address the basis for the SF-mediated K16 and K17 induction in mouse epidermis in vivo. As expected, induction of K16 partly depends on the transcription factor Nrf2, which is activated by SF exposure. Strikingly, K17 induction occurs independently of Nrf2 activity and parallels the decrease in glutathione occurring shortly after epidermal exposure to SF. Pharmacological manipulation of glutathione levels in mouse epidermis in vivo alters K17 and K16 expression in the expected manner. We present findings suggesting that select MAP kinases participate in mediating the Nrf2- and glutathione-dependent alterations in K16 and K17 levels in SF-treated epidermis. These findings advance our understanding of the effect of SF on gene expression in epidermis, point to a role for glutathione in mediating some of these effects, and establish that SF induces the expression of two contiguous and highly related genes, K16 and K17, via distinct mechanisms.
INTRODUCTION
Diets rich in cruciferous vegetables correlate with a reduced risk of developing various cancers in the human population (Talalay and Fahey, 2001; Hayes et al., 2008; Zhang et al., 2009). The general mechanism accounting for this benefit is thought to involve the inhibition and activation of phase 1 and phase 2 metabolic enzymes, respectively, causing a deactivation and/or hastened disposal of potential carcinogens (Kobayashi and Yamamoto, 2006; Myzak and Dashwood, 2006; Tan and Spivack, 2009). Isolation and identification of the isothiocyanate sulforaphane (SF) as the chemopreventive agent in a precursor form, in broccoli sprout extracts (Zhang et al., 1992) paved the way for the discovery of the Nrf2-Keap1-ARE pathway of transcriptional regulation, which mediates these effects at the molecular level (Talalay and Fahey, 2001; Kobayashi and Yamamoto, 2006). Nrf2, a cap'n'collar bZIP family transcription factor (Moi et al., 1994), activates gene expression after binding to antioxidant response elements (AREs) located in or near the promoter region of its target genes (McMahon et al., 2001; Wakabayashi et al., 2004). Nrf2 is normally sequestered in the cytoplasm by the cysteine-rich Keap1 protein, a substrate adaptor protein for a Cul3-dependent ubiquitin ligase complex that targets Nrf2 for proteasome-mediated degradation (Zhang and Gordon, 2004). SF and other isothiocyanates allow for Nrf2 translocation to the nucleus (and Nrf2-dependent transcription) by reacting with thiol groups in Keap1, inactivating it (Dinkova-Kostova et al., 2002). The full extent of the transcriptional program regulated by SF or Nrf2-Keap1-ARE and its biological implications and therapeutic potential appear underestimated at present.
We reported that treatment with SF markedly reduces the skin blistering and morbidity associated with a keratin 14 (K14) deficiency in neonatal mice (Kerns et al., 2007). Normally, K14 copolymerizes with its obligatory partner protein K5 to yield 10-nm intermediate filaments that, once organized into a pan-cytoplasmic network, provide crucial structural support in basal keratinocytes of epidermis and related stratified epithelia (Coulombe et al., 2009). Partial or complete loss of either K5 or K14 function (e.g., through null mutations or dominantly acting missense alleles) renders basal keratinocytes fragile and accounts for epidermolysis bullosa simplex, a rare inherited bullous disease in which the basal layer of epidermis, and occasionally related surface epithelia, rupture readily in response to trivial mechanical trauma (Fine et al., 2008; Coulombe et al., 2009). The benefit afforded by SF treatment in K14 null neonatal mice likely involves the induction of K16 and K17, two keratins highly related to K14 both in primary structure and properties (Kerns et al., 2007). In this setting, K16 and especially K17 are thought to act as surrogate keratins which, owing to their ability to copolymerize efficiently with partner K5, compensate for the loss of K14 protein (Kerns et al., 2007; Coulombe et al., 2009).
Broccoli sprout extracts rich in SF, the natural product form in which known amounts of the latter can be safely delivered to humans (Shapiro et al., 2006; Cornblatt et al., 2007), represents a form of therapy worth considering for epidermolysis bullosa simplex and related conditions (Kerns et al., 2007; Coulombe et al., 2009). Several issues must be addressed in preparation for clinical trials testing this idea. Here, we investigate the mechanisms that account for the induction of K16 and K17 expression in mouse skin keratinocytes treated with SF in vivo.
MATERIALS AND METHODS
Animals, Antibodies, and Chemicals
Studies involving mice were approved by the Johns Hopkins University Institutional Animal Care and Use Committee. SKH-1 hairless mice were purchased (Charles River Laboratories, Wilmington, MA). Nrf2−/− and hypomorphic Keap1 mice were maintained as described (Chan et al., 1996; Osburn et al., 2008). Antibodies used included rabbit polyclonals against K6, K16, and K17 (P.A.C. laboratory) and a mouse monoclonal anti-actin (Sigma, St. Louis, MO). Fluorochrome-conjugated goat anti-rabbit antibodies were from Kirkegaard & Perry Laboratories (Gaithersburg, MD). SF [1-isothiocyanato-4(R,S)-(methylsulfinyl)butane] was obtained from LKT Laboratories (St. Paul, MN). Solutions of SF in jojoba oil (MP Biomedicals, Solon, OH) were prepared on the day of application. Glutathione monoethyl ester, c-Jun N-terminal kinase (JNK) II inhibitor SP60015, p38 inhibitor SB202190, and extracellular signal–regulated kinase (ERK)1/2 inhibitor UO126 were from Calbiochem (San Diego, CA). Mitogen-activated protein (MAP) kinase inhibitors were diluted in 1:250 acetone:oil and made fresh before each application. l-Buthionine-sulfoximine was from Sigma (St. Louis, MO).
Mouse Studies
Seven-week-old female Nrf2−/− SKH-1 hairless were topically treated with 1 μmol SF in 100 μl jojoba oil once a day for 4 d and killed on day 5. For keratin expression studies mice were treated four times, 48 h apart, and killed 24 h after the last treatment. Neonatal Nrf2−/− mice in the C57BL/6 background were treated with 1 μmol of SF in 100 μl jojoba oil daily on postnatal day 5 (P5), P6, and P7 and killed on P8, and their dorsal skin was harvested for study. Seven-week-old SKH-1 female hairless mice were given four i.p. injections of either 50 μmol l-buthionine-sulfoximine (BSO) in 100 μl jojoba oil or 5 μmol glutathione monoethyl ester (GME) in 100 μl jojoba oil, whereas a subset received a topical treatment of 1 μmol SF in 100 μl jojoba oil on the second, third, and fourth day. On the fifth day dorsal skin was harvested for analysis. Seven-week-old female SKH-1 hairless mice and Nrf2−/− SKH-1 hairless mice were treated with 10 μM SB20219, 100 μM SP600125, 100 μM UO126, or vehicle control (100 μl of acetone:oil 1:250) 30 min before and 30 min after topical treatment with 1 μmol SF in 100 μl jojoba oil daily for 2 d and were killed on day 3. Five-week-old female hypomorphic Keap1 C57BL/6 mice were killed, and tissue was analyzed as described.
Biochemical Analyses
RNA was extracted from full-thickness skin of wild-type C57BL/6 and hypomorphic Keap1 C57BL/6 mice using TRIzol according to the manufacturer's instructions (Invitrogen, Carlsbad, CA). After DNAse treatment (RNase free DNase kit; Qiagen, Valencia, CA), 1 μg of total RNA from each phenotype was reverse-transcribed (Advantage RT-for PCR kit; Clontech, Mountain View, CA). First-strand cDNA was used for PCR analysis of gene expression using Invitrogen Taq. Target mRNAs and oligonucleotide primer sets are described in Kerns et al. (2007). Intensity of electrophoresed, ethidium bromide–stained PCR products was quantified using the NIH ImageJ freeware (http://rsb.info.nih.gov/ij/). For protein analysis, dorsal skin was harvested from control and treated Nrf2−/− SKH-1 hairless mice, and total protein was extracted (Paladini and Coulombe, 1999). Protein concentrations were obtained by using a Bradford Assay Kit (Bio-Rad, Hercules, CA). Equal amounts of protein (10–20 μg) were resolved on 8.5% SDS/PAGE gels and blotted onto nitrocellulose. Bound primary antibodies were detected via enhanced chemiluminescence (Amersham Pharmacia Biotech, Pittsburgh, PA). For total glutathione measurements, full-thickness dorsal skin was flash-frozen in liquid nitrogen and pulverized into a fine powder. A total of 10 volumes of 5% 5-sulfosalicylic acid solution was added to 0.1–0.3 g of tissue powder. Samples were then homogenized with 3 ml PTFE pestle in glass tube, stored at 4°C for 10 min, and then centrifuged at 10,000 × g at 4°C for 10 min to remove the denatured protein precipitate. The samples were analyzed using a commercial kit (Sigma) based on the original method of Sedlak and Lindsay (1968), a kinetic assay in which GSH (reduced glutathione) continuously reduces 5,5′-dithiobis(2-nitrobenzoic acid) (DTNB) to 5-thio-2-nitrobenzoic acid (TNB), and the resulting GSSG (oxidized glutathione) is recycled by glutathione reductase and NADPH. TNB was spectrophotometrically measured at 412 nm. The levels of total and phosphorylated MAP kinases and their targets were analyzed using a commercial Sandwich ELISA kit from Cell Signaling (St. Louis, MO).
Morphological Analyses
Five-micrometer sections were obtained from surgically harvested back skin tissue samples, embedded, and frozen in OCT (Sakura, Torrance, CA). For indirect immunofluorescence, they were thawed, postfixed with neutral buffer Formalin for 10 min at room temperature, and incubated with primary antibodies to K17, K16, and K6. Bound primary antibodies were revealed by incubation with FITC-conjugated secondary antibodies (Kirkegaard & Perry Laboratories). The samples were also stained with Hoechst (Sigma) stain following the manufacturer's instructions.
Quantitation of Immunofluorescence
Micrographs were recorded using a Zeiss AxioPlan2 upright microscope equipped with a Zeiss HRC high-resolution digital camera (Thornwood, NY). Control and experimental skin samples were examined consecutively, and micrographs of stained preparations were recorded using the same exposure conditions. Immunofluorescence intensity was determined by measuring the mean pixel density of immunofluorescence in three 25-μm2 areas of epidermis per stained preparation of skin tissue, using Zeiss' Axiovision software. Each section selected was representative of its experimental group, and the 25-μm2 areas were selected from predetermined sites of epidermis (center, left, and right of the section) to foster unbiased sampling.
Statistical Analyses
Results were expressed as means ± SEM. The Student's t test was used for comparison between groups. p < 0.05 was considered statistically significant.
RESULTS
Nrf2 Plays a Partial Role in Keratin Regulation by SF in Skin Epidermis
The Nrf2-Keap-ARE pathway represents the best characterized mechanism to account for the SF-dependent transcriptional induction of specific genes (Talalay and Fahey, 2001; Kobayashi and Yamamoto, 2006). Several AREs matching the consensus for Nrf2 binding can be found in the area of the mouse and human genome that houses the clustered and highly conserved K14, K16, and K17 genes (data not shown). Recent studies have shown that K6, a polymerization partner for K16/K17 in vivo (McGowan and Coulombe, 1998b), and K16 are bona fide Nrf2 target genes (Wakabayashi et al., 2003; Endo et al., 2008). To examine the role of the Nrf2-Keap-ARE pathway in SF-mediated induction of K16 and K17 expression in the epidermis, studies were conducted in a mouse strain carrying a Nrf2 null mutation (Wakabayashi et al., 2003), and another one in which Nrf2-dependent transcription is enhanced secondary to a Keap1 hypomorph allele (Osburn et al., 2008). The Nrf2 null allele was transferred to the SKH-1 hairless strain (which is euthymic and immunocompetent; Benavides et al., 2009) to augment the ease and consistency of SF topical delivery. A dosage of 1 μmol SF, applied to the middorsum of mice, was used throughout this study because it rescues the skin fragility characteristic of K14 null mouse skin without significantly impacting proliferation or apoptosis in vivo (Kerns et al., 2007). Because K16 and especially K17 are robustly expressed in hair follicles under normal conditions, whereas the epidermis represents the exclusive interest of our study, the primary mean of sample analysis used was indirect immunofluorescence using well-characterized primary antibodies coupled with software-based fluorescence quantitation (see Materials and Methods).
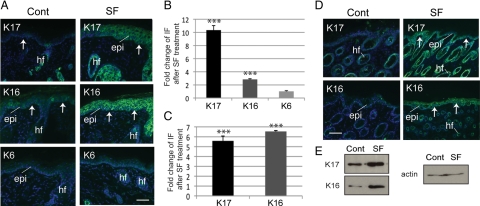
Relative to vehicle treatment, Nrf2 null mice topically treated with 1 μmol SF consistently showed a significant increase of K17 and K16 immunoreactivity in the epidermis (Figure 1, A and B; see Supplemental Table S1a for numerical data and p values) and Western blotting (Figure 1E). A minimal impact was seen on K6 expression (Figure 1, A and B). The epidermis of SF-treated Nrf2 null mice in the SKH-1 background was thicker than in controls. Epidermal thickening was previously observed after chronic SF treatment of SKH-1 hairless mice (Kerns et al., 2007). A similar change in keratin expression was observed after SF treatment of Nrf2 null pups in the C57BL6 strain background (e.g., Figure 1, C and D; Supplemental Table S1b), indicating that this outcome was not a function of the genetic background.
Figure 1.
Nrf2-independent modulation of keratin expression in skin topically treated with SF. (A) Indirect immunofluorescence for K6, K16, and K17 (as indicated in the top left corner) in frozen sections prepared from adult SKH-1/Nrf2−/− mouse skin treated either with vehicle (Cont, control) or SF. Sections were lightly stained with Hoechst dye to reveal nuclei. Arrows depict the dermo-epidermal interface. Epi, epidermis; hf, hair follicle. Bar, 100 μm. (B) Quantification of fold-change in immunofluorescence signal in the epidermis of SF-treated adult SKH-1/Nrf2−/− mice, relative to treated controls (see A). (C) Quantification of fold-change in immunofluorescence signal in the epidermis of Nrf2−/− mice in the C57/Bl6 background, relative to treated controls (see D). (D) Pattern of keratin expression in 8-d-old control and SF-treated Nrf2−/− mice in the C57/Bl6 background. Bar, 100 μm. (E) Western immunoblots depicting levels of K16 and 17 antigens (proteins) in response to control or SF treatment of adult Nrf2−/− mice. Actin was used as a loading control. Student's t test: *p < 0.05, **p < 0.01, ***p < 0.001.
We examined the mRNA levels for select genes in backskin tissue of adult C57BL6 mice carrying a hypomorphic Keap1 allele. Previous studies of these mice showed enhanced Nrf2-dependent transcription in several tissues (Osburn et al., 2008). Relative to wild type, Keap1 mRNA levels were reduced to ∼60% in Keap1 mutant skin, after normalization to HPRT (hypoxanthine phosphoribosyltransferase) levels (Supplementary Figure S1A). K16 and K6 mRNAs were elevated ∼2.1- and ∼1.4-fold, respectively, in Keap1 mutant skin. K17 mRNA, in contrast, remained unaltered, whereas the K14 mRNA was slightly decreased (Supplemental Figure S1A). An increase in K6 and K16 immunoreactivity, but not K17, was detected in Keap1 mutant epidermis (Supplemental Figure S1B). The findings garnered from Nrf2 null and Keap1 mutant mice suggest that the induction of K16 in SF-exposed epidermal keratinocytes proceeds through Nrf2-dependent and -independent mechanisms, whereas K17 induction occurs independently from Nrf2.
Although not a primary concern in this study, the issue of how the type I-to-type II keratin ratio is maintained after SF treatment is of interest. Preliminary studies revealed modest increases in the steady-state levels of K5 and K1, but no change in K6 antigens, in extracts prepared from SF-treated skin (data not shown). Follow-up studies are required to address this issue in full.
Correlation of Glutathione Levels and Induction of Keratins by SF in Skin Epidermis
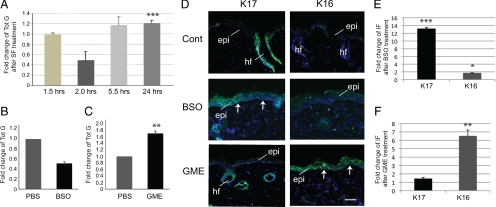
In cultured hepatocytes, SF directly conjugates with GSH, an antioxidant with a critical role in the cellular response to stress, resulting in lowered GSH levels 30 min after treatment (Zhang, 2000), and a subsequent elevation of GSH levels 24 h after treatment (Ye and Zhang, 2001). Similar findings were recently reported in SF-treated mouse embryonic fibroblasts (Higgins et al., 2009). We tested whether SF impacts glutathione levels in a similar manner in mouse skin tissue. Total glutathione levels were measured at 1.5, 2, 5.5, and 24 h after topical treatment of SKH-1 hairless mice with 1 μmol SF or vehicle. There was no significant change in glutathione levels at 1.5 h after SF treatment. At 2 h, glutathione levels had fallen to 53 ± 11% of those seen in control-treated skin. This was followed by a rise in glutathione levels to 117 ± 17 and 121 ± 06% of controls by 5.5 and 24 h, respectively (Figure 2A; see Supplemental Table S2a for absolute values and statistics).
Figure 2.
Role of glutathione in SF-mediated alterations in keratin expression. (A) Time-dependent changes in cellular levels of glutathione in skin after exposure to SF. Reported data (mean ± SEM) are from three independent experiments. (B and C) Modulation of glutathione levels in the skin of 7-wk-old SKH-1 mice that received four i.p. injections of 50 μM BSO or 5 μM GME on consecutive days and were harvested 24 h after the final treatment. Data (mean ± SEM) are expressed as fold change relative to mice injected with PBS. (D) Indirect immunofluorescence for keratins 16 and 17 (as indicated in the top left corner) on frozen sections prepared from mice treated with PBS (Cont), BSO, or GME as described for B and C. Sections were lightly stained with Hoechst dye to reveal nuclei. Arrows depict the dermo-epidermal interface. Small bright dots are staining artifacts. Epi, epidermis; hf, hair follicle. Bar, 100 μm. (E and F) Quantitation of fold-change in immunofluorescence, relative to control, in the epidermis of 7-wk-old SKH-1 mice after BSO or GME treatment, as described for B and C. Student's t test: *p < 0.05, **p < 0.01, ***p < 0.001.
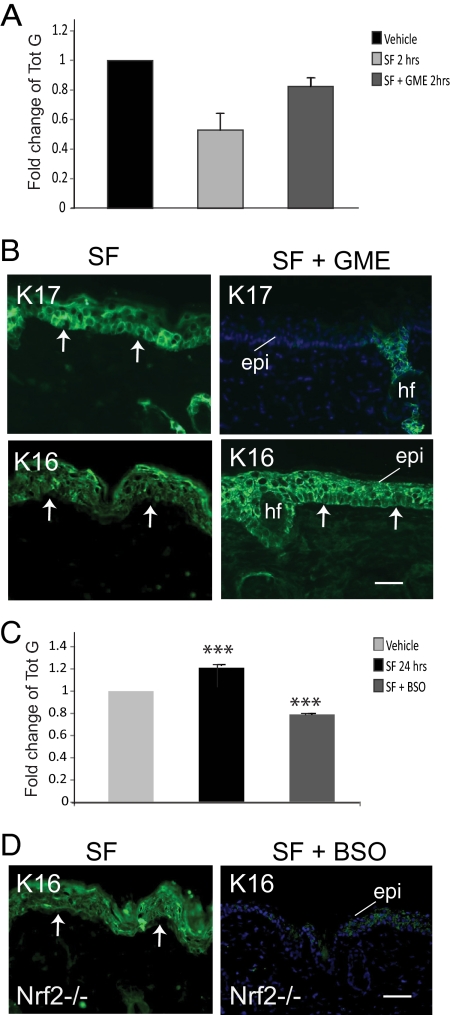
Next, we tested whether the modulation of glutathione levels impacts K16 and K17 expression in mouse skin epidermis. Female SKH-1 hairless mice were treated systemically (see Materials and Methods) with BSO, an inhibitor of glutathione synthesis, or GME, a form of glutathione readily taken up by cells. Total glutathione levels were measured in skin samples. Systemic treatment with BSO of SKH-1 hairless mice lowered glutathione levels in the backskin to 51 ± 4% of vehicle-treated control (Figure 2B; Supplemental Table S2b) and was associated with a significant increase in K17 and a smaller, but statistically significant increase in K16 expression as shown by immunofluorescence (Figure 2, D and E; Supplemental Table S1c). Likewise, systemic treatment with GME significantly raised glutathione levels in the backskin of SKH-1 hairless mice to 167 ± 7% relative to vehicle-treated control (Figure 2C; Supplemental Table S2c). The epidermis of the GME-treated mice was immunoreactive for K16 but showed no K17 signal beyond hair follicles (Figure 2, D and F; Supplemental Table S1c), where K17 is constitutively expressed (McGowan and Coulombe, 1998a,b).
Systemic treatment with GME partially prevented the decrease in glutathione levels 2 h after treatment of skin tissue with SF, creating an opportunity to assess whether this drop plays a role in K17 and/or K16 induction. Glutathione levels in dorsal skin of SKH-1 mice treated with both GME and SF is 83 ± 6% of vehicle-treated mice, compared with 50 ± 11% when treated with SF alone (Figure 3A; Supplemental Table S2d). Remarkably, no induction of K17 was observed in the epidermis of mice treated with both SF and GME (Figure 3B; Supplemental Figure S2A and Supplemental Table S1d). In contrast, the induction of K16 was unaltered in this context (Figure 3B). Next we wanted to test whether the SF-mediated induction of K16 treatment shows a dependency on the late-onset rise in the glutathione levels, in the absence of Nrf2. In SKH-1 null mice, systemic treatment of SKH-1 mice with BSO partly prevents the rise in glutathione levels 24 h after SF treatment. Glutathione levels were 74 ± 1% of vehicle-treatment, compared with 121 ± 6% seen with SF treatment alone, in such mice (Figure 3C; Supplemental Table S2e). When examined in Nrf2−/− SKH-1 mouse epidermis, induction of K16 is markedly attenuated after combined treatment with SF and BSO relative to SF alone (Figure 3D, Supplemental Figure S2B and Supplemental Table S1e). Together these results suggest that the modulation of total glutathione levels, in either direction, is poised to play a key role in the SF-mediated induction of K16 and K17 in skin tissue.
Figure 3.
SF-induced fluctuations in glutathione levels by SF play a role in keratin induction. (A) Fold-change in total glutathione levels (mean ± SEM from three experiments) measured in female SKH-1 hairless mouse skin tissue at 2 h after treatment with vehicle alone (adjusted to 1), SF, or SF combined with GME. (B) Indirect immunofluorescence for K17 and K16 in skin tissue sections from SKH-1 mice after three treatments with SF or SF in combination with GME. (C) Fold-change in total glutathione levels (mean ± SEM from three experiments) measured in female SKH-1 hairless mouse skin tissue at 24 h after treatment with vehicle alone (adjusted to 1), SF, or SF combined with BSO. (D) Indirect immunofluorescence for K16 in skin tissue sections from Nrf2−/− mice after three treatments with SF or SF in combination with GME. In A and C, Student's t test: *p < 0.05, **p < 0.01, ***p < 0.001. For B and D, sections were lightly stained with Hoechst dye to reveal nuclei. Arrows depict the dermo-epidermal interface. Epi, epidermis; hf, hair follicle. Bars, 100 μm.
SF-induced Keratin Expression Involves MAP Kinase Activation
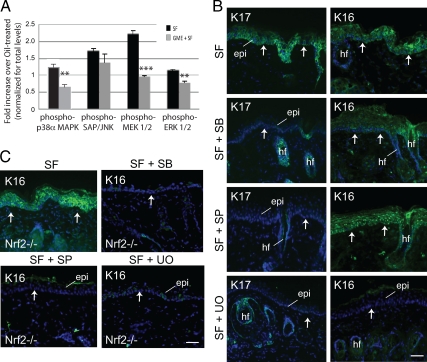
Previous studies have implicated the MAP kinases p38, ERK, and JNK in SF-mediated chemoprevention (e.g., Cho et al., 2005; Hwang et al., 2005; Jo et al., 2007). In addition, glutathione has been shown to modulate several MAP kinase cascades, including ERK, p38, and JNK (Limon-Pacheco et al., 2007). Each of these MAP kinases converge on AP-1, a transcription factor that regulates the differential expression of K6 among other keratin genes (Navarro et al., 1995; Ma et al., 1997), raising the possibility that they could be involved in the SF-induced, Nrf2-independent expression of K17 and/or K16. We treated SKH1- mice with 1 μmol SF (or vehicle control) and measured the levels of total and phosphorylated forms of key MAP kinases using ELISA. At 2 h after SF treatment, the time at which glutathione levels are decreased (Figure 2A), there were statistically significant increases in phospho-p38α (∼1.2-fold) and phospho-MEK1/2 (∼2.2-fold), an increase in phospho-SAP/JNK (∼1.7-fold), and little if any change in phospho-ERK1/2 (Figure 4A, data expressed relative to vehicle-treated skin; see Supplemental Table S3a for corresponding absolute values and p values). Compared with controls, systemic GME treatment reduced the activation of phospho-p38α (by 45%) and phospho-MEK1/2 (by 56%) at 2 h after SF treatment (Figure 4A; Supplemental Table S3a). At 24 h after SF treatment, a time at which glutathione levels are slightly above normal (Figure 2A), the levels of phospho-p38α (∼0.93-fold), phospho-SAP/JNK (∼1.03-fold), phospho-MEK1/2 (∼1.2-fold), and phospho-ERK1/2 (∼0.90-fold) are similar to levels observed of vehicle-treated mice (Supplemental Table S3b). These findings suggest that the transient activation of p38 and MEK1/2 kinases shortly after skin keratinocyte exposure to SF is partly due to the depletion in glutathione levels, whereas JNK activation occurs independent of the glutathione status.
Figure 4.
Role of MAP kinases in the modulation of keratin expression by SF. (A) Dependence of MAP kinase activation on alteration of glutathione levels by SF in skin. Mice were treated with either SF alone or SF in combination with GME, and the activation status of MAP kinases in skin was assessed via ELISA. Data (mean ± SEM) are reported as fold change over treatment with SF alone (adjusted to unity). (B) Indirect immunofluorescence for K17 and K16 in skin tissue sections after treatment with SF alone or in combination with inhibitors to p38 (SF + SB), JNK (SF + SP), or ERK (SF + UO). (C) Indirect immunofluorescence for K16 in Nrf2−/− mouse skin after treatment with SF alone, or in combination with inhibitors to p38 (SF + SB), JNK (SF + SP), or ERK (SF + UO). Sections were lightly stained with Hoechst dye to reveal nuclei. Arrows depict the dermo-epidermal interface. Epi, epidermis; hf, hair follicle. Bars, 100 μm. Student's t test: *p < 0.05, **p < 0.01, ***p < 0.001.
To directly test the role of these MAP kinases in the induction of K17 and K16 by SF, SKH-1 hairless mice were topically treated with inhibitors of p38 (100 μl of SB20219 at 10 μM), JNK (100 μl of SP600125 at 100 μM), or ERK1/2 (100 μl of UO126 at 100 μM) at 30 min before and 30 min after topical treatment with 1 μmol SF formulated in jojoba oil. These inhibitors have been used topically in mice by many other groups (Jaffee et al., 2000; Takanami-Ohnishi et al., 2002; Bachelor et al., 2005; Lee et al., 2007). An ELISA assay was used to confirm the effectiveness of treatment with these inhibitors (Supplemental Figure S3, Supplemental Table S3c). Significantly reduced immunoreactivity for K17 occurred in the epidermis of mice treated with SF and any of these inhibitors, compared with mice treated with SF alone (Figure 4B, Supplemental Figure S4A, Supplemental Table S1f). On the other hand, only UO treatment reduced K16 immunoreactivity that arises after SF treatment (Figure 4B, Supplemental Figure S4B, Supplemental Table S1f). When ERK1/2 is inhibited, we find that both the Nrf2-dependent and -independent pathways are both blocked and the SF-mediated induction of K16 is lost. In the absence of Nrf2 signaling all inhibitor treatments are able to attenuate induction of K16 gene expression (Figure 4C, Supplemental Figure S4C, Supplemental Table S1g).
DISCUSSION
The main findings of our study are as follows. First, we identify a gene, namely K17, that is induced in an Nrf2-independent manner in skin epidermis treated with SF. Second, we show that SF exposure causes a transient decrease in total glutathione levels in mouse skin. Such a decrease in glutathione has been reported for select cell lines in culture (Zhang et al., 2000; Higgins et al., 2009). Third, we show that K17 induction closely parallels the drop in glutathione levels that follows treatment of skin with SF and that this process also involves the activation of MAP kinases. The latter are known to converge on AP-1, a transcription factor already known to participate in the regulation of K6 (Navarro et al., 1995; Ma et al., 1997) and K16 (Wang and Chang, 2003) in other biological settings. Fourth, we show that a topical treatment that reduces glutathione levels induces K17 expression in mouse epidermis, independently of SF exposure. Finally, we show that the SF-mediated epidermal induction of K16 and K17, two neighboring keratin genes that exhibit a high degree of sequence homology and an overlapping regulation (McGowan and Coulombe, 1998b), occurs through distinct mechanisms in SF-treated keratinocytes (i.e., it entails a differential involvement of Nrf2, glutathione, and MAP kinases).
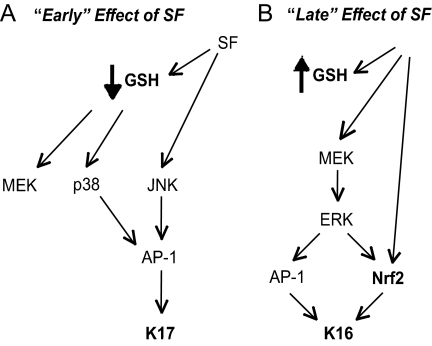
Our findings suggest a temporal sequence of molecular events that ultimately results in the stepwise activation of select keratin genes in SF-treated epidermis (Figure 5). Early on (e.g., <3 h; Figure 5A), SF induces K17, coinciding with the transient drop in glutathione levels and its associated impact on p38 kinase activity. K17 induction may also involve SF's ability to activate JNK kinase, which, like other MAP kinases including p38, can activate AP-1 and (presumably) contribute to activating K17 gene transcription. Later on (e.g., ∼24 h; Figure 5B), glutathione levels have rebounded, and activated Nrf2 may at this point represent the main mechanism through which SF impacts gene expression in epidermal keratinocytes. Activation of Nrf2 could also result from the MEK-mediated activation of ERK kinase, in addition to Keap1 inactivation, at this later time (Kang et al., 2007). Once activated, Nrf2 and AP-1 can each, or tandemly, activate K16 gene transcription (Figure 5B). This model could apply to the regulation of additional genes after exposure to SF.
Figure 5.
Model depicting the cascade of signaling events leading to keratin induction “early” (A) and “late” (B) after treatment of mouse epidermis with SF. (A) Within 3 h after SF exposure, a marked decrease in total glutathione levels occurs, coinciding in the activation of MAP kinases. This culminates in activation of AP-1, a transcription factor able to activate K17 expression. (B) Later on, SF can directly stimulate Nrf2 by alleviating its tethering to Keap1 in the cytoplasm. A portion of K16 induction is directly due to Nrf2 transcriptional activation. In addition, glutathione levels have rebounded and exceed normal baseline levels, stimulating MEK and ERK signaling and leading to AP-1 activation and K16 induction. See Discussion for details.
The lack of uniformity with which SF impacts the expression of K17, K16, and the K6 isoforms in adult mouse epidermis (Kerns et al., 2007) was unexpected. The type II K6a and/or K6b are usually cotranscribed with the type I K16 in adult epithelia and sometimes with K17 as well (McGowan and Coulombe, 1998a; Smith et al., 1998). Our finding that K6a and K6b are not significantly induced in Keap1 hypomorph mutant mice extends our earlier study (Kerns et al., 2007). At first glance, this finding may appear at odds with the report that the K6a gene contains a functional ARE and is robustly induced in the markedly hyperkeratotic esophageal epithelium of Keap1 null mice (Wakabayashi et al., 2003). This said, SF has been shown to directly inhibit nuclear factor κB (NF-κB; Heiss et al., 2001), a transcription factor required for K6 gene expression in at least some settings (alone or in combination for other factors such as AP-1; see Navarro et al., 1995; Ma et al., 1997). It could be, therefore, that NF-κB inhibition prevents the induction of K6 gene expression after SF treatment. Additional studies are required to further probe this intriguing phenomenon.
There is microarray-based evidence for SF-induced gene expression in the absence of Nrf2 in select cell models (Kraft et al., 2004; Hu et al., 2006), though to our knowledge this has not been shown for epidermal keratinocytes. The biochemical mechanism(s) involved have not been elucidated. The findings reported here bring forth several issues of interest. For instance, which genes other than K17 are turned on in an Nrf2-independent manner after keratinocyte exposure to SF and what is their relevance to the overall response mounted by the cell? In this regard several intermediate filaments genes are rapidly induced whenever the cells and tissues expressing them are subject to environmental challenges. For the K6/K16/K17 subgrouping of keratin genes, such adverse conditions include wounding, viral infection, UV exposure, and disease processes that result in aberrant differentiation within stratified epithelia (Weiss et al., 1984; Stoler et al., 1988; Jiang et al., 1994; Leigh et al., 1995; Paladini et al., 1996). In a few cases, the role of the IF protein being induced has been defined and includes protection against apoptosis in a signal-specific manner (see Kim and Coulombe, 2007), stimulation of protein synthesis and cell growth (Kim et al., 2006), providing a phosphate sink for hyperactive, stress-related kinases (Ku and Omary, 2006), and regulation of reactive oxygen species (ROS)-induced and mitochondria-mediated death in hepatic cells (Mathew et al., 2008). The role that K17 might fulfill in a cellular context where glutathione levels are low is a question worth pursuing. Another issue of interest is the relationship between glutathione and K17 expression. Regulation of the balance between reduced and oxidized forms of glutathione (GSH/GSSG) in the cell, and the associated impact, represent complex issues (MacLeod et al., 2009).
Treatment with SF has been shown to be very effective in alleviating skin blistering in a mouse model for K14 deficiency, thus showing promise as a therapeutic agent for a subset of epidermolysis bullosa simplex cases (Kerns et al., 2007). The list of conditions that stand to benefit from treatment with natural products enriched in this remarkable molecule (Shapiro et al., 2006; Dinkova-Kostova et al., 2007) is growing steadily. The findings reported here shed new light into the complexity of the cellular pathways that are impacted by SF and related molecules and as such may assist in efforts to use them appropriately in a clinical setting.
Supplementary Material
ACKNOWLEDGMENTS
The authors are grateful to Dr. Paul Talalay (Johns Hopkins School of Medicine) for advice. These studies were supported by grants to P.A.C. from the March of Dimes Birth Defect Research Foundation and the National Institutes of Health (AR44232, CA123530).
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E10-02-0153) on October 6, 2010.
REFERENCES
- Bachelor M. A., Cooper S. J., Sikorski E. T., Bowden G. T. Inhibition of p38 mitogen-activated protein kinase and phosphatidylinositol 3-kinase decreases UVB-induced activator protein-1 and cyclooxygenase-2 in a SKH-1 hairless mouse model. Mol. Cancer Res. 2005;3:90–99. doi: 10.1158/1541-7786.MCR-04-0065. [DOI] [PubMed] [Google Scholar]
- Benavides F., Oberyszyn T. M., Van Buskirk A. M., Reeve V. E., Kusewitt D. F. The hairless mouse in skin research. J. Dermotol. Sci. 2009;53:10–18. doi: 10.1016/j.jdermsci.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan K., Lu R., Chang J. C., Kan Y. W. NRF2, a member of the NFE2 family of transcription factors, is not essential for murine erythropoiesis, growth, and development. Proc. Natl. Acad. Sci. USA. 1996;93:13943–13948. doi: 10.1073/pnas.93.24.13943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho S. D., Li G., Hu H., Jiang C., Kang K. S., Lee Y. S., Kim S. H., Lu J. Involvement of c-Jun N-terminal kinase in G2/M arrest and caspase-mediated apoptosis induced by sulforaphane in DU145 prostate cancer cells. Nutr. Cancer. 2005;52:213–224. doi: 10.1207/s15327914nc5202_11. [DOI] [PubMed] [Google Scholar]
- Cornblatt B. S., et al. Preclinical and clinical evaluation of sulforaphane for chemoprevention in the breast. Carcinogenesis. 2007;28:1485–1490. doi: 10.1093/carcin/bgm049. [DOI] [PubMed] [Google Scholar]
- Coulombe P. A., Kerns M. L., Fuchs E. Epidermolysis bullosa simplex: a paradigm for disorders of tissue fragility. J. Clin. Invest. 2009;119:1784–1793. doi: 10.1172/JCI38177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinkova-Kostova A. T., Fahey J. W., Wade K. L., Jenkins S. N., Shapiro T. A., Fuchs E. J., Kerns M. L., Talalay P. Induction of the phase 2 response in mouse and human skin by sulforaphane-containing broccoli sprout extracts. Cancer Epidemiol. Biomarkers Prev. 2007;16:847–851. doi: 10.1158/1055-9965.EPI-06-0934. [DOI] [PubMed] [Google Scholar]
- Dinkova-Kostova A. T., Holtzclaw W. D., Cole R. N., Itoh K., Wakabayashi N., Katoh Y., Yamamoto M., Talalay P. Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc. Natl. Acad. Sci. USA. 2002;99:11908–11913. doi: 10.1073/pnas.172398899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo H., Sugioka Y., Nakagi Y., Saijo Y., Yoshida T. A novel role of the NRF2 transcription factor in the regulation of arsenite-mediated keratin 16 gene expression in human keratinocytes. Environ. Health Perspect. 2008;116:873–879. doi: 10.1289/ehp.10696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine J. D., Eady R. A., Bauer E. A., Bauer J. W., Bruckner-Tuderman L., Heagerty A., Hintner H., Hovnanian A., Jonkman M. F., Leigh I., et al. The classification of inherited epidermolysis bullosa (EB): Report of the Third International Consensus Meeting on Diagnosis and Classification of EB. J. Am. Acad. Dermatol. 2008;58:931–950. doi: 10.1016/j.jaad.2008.02.004. [DOI] [PubMed] [Google Scholar]
- Hayes J. D., Kelleher M. O., Eggleston I. M. The cancer chemopreventive actions of phytochemicals derived from glucosinolates. Eur. J. Nutr. 2008;47(Suppl. 2):73–88. doi: 10.1007/s00394-008-2009-8. [DOI] [PubMed] [Google Scholar]
- Heiss E., Herhaus C., Klimo K., Bartsch H., Gerhäuser C. Nuclear factor kappa B is a molecular target for sulforaphane-mediated anti-inflammatory mechanisms. J. Biol. Chem. 2001;276((34)):32008–32015. doi: 10.1074/jbc.M104794200. [DOI] [PubMed] [Google Scholar]
- Higgins L. G., Kelleher M. O., Eggleston I. M., Itoh K., Yamamoto M., Hayes J. D. Transcription factor Nrf2 mediates an adaptive response to sulforaphane that protects fibroblasts in vitro against the cytotoxic effects of electrophiles, peroxides and redox-cycling agents. Toxicol. Appl. Pharmacol. 2009;237:267–280. doi: 10.1016/j.taap.2009.03.005. [DOI] [PubMed] [Google Scholar]
- Hu R., Xu C., Shen G., Jain M. R., Khor T. O., Gopalkrishnan A., Lin W., Reddy B., Chan J. Y., Kong A. N. Gene expression profiles induced by cancer chemopreventive isothiocyanate sulforaphane in the liver of C57BL/6J mice and C57BL/6J/Nrf2 (−/−) mice. Cancer Lett. 2006;243:170–192. doi: 10.1016/j.canlet.2005.11.050. [DOI] [PubMed] [Google Scholar]
- Hwang J. W., Park J. S., Jo E. H., Kim S. J., Yoon B. S., Kim S. H., Lee Y. S., Kang K. S. Chinese cabbage extracts and sulforaphane can protect H2O2-induced inhibition of gap junctional intercellular communication through the inactivation of ERK1/2 and p38 MAP kinases. J. Agric. Food Chem. 2005;53:8205–8210. doi: 10.1021/jf051747h. [DOI] [PubMed] [Google Scholar]
- Jaffee B. D., Manos E. J., Collins R. J., Czerniak P. M., Favata M. F., Magolda R. L., Scherle P. A., Trzaskos J. M. Inhibition of MAP kinase kinase (MEK) results in an anti-inflammatory response in vivo. Biochem. Biophys. Res. Commun. 2000;268:647–651. doi: 10.1006/bbrc.2000.2184. [DOI] [PubMed] [Google Scholar]
- Jiang C. K., Flanagan S., Ohtsuki M., Shuai K., Freedberg I. M., Blumenberg M. Disease-activated transcription factor: allergic reactions in human skin cause nuclear translocation of STAT-91 and induce synthesis of keratin K17. Mol. Cell. Biol. 1994;14:4759–4769. doi: 10.1128/mcb.14.7.4759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo E. H., Kim S. H., Ahn N. S., Park J. S., Hwang J. W., Lee Y. S., Kang K. S. Efficacy of sulforaphane is mediated by p38 MAP kinase and caspase-7 activations in ER-positive and COX-2-expressed human breast cancer cells. Eur. J. Cancer Prev. 2007;16:505–510. doi: 10.1097/01.cej.0000243856.97479.3b. [DOI] [PubMed] [Google Scholar]
- Kang K. A., Lee K. H., Park J. W., Lee N. H., Na H. K., Surh Y. J., You H. J., Chung M. H., Hyun J. W. Triphlorethol-A induces heme oxygenase-1 via activation of ERK and NF-E2 related factor 2 transcription factor. FEBS Lett. 2007;581:2000–2008. doi: 10.1016/j.febslet.2007.04.022. [DOI] [PubMed] [Google Scholar]
- Kerns M. L., DePianto D., Dinkova-Kostova A. T., Talalay P., Coulombe P. A. Reprogramming of keratin biosynthesis by sulforaphane restores skin integrity in epidermolysis bullosa simplex. Proc. Natl. Acad. Sci. USA. 2007;104:14460–14465. doi: 10.1073/pnas.0706486104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S., Coulombe P. A. Intermediate filament scaffolds fulfill mechanical, organizational, and signaling functions in the cytoplasm. Genes Dev. 2007;21:1581–1597. doi: 10.1101/gad.1552107. [DOI] [PubMed] [Google Scholar]
- Kim S., Wong P., Coulombe P. A. A keratin cytoskeletal protein regulates protein synthesis and epithelial cell growth. Nature. 2006;441:362–365. doi: 10.1038/nature04659. [DOI] [PubMed] [Google Scholar]
- Kobayashi M., Yamamoto M. Nrf2-Keap1 regulation of cellular defense mechanisms against electrophiles and reactive oxygen species. Adv. Enzyme Regul. 2006;46:113–140. doi: 10.1016/j.advenzreg.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Kraft A. D., Johnson D. A., Johnson J. A. Nuclear factor E2-related factor 2-dependent antioxidant response element activation by tert-butylhydroquinone and sulforaphane occurring preferentially in astrocytes conditions neurons against oxidative insult. J. Neurosci. 2004;24:1101–1112. doi: 10.1523/JNEUROSCI.3817-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku N. O., Omary M. B. A disease- and phosphorylation-related nonmechanical function for keratin 8. J. Cell Biol. 2006;174:115–125. doi: 10.1083/jcb.200602146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. C., Kundu J. K., Hwang D. M., Na H. K., Surh Y. J. Humulone inhibits phorbol ester-induced COX-2 expression in mouse skin by blocking activation of NF-kappaB and AP-1, IkappaB kinase and c-Jun-N-terminal kinase as respective potential upstream targets. Carcinogenesis. 2007;28:1491–1498. doi: 10.1093/carcin/bgm054. [DOI] [PubMed] [Google Scholar]
- Leigh I. M., Navsaria H., Purkis P. E., McKay I. A., Bowden P. E., Riddle P. N. Keratins (K16 and K17) as markers of keratinocyte hyperproliferation in psoriasis in vivo and in vitro. Br. J. Dermatol. 1995;133:501–511. doi: 10.1111/j.1365-2133.1995.tb02696.x. [DOI] [PubMed] [Google Scholar]
- Limon-Pacheco J. H., Hernandez N. A., Fanjul-Moles M. L., Gonsebatt M. E. Glutathione depletion activates mitogen-activated protein kinase (MAPK) pathways that display organ-specific responses and brain protection in mice. Free Radic. Biol. Med. 2007;43:1335–1347. doi: 10.1016/j.freeradbiomed.2007.06.028. [DOI] [PubMed] [Google Scholar]
- Ma S., Rao L., Freedberg I. M., Blumenberg M. Transcriptional control of K5, K6, K14, and K17 keratin genes by AP-1 and NF-kappaB family members. Gene Expr. 1997;6:361–370. [PMC free article] [PubMed] [Google Scholar]
- MacLeod A. K., McMahon M., Plummer S. M., Higgins L. G., Penning T. M., Igarashi K., Hayes J. D. Characterization of the cancer chemopreventive NRF2-dependent gene battery in human keratinocytes: demonstration that the KEAP1-NRF2 pathway, and not the BACH1-NRF2 pathway, controls cytoprotection against electrophiles as well as redox-cycling compounds. Carcinogenesis. 2009;30:1571–1580. doi: 10.1093/carcin/bgp176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew J., Galarneau L., Loranger A., Gilbert S., Marceau N. Keratin-protein kinase C interaction in reactive oxygen species-induced hepatic cell death through mitochondrial signaling. Free Radic. Biol. Med. 2008;45:413–424. doi: 10.1016/j.freeradbiomed.2008.04.031. [DOI] [PubMed] [Google Scholar]
- McGowan K. M., Coulombe P. A. Onset of keratin 17 expression coincides with the definition of major epithelial lineages during skin development. J. Cell Biol. 1998a;143:469–486. doi: 10.1083/jcb.143.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan K. M., Coulombe P. A. The wound repair associated keratins 6, 16, and 17: Insights into the role of intermediate filaments in specifying cytoarchitecture. In: Harris J. R., Herrmann H., editors. Subcellular Biochemistry: Intermediate Filaments. London: Plenum Publishing; 1998b. pp. 141–165. [PubMed] [Google Scholar]
- McMahon M., Itoh K., Yamamoto M., Chanas S. A., Henderson C. J., McLellan L. I., Wolf C. R., Cavin C., Hayes J. D. The cap‘n’collar basic leucine zipper transcription factor Nrf2 (NF-E2 p45-related factor 2) controls both constitutive and inducible expression of intestinal detoxification and glutathione biosynthetic enzymes. Cancer Res. 2001;61:3299–3307. [PubMed] [Google Scholar]
- Moi P., Chan K., Asunis I., Cao A., Kan Y. W. Isolation of NF-E2-related factor 2 (Nrf2), a NF-E2-like basic leucine zipper transcriptional activator that binds to the tandem NF-E2/AP1 repeat of the beta-globin locus control region. Proc. Natl. Acad. Sci. USA. 1994;91:9926–9930. doi: 10.1073/pnas.91.21.9926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myzak M. C., Dashwood R. H. Chemoprotection by sulforaphane: keep one eye beyond Keap1. Cancer Lett. 2006;233:208–218. doi: 10.1016/j.canlet.2005.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro J. M., Casatorres J., Jorcano J. L. Elements controlling the expression and induction of the skin hyperproliferation-associated keratin K6. J. Biol. Chem. 1995;270:21362–21367. doi: 10.1074/jbc.270.36.21362. [DOI] [PubMed] [Google Scholar]
- Osburn W. O., Yates M. S., Dolan P. D., Chen S., Liby K. T., Sporn M. B., Taguchi K., Yamamoto M., Kensler T. W. Genetic or pharmacologic amplification of nrf2 signaling inhibits acute inflammatory liver injury in mice. Toxicol. Sci. 2008;104:218–227. doi: 10.1093/toxsci/kfn079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paladini R. D., Takahashi K., Bravo N. S., Coulombe P. A. Onset of re-epithelialization after skin injury correlates with a reorganization of keratin filaments in wound edge keratinocytes: defining a potential role for keratin 16. J. Cell Biol. 1996;132:381–397. doi: 10.1083/jcb.132.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paladini R., Coulombe P. A. The functional diversity of epidermal keratins revealed by the partial rescue of the keratin 14 null phenotype by keratin 16. J. Cell Biol. 1999;146:1185–1201. doi: 10.1083/jcb.146.5.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedlak J., Lindsay R. H. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissues with Ellman's reagent. Anal. Biochem. 1968;25:192–205. doi: 10.1016/0003-2697(68)90092-4. [DOI] [PubMed] [Google Scholar]
- Shapiro T. A., Fahey J. W., Dinkova-Kostova A. T., Holtzclaw W. D., Stephenson K. K., Wade K. L., Ye L., Talalay P. Safety, tolerance, and metabolism of broccoli sprout glucosinolates and isothiocyanates: a clinical phase I study. Nutr. Cancer. 2006;55:53–62. doi: 10.1207/s15327914nc5501_7. [DOI] [PubMed] [Google Scholar]
- Smith F. J., Jonkman M. F., van Goor H., Coleman C. M., Covello S. P., Uitto J., McLean W. H. A mutation in human keratin K6b produces a phenocopy of the K17 disorder pachyonychia congenita type 2. Hum. Mol. Genet. 1998;7:1143–1148. doi: 10.1093/hmg/7.7.1143. [DOI] [PubMed] [Google Scholar]
- Stoler A., Kopan R., Duvic M., Fuchs E. Use of monospecific antisera and cRNA probes to localize the major changes in keratin expression during normal and abnormal epidermal differentiation. J. Cell Biol. 1988;107:427–446. doi: 10.1083/jcb.107.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takanami-Ohnishi Y., Amano S., Kimura S., Asada S., Utani A., Maruyama M., Osada H., Tsunoda H., Irukayama-Tomobe Y., Goto K., et al. Essential role of p38 mitogen-activated protein kinase in contact hypersensitivity. J. Biol. Chem. 2002;277:37896–37903. doi: 10.1074/jbc.M207326200. [DOI] [PubMed] [Google Scholar]
- Talalay P., Fahey J. W. Phytochemicals from cruciferous plants protect against cancer by modulating carcinogen metabolism. J. Nutr. 2001;131:3027S–3033S. doi: 10.1093/jn/131.11.3027S. [DOI] [PubMed] [Google Scholar]
- Tan X. L., Spivack S. D. Dietary chemoprevention strategies for induction of phase II xenobiotic-metabolizing enzymes in lung carcinogenesis: a review. Lung Cancer. 2009;65:129–137. doi: 10.1016/j.lungcan.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi N., Dinkova-Kostova A. T., Holtzclaw W. D., Kang M. I., Kobayashi A., Yamamoto M., Kenster T. W., Talalay P. Protection against electrophile and oxidant stress by induction of the phase 2 response: fate of cysteines of the Keap1 sensor modified by inducers. Proc. Natl. Acad. Sci. USA. 2004;101:2040–2045. doi: 10.1073/pnas.0307301101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi N., Itoh K., Wakabayashi J., Motohashi H., Noda S., Takahashi S., Imakado S., Kotsuji T., Otsuka F., Roop D. R., et al. Keap1-null mutation leads to postnatal lethality due to constitutive Nrf2 activation. Nat. Genet. 2003;35:238–245. doi: 10.1038/ng1248. [DOI] [PubMed] [Google Scholar]
- Wang Y. N., Chang W. C. Induction of disease-associated keratin 16 gene expression by epidermal growth factor is regulated through cooperation of transcription factors Sp1 and c-Jun. J. Biol. Chem. 2003;278:45848–45857. doi: 10.1074/jbc.M302630200. [DOI] [PubMed] [Google Scholar]
- Weiss R. A., Eichner R., Sun T. T. Monoclonal antibody analysis of keratin expression in epidermal diseases: a 48- and 56-kdalton keratin as molecular markers for hyperproliferative keratinocytes. J. Cell Biol. 1984;98:1397–1406. doi: 10.1083/jcb.98.4.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye L., Zhang Y. Total intracellular accumulation levels of dietary isothiocyanates determine their activity in elevation of cellular glutathione and induction of Phase 2 detoxification enzymes. Carcinogenesis. 2001;22:1987–1992. doi: 10.1093/carcin/22.12.1987. [DOI] [PubMed] [Google Scholar]
- Zhang C. X., Ho S. C., Chen Y. M., Fu J. H., Cheng S. Z., Lin F. Y. Greater vegetable and fruit intake is associated with a lower risk of breast cancer among Chinese women. Int. J. Cancer. 2009;125:181–188. doi: 10.1002/ijc.24358. [DOI] [PubMed] [Google Scholar]
- Zhang Y. Role of glutathione in the accumulation of anticarcinogenic isothiocyanates and their glutathione conjugates by murine hepatoma cells. Carcinogenesis. 2000;21:1175–1182. [PubMed] [Google Scholar]
- Zhang Y., Gordon G. B. A strategy for cancer prevention: stimulation of the Nrf2-ARE signaling pathway. Mol. Cancer Ther. 2004;3:885–893. [PubMed] [Google Scholar]
- Zhang Y., Talalay P., Cho C. G., Posner G. H. A major inducer of anticarcinogenic protective enzymes from broccoli: isolation and elucidation of structure. Proc. Natl. Acad. Sci. USA. 1992;89:2399–2403. doi: 10.1073/pnas.89.6.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.