Abstract
The morphology of alphaherpesviruses during anterograde axonal transport from the neuron cell body towards the axon terminus is controversial. Reports suggest that transport of herpes simplex virus type 1 (HSV-1) nucleocapsids and envelope proteins occurs in separate compartments and that complete virions form at varicosities or axon termini (subassembly transport model), while transport of a related alphaherpesvirus, pseudorabies virus (PRV) occurs as enveloped capsids in vesicles (assembled transport model). Transmission electron microscopy of proximal and mid-axons of primary superior cervical ganglion (SCG) neurons was used to compare anterograde axonal transport of HSV-1, HSV-2 and PRV. SCG cell bodies were infected with HSV-1 NS and 17, HSV-2 2.12 and PRV Becker. Fully assembled virus particles were detected intracellularly within vesicles in proximal and mid-axons adjacent to microtubules after infection with each virus, indicating that assembled virions are transported anterograde within axons for all three alphaherpesviruses.
Keywords: Alphaherpesviruses, HSV-1, HSV-2, PRV, anterograde axonal transport, transmission electron microscopy
Introduction
Herpes simplex virus type 1 (HSV-1), herpes simplex virus type 2 (HSV-2) and pseudorabies virus (PRV) are neuroinvasive alphaherpesviruses. The lifecycle for each virus involves transport along axons in two directions, retrograde (from the axon terminus towards the neuron cell body) and anterograde (from the neuron cell body towards the axon terminus). Transport in both directions is microtubule-dependent. Retrograde axonal transport is mediated by dynein, while anterograde axonal transport is kinesin-dependent (Ch'ng and Enquist, 2005b; Holland et al., 1999; Miranda-Saksena et al., 2000; Miranda-Saksena et al., 2009; Radtke et al., 2010; Wolfstein et al., 2006). HSV-1 and HSV-2 infect neurons innervating the skin and mucosal sites and virion components travel by retrograde axonal transport to nuclei in sensory ganglia where latency is established. Upon reactivation, virion components travel by anterograde axonal transport to infect cells innervated by the neurons.
The morphology of alphaherpesviruses during anterograde axonal transport is controversial. Two models have been proposed based on studies with HSV-1 and PRV. The “separate” or subassembly model is based on transmission electron microscopy (TEM), transmission immune electron microscopy (TIEM), and immunofluorescent observations of HSV-1 anterograde axonal transport. This model suggests that tegument-coated nucleocapsids are transported separately from envelope proteins and that virions are assembled at exit sites from the axon, such as varicosities and growth cones (Holland et al., 1999; LaVail et al., 2003; LaVail et al., 2005; LaVail et al., 2007; Miranda-Saksena et al., 2000; Penfold, Armati, and Cunningham, 1994; Snyder et al., 2007). The “married” or assembled model derives from TEM, TIEM and immunofluorescent observations of PRV and proposes that virus-like particles are assembled prior to entry into the axon and are transported as enveloped capsids within vesicles (Antinone and Smith, 2006; Ch'ng and Enquist, 2005b; del Rio et al., 2005; Feierbach et al., 2007; Lyman et al., 2007; Maresch et al., 2010).
The central question in the controversy is whether anterograde axonal transport of HSV differs from PRV, with HSV tegument-coated nucleocapsids and envelope proteins forming virions at exit sites from axons while PRV nucleocapsids and envelope proteins form virions prior to entering the axon. Studies that address anterograde axonal transport of alphaherpesviruses have been performed in different laboratories using HSV-1 or PRV, different sources of neurons, including chick embryo dorsal root ganglion (DRG) neurons, rat DRG neurons, rat superior cervical ganglion (SCG) neurons, human DRG neurons, or human neuroblastoma cells, and different detection methods, such as TEM, TIEM, and immunofluorescence (Ch'ng and Enquist, 2005b; Holland et al., 1999; LaVail et al., 2003; LaVail et al., 2005; LaVail et al., 2007; Maresch et al., 2010; Miranda-Saksena et al., 2000; Miranda-Saksena et al., 2009; Penfold, Armati, and Cunningham, 1994; Smith, Gross, and Enquist, 2001; Snyder et al., 2007; Tomishima and Enquist, 2002). Here, we compare anterograde axonal transport of three alphaherpesviruses in a single laboratory using similar culture conditions and approach. HSV-1 NS and 17, HSV-2 2.12 and PRV Becker were evaluated in rat and mouse SCG neuron cultures by TEM to observe virion morphology within axons.
Results
Campenot chamber cultures
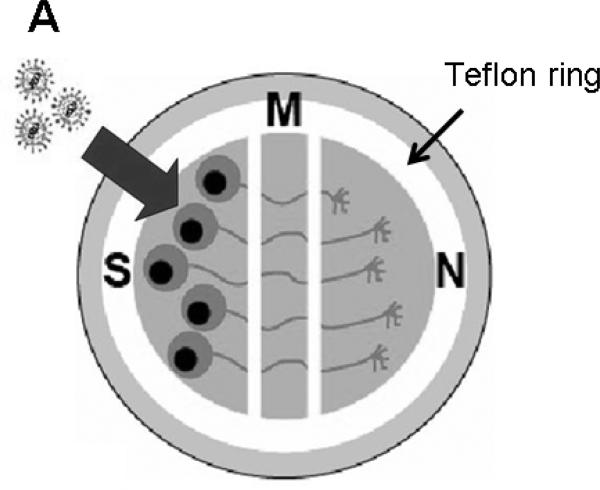
Campenot chambers were used to assess anterograde axonal transport (Figure 1). Dissociated cells from one-half of a rat or mouse SCG were plated into the soma (S) compartment. Upon differentiation, axons from these sympathetic neurons penetrate into the middle (M) chamber and reach the neurite (N) compartment by 2-3 weeks. After infection of S chamber neurons, virion components can only reach the M chamber by transport within axons, since the chambers are sealed from one another by silicone grease. Methylcellulose placed in the M chamber forms an additional barrier to leakage of cell-free virus between chambers (McGraw and Friedman, 2009). Detecting enveloped virions within axons in the M chamber at sites other than in growth cones indicates anterograde axonal transport of fully assembled virus particles, since virus particles that exit axons in the M chamber and subsequently re-infect neurons lose their envelope after fusing with the plasma membrane upon virus entry (Lycke et al., 1984; Nicola et al., 2005; Saksena et al., 2006; Smith et al., 2004).
Figure 1.
Model of SCG neuron cultures in Campenot chambers. A Teflon ring separates the cultures into three compartments; S is the soma, M the middle, and N the neurite (axon) chamber. Neurons are seeded into the S chamber and over 2-3 weeks neurites (axons) extend into the M and N chambers. Large arrow indicates virions added to the S chamber.
Ultrastructure of PRV, HSV-1 and HSV-2 in infected neurons
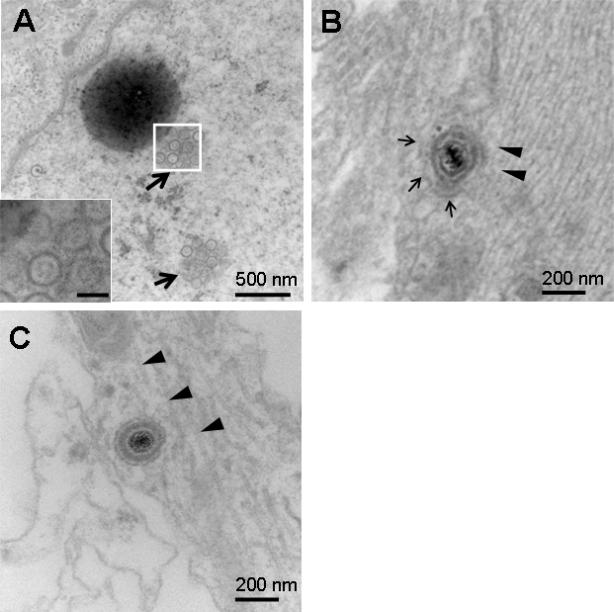
Rat SCG cell bodies in the S chamber were infected with PRV Becker. S and M chamber neurons were processed by TEM at 24 hours post infection (hpi). Numerous nucleocapsids were detected in the S chamber nuclei (Figure 2A). An enveloped capsid within a vesicle is shown in the proximal axon (S chamber) (Figure 2B) and in the mid-axon (M chamber) (Figure 2C). The virus-like particles were detected intracellularly within axons in close proximity to microtubules. These results support reports by others that complete virions are detected during anterograde transport of PRV (Ch'ng and Enquist, 2005b; del Rio et al., 2005; Feierbach et al., 2007; Lyman et al., 2007; Maresch et al., 2010).
Figure 2.
Rat SCG neurons in the S chamber infected with 1×105 PFU of PRV Becker and examined by TEM at 24 hpi. (A) Nucleocapsids 115 -120 nm in diameter were detected in nuclei (arrows). Boxed area is enlarged in inset. Bar in inset is 100 nm. (B) Anterograde axonal transport of PRV virions. A virion measuring 240 nm within a vesicle (thin arrows) was detected adjacent to microtubules (arrowheads) within a proximal axon (S chamber). (C) A virion measuring 235 nm was detected in close proximity to microtubules (arrowheads) within a mid-axon (M chamber).
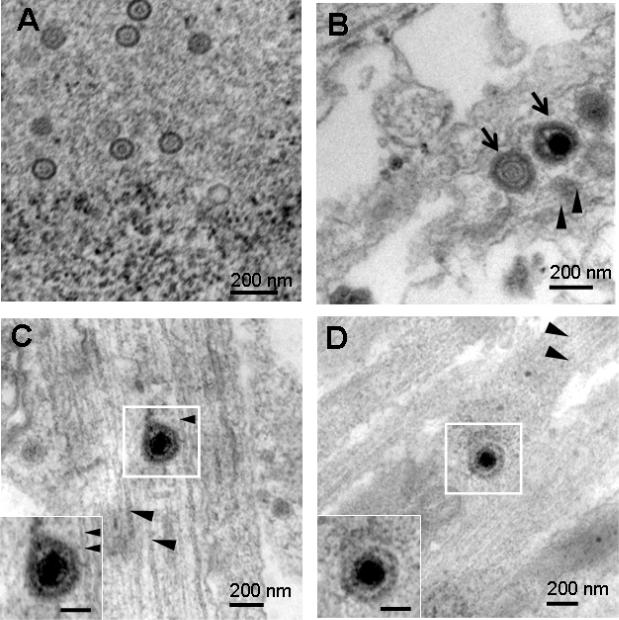
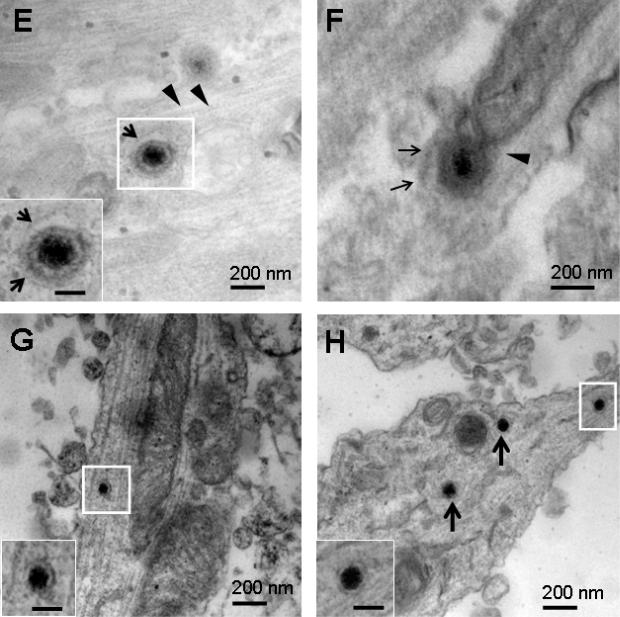
SCG neurons in the S chamber were infected with HSV-1 NS and at 48 hpi, S and M chambers were processed for TEM. Nucleocapsids were detected in SCG nuclei (Figure 3A). Enveloped capsids were detected within a vesicle in the proximal axon (S chamber) of a rat SCG neuron (Figure 3B), and in the mid-axon (M chamber) of a mouse SCG neuron (Figure 3C). HSV-1 17 was used to infect rat SCG neurons. Virions in vesicles in the mid-axon (M chamber) were detected intracellularly in close proximity to microtubules (Figures 3D, E). Rat SCG neurons were infected with HSV-2 2.12 and samples processed for TEM at 48 hpi. An enveloped capsid within a vesicle was apparent in the mid-axon adjacent to microtubules (Figure 3F). No differences in morphology of virus-like particles in proximal and mid-axons were detected comparing HSV-1, HSV-2 and PRV.
Figure 3.
Rat or mouse SCG neurons that were mock infected, or infected with 1×105 PFU of HSV-1 or HSV-2 and examined by TEM at 48 hpi. (A) Nucleocapsids ~ 110 nm were detected in rat SCG nuclei after infection with HSV-1 NS. (B) Multiple virions (arrows) measuring 220 to 225 nm possibly within a large vesicle were detected adjacent to microtubules (arrowheads) in a proximal axon (S chamber) of a rat SCG neuron after infection with HSV-1 NS. (C) A virion measuring 235 nm was detected in the mid-axon of a mouse SCG neuron adjacent to microtubules after infection with HSV-1 NS. (D, E) Virions measuring 213 nm and 220 nm within vesicles (arrows) were detected adjacent to microtubules (arrowheads) in rat SCG mid-axons (M chamber) after infection with HSV-1 17. (F) A virion measuring 255 nm in a vesicle (arrows) in close proximity to microtubules after infection with HSV-2 2.12. (G) A dense core vesicle measuring 102 nm was detected in a mid-axon (M chamber) of a mock infected rat SCG culture. (H) Three dense core vesicles 110 nm in diameter were noted in a mid-axon (M chamber, boxed particle) and growth cone (arrows). Boxed areas are enlarged in insets. Bars in insets are 100 nm.
As a control, TEM was performed on M chamber axons 48 h after mock infection of S chamber neurons. No fully assembled virions were noted; however, multiple dense core vesicles were detected in axons and growth cones that were generally 100 to 120 nm in diameter (range 80 to 170 nm) (Figures 3G, H). Based on size and morphology, some of these structures were difficult to distinguish from nucleocapsids, but none resembled fully assembled virions that ranged in size from 213-255 nm and were enclosed within vesicles. Therefore, we conclude that fully assembled virus particles are present within regions of the axon clearly distinguishable from the axon growth cone.
The number of virions detected in proximal and mid-axons was determined by examining every field (several hundred) of duplicate EM grids. Enveloped capsids were detected in rat SCG proximal and mid-axons after infection with HSV-1 NS (78 virions), HSV-1 17 (65 virions), or HSV-2 2.12 (68 virions), while 108 virions were detected after infection with PRV Becker and none was noted after mock infection. Virus-like particles were more numerous in proximal axons in S chambers; however, 10 to 20% of virions were detected in mid-axons in M chambers, indicating anterograde transport of assembled virions.
Discussion
Previous studies of anterograde axonal transport have not directly compared PRV, HSV-1 and HSV-2 using similar neuron cultures and detection techniques (Ch'ng and Enquist, 2005b; Feierbach et al., 2007; Holland et al., 1999; LaVail et al., 2005; Lyman et al., 2007; Maresch et al., 2010; Miranda-Saksena et al., 2009; Snyder et al., 2007). In fact, this report is the first to assess the morphology of HSV-2 during anterograde axonal transport. We chose to evaluate SCG neurons in Campenot chambers for the following reasons: i) rat SCG neurons are permissive to HSV-1, HSV-2 and PRV; ii) the purity of SCG neuron cultures approaches 100%, which minimizes contamination by non-neuronal cells; iii) the culture system includes physical barriers of silicon grease and methylcellulose that provide a leak-proof seal, thereby assuring that virion spread from one chamber to another is occurring by axonal transport; and iv) when virus is added to the S chamber, detecting virions in M chamber axons at sites other than growth cones permits a conclusion that anterograde axonal transport of assembled virions occurred (Ch'ng and Enquist, 2005b; Curanovic et al., 2009; McGraw et al., 2009; McGraw and Friedman, 2009).
We evaluated whether virion morphology during anterograde axonal transport is similar for HSV-1, HSV-2 and PRV using TEM as the detection assay. Our results with PRV are consistent with findings reported by others that detected virions within vesicles in axons during anterograde transport (Ch'ng and Enquist, 2005b; Feierbach et al., 2007; Lyman et al., 2007; Maresch et al., 2010). Our findings with HSV-1 are also consistent with a recent report that used live-cell imaging to assess HSV-1 and PRV anterograde axonal transport in chick embryo and rat dorsal root ganglia neurons (Antinone, Zaichick, and Smith, 2010). The live-cell imaging study concluded that capsids were mostly enveloped during anterograde transport of both PRV and HSV-1. We chose not to address the relative proportion of enveloped to non-enveloped capsids using TEM since static images cannot determine the direction of transport. In addition, dense core vesicles are morphologically difficult to distinguish from nucleocapsids (Bauer et al., 2004; Goldman, Kim, and Schwartz, 1976; Harvey, 1975; Maresch et al., 2010; Saksena et al., 2006). We detected assembled virions within vesicles adjacent to microtubules in M chamber axons after infection of S chamber neurons, which led us to conclude that anterograde transport of complete virions occurs for all three alphaherpesviruses examined to date.
Materials and methods
Cells and viruses
HSV-1 strains NS and 17, and HSV-2 strain 2.12 were grown in Vero (African green monkey kidney epithelial) cells, while PRV strain Becker was propagated on PK15 (porcine kidney epithelial) cells (Friedman et al., 1981; Hook et al., 2006; Keeler, Whealy, and Enquist, 1986). Dissociated embryonic superior cervical ganglion (SCG) neurons were derived from Sprague-Dawley rats (Charles River) on gestation day E17 or from BALB/c mice (Charles River) on gestation day E15 and approximately 5,000 neurons from one half of a SCG ganglion was cultured in each Campenot chamber (Ch'ng and Enquist, 2005a; Ch'ng and Enquist, 2005b; del Rio et al., 2005; McGraw et al., 2009; McGraw and Friedman, 2009; Wang et al., 2005). A Teflon ring separated the culture into three compartments; S is the soma, M the middle, and N the neurite (axon) chamber. The Teflon ring was held to the culture dish by silicon grease which formed a barrier to prevent free flow of virus between chambers. Methylcellulose was added to the M chamber prior to infection as another barrier to free flow. Movement of virus from one chamber to another requires axonal transport of virion components. Prior to infection, the neurons were grown for 2 to 3 weeks in neuron culture medium supplemented with 4.6 mg/ml glucose, 2 mM L-glutamine, 16 μg/ml putrescine (Sigma), 100 μg/ml holo-transferrin (Sigma), 10 μg/ml insulin (Sigma), 50 U/ml penicillin-streptomycin, 100 ng/ml nerve growth factor (Promega), 30 nM selenium (Sigma), and 20 nM progesterone (Sigma). Cultures were maintained in a humidified incubator at 37°C with 6% CO2 and the culture medium was replaced every 3 to 4 days.
Infection of SCG neurons
To evaluate anterograde axonal transport, neurons in the S chamber were infected with 1×105 PFU of virus in 50 μl of neuronal culture medium (MOI of 20) (Ch'ng and Enquist, 2005a; Ch'ng and Enquist, 2005b; McGraw et al., 2009; McGraw and Friedman, 2009). At 24 hpi with PRV or 48 hpi with HSV-1 or HSV-2, the cells were fixed in 2.5% glutaraldehyde buffer (pH 7.2). For mock infection, 50 μl of neuronal culture medium was added without virus.
Transmission electron microscopy
After several buffer washes the fixed samples were post-fixed in 2% osmium tetroxide containing 1.5% potassium ferricyanide for 1 h at room temperature. Samples were en bloc stained with 2% uranyl acetate for 30 min and subsequently dehydrated through a graded ethanol series prior to infiltrating and embedding with EMbed-812 (Electron Microscopy Sciences). Ultrathin (60 nm), longitudinal sections were cut and stained with uranyl acetate and bismuth subnitrite, and examined in a JEOL 1010 electron microscope fitted with a Hamamatsu digital camera and AMT Advantage image capture software. S and M chambers were processed separately. Many sections prepared from each chamber were screened to determine the best preparations. Axon segments in the S chamber were considered proximal axons, while those in the M chamber were considered mid-axons, based on distance (approximately 5 mm) from the neuronal cell body. Experiments were performed in duplicate and particle quantities in proximal and mid-axons were determined by counting virus-like particles in all fields of the TEM grid.
Acknowledgement
We thank Ray Meade for TEM technical assistance. This study was supported by NIH grant RO1 AI033063.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Antinone SE, Smith GA. Two modes of herpesvirus trafficking in neurons: membrane acquisition directs motion. J Virol. 2006;80(22):11235–40. doi: 10.1128/JVI.01441-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antinone SE, Zaichick SV, Smith GA. Resolving the assembly state of herpes simplex virus during axon transport by live-cell imaging. Journal of Virology Accepts, published online ahead of print. 2010 doi: 10.1128/JVI.01296-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer RA, Khera RS, Lieber JL, Angleson JK. Recycling of intact dense core vesicles in neurites of NGF-treated PC12 cells. FEBS Lett. 2004;571(1-3):107–11. doi: 10.1016/j.febslet.2004.05.086. [DOI] [PubMed] [Google Scholar]
- Ch'ng TH, Enquist LW. Efficient axonal localization of alphaherpesvirus structural proteins in cultured sympathetic neurons requires viral glycoprotein E. J Virol. 2005a;79(14):8835–46. doi: 10.1128/JVI.79.14.8835-8846.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ch'ng TH, Enquist LW. Neuron-to-cell spread of pseudorabies virus in a compartmented neuronal culture system. J Virol. 2005b;79(17):10875–89. doi: 10.1128/JVI.79.17.10875-10889.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curanovic D, Ch'ng TH, Szpara M, Enquist L. Compartmented neuron cultures for directional infection by alpha herpesviruses. Curr Protoc Cell Biol. 2009 doi: 10.1002/0471143030.cb2604s43. Chapter 26, Unit 26 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Rio T, Ch'ng TH, Flood EA, Gross SP, Enquist LW. Heterogeneity of a fluorescent tegument component in single pseudorabies virus virions and enveloped axonal assemblies. J Virol. 2005;79(7):3903–19. doi: 10.1128/JVI.79.7.3903-3919.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feierbach B, Bisher M, Goodhouse J, Enquist LW. In vitro analysis of transneuronal spread of an alphaherpesvirus infection in peripheral nervous system neurons. J Virol. 2007;81(13):6846–57. doi: 10.1128/JVI.00069-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman HM, Macarak EJ, MacGregor RR, Wolfe J, Kefalides NA. Virus infection of endothelial cells. Journal of Infectious Diseases. 1981;143(2):266–73. doi: 10.1093/infdis/143.2.266. [DOI] [PubMed] [Google Scholar]
- Goldman JE, Kim KS, Schwartz JH. Axonal transport of [3H]serotonin in an identified neuron of Aplysia californica. J Cell Biol. 1976;70(2 pt 1):304–18. doi: 10.1083/jcb.70.2.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey FH. The large dense core vesicle: a normal organelle of the central nervous system myelinated axon. Acta Neuropathol. 1975;31(2):91–6. doi: 10.1007/BF00688142. [DOI] [PubMed] [Google Scholar]
- Holland DJ, Miranda-Saksena M, Boadle RA, Armati P, Cunningham AL. Anterograde transport of herpes simplex virus proteins in axons of peripheral human fetal neurons: an immunoelectron microscopy study. Journal of Virology. 1999;73(10):8503–11. doi: 10.1128/jvi.73.10.8503-8511.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hook LM, Lubinski JM, Jiang M, Pangburn MK, Friedman HM. Herpes simplex virus type 1 and 2 glycoprotein C prevents complement-mediated neutralization induced by natural immunoglobulin M antibody. J Virol. 2006;80(8):4038–46. doi: 10.1128/JVI.80.8.4038-4046.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeler CL, Jr., Whealy ME, Enquist LW. Construction of an infectious pseudorabies virus recombinant expressing a glycoprotein gIII-beta-galactosidase fusion protein. Gene. 1986;50(1-3):215–24. doi: 10.1016/0378-1119(86)90326-4. [DOI] [PubMed] [Google Scholar]
- LaVail JH, Tauscher AN, Aghaian E, Harrabi O, Sidhu SS. Axonal transport and sorting of herpes simplex virus components in a mature mouse visual system. Journal of Virology. 2003;77(11):6117–26. doi: 10.1128/JVI.77.11.6117-6126.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaVail JH, Tauscher AN, Hicks JW, Harrabi O, Melroe GT, Knipe DM. Genetic and molecular in vivo analysis of herpes simplex virus assembly in murine visual system neurons. J Virol. 2005;79(17):11142–50. doi: 10.1128/JVI.79.17.11142-11150.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaVail JH, Tauscher AN, Sucher A, Harrabi O, Brandimarti R. Viral regulation of the long distance axonal transport of herpes simplex virus nucleocapsid. Neuroscience. 2007;146(3):974–85. doi: 10.1016/j.neuroscience.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lycke E, Kristensson K, Svennerholm B, Vahlne A, Ziegler R. Uptake and transport of herpes simplex virus in neurites of rat dorsal root ganglia cells in culture. J Gen Virol. 1984;65(Pt 1):55–64. doi: 10.1099/0022-1317-65-1-55. [DOI] [PubMed] [Google Scholar]
- Lyman MG, Feierbach B, Curanovic D, Bisher M, Enquist LW. Pseudorabies virus Us9 directs axonal sorting of viral capsids. J Virol. 2007;81(20):11363–71. doi: 10.1128/JVI.01281-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maresch C, Granzow H, Negatsch A, Klupp BG, Fuchs W, Teifke JP, Mettenleiter TC. Ultrastructural analysis of virion formation and anterograde intraaxonal transport of the alphaherpesvirus pseudorabies virus in primary neurons. J Virol. 2010;84(11):5528–39. doi: 10.1128/JVI.00067-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGraw HM, Awasthi S, Wojcechowskyj JA, Friedman HM. Anterograde spread of herpes simplex virus type 1 requires glycoprotein E and glycoprotein I but not Us9. J Virol. 2009;83(17):8315–26. doi: 10.1128/JVI.00633-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGraw HM, Friedman HM. Herpes simplex virus type 1 glycoprotein E mediates retrograde spread from epithelial cells to neurites. J Virol. 2009;83(10):4791–9. doi: 10.1128/JVI.02341-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda-Saksena M, Armati P, Boadle RA, Holland DJ, Cunningham AL. Anterograde transport of herpes simplex virus type 1 in cultured, dissociated human and rat dorsal root ganglion neurons. Journal of Virology. 2000;74(4):1827–39. doi: 10.1128/jvi.74.4.1827-1839.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda-Saksena M, Boadle RA, Aggarwal A, Tijono B, Rixon FJ, Diefenbach RJ, Cunningham AL. Herpes simplex virus utilizes the large secretory vesicle pathway for anterograde transport of tegument and envelope proteins and for viral exocytosis from growth cones of human fetal axons. J Virol. 2009;83(7):3187–99. doi: 10.1128/JVI.01579-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicola AV, Hou J, Major EO, Straus SE. Herpes Simplex Virus Type 1 Enters Human Epidermal Keratinocytes, but Not Neurons, via a pH-Dependent Endocytic Pathway. J. Virol. 2005;79(12):7609–7616. doi: 10.1128/JVI.79.12.7609-7616.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penfold ME, Armati P, Cunningham AL. Axonal transport of herpes simplex virions to epidermal cells: evidence for a specialized mode of virus transport and assembly. Proceedings of the National Academy of Sciences of the United States of America. 1994;91(14):6529–33. doi: 10.1073/pnas.91.14.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radtke K, Kieneke D, Wolfstein A, Michael K, Steffen W, Scholz T, Karger A, Sodeik B. Plus- and minus-end directed microtubule motors bind simultaneously to herpes simplex virus capsids using different inner tegument structures. PLoS Pathog. 2010;6(7):e1000991. doi: 10.1371/journal.ppat.1000991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saksena MM, Wakisaka H, Tijono B, Boadle RA, Rixon F, Takahashi H, Cunningham AL. Herpes simplex virus type 1 accumulation, envelopment, and exit in growth cones and varicosities in mid-distal regions of axons. J Virol. 2006;80(7):3592–606. doi: 10.1128/JVI.80.7.3592-3606.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GA, Gross SP, Enquist LW. Herpesviruses use bidirectional fast-axonal transport to spread in sensory neurons. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(6):3466–70. doi: 10.1073/pnas.061029798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GA, Pomeranz L, Gross SP, Enquist LW. Local modulation of plus-end transport targets herpesvirus entry and egress in sensory axons. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(45):16034–9. doi: 10.1073/pnas.0404686101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder A, Bruun B, Browne HM, Johnson DC. A herpes simplex virus gD-YFP fusion glycoprotein is transported separately from viral capsids in neuronal axons. J Virol. 2007;81(15):8337–40. doi: 10.1128/JVI.00520-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomishima MJ, Enquist LW. In vivo egress of an alphaherpesvirus from axons. Journal of Virology. 2002;76(16):8310–7. doi: 10.1128/JVI.76.16.8310-8317.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Tang W, McGraw HM, Bennett J, Enquist LW, Friedman HM. Herpes simplex virus type 1 glycoprotein E is required for axonal localization of capsid, tegument, and membrane glycoproteins. J Virol. 2005;79(21):13362–72. doi: 10.1128/JVI.79.21.13362-13372.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfstein A, Nagel CH, Radtke K, Dohner K, Allan VJ, Sodeik B. The inner tegument promotes herpes simplex virus capsid motility along microtubules in vitro. Traffic. 2006;7(2):227–37. doi: 10.1111/j.1600-0854.2005.00379.x. [DOI] [PubMed] [Google Scholar]