Abstract
Melanoma cell lines are useful tools for the analysis of tumor-specific lymphocytes which are injected to patients treated by adoptive immunotherapy. So they have been established previously (with an efficacy of 47%) in Roswell Park Memorial Institute (RPMI) medium enriched with fetal calf serum (FCS). In order to improve the probability of establishing melanoma cell lines, we compared two FCS-free media with the original FCS medium. Ten melanoma-invaded lymph nodes were tested for their ability to grow in three different culture media: RPMI with FCS; RPMI with human serum (HS); serum-free X-vivo 15 (X15). For each medium, we compared the following criteria: percentage of lines obtained; period of establishment; cell morphology; expression of melanoma-associated antigens and surface molecules. More cell lines were obtained with HS and X15 media compared to FCS medium (7/10, 5/10 and 4/10, respectively). The time period to establish a stable line was similar for the three media. No morphological differences were observed in cells derived from the same tumor sample in the different media. With the X15 medium, cells generally expressed lower levels of melanocytic differentiation antigens and surface molecules. The growth of melanoma cell lines in FCS-free culture media appears possible and advantageous, with an increased probability of obtaining autologous tumor cell lines. Furthermore the cells obtained could be used as multiple antigenic sources in active or adoptive immunotherapy protocols.
Keywords: Cell-based therapy, Melanoma, Serum-free cell culture medium, Tumor cell line growth
Introduction
Continuous cell lines and short term cultures of tumor cells are important tools for testing and improving new diagnostic and therapeutic strategies. In active immunotherapy, cell lines can be used directly (Dillman et al. 2005), either after genetic manipulation in order to increase the antigenic response (Zhou et al. 2005), or in the form of tumor lysate presented by the autologous dendritic cells (Zitvogel et al. 2000). In adoptive immunotherapy protocols, cell lines enable the selection of populations of Tumor Infiltrating Lymphocytes (TILs) rich in T lymphocytes specific to the autologous tumor before their expansion (Dudley and Rosenberg 2003). After TILs expansion, they are used in the evaluation of the relative number of TILs specific to the autologous melanoma line that has been injected into the patients (Pandolfino et al. 2001; Labarrière et al. 2002). They can also contribute to identifying prognostic factors in the therapeutic response (Lacreusette et al. 2007; Lacreusette et al. 2008; Lacreusette et al. 2009) and new antigens (Godet et al. 2008).
Since 1994, at our cell engineering unit (UTCG, CHU Nantes, France) which follows Good Manufacturing Practices (GMP), we have regularly established autologous tumor lines derived from metastatic tumor fragments, from which TILs are extracted. Fetal calf serum (FCS) is mainly used for the in vitro culture of tumor lines as a supplement to synthetic culture media (such as RPMI, DMEM, L15 …). However, the non-standardized composition of this non-human serum, which includes a large variety of unidentified mediators, can influence the results of the lines obtained. In addition, the setting up and growth of tumor cells in FCS medium can induce artifacts in the immune response analysis, since the proteins present in the FCS during the in vitro culture are a source of antigenic peptides which can be presented to the T cells (Sulit et al. 1976; Le Dréan et al. 1995).
In this context, we have compared our normal FCS-containing medium with two other FCS-free culture media; one of these media (the X-vivo15 medium) is serum-free and has never been used before for this indication. Before carrying out the second step which consists in testing the autologous TILs, we characterized and compared the cell lines established in these different media on the basis of melanoma-associated tumor antigens (MAA), major histocompatibility complex (MHC) class I and class II molecules, and adhesion molecules (CD54 and CD58), in order to see if the composition of one medium could modify the recognition of the melanoma cells by the immune system. All these markers are important in the TILs response to the autologous tumor cell.
Materials and methods
Culture media
To establish autologous melanoma cell lines, we used three different culture media: the first culture medium consisted of Roswell Park Memorial Institute (RPMI) 1640 medium (Sigma Chemical, St Louis, MO) supplemented with 10% FCS (Biowest, Nuaillé, France), 1 mM glutamine (Lonza, Walkersville, MD) (called FCS medium in the text); the second culture medium consisted of RPMI 1640 medium (Sigma) supplemented with 8% human diseased serum specimen from hemochromatosis patients (HS) (Transfusion Center, Nantes, France), 1 mM glutamine (Lonza) (called HS medium in the text); the third culture medium was X-vivo15 serum-free medium (Lonza) supplemented with 1 mM l-glutamine only (Lonza) (called X15 medium in the text). FCS and HS media unlike X15 medium did not contain antibiotics.
Tumor samples
Tumor samples were obtained from 10 melanoma-invaded lymph nodes (LNs). All patients signed an informed consent approved by the Ethical Committee (Pays de La Loire, France) for the use of surgical samples for research. Following surgical resection, all samples were immediately transferred to our cell engineering unit UTCG (Unit of Cell and Gene Therapy, Nantes, France). One half of the samples were used for these studies, and the rest of the samples were processed for pathologic examination.
Establishment of melanoma cell lines
Melanoma cell lines were established as previously described (Gervois et al. 1990). Briefly, fresh LNs with metastasis were minced into small tumor microexplants (approximately 1–2 mm3) using scissors and biopsy punch. The resultant fragments suspension was centrifuged and then pieces were inoculated (at a rate of two or three per well) in wells of 24-well plates (NUNC) and 1.5 mL of different media (described above) was added in each well. The plates were placed at 37 °C in a humidified incubator with 5% CO2. The plates were observed under a light microscope every week and subcultured if necessary.
Phenotypic characterization of tumor cell lines
Once the melanoma cell lines were established, cell surface expression of HLA, MAA and other surface antigens (Ags) was determined by flow cytometry. MAA expression was assessed by intracellular staining. The process consisted in fixing cells in 200 μL PBS 4% paraformaldehyde (Sigma–Aldrich) at room temperature for 10 min and washed before labeling. Cells were then aliquoted and incubated separately with anti-Melan-A (A103, DakoCytomation, Carpinteria, CA), anti-gp100 (HMB45, DakoCytomation), anti-tyrosinase (T311, Novocastra laboratories Ltd, Newcastle upon Tyne, UK), anti-MAGES (6C1: recognized MAGE 1.2.3.4.6. 10 and 12; Santa Cruz) and anti-NY-ESO1 (E978, Santa Cruz) antibodies. They were then incubated for 30 min at room temperature at an antibody concentration of 5 μg/mL. After two washes with perm wash, cells were subjected to a second incubation with fluorescein isothiocyanate (FITC)-labeled Fab’2 fragments of goat anti-mouse immunoglobulin G (IgG) (Beckman Coulter). Negative controls were performed by incubating the cells with isotype-matched control antibody (all from BD Pharmingen). Reagent dilutions and washes were performed with PBS containing 0.1% BSA and 0.1% saponine (Sigma–Aldrich). In order to detect surface molecules, approximately 5–10 × 104 cells were incubated with the first monoclonal antibody (mAb, used at a concentration of 5 μg/mL) for 30 min at 4 °C: anti-MHC class I (A, B, C) (G46-222.6; BD Pharmingen, San Diego, USA); anti-MHC class II (DR, DQ, DP) (TÜ39; BD Pharmingen); anti-CD54 (ICAM-1) (84H10; Immunotech, Marseille, France); anti-CD58 (LFA-3) (BRIC-5; Serotec, Oxford, UK). Then cells were incubated for 30 min with FITC-labeled Fab’2 fragments of goat anti-mouse IgG (Beckman Coulter). Negative controls were performed by incubating the cells with isotype-matched control antibodies (all from BD Pharmingen). Reagent dilutions and washes were performed with PBS containing 0.2% bovine serum albumin (BSA) (Sigma–Aldrich). Then fluorescence was measured on a FACScan flow cytometer and was analyzed with CellQuestPro software (both from BD Bioscience, San Diego, CA, USA). 10 × 103 cells were gated with FSC/SSC parameters and analyzed.
Semi quantitative polymerase chain reaction (PCR)
This technique was used by the laboratory of Immuno-Dermatology of Nantes University Hospital (CHU Nantes, France). Total RNA was extracted from melanoma cell lines by the guanidium/cesium chloride procedure. Reverse transcription was performed on 2 μg of total RNA in a reaction volume of 20 μL of reverse transcription buffer (Invitrogen Life Technologies, Cergy Pontoise, France), 2 μL of 20 μM oligo (dT-15) primer solution (Invitrogen), 4 μL of 10 mM dNTP mix (Boehringer Mannheim, GmbH, Mannheim, Germany), 20 U of RNAsin (Promega, Madison, WI), 2 μL DTT 100 μM and 200 U of M-MLV reverse transcriptase (Invitrogen). The reaction was incubated for 60 min at 42 °C, and the volume was then adjusted to 100 μL with distilled water. PCR amplification of human β-actin cDNA was then performed with 2.5, 5 or 10 μL of the cDNA solution supplemented with 5 μL of PCR buffer, 3 μL of MgCl2 25 mM (Invitrogen), 4 μL of dNTP mix 10 mM, 1 μL of each 20 μM solution of primers, 0.5 U of Taq polymerase (Invitrogen) and water to a final volume of 50 μL. PCR was run for 21 cycles of 1 min at 94 °C, 1 min at 65 °C and 1 min at 72 °C. The primers used were: Melan-A: sense: 5′ CTGACCCTACAAGATGCCAAGAG 3′, antisense: 5′ CTCTACTTCCTCCTGATTGTTGAG 3′; Tyrosinase: sense: 5′GGATAGCGGATGCCTCTAAAG 3′, antisense: 5′ GGGCCAGGCTCCAGGTAAGTAT 3; MAGE1: sense: 5′ GGCCGAAGGAACCTGACCCAG 3′, antisense: 5′GCTGGAACCCTCACT GGGTTGC 3′, MAGE3: sense: 5′ TGGAGGACCAGAGGCCCCC 3′, antisense: 5′ GGACGATTATCAGGAGGCCTGC 3′; NY-ESO1: sense: 5′ CCCCACCGCTTCCCGTG 3′, antisense: 5′ CTGGCCACTCGTGCTGGGA 3′; Na17-A: sense: 5′GATGTGTTCAT ACGCTGTGTGGT 3′, antisense: 5′ CTCTACTTCCTCCTGATTGTTGAGA 3′. Ten microlitre of PCR products were size-fractionated on 1% agarose gel, and the signal intensity was evaluated. For the amplification of melanoma antigens, cDNA volumes were chosen which gave the same signal intensity after actin amplification. For the amplification of Na17-A and MAGE-1, MAGE-3 cDNA, the PCR assay was performed for 30 cycles, respectively, 1 min at 94 °C, 1 min at 62 °C, 1 min at 72 °C; and 1 min at 92 °C, 2 min at 72 °C and 5 min at 72 °C. Amplification of tyrosinase cDNA was run for 25 cycles (1 min at 94 °C, 1 min at 65 °C, 1 min at 72 °C). For the amplification of Melan-A/MART-1 cDNA, the PCR assay was performed for 24 cycles (1 min at 94 °C, 1 min at 60 °C, 1 min at 72 °C) and, finally, for the amplification of NY-ESO1 cDNA, the PCR assay was performed for 30 cycles: 30 s at 95 °C, 1 min at 62 °C and 2 min at 72 °C. PCR products were then size-fractionated on 1% agarose gel. Positive and negative controls were used. Visual estimation of gene expression was performed by comparison with the controls and expressed as percentage of no gene expression (0), faintly visible band (<25), visible band (25–50) and strongly visible band (>50).
Results
Establishment of melanoma cell lines in different culture media
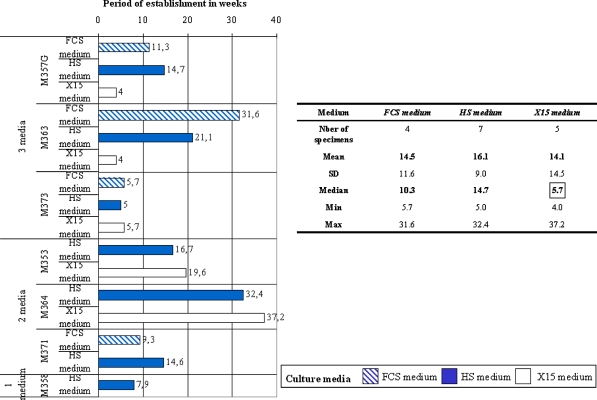
Seven of the ten specimens could thus be established as stable cell lines with either one (n = 1, 14%), two (n = 3, 43%) or three culture media (n = 3, 43%; Fig. 1). The analysis of the number of tumor cell lines obtained with each of the media showed that the HS medium was the most effective medium for establishing a melanoma cell line (7/10), followed by the X15 medium (5/10), and the FCS medium (4/10) (Table 1). We compared the period of establishment of melanoma cell lines in the different culture media for the seven tumor biopsies for which melanoma cell lines were obtained. The establishment period, presented in Fig. 1, was expressed in weeks and corresponded to the time interval between the seeding of tumor fragments and the confluence of a culture flask of 25 cm2 containing only tumor cells (light microscope) and ready to freeze. The establishment period of stable cell-lines was on average the same for the three culture media (FCS medium: 14.5 ± 11.6 weeks, n = 4; HS medium: 16.1 ± 9 weeks, n = 7; X15 medium: 14.1 ± 14.5 weeks, n = 5). However, it was interesting to note that for two melanoma-invaded LNs (M357G and M363), the establishment period of the cell line using the X15 medium was considerably shortened in comparison to the two other culture media used (Fig. 1).
Fig. 1.
The difference between culture media* as to the establishment period of melanoma cell lines. The establishment period corresponded to the time interval between the seeding of tumor fragments and the confluence of a culture flask of 25 cm2 containing only tumor cells and ready to freeze; the establishment period is expressed in weeks
Table 1.
The difference between culture media as to the establishment efficiency of melanoma cell lines
| Medium | Establishment efficiency |
|---|---|
| FCS medium | 4/10 |
| HS Medium | 7/10 |
| X15 Medium | 5/10 |
Growth characteristics
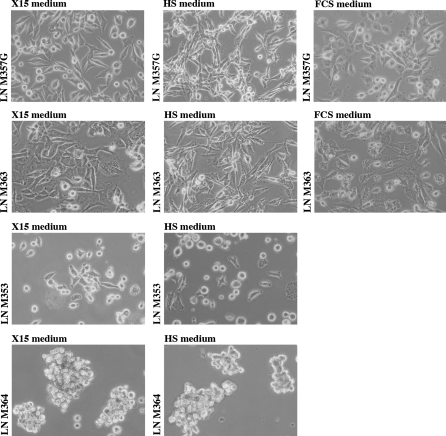
When the tumor specimens were plated in the different media, melanoma cells differed from the other contaminant cells contained in the LN by their typical epithelioid or spindle refractile appearance. We observed that the fibroblasts and lymphocytes in the melanoma-invaded LNs tended to have reduced proliferation and reduced activation in the HS medium and especially in the X15 medium, compared to those in the FCS medium. No morphological difference was observed between the cells originating from the same tumor sample in the three different culture media (Fig. 2). In one instance, the pellet after centrifugation of the M364 line obtained with the X15 medium was more pigmented than the M364 line established in the HS medium (data not shown).
Fig. 2.
Appearances of melanoma cell lines established from 4 tumor-invaded LNs with 2 or 3 culture media. (Phase contrast, ×40). Cultures have less than 10 passages
To ascertain the melanocytic origin and confirm that the cell cultures consist exclusively of melanoma cells, we studied the expression of specific tumor markers by FACS analysis and semi-quantitative RT-PCR.
MAA of the melanoma cell lines established in the different culture media
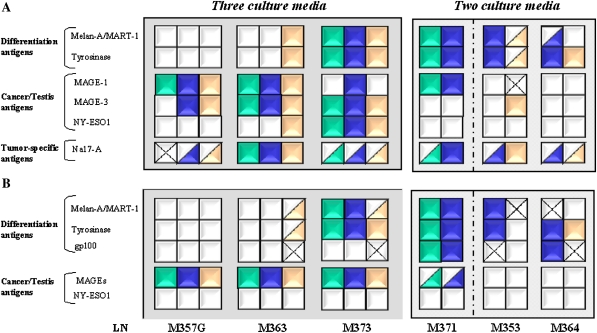
Tumor lines for six of the seven melanoma-invaded LNs were obtained, either from all three media (n = 3) or from two media (n = 3), thus allowing the Ag expression determined by semi-quantitative reverse transcription polymerase chain reaction (RT–PCR) and FACS to be compared. The overall results are presented in Fig. 3.
Fig. 3.
The transcriptional and proteic expression of melanoma-associated antigens in melanoma cell cultures established in different growth media. a The transcriptional expression of tumor antigens was determined by semi-quantitative RT–PCR. Normalized scores were determined by comparison with the positive and negative controls expressed as a percentage. b The expression of intracellular tumor antigens was determined by FACS analysis. Normalized scores were calculated by subtracting the number of cells stained for the relevant Ag by the background of cells stained by the relevant isotype control mAb. Normalized scores for (a) and (b) were represented by:
By FACS analysis, we evaluated the expression of Melan-A, Tyrosinase, gp100, MAGEs (by 6C1 Ab which recognized MAGE 1.2.3.4.6. 10 and 12) and NY-ESO1. Figure 3 B shows the percentages, for each tumor antigen studied, of positive cells within a melanoma cell population obtained with one culture medium. In the case of four out of the six tumor-invaded LNs, the melanoma cell lines derived from the same tissue sample displayed a different MAA expression profile reflected in a distinct expression pattern of the differentiation antigens. This related to the melanoma cell lines established in the X15 medium compared to those obtained in the HS medium and or FCS medium. At the transcriptional level, except for LN M373, we found variations in the expression of mRNA specific Melan-A and Tyrosinase differentiation antigens (Fig. 3a). No difference was detected in the percentage of expression of positive cells of Cancer/Testis antigens per melanoma cell line established in different culture media (Fig. 3b). However, we observed differences in MAA-specific mRNA expression of Cancer/Testis antigens and Na17-A between the cell lines established in different culture media and these differences could not be associated with a culture medium in particular (Fig. 3a).
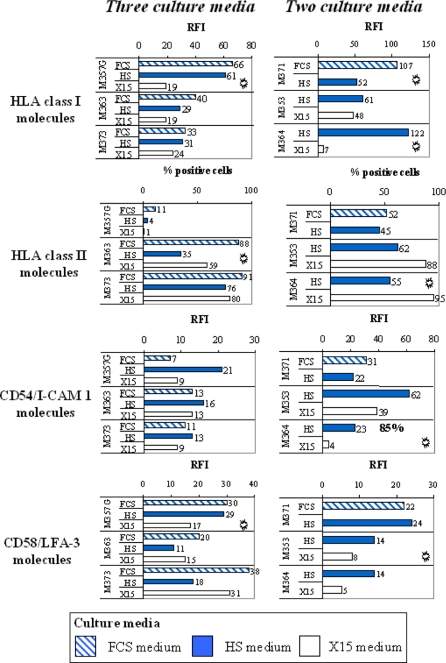
Expression of MHC and adhesion molecules on melanoma cell lines
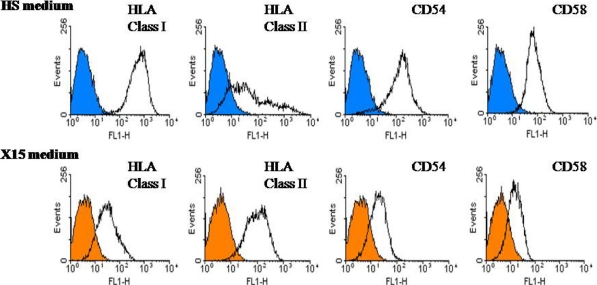
All the cells within the tumor cell lines established in this study expressed on their surface HLA class I, CD58 and CD54 molecules (except for the M364 line established in the HS medium). In order to compare cell lines established from the same sample in two or three culture media, we calculated a ratio of fluorescence intensity (RFI) for antigen expression as follows: mean fluorescence intensity with the specific mAb divided by mean fluorescence intensity obtained with isotype control mAb. The analysis of surface molecules is presented in Fig. 4. There were differences in expression, but less than for the tumor Ags. Overall, compared to the other media, the cell lines established in the X15 medium expressed HLA class I molecules slightly less. The lines established from five out of six LNs expressed HLA class II molecules on a variable fraction of tumor cells within melanoma population, which is not uncommon for melanoma. The cell lines established in the HS medium expressed HLA class II molecules less. Tumor cell lines established from LN M364 in the HS and X15 media showed differences in the expression of surface molecules in Fig. 5.
Fig. 4.
The expression of surface markers on melanoma cell lines as determined by FACS analysis. Surface expression was determined by flow cytometry using specific mAbs. For HLA class II molecules, the percentage of positive cells within a population was calculated to relative isotype control. The ratio RF I = mean fluorescence intensity with the specific mAb divided by its isotype control was calculated for HLA Class I, CD54 and CD58 molecules
.  Representative of two independent experiments
Representative of two independent experiments
Fig. 5.
The heterogeneous expression of surface molecules per melanoma cell line established in two culture media from the same tumor-invaded LN M364. The empty histograms show staining with the mAb specific for the cell surface molecule indicated, while the overlaid filled histograms (blue for cell line established in HS medium and orange in X15 medium) represent negative control staining with isotype matched control antibody
Discussion
In this study, we showed that it was possible to use two FCS-free culture media to increase the probability of establishing a melanoma cell line from each lymph node sample invaded by the tumor. The results show that the most favorable culture medium for obtaining an autologous tumor line, by comparison with the FCS medium, is the HS medium, followed by the X15 medium. The use of these two other media enabled us to obtain three additional lines with the HS medium and two additional lines with the X15 medium; this was in addition to the four lines obtained with the usual FCS-supplemented medium. Overall, compared to the FCS medium, the use of the HS and X15 media did not result in a quicker development of melanoma lines. However, in two out of the five melanoma-invaded LNs treated in the X15 medium, a cell line appeared more rapidly compared to those in the HS and FCS media. The cell composition of the tumor sample was an important factor to determine the rate of success in obtaining initial growth of tumor cells from microexplants and it has become apparent that there is no optimal growth medium and that a particular melanoma cell line may be established well and quickly in one medium but not another. A major problem with adapting fresh melanoma-invaded LNs to grow in vitro is contamination by connective tissue containing non-transformed cells especially fibroblasts and, for some LNs, by activated TILs which destroy tumor cells. One advantage of using these two media, and particularly the X15 medium, is that not only fibroblasts but also lymphocytes proliferated less. We frequently observed, at the bottom of culture wells containing tumor fragments grown in the X15 medium, normal adherent cells which containing a variable amount of melanin granules and presenting the appearance of dendritic cells or macrophages. This is perhaps due to the fact that this medium could be also used to support the growth of human monocytes and macrophages (distributor’s information). We already knew that melanoma lines established in the FCS medium could adapt to the HS medium (Le Dréan et al. 1995). Here, we have shown for the first time that the use of HS-supplemented RPMI 1640 medium could definitively increase the direct establishment rate of a melanoma cell line compared to the FCS medium (from approximately 40 to 70%). Only one study mentions using HS to establish melanoma lines directly from fine needle aspirates of melanoma metastases (from lymph node or of subcutaneous origin; Panelli et al. 2000); the efficacy in that study was around 44%, i.e. weaker than ours. The use of HS to establish or grow tumor lines has been slowed due to its lack of availability and even more so to its cost. We have access to HS produced by INSERM Unit 892 (Nantes, France) and originating from a large number of more than 20 hemochromatosis donors (Transfusion Center, Nantes, France).
As far as we know, this is also the first description of the synthetic serum-free medium X-vivo 15 being used to establish tumor cell lines directly. This medium, which was developed for the in vitro production of TILs under safety conditions, has only been described previously as providing a serum-free environment for various previously established tumor cell lines [distributor’s information, Sezary syndrome, osteosarcoma (Bruserud et al. 2005)]. The cell lines directly established and grown in X15 medium are particularly interesting for clinical use; since these cells have never been in contact with FCS, there is no risk that they have been contaminated by pathogens, such as viruses and prions, originating from FCS. In addition, the use of the X15 medium avoids problems encountered with FCS or with HS due to batch-to-batch variability, which can influence the characteristics of the cultivated tumor cells, and impedes the standardization of experimental protocols.
The tumor cell lines, which we have produced up to now in FCS medium, have enabled us to detect the presence of T lymphocytes specific to the autologous melanoma cell line in the therapeutic products (TILs) produced at UTCG (Pandolfino et al. 2001; Labarrière et al. 2002). In addition, they have enabled the discovery of new tumor Ags (Godet et al. 2008), and the investigation of new therapeutic response biomarkers (Lacreusette et al. 2007; Lacreusette et al. 2008). For these reasons, we evaluated the expression of tumor Ags to detect the possible impact that a culture medium could have and we targeted the tumor Ags which can be used in anti-melanoma therapies. It emerges, from our study of cell lines established from six melanoma-invaded LNs, that the lines obtained in HS medium had an antigenic profile which was relatively similar to that of the lines obtained in FCS medium. On the other hand, the lines established in X15 medium had different expression levels of differentiation Ags, compared to those obtained in the FCS or HS medium. Regarding the cancer-germline antigens, it is more difficult to come to a conclusion as to the influence of a particular medium since our means of detection (semi-quantitative PCR, and Abs that do not discriminate between MAGE-1 and MAGE-3) are not accurate enough. This is also the case for the Na17-A Ag.
The expression level of the surface molecules involved in the lymphocyte-tumor cell interaction (i.e. the HLA and adhesion molecules) was weaker in the X15 medium compared to that seen on the cell lines grown in the FCS and HS media. This could have a knock-on effect on the T lymphocyte response against the autologous tumor cell. Adhesion molecules play an important role in the initial lymphocyte activation phase. CD54 is important for the lysis of melanoma cell lines by cytotoxic T lymphocytes (CTLs) (Labarriere et al. 1997), and a minimal expression of CD58 is needed for the optimal activation and correct functioning of melanoma-specific CTL clones (Labarriere et al. 1997). A high level of CD58 increases cytokine production by CTL clones, thus indicating that CD58 is needed for effective specific immune reactions against melanoma cells (Le Guiner et al. 1998). Additional studies on a larger sample are now necessary, associated with functional assays to evaluate the impact of culture medium on the recognition of the melanoma cells by the immune system.
Our analysis was carried out on cultures having less than ten passages, in order to avoid effects related to prolonged culture (Panelli et al. 2000; Lotem et al. 2003; Vogl et al. 2005). We cannot, however, exclude the possibility that the culture media and culture conditions affected the proliferation of some subclones compared to others. After mechanical separation of around 10% of the LN, the tumor microexplants were distributed into several wells of 24-well plates depending on the size of the starting material, in a random manner; subclones derived from a small tumor zone with a phenotype differing from the rest of the LN could therefore emerge and promote the growth of one subclone. To distinguish between the influence of the X15 medium and the random distribution, it would be interesting to thaw parts of the same LN and then analyze and compare the cell lines obtained on the basis of tumor Ag expression. Mechanic dissociation seems better suited to establish melanoma cell lines for therapeutic use than “well-mixed” single cell suspension obtained after enzymatic digestion.
The results of this study lead us to conclude that melanoma cell lines can be easily established in culture media which are adapted for lymphocyte proliferation and do not contain FCS. Antitumor immune response studies could thus be carried out under standardized conditions where the tumor cells only present self peptides to the T lymphocytes without interference from foreign peptides contained in FCS. In addition, these directly usable clinical grade media are of interest for carrying out short-term cultures or establishing and growing autologous or allogenic melanoma cell lines, under GMP conditions. After irradiation, these cell lines could be used in clinical situations such as vaccination protocols or adoptive immunotherapy protocols (where they could be used in the autologous or allogenic stimulation of lymphocytes).
Acknowledgments
We would like to thank M. Yviquiel and S. Peltier for their technical support in semi-quantitative RT–PCR. We also would like to acknowledge C. Holmes who translated parts of this article. This paper is supported by Cancer Immunotherapy SIXTH FRAMEWORK PROGRAMME.
References
- Bruserud O, Tronstad KJ, Berge R (2005) In vitro culture of human osteosarcoma cell lines: a comparison of functional characteristics for cell lines cultured in medium without and with fetal calf serum. J Cancer Res Clin Oncol 131:377–384. doi:10.1007/s00432-004-0650-z [DOI] [PubMed]
- Dillman RO, Beutel L, Nayak S, Depriest C, Selvan S, Schiltz P. Cancer vaccine potency: is there a dose/response relationship for patient-specific vaccines and clinical outcomes? Cancer Biother Radiopharm. 2005;20:373–378. doi: 10.1089/cbr.2005.20.373. [DOI] [PubMed] [Google Scholar]
- Dudley ME, Rosenberg SA (2003) Adoptive-cell-transfer therapy for the treatment of patients with cancer. Nat Rev Cancer 3:666–675. doi:10.1038/nrc1167 [DOI] [PMC free article] [PubMed]
- Gervois N, Heuze F, Diez E, Jotereau F. Selective expansion of a specific anti-tumor CD8 + cytotoxic T lymphocyte clone in the bulk culture of tumor-infiltrating lymphocytes from a melanoma patient: cytotoxic activity and T cell receptor gene rearrangements. Eur J Immunol. 1990;20:825–831. doi: 10.1002/eji.1830200417. [DOI] [PubMed] [Google Scholar]
- Godet Y, Moreau-Aubry A, Guilloux Y, Vignard V, Khammari A, Dreno B, Jotereau F, Labarriere N (2008) MELOE-1 is a new antigen overexpressed in melanomas and involved in adoptive T cell transfer efficiency. J Exp Med 205:2673–2682. doi:10.1084/jem.20081356 [DOI] [PMC free article] [PubMed]
- Labarriere N, Diez E, Pandolfino MC, Viret C, Guilloux Y, Le Guiner S, Fonteneau JF, Dreno B, Jotereau F. Optimal T cell activation by melanoma cells depends on a minimal level of antigen transcription. J Immunol. 1997;158:1238–1245. [PubMed] [Google Scholar]
- Labarrière N, Pandolfino MC, Gervois N, Khammari A, Tessier MH, Dréno B, Jotereau F (2002) Therapeutic efficacy of melanoma-reactive TIL injected in stage III melanoma patients. Cancer Immunol Immunother CII 51:532–538. doi:10.1007/s00262-002-0313-3 [DOI] [PMC free article] [PubMed]
- Lacreusette A, Nguyen J-M, Pandolfino M-C, Khammari A, Dreno B, Jacques Y, Godard A, Blanchard F (2007) Loss of oncostatin M receptor beta in metastatic melanoma cells. Oncogene 26:881–892. doi:10.1038/sj.onc.1209844 [DOI] [PubMed]
- Lacreusette A, Lartigue A, Nguyen J-M, Barbieux I, Pandolfino M-C, Paris F, Khammari A, et al. (2008) Relationship between responsiveness of cancer cells to Oncostatin M and/or IL-6 and survival of stage III melanoma patients treated with tumour-infiltrating lymphocytes. J Pathol 216:451–459. doi:10.1002/path.2416 [DOI] [PubMed]
- Lacreusette A, Barbieux I, Nguyen JM, Pandolfino MC, Dréno B, Jacques Y, Godard A, Blanchard F (2009) Defective activations of STAT3 Ser727 and PKC isoforms lead to oncostatin M resistance in metastatic melanoma cells. J Pathol 217:665–676. doi:10.1002/path.2490 [DOI] [PubMed]
- Le Dréan E, Gervois N, Diez E, Semana G, Dreno B, Jotereau F. HLA class II-restricted recognition of common tumor epitopes on human melanoma cells by CD4 + melanoma-infiltrating lymphocytes. Eur J Immunol. 1995;25:2732–2736. doi: 10.1002/eji.1830251003. [DOI] [PubMed] [Google Scholar]
- Le Guiner S, Le Dréan E, Labarrière N, Fonteneau JF, Viret C, Diez E, Jotereau F. LFA-3 co-stimulates cytokine secretion by cytotoxic T lymphocytes by providing a TCR-independent activation signal. Eur J Immunol. 1998;28:1322–1331. doi: 10.1002/(SICI)1521-4141(199804)28:04<1322::AID-IMMU1322>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Lotem M, Yehuda-Gafni O, Butnaryu E, Drize O, Peretz T, Abeliovich D. Cytogenetic analysis of melanoma cell lines: subclone selection in long-term melanoma cell cultures. Cancer Genet Cytogenet. 2003;142:87–91. doi: 10.1016/S0165-4608(02)00798-7. [DOI] [PubMed] [Google Scholar]
- Pandolfino MC, Labarrière N, Tessier MH, Cassidanius A, Bercegeay S, Lemarre P, Dehaut F, Dréno B, Jotereau F. High-scale expansion of melanoma-reactive TIL by a polyclonal stimulus: predictability and relation with disease advancement. Cancer Immunol Immunother CII. 2001;50:134–140. doi: 10.1007/PL00006683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panelli MC, Riker A, Kammula U, Wang E, Lee KH, Rosenberg SA, Marincola FM. Expansion of tumor-T cell pairs from fine needle aspirates of melanoma metastases. J Immunol. 2000;164:495–504. doi: 10.4049/jimmunol.164.1.495. [DOI] [PubMed] [Google Scholar]
- Sulit HL, Golub SH, Irie RF, Gupta RK, Grooms GA, Morton D (1976) Human tumor cells grown in fetal calf serum and human serum: influences on the tests for lymphocyte cytotoxicity, serum blocking and serum arming effects. Int J Cancer 17:461–468 [DOI] [PubMed]
- Vogl A, Sartorius U, Vogt T, Roesch A, Landthaler M, Stolz W, Becker B (2005) Gene expression profile changes between melanoma metastases and their daughter cell lines: implication for vaccination protocols. J Invest Dermatol 124:401–404. doi:10.1111/j.0022-202X.2004.23603.x [DOI] [PubMed]
- Zhou X, Jun DY, Thomas AM, Huang X, Huang L, Mautner J, Mo W, Robbins PF, Pardoll DM, Jaffee EM (2005) Diverse CD8 + T-cell responses to renal cell carcinoma antigens in patients treated with an autologous granulocyte-macrophage colony-stimulating factor gene-transduced renal tumor cell vaccine. Cancer Res 65:1079–1088 [PubMed]
- Zitvogel L, Angevin E, Tursz T (2000) Dendritic cell-based immunotherapy of cancer. Ann Oncol 11 Suppl 3:199–205 [DOI] [PubMed]