Abstract
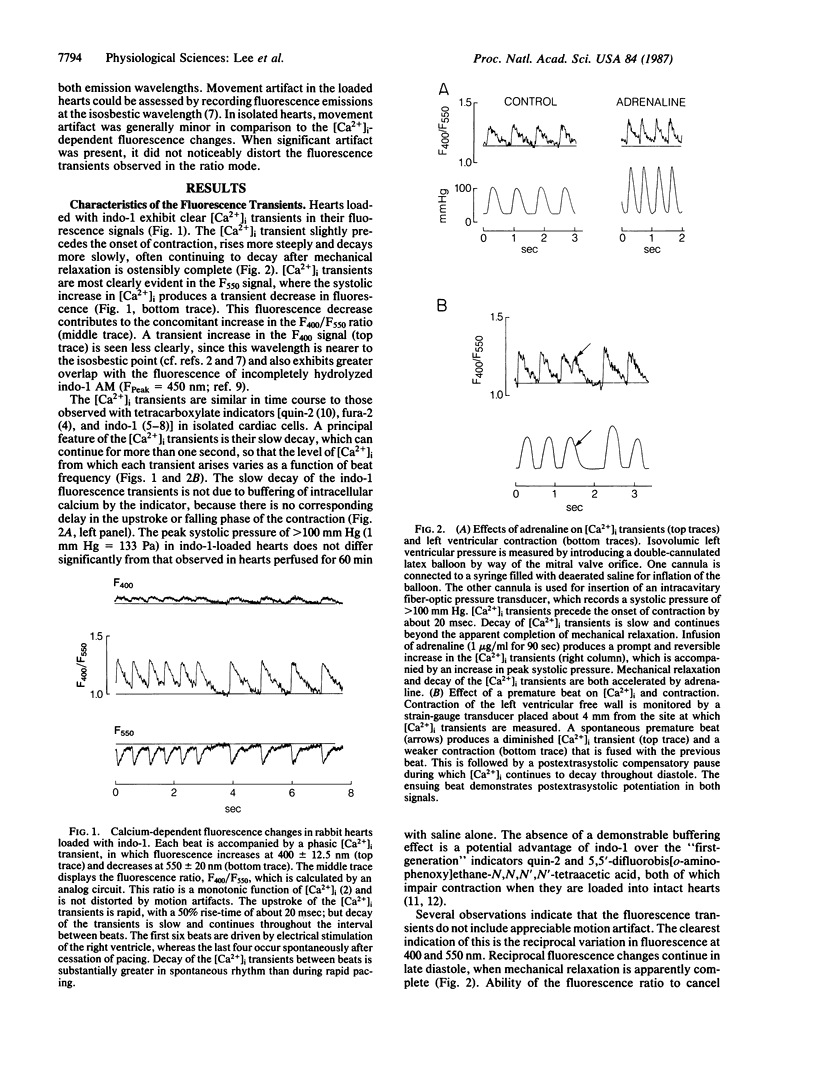
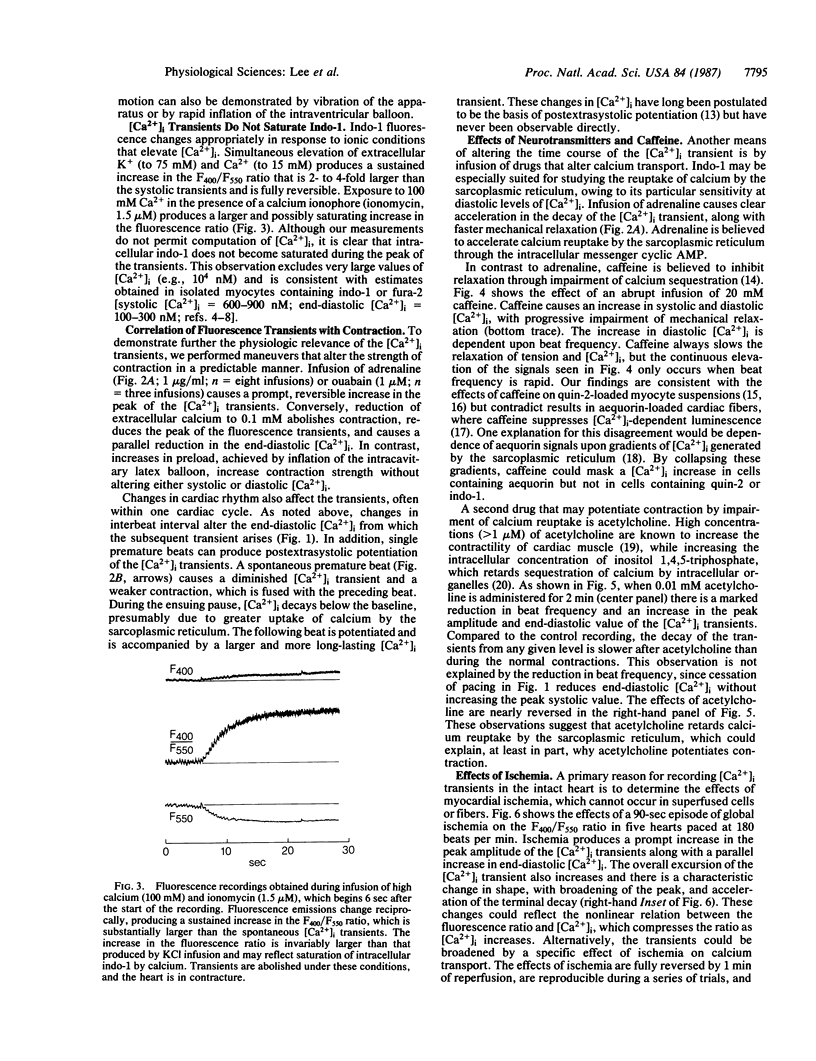
To elucidate the role of cytosolic calcium, [Ca2+]i, in the physiology of the normal and ischemic heart, we have developed a method for recording [Ca2+]i transients from the epicardial surface of the rabbit ventricle after arterial perfusion with the cell-permeant cytosolic calcium indicator indo-1 AM. Hearts were illuminated at 360 nm, and fluorescence was recorded simultaneously at 400 and 550 nm. The F400/F550 fluorescence ratio was calculated by an analog circuit that allowed cancelation of small movement artifacts that were present at single wavelengths. Clear [Ca2+]i transients were present in the F400/F550 signal and were remarkable for their slow decay. Slow decay of the transients was not due to buffering of [Ca2+]i by indo-1, since there was no associated impairment of contraction or relaxation. The peak amplitude of the [Ca2+]i transients was increased by ouabain, adrenaline, postextrasystolic potentiation, and acetylcholine. The extent to which the transients decayed diminished with shortening of the interbeat interval, but decay of the transients could be further diminished by acetylcholine or caffeine. A major advantage of the intact heart over isolated myocytes is the ability to measure changes in [Ca2+]i during ischemia. Ischemia produced a marked increase in both peak systolic and end-diastolic [Ca2+]i, which was most rapid during the first 30 sec, and approached a plateau value after 90 sec. This increase in [Ca2+]i was associated with a characteristic broadening of the peak of the transient. The increase in [Ca2+]i during ischemia is consistent with a proposed causative role of [Ca2+]i in mediating early electrophysiological abnormalities.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen D. G., Blinks J. R. Calcium transients in aequorin-injected frog cardiac muscle. Nature. 1978 Jun 15;273(5663):509–513. doi: 10.1038/273509a0. [DOI] [PubMed] [Google Scholar]
- Allen D. G., Eisner D. A., Lab M. J., Orchard C. H. The effects of low sodium solutions on intracellular calcium concentration and tension in ferret ventricular muscle. J Physiol. 1983 Dec;345:391–407. doi: 10.1113/jphysiol.1983.sp014984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen D. G., Orchard C. H. Intracellular calcium concentration during hypoxia and metabolic inhibition in mammalian ventricular muscle. J Physiol. 1983 Jun;339:107–122. doi: 10.1113/jphysiol.1983.sp014706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake K., Smith N. A., Clusin W. T. Rate dependence of ischaemic myocardial depolarisation: evidence for a novel membrane current. Cardiovasc Res. 1986 Aug;20(8):557–562. doi: 10.1093/cvr/20.8.557. [DOI] [PubMed] [Google Scholar]
- Blinks J. R., Wier W. G., Hess P., Prendergast F. G. Measurement of Ca2+ concentrations in living cells. Prog Biophys Mol Biol. 1982;40(1-2):1–114. doi: 10.1016/0079-6107(82)90011-6. [DOI] [PubMed] [Google Scholar]
- Bloom S., Brady A. J., Langer G. A. Calcium metabolism and active tension in mechanically disaggregated heart muscle. J Mol Cell Cardiol. 1974 Apr;6(2):137–147. doi: 10.1016/0022-2828(74)90017-0. [DOI] [PubMed] [Google Scholar]
- Clusin W. T., Buchbinder M., Ellis A. K., Kernoff R. S., Giacomini J. C., Harrison D. C. Reduction of ischemic depolarization by the calcium channel blocker diltiazem. Correlation with improvement of ventricular conduction and early arrhythmias in the dog. Circ Res. 1984 Jan;54(1):10–20. doi: 10.1161/01.res.54.1.10. [DOI] [PubMed] [Google Scholar]
- Clusin W. T., Buchbinder M., Harrison D. C. Calcium overload, "injury" current, and early ischaemic cardiac arrhythmias--a direct connection. Lancet. 1983 Feb 5;1(8319):272–274. doi: 10.1016/s0140-6736(83)91688-4. [DOI] [PubMed] [Google Scholar]
- Clusin W. T. Correlation between relaxation and automaticity in embryonic heart cell aggregates. Proc Natl Acad Sci U S A. 1980 Jan;77(1):679–683. doi: 10.1073/pnas.77.1.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clusin W. T. Do caffeine and metabolic inhibitors increase free calcium in the heart? Interpretation of conflicting intracellular calcium measurements. J Mol Cell Cardiol. 1985 Mar;17(3):213–220. doi: 10.1016/s0022-2828(85)80004-3. [DOI] [PubMed] [Google Scholar]
- Clusin W. T., Hamilton W. E., Nelson D. V. The mechanical activity of chick embryonic myocardial cell aggregates. J Physiol. 1981 Nov;320:149–174. doi: 10.1113/jphysiol.1981.sp013941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobbold P. H., Bourne P. K. Aequorin measurements of free calcium in single heart cells. 1984 Nov 29-Dec 5Nature. 312(5993):444–446. doi: 10.1038/312444a0. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Neher E., Reuter H., Stevens C. F. Inward current channels activated by intracellular Ca in cultured cardiac cells. Nature. 1981 Dec 24;294(5843):752–754. doi: 10.1038/294752a0. [DOI] [PubMed] [Google Scholar]
- Gilmour R. F., Jr, Zipes D. P. Positive inotropic effect of acetylcholine in canine cardiac Purkinje fibers. Am J Physiol. 1985 Oct;249(4 Pt 2):H735–H740. doi: 10.1152/ajpheart.1985.249.4.H735. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Jackson A. P., Timmerman M. P., Bagshaw C. R., Ashley C. C. The kinetics of calcium binding to fura-2 and indo-1. FEBS Lett. 1987 May 25;216(1):35–39. doi: 10.1016/0014-5793(87)80752-4. [DOI] [PubMed] [Google Scholar]
- KOCH-WESER J., BLINKS J. R. THE INFLUENCE OF THE INTERVAL BETWEEN BEATS ON MYOCARDIAL CONTRACTILITY. Pharmacol Rev. 1963 Sep;15:601–652. [PubMed] [Google Scholar]
- Kass R. S., Lederer W. J., Tsien R. W., Weingart R. Role of calcium ions in transient inward currents and aftercontractions induced by strophanthidin in cardiac Purkinje fibres. J Physiol. 1978 Aug;281:187–208. doi: 10.1113/jphysiol.1978.sp012416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusuoka H., Weisfeldt M. L., Zweier J. L., Jacobus W. E., Marban E. Mechanism of early contractile failure during hypoxia in intact ferret heart: evidence for modulation of maximal Ca2+-activated force by inorganic phosphate. Circ Res. 1986 Sep;59(3):270–282. doi: 10.1161/01.res.59.3.270. [DOI] [PubMed] [Google Scholar]
- Lattanzio F. A., Jr, Pressman B. C. Alterations in intracellular calcium activity and contractility of isolated perfused rabbit hearts by ionophores and adrenergic agents. Biochem Biophys Res Commun. 1986 Sep 14;139(2):816–821. doi: 10.1016/s0006-291x(86)80063-8. [DOI] [PubMed] [Google Scholar]
- Lee H. C., Clusin W. T. Na+/Ca2+ exchange in cardiac myocytes. Effect of ouabain on voltage dependence. Biophys J. 1987 Feb;51(2):169–176. doi: 10.1016/S0006-3495(87)83322-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London B., Krueger J. W. Contraction in voltage-clamped, internally perfused single heart cells. J Gen Physiol. 1986 Oct;88(4):475–505. doi: 10.1085/jgp.88.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lückhoff A. Measuring cytosolic free calcium concentration in endothelial cells with indo-1: the pitfall of using the ratio of two fluorescence intensities recorded at different wavelengths. Cell Calcium. 1986 Aug;7(4):233–248. doi: 10.1016/0143-4160(86)90003-5. [DOI] [PubMed] [Google Scholar]
- Marban E., Kitakaze M., Kusuoka H., Porterfield J. K., Yue D. T., Chacko V. P. Intracellular free calcium concentration measured with 19F NMR spectroscopy in intact ferret hearts. Proc Natl Acad Sci U S A. 1987 Aug;84(16):6005–6009. doi: 10.1073/pnas.84.16.6005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura D. S., Biedert S., Barry W. H. Effects of calcium overload on relaxation in cultured heart cells. J Mol Cell Cardiol. 1981 Nov;13(11):949–961. doi: 10.1016/0022-2828(81)90471-5. [DOI] [PubMed] [Google Scholar]
- Sheu S. S., Sharma V. K., Banerjee S. P. Measurement of cytosolic free calcium concentration in isolated rat ventricular myocytes with quin 2. Circ Res. 1984 Dec;55(6):830–834. doi: 10.1161/01.res.55.6.830. [DOI] [PubMed] [Google Scholar]
- Sperelakis N., Schneider J. A. A metabolic control mechanism for calcium ion influx that may protect the ventricular myocardial cell. Am J Cardiol. 1976 Jun;37(7):1079–1085. doi: 10.1016/0002-9149(76)90428-8. [DOI] [PubMed] [Google Scholar]
- Thomas A. P., Selak M., Williamson J. R. Measurement of electrically-induced Ca2+ transients in Quin2-loaded cardiac myocytes. J Mol Cell Cardiol. 1986 May;18(5):541–545. doi: 10.1016/s0022-2828(86)80919-1. [DOI] [PubMed] [Google Scholar]
- Tsuji Y., Tajima T., Yuen J., Pappano A. J. Positive inotropic effects of acetylcholine and BAY K 8644 in embryonic chick ventricle. Am J Physiol. 1987 Apr;252(4 Pt 2):H807–H815. doi: 10.1152/ajpheart.1987.252.4.H807. [DOI] [PubMed] [Google Scholar]
- Wier W. G. Calcium transients during excitation-contraction coupling in mammalian heart: aequorin signals of canine Purkinje fibers. Science. 1980 Mar 7;207(4435):1085–1087. doi: 10.1126/science.7355274. [DOI] [PubMed] [Google Scholar]
- Wier W. G., Cannell M. B., Berlin J. R., Marban E., Lederer W. J. Cellular and subcellular heterogeneity of [Ca2+]i in single heart cells revealed by fura-2. Science. 1987 Jan 16;235(4786):325–328. doi: 10.1126/science.3798114. [DOI] [PubMed] [Google Scholar]


