Abstract
Type I allergy is characterized by the development of an initial Th2-dependent allergen-specific IgE response, which is boosted upon subsequent allergen encounter. While the immediate symptoms of allergy are mainly IgE-mediated, allergen-specific T cell responses contribute to the late-phase as well as chronic manifestations of allergy. This study investigates the potential of co-stimulation blockade with CTLA4Ig and anti-CD154 monoclonal antibody for modifying the allergic immune response to the major timothy grass pollen allergen Phl p 5 in a mouse model. BALB/c mice where treated with the co-stimulation blockers at the time of primary sensitization to the Phl p 5 allergen or at the time of a secondary allergen challenge. Co-stimulation blockade (CTLA4Ig plus anti-CD154 or anti-CD154 alone) at the time of sensitization prevented the development of allergen-specific IgE, IgM, IgG, and IgA responses compared to untreated, but sensitized mice. However, co-stimulation blockade had no influence on established IgE responses in sensitized mice. Immediate type reactions as analyzed by a rat basophil leukemia cell mediator release assay were only suppressed by early treatment but not by co-stimulation blockade after sensitization. CTLA4Ig given alone failed to suppress both the primary and secondary allergen-specific antibody responses. Allergen-specific T cell activation was suppressed in mice by early as well as late co-stimulation blockade suggesting that IgE-responses in sensitized mice are independent of T cell help. Our results indicate that T cell suppression alone without active immune regulation or shifting of the Th2/Th1 balance is not sufficient for the treatment of established IgE responses in allergy.
Keywords: Allergy, Antigen/Peptides/Epitopes, Th1/Th2 Cells
Introduction
IgE-mediated allergy affects more than 25% of the population worldwide with a continuously increasing prevalence (1). Several studies suggest that the induction of IgE responses leading to allergic sensitization occurs very early in childhood, perhaps even in pregnancy upon allergen encounter (2). Almost all important allergens are protein/peptide antigens which after presentation to the immune system of atopic individuals under certain conditions (e. g., environmental factors, antigen dose, mode of antigen encounter) preferentially activate TH2 cells to produce cytokines such as IL-4 and IL-13 (3-5). These cytokines together with CD40/CD154 (CD40L) interactions are required for class switch recombination of B cells to IgE (6). This process results in the formation of allergen-specific IgE antibodies, which bind to Fcε receptors on a variety of inflammatory cells and after repeated allergen contact mediate the immediate and late symptoms of allergic disease (7,8). After the primary sensitization event has taken place repeated encounter with allergens leads to a strong increase of systemic allergen-specific IgE antibody levels and IgE-mediated symptoms (9). The continuous boosting of secondary IgE responses may be one of the important mechanisms that promotes the transition from mild (e.g. rhinoconjunctivitis) to severe allergic manifestations (e.g. asthma) (10).
TH2 cells play a central role in the initial allergic sensitization and in the chronic inflammatory cascades of allergic diseases. Several approaches for the suppression or modulation of T cell activity have therefore been developed in order to prevent and treat allergy. They include the induction of T cell tolerance with allergen-derived T cell epitope containing peptides, the use of IL-4 antagonists, treatment with anti-CD4 antibodies or immunosuppressive agents such as cyclosporine and experimental strategies aiming at the induction of tolerance via the activation of tolerogenic antigen presenting cells or T regulatory cells (4, 11, 12).
Another possible option for the prevention and treatment of allergic immune responses may be interference at the level of co-stimulation (13). Here we studied the effect of co-stimulation blockade on the immune responses to the major timothy grass (Phleum pratense) pollen allergen Phl p 5 in a murine model of grass pollen allergy (14). CTLA4Ig, a soluble fusion protein consisting of the extracellular domain of CTLA4 linked to the IgG1 Fc region, is known to block the CD28-B7 pathway (15). Anti-CD154 (CD40L) mAbs interfere with the CD154-CD40 pathway, mainly by blocking engagement of CD40 (16), but potentially also through other mechanisms (17). CTLA4Ig and anti-CD154 antibodies have been shown to be powerful co-stimulatory blocking reagents in numerous models of transplantation and autoimmunity (13). CTLA4Ig (abatacept) has recently been approved for the treatment of rheumatoid arthritis (18), and a second generation version (LEA29Y, belatacept) is currently being evaluated in renal transplantation (19). Little is known, however, about the potential effect of these co-stimulation blockers on allergic immune responses, in particular no studies dissecting the humoral response to defined clinically relevant allergens have been reported. Our results demonstrate that co-stimulation is essential for the establishment of an allergen-specific immune response at the time of allergic sensitization. In contrast, co-stimulation blockade did not affect an already established IgE antibody response. These findings have important implications for the development of therapeutic strategies for allergic diseases.
Materials and Methods
Recombinant allergens, antibodies
Purified recombinant timothy grass pollen allergens (rPhl p 2, rPhl p 5) (20) were obtained from BIOMAY (Vienna, Austria). The hamster anti-mouse CD154 (MR1) antibody was purchased from Bioexpress Inc. (New Hampshire, USA). Human CTLA4Ig was generously provided by Bristol-Myers, Squibb Pharmaceuticals (Princeton, New Jersey). Both reagents have been used to successfully block T cell responses in several mouse models of transplantation (21-24). For control experiments, purified human IgG1 and hamster IgG were obtained from Sigma (Saint Louis, Missouri, USA) and ICN Biomedicals (Ohio, USA), respectively.
Animals
Female, 5-8 week old BALB/c and C57BL/6 (B6) mice were purchased from Charles River (Germany) and were kept under specific pathogen-free conditions. All experiments were approved by the local review board of the Medical University of Vienna, and were performed in accordance with national and international guidelines of laboratory animal care.
Immunization and treatment of BALB/c mice
Groups of mice (n=6) were sensitized subcutaneously (day 0 and day 21) with 5 μg rPhl p 5, adsorbed to Al(OH)3 (Alu-Gel-S, Serva, Ingelheim, Germany) (14). Treatment (anti-CD154 mAb; human CTLA4Ig) (0.5 mg / mouse) was given intraperitoneally on d 0, 2, 4, (designated as ‘early’) or on d 21, 23, 25 (designated as ‘late’). I.p. treatment with corresponding amounts of hamster IgG or huIgG1 was performed for control purpose. The sensitization and treatment schedules for each group of mice are displayed in Table I. Blood samples were taken from the tail veins and serum was stored at −20°C until analysis.
Table I.
Immunization and Treatment protocol.a
| Sensitization | Treatment | |||
|---|---|---|---|---|
|
| ||||
| group | rPhl p 5 | anti-CD154 | CTLA4 | Ig |
| A | + | - | - | |
| B | + | d 0,2,4 | - |
|
| C | + | - | d 0,2,4 | |
| D | + | d 0,2,4 | d 0,2,4 | |
| E | + | d 21,23,25 | - |
|
| F | + | - | d 21,23,25 | |
| G | + | d 21,23,25 | d 21,23,25 | |
Groups of 6 BALB/c mice (A-G) were sensitized with aluminium hydroxide-adsorbed recombinant timothy grass pollen allergen, Phl p 5, on days 0 and 21. Treatment with anti-CD154 and / or CTLA4Ig was given at the time of sensitization (groups B-D, ‘early’) (days 0, 2, 4) or second immunization (groups E-G, ‘late’) (days 21, 23, 25) as shown in the Table.
B cell epitope mapping using recombinant allergen fragments
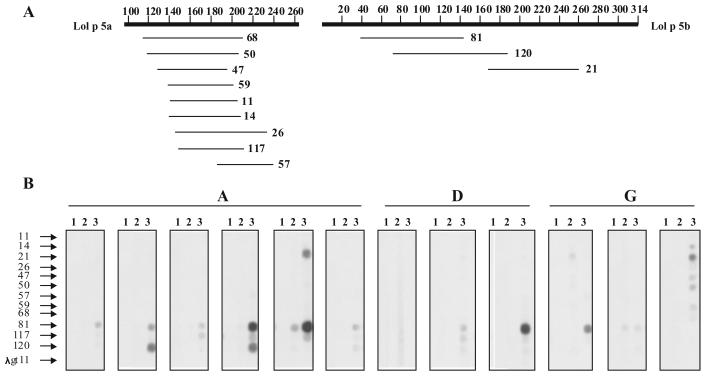
λgt11 clones expressing B cell epitopes of the major rye grass pollen allergens Lol p 5A and Lol p 5B and empty wild-type phage were probed for reactivity with mouse IgG1 antibodies induced by sensitization to Phl p 5 as described (14, 25). E. coli Y1090 were grown overnight in LB medium containing 0.4 % w/v maltose and 50 μg/ml ampicillin, harvested by centrifugation and resuspended in 10 mM MgSO4. Cells were dissolved in 0.6 % w/v agarose and plated onto LB plates containing 50 mg/L ampicillin. Two μl aliquots of phage lysates containing > 105 Pfu were dotted onto the plates. Plates were incubated at 43°C until plaques became visible and protein synthesis was induced by overlay with nitrocellulose filters (Schleicher & Schull, Dassel, Germany) soaked with 10 mM IPTG for 4 h at 37°C. Filters were cut into stripes. Stripes, containing the expressed allergen fragments from clones 11,14, 21,26, 47, 50, 57, 59, 68, 81, 117, and 120, and the phage λgt11 as negative control, or 1 μg rPhl p 5 as positive control, were incubated with mouse sera diluted 1:1000 overnight, a monoclonal rat anti-mouse IgG1 antibody (Pharmingen, San Diego, USA) diluted 1:1000 for 5 h, and a 125I-labelled goat anti-rat IgG antibody (Sigma-Aldrich, St.Louis, MO, USA) diluted 1:2000 for 2 h. Reactivity with the allergen fragments was detected by autoradiography. The intensities of the signals where determined by densitometry using the AlphaEaseFC™ ChemiImager 4400 software.
ELISA experiments
To measure antigen-specific antibodies in the sera of immunized mice an ELISA was performed as described earlier (14, 26). Plates were coated with rPhl p 5 (5μg/ml), sera were diluted 1:10 for IgE, 1:100 for IgM, IgA, and IgG2a, and 1:1000 for IgG1 and bound antibodies where detected with monoclonal rat anti-mouse IgM, IgG1, IgE, IgA, and IgG2A antibodies (Pharmingen, San Diego, USA) diluted 1:1000 and a HRP-coupled goat anti-rat antiserum (Amersham, Biosciences, U.K.) diluted 1:2000.
T cell proliferation assay
Spleens were removed under aseptic conditions (day 100) and homogenized. After lysis of erythrocytes, cells were washed and re-suspended in complete medium (RPMI, 10% fetal calf serum, 0.1 mg/ml gentamicin, 2mM glutamine). Single cell suspensions were plated into 96-well round-bottom plates at a concentration of 2 × 105 cells / well (200 μl) in triplicates and stimulated with or without concavalin A (0.5μg/well), rPhl p 2 (3μg/well), and rPhl p 5 (3μg/well) for 4 days. The cultures were pulsed with 0.5 μCi / well tritiated thymidine for 16 h and harvested. The proliferation responses were measured by scintillation counting. The ratio of the mean proliferation after antigen stimulation and medium control values, i.e. the stimulation index (SI), was calculated.
RBL assay
For the quantification of IgE antibody-mediated immediate type reactions, rat basophil leukemia (RBL) cell mediator release assays were performed as previously described (27). RBL-2H3 cells were cultivated in 96 well tissue culture plates (4×104 cells/well) for 24 h at 37° C using 7% CO2. Passive sensitization was performed by incubation with 1:30 diluted murine sera for 2 h. Cells were washed twice with Tyrode's buffer (137 mM NaCl, 2.7 mM KCl, 0.5 mM MgCl2, 1.8 mM CaCl2, 0.4 mMNaH2PO4, 5.6 mM D-glucose, 12 mM NaHCO3, 10 mM HEPES, and 0.1% w/v BSA, pH 7.2) to remove unbound antibodies. Degranulation of RBL cells was induced by adding 0.3 μg/ml rPhl p 5. After 30 min, ß-hexosaminidase release was analysed. Results are expressed as percentages of total ß-hexosaminidase released after addition of 1% Triton X-100 and represent mean of triplicate determinations.
Mixed lymphocyte reaction (MLR)
As a positive control for the biological activity of CTLA4Ig, an MLR was performed in the presence of 100 μg/ml CTLA4Ig. Spleen cells were washed with PBS and resuspended in MLR-Medium (42.5 ml RPMI 1640 (Bio-Whittaker), 7.5 ml CPSR-2 (Sigma), 0.5 ml HEPES buffer (ICN, Biomedica, Vienna Austria), 1.55 ml Nutrient mixture (10 ml Aqua dest., 5 ml Non-essential amino acids, 0.055 g Sodium pyruvate, 0.146 g L-Glutamine, 5000 U Penicillin, 5000 μg Streptomycin) and 100 μl 2-Mercaptoethanol (dilution 1:3000)). Four × 105 B6 responder cells were incubated at 37°C, 5 % CO2 with 4 × 105 irradiated (30 Gy) stimulator cells of either BALB/c or B6 mice or with medium in triplicates. After 3 or 4 days, cells were pulsed with 3H-thymidine and incubated for 12 to 18 hours. After harvesting on filter plates, they were analysed with a Microbeta 1450 beta-counter (Wallac, HVD Life Science, Vienna, Austria). Stimulation indices (SI) were calculated by dividing the mean counts per minute (c.p.m.) from responses against self (B6), and against the allogeneic stimulator (BALB/c) by mean background c.p.m. (i.e., c.p.m. with no stimulator population).
Statistical analysis
Antibody levels are illustrated by box and whisker plots. Differences between groups B to G and the control group A were assessed with Wilcoxon-Mann-Whitney-U test, exact significances were determined and adjusted using the Bonferroni-Holm method. SPSS statistical software system (SPSS 14.0 Inc., Chicago, IL, USA) was used for calculations. The reported p-values are results of two-sided tests. P-values less than or equal to 0.05 were considered statistically significant.
Results
Establishment of a murine model for grass pollen allergy by sensitization of mice with the timothy grass pollen allergen Phl p 5
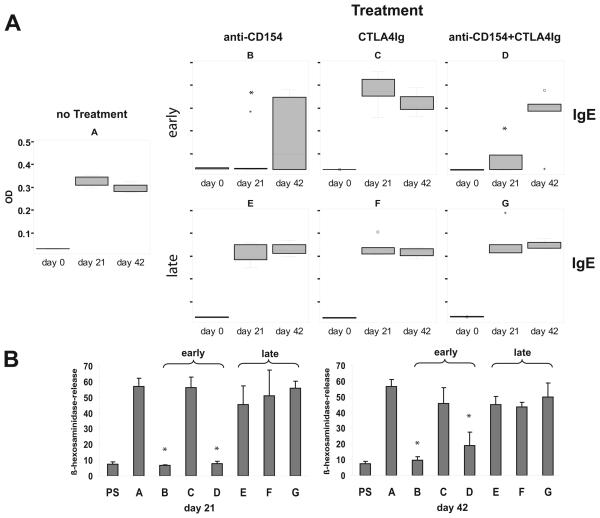
Mice sensitized with Phl p 5 developed an IgE antibody response specific for Phl p 5 already at day 21 (Fig. 1 A, group A). Passive transfer of serum IgE from the sensitized mice but not of their pre-immune serum led to sensitization of RBL cells and the release of ß-hexosaminidase after challenge with Phl p 5 (Fig. 1 B, group A). Sensitized mice developed also Phl p 5-specific IgG1 antibodies, which were detectable at day 21 and continued to increase after repeated immunization (Fig. 2, group A). Phl p 5-sensitized mice cross-reacted with group 5 allergens from other grass species including Lol p 5 from rye grass (Lolium perenne) (data not shown). We could therefore analyze the epitope spectrum recognized by sensitized mice using phage clones expressing recombinant fragments of the two isoforms (Lol p 5A, Lol p 5B) of the rye grass allergen Lol p 5 which had been identified with IgE antibodies from grass pollen allergic patients (Fig. 3). As determined by densitometry more than 75 % (average 78 %) of Phl p 5-specific antibodies bound to the Phl p 5-derived fragments. Most of the sensitized mice developed antibody responses against the clone 81-defined Lol p 5B fragment (Fig. 3) which corresponds to an N-terminal fragment of Phl p 5 (timothy grass-Phleum pratense) and which is highly homologous to group 5 allergens from other grasses, and group 6 allergens from timothy grass. The clone 81-defined fragment corresponds to a highly allergenic domain of Phl p 5, which is recognized by most grass pollen allergic patients (25), underscoring the clinical relevance of our model.
FIGURE 1.
Co-stimulation blockade with CTLA4Ig plus anti-CD154 inhibits allergic sensitization but does not affect an already established allergic secondary IgE response. (A) Serum levels of rPhl p 5-specific IgE measured by ELISA on days 0, 21 and 42 are shown in box-and-whiskers plots. (B) The biological activity of allergen-specific IgE was studied by rat basophil leukemia cell (RBL) degranulation assays. RBL cells were loaded with serum IgE of Phl p 5- sensitized mice (groups A-G) obtained on day 21 and 42 or with the corresponding pre-immune sera (PS) and challenged with the allergen. The mean β–hexosaminidase release ±SD of each group of mice is shown on the y-axis. * indicates a significant reduction (p<0.05) compared to the control group A.
FIGURE 2.
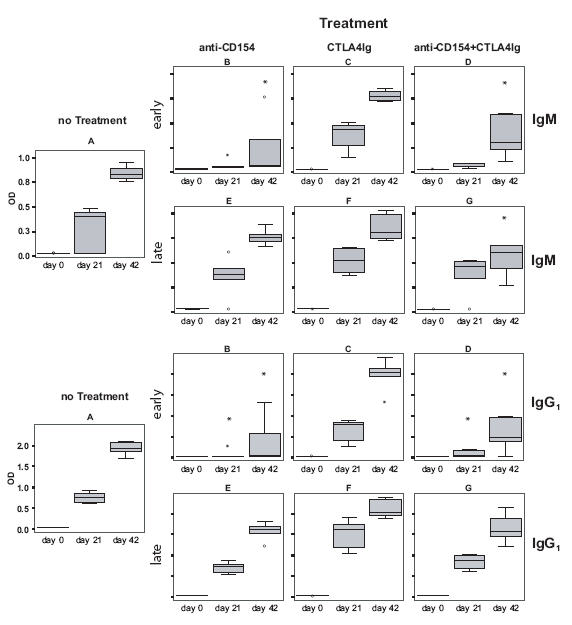
Early treatment with CTLA4Ig plus anti-CD154 but not late treatment prevents the induction of IgM and IgG1 antibody responses to the major timothy grass pollen allergen Phl p 5. Serum samples, collected at day 0, 21, and 42 after primary immunization were analyzed for Phl p 5-specific IgM and IgG1 antibodies by ELISA. Antibody levels are displayed as box-and-whiskers plots, * indicates a significant reduction (p<0.05) compared to the untreated but sensitized group A.
FIGURE 3.
Epitope-mapping of the Phl p 5-specific IgG1 response in immunized mice. (A) Schematic representation of Lol p 5A and Lol p 5B fragments expressed by the phage clones. (B) Lol p 5A- and Lol p 5B-derived clones (11-120) and a phage clone without insert (λgt11) were immobilized to a nitrocellulose filter in the order given and probed with mouse sera from groups A, D, and G obtained before sensitization (lane 1), on day 21 (lane 2), and on day 42 (lane 3).
Co-stimulation blockade inhibits allergic sensitization
The effects of co-stimulation blockage on allergen-specific primary immune responses (i.e., allergic sensitization) and on the secondary allergen-specific immune responses (i.e., boosting of an allergen-specific immune response) was investigated by the administration of co-stimulation blockers CTLA4Ig and / or an anti-CD154 monoclonal antibody at the time of sensitization or at the time of the secondary allergen challenge (i.e., 3 weeks after sensitization) (for group descriptions see Table 1).
First, we investigated the effect of co-stimulation blockade on allergic sensitization in our murine allergy model (‘early’ treatment). In BALB/c mice, which had been sensitized to the major grass pollen allergen Phl p 5 (group A), allergen-specific IgE could be detected 3 weeks after sensitization by ELISA. Almost no Phl p 5-specific IgE responses could be detected in groups B and D, which had received treatment with anti-CD154 or anti-CD154 plus CTLA4Ig at the time of sensitization. The effect of early co-stimulatory blockade at days 0, 2 and 4 was limited because it was possible to sensitize mice with a second injection of Phl p 5 three weeks later This second immunization induced an allergen-specific IgE antibody response in groups B and D similar to that observed for the untreated group after primary immunization (Fig. 1A). The inhibition of allergic sensitization seemed to depend mainly on the anti-CD154 antibody treatment, because the application of this antibody alone (group B) yielded a similar result as treatment with both antibodies (group D). This assumption is supported by the finding that treatment with CTLA4Ig alone (group C) did not suppress allergic sensitization to rPhl p 5 (Fig. 1A). Despite treatment with CTLA4Ig the induction of a Phl p 5-specific IgE response was comparable to the untreated, sensitized control group A, although the activity of the CTLA4Ig fusion protein had been confirmed in a mixed lymphocyte reaction using the same batch of protein (data not shown).
The impact of co-stimulation blockade on IgE antibody-mediated immediate allergic reactions was also demonstrated using rat basophil leukemia cell degranulation assays. When mouse sera of the sensitized, but untreated group A were loaded on RBL cells a strong degranulation of basophils upon allergen challenge (57% ß-hexosaminidase-release 3 and 6 weeks after sensitization) was observed confirming the presence of allergen-specific IgE in the serum (Fig. 1 B). By contrast, sera collected at day 21 from groups B and D who had received co-stimulation blockade at the time of sensitization failed to elicit degranulation. The data obtained with the RBL assay were thus in agreement with the measured levels of IgE in the serum. Administration of the CTLA4Ig at the time of sensitization failed to prevent allergic sensitization. Furthermore, mice (groups B and D) that had been been sensitized with a second injection of Phl p 5 21 days after co-stimulation blockade exhibited a ß-hexosaminidase release of 10 and 19 %, respectively after 6 weeks (Fig. 1B).
Co-stimulation blockade has no influence on a secondary IgE response
In order to analyse the effect of co-stimulation blockade on an already established allergic immune response (i.e., secondary immune response or boosting of an established response), groups of mice which had been sensitized to rPhl p 5 received three weeks after sensitization treatment with anti-CD154 alone (group E), CTLA4Ig alone (group F) or a combination of anti-CD154 plus CTLA4Ig (group G) (Table I). Figure 1A-‘late’ shows the development of Phl p 5-specific IgE antibody titers in these groups 3 and 6 weeks after sensitization. On day 21 after the first allergen contact mice of groups E-G had developed Phl p 5 specific IgE antibodies comparable to the sensitized and untreated controls of group A. Upon secondary allergen contact (i.e., second injection of Phl p 5 at day 21), the IgE antibody levels in the treated groups E, F, and G were almost identical to those in untreated, sensitized control group A indicating that the IgE response to Phl p 5 was not influenced by the co-stimulation blockade after sensitization. We again determined the allergenic activity of these Phl p 5-specific IgE antibodies in an RBL degranulation assay, confirming that there was no effect of late co-stimulation blockade on allergic immune responses because there was no significant difference of the ß-hexosaminidase-release from rat basophils between the untreated group A and mice of groups E, F, and G (Fig. 1 B).
Co-stimulation blockade prevents the initiation of allergen-specific IgM, IgG and IgA responses but does not affect allergen-specific secondary antibody responses
BALB/c mice, which had been sensitized with the major grass pollen allergen, Phl p 5, on day 0 and 21, had Phl p 5-specific IgM, IgG1, IgG2A, and IgA antibody responses already at day 21 (Figs. 2, 4). Treatment with anti-CD154 antibody alone and with the combination of both co-stimulation blockers given at the time of sensitization blocked the development of Phl p 5-specific IgM, as well as IgG1, IgG2a and IgA responses. As observed for IgE, CTLA4Ig alone was not able to suppress allergen-specific antibody responses. The suppressive effect of co-stimulation blockade on humoral responses had disappeared at day 21, because the second injection of Phl p 5 at day 21 could induce Phl p 5-specific antibody responses in mice of groups B and D (Figs. 2, 4).
FIGURE 4.
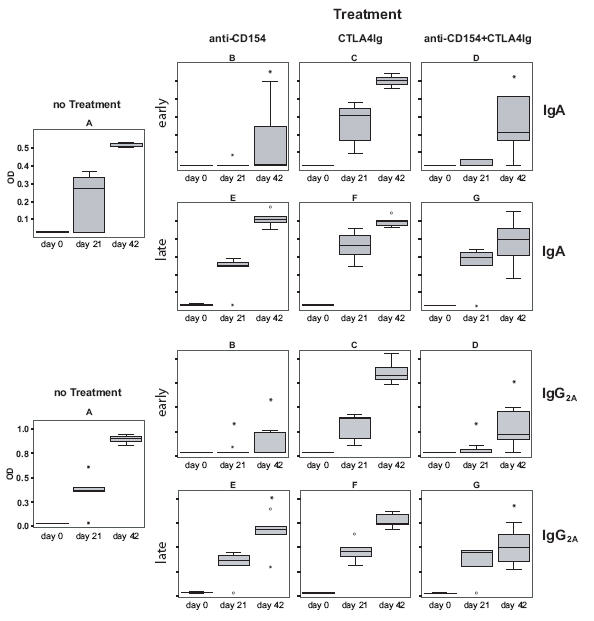
Effects of co-stimulation blockade on rPhl p 5-specific IgA and IgG2A antibody responses. Serum samples, collected at day 0, 21, and 42 from the mice of groups A-G were analyzed for Phl p 5-specific IgA and IgG2A antibodies by ELISA. Antibody levels (OD values) are displayed as box-and-whiskers plots, * indicates a significant reduction (p<0.05) compared to the untreated but sensitized group A.
Similar as observed for IgE, co-stimulation blockade given after the development of an allergen-specific antibody response did not suppress allergen-specific IgM, IgG, and IgA secondary immune responses in mice of groups E, F, and G (Figs. 2, 4). Animals from groups E, F and G had already developed a Phl p 5-specific antibody response comparable to the untreated but sensitized control group A. This allergen-specific antibody response could be boosted upon subsequent immunization with Phl p 5, which resulted in an increase of allergen-specific antibody levels 6 weeks after primary immunization (Fig. 2, 4). Thus no strong effect of co-stimulation blockade on secondary antibody responses became apparent. Possible subtle differences between the antibody levels on day 42 were not analysed in detail. Mice, which were not sensitized to Phl p 5 but had been treated with CTLA4Ig and anti-CD154 or isotype control antibodies did not mount any detectable humoral response to the Phl p 5 allergen (data not shown).
Co-stimulation blockade induces allergen-specific non-responsiveness of T cells
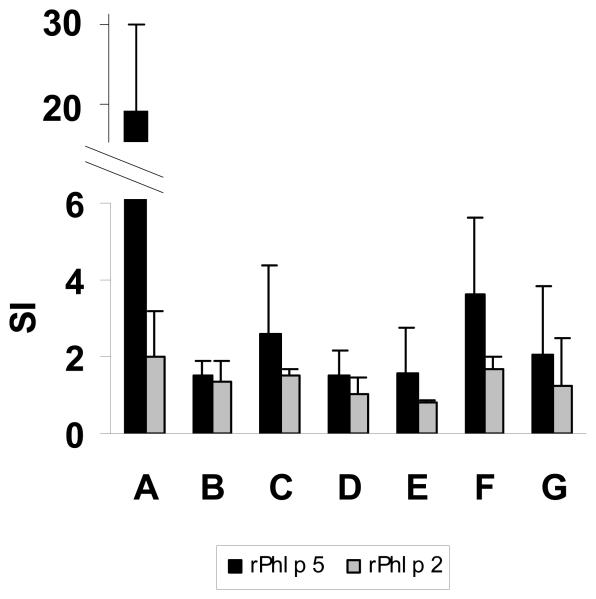
We further investigated whether spleen-derived T cells isolated from the various groups of mice were able to proliferate upon stimulation with rPhl p 5 (Fig. 5). Spleen cells from untreated, Phl p 5-sensitized mice strongly proliferated in response to Phl p 5 (mean SI: 18.5). Phl p 5-specific proliferation of splenocytes was substantially inhibited in treated groups B, D, E, and G resulting in mean stimulation indices between 1.5 and 2. In our model CTLA4Ig treatment alone (groups C and F) was not sufficient for the complete suppression of allergen-specific T cell responses, although T cell responses were considerably reduced (mean SI: 2.6 and 3.6). Spleen cells of mice from the various groups did not proliferate in response to an immunologically unrelated allergen, Phl p 2 (20) (Fig. 5).
FIGURE 5.
Treatment with CTLA4Ig and/or anti-CD154 reduces lymphoproliferative responses to Phl p 5. T cell proliferation was measured at day 100 in spleen cell cultures after in vitro stimulation with timothy grass pollen allergens Phl p 5 (black bars) or Phl p 2, an immunologically unrelated grass pollen allergen (negative control) (grey bars). The bars represent the mean stimulation indices (SI±SD) for the different groups of mice.
Discussion
Sensitization, i.e., the induction of allergen-specific IgE antibodies, is the initial event in the establishment of allergic disease. Subsequent allergen contact leads to a boost of allergen-specific IgE and T cell responses as well as to allergic inflammation (28). Allergic inflammation can be elicited via IgE-mediated activation of different inflammatory cells but may also occur via IgE-independent T cell activation (11, 28).
In this study we investigated the effects of co-stimulation blockade on allergic sensitization and allergen-specific secondary IgE responses. CTLA4Ig and anti-CD154 mAb that we used for suppression of T cell help have previously been described as potent co-stimulatory blockade reagents and were effective in numerous models of transplantation and autoimmunity, but also in clinical trials for rheumatoid arthritis and psoriasis (18, 21-23, 29). When these reagents were given in combination in the course of allergic sensitization, they efficiently prevented the induction of a primary IgE response to the major timothy grass pollen allergen Phl p 5. This result confirms the pivotal role of the CD40-CD40L interaction (30-32) in the induction of an allergen-specific IgE response and also in T cell-dependent B cell responses in general, as the induction of an IgM and IgG response to rPhl p 5 was also inhibited. CTLA4Ig, when given alone at the time of sensitization with allergen did not effectively prevent a primary IgE response, while dampening allergen-specific T cell reactivity to some degree. Several studies have suggested that CD28-blockade through CTLA4Ig can have distinct effects on Th1 vs. Th2 responses, but results differ depending on the specific experimental system investigated (13). In several models Th2 responses were less susceptible to CTLA4Ig treatment (33-35), which is compatible with our observation that in the presented allergy model a Th2-driven humoral response was not blocked by CTLA4Ig.
Interestingly, CTLA4Ig and anti-CD154 failed to inhibit secondary IgE responses and IgE-mediated, allergen-specific degranulation of basophils in mice with an already established allergy although T cell responses had been strongly suppressed (as revealed by results of the proliferation assays). Our data thus suggest that established secondary IgE responses are difficult to control via T cell-based therapeutic approaches. In fact, it is well established that priming of naive T cells during sensitization is crucial in order to provide help for B cell differentiation but the role of T cells in secondary immune responses of memory B cells is a matter of controversy (36-40). Previous studies in mice, using T cell depleting anti-CD4 antibodies, suggested, that memory B cell persistence needs little or no antigen-mediated T cell help (36). Also the influence of T cells on the activation of memory B cells has recently been investigated. Using a model of T and B cell deficient RAG−/− mice for adoptive transfer experiments, the activation of virus-specific memory B cells to secrete IgG was shown to be independent of cognate or bystander T cell help (37).
The lack of effect of co-stimulation blockade on secondary IgE responses and IgE-mediated allergic responses in already sensitized mice does not contradict earlier studies showing that co-stimulation blockade can suppress T cell-mediated allergic inflammation because several reports have provided convincing evidence for the co-existence of IgE-mediated as well as IgE-independent, T cell-mediated reactions in allergic patients (4, 11). This may also explain why attempts to treat allergic symptoms by down-regulating allergen-specific T cell responses in asthmatic patients using allergen-derived T cell peptides led to controversial results (41, 42).
In fact, blockade of the CD28/CTLA4-CD80/CD86 pathway could block allergen-induced peripheral blood T cell proliferation as well as IL-5 production of asthmatic subjects (43), and abrogate airway hyper-responsiveness in a murine model of allergic asthma (44). Furthermore, it has been demonstrated that the aerosol-induced effector functions of memory T cells could be inhibited by blocking the function of the B7-1 or B7-2 ligands (45-47). As a consequence, the blockade of co-stimulation at the time of allergen exposure has been proposed as a potential strategy for the treatment of atopic disease (48).
Costimulation blockers exert their effects through several mechanisms. Both anti-CD154 and CTLA4Ig have been shown to be able to induce anergy, deletion and regulation (24, 49). The contribution of each of these mechanisms in vivo depends on the specifics of the model used (13). It is likely, that regulation was induced by anti-CD154 mAb and CTLA4Ig in the presented study, but if so, it was not sufficient to suppress the secondary IgE response.
We think that the differential effects of co-stimulation blockade on allergic inflammation can be explained by the existence of at least two pathomechanisms, one involving IgE-mediated allergic inflammation which seems to be less susceptible to T cell-mediated control and non-IgE-mediated but T cell-dependent allergic inflammation, a mechanism which can be controlled by T cell epitope-derived peptides, anti-CD4 antibodies, cyclosporine and perhaps regulatory T cells (4, 11). In addition there may be a link between the latter two mechanisms because it has been shown that T cell activation in allergic patients can be regulated by IgE-facilitated antigen presentation (8).
Our data therefore suggest that it will be difficult to treat all facets of established allergic disease using strategies focusing only on the induction of T cell tolerance. It will rather be necessary to regulate established allergic immune responses using strategies, which actively antagonize the established immune response (e.g. regulatory immune responses, shifting the balance towards Th1 or induction of counter-immune responses) (11, 50). The dissection of the various pathways and their importance for allergic inflammation as well as the determination of their sensitivity for various immunological treatment strategies should conceivably result in the selection of optimal immunological strategies for the treatment of allergic diseases.
Footnotes
This study was supported by grants from the FFG (Forschungsförderungsgesellschaft) Austria (Bridge grant 810105-SCK/SAI to R.V.) and from the Austrian Science Fund (grants SFB F2310-B13 to T.W., F1815 to R.V. and S8811 to J.T.).
References
- 1.Holt PG, Thomas WR. Sensitization to airborne environmental allergens: unresolved issues. Nat. Immunol. 2005;6:957–960. doi: 10.1038/ni1005-957. [DOI] [PubMed] [Google Scholar]
- 2.Kulig M, Bergmann R, Klettke U, Wahn V, Tacke U, Wahn U. Natural course of sensitization to food and inhalant allergens during the first six years of life. J. Allergy Clin. Immunol. 1999;103:1173–1179. doi: 10.1016/s0091-6749(99)70195-8. [DOI] [PubMed] [Google Scholar]
- 3.Valenta R, Kraft D. From allergen structure to new forms of allergen-specific immunotherapy. Curr. Opin. Immunol. 2002;14:718–727. doi: 10.1016/s0952-7915(02)00402-8. [DOI] [PubMed] [Google Scholar]
- 4.Valenta R, Ball T, Focke M, Linhart B, Mothes N, Niederberger V, Spitzauer S, Swoboda I, Vrtala S, Westritschnig K, Kraft D. Immunotherapy of allergic disease. Adv. Immunol. 2004;82:105–153. doi: 10.1016/S0065-2776(04)82003-0. [DOI] [PubMed] [Google Scholar]
- 5.Akdis M, Verhagen J, Taylor A, Karamloo F, Karagiannidis C, Crameri R, Thunberg S, Deniz G, Valenta R, Fiebig H, Kegel C, Disch R, Schmidt-Weber CB, Blaser K, Akdis CA. Immune responses in healthy and allergic individuals are characterized by a fine balance between allergen-specific T regulatory 1 and T helper 2 cells. J. Exp. Med. 2004;199:1567–1575. doi: 10.1084/jem.20032058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Geha RS, Jabara HH, Brodeur SR. The regulation of immunoglobulin E class-switch recombination. Nat. Rev. Immunol. 2003;3:721–732. doi: 10.1038/nri1181. [DOI] [PubMed] [Google Scholar]
- 7.Kawakami T, Galli SJ. Regulation of mast-cell and basophil function and survival by IgE. Nat. Rev. Immunol. 2002;2:773–786. doi: 10.1038/nri914. [DOI] [PubMed] [Google Scholar]
- 8.van Neerven RJ, Knol EF, Ejrnaes A, Wurtzen PA. IgE-Mediated Allergen Presentation and Blocking Antibodies: Regulation of T-Cell activation in Allergy. Int. Arch. Allergy Immunol. 2006;141:119–129. doi: 10.1159/000094714. [DOI] [PubMed] [Google Scholar]
- 9.Naclerio RM, Adkinson NF, Moylan B, Baroody FM, Proud D, Kagey-Sobotka A, Lichtenstein LM, Hamilton R. Nasal provocation with allergen induces a secondary serum IgE antibody response. J. Allergy Clin. Immunol. 1997;100:505–510. doi: 10.1016/s0091-6749(97)70143-x. [DOI] [PubMed] [Google Scholar]
- 10.Illi S, von Mutius E, Lau S, Niggemann B, Gruber C, Wahn U, Multicentre Allergy Study (MAS) group Perennial allergen sensitisation early in life and chronic asthma in children: a birth cohort study. Lancet. 2006;368:763–770. doi: 10.1016/S0140-6736(06)69286-6. [DOI] [PubMed] [Google Scholar]
- 11.Larche M, Akdis CA, Valenta R. Immunological mechanisms of allergen-specific immunotherapy. Nat. Rev. Immunol. 2006;6:761–771. doi: 10.1038/nri1934. [DOI] [PubMed] [Google Scholar]
- 12.Hahn C, Teufel M, Herz U, Renz H, Erb KJ, Wohlleben G, Brocker EB, Duschl A, Sebald W, Grunewald SM. Inhibition of the IL-4/IL-13 receptor system prevents allergic sensitization without affecting established allergy in a mouse model for allergic asthma. J. Allergy Clin. Immunol. 2003;111:1361–1369. doi: 10.1067/mai.2003.1527. [DOI] [PubMed] [Google Scholar]
- 13.Wekerle T, Kurtz J, Bigenzahn S, Takeuchi Y, Sykes M. Mechanisms of transplant tolerance induction using costimulatory blockade. Curr. Opin. Immunol. 2002;14:592–600. doi: 10.1016/s0952-7915(02)00378-3. [DOI] [PubMed] [Google Scholar]
- 14.Vrtala S, Ball T, Spitzauer S, Pandjaitan B, Suphioglu C, Knox B, Sperr WR, Valent P, Kraft D, Valenta R. Immunization with purified natural and recombinant allergens induces mouse IgG1 antibodies that recognize similar epitopes as human IgE and inhibit the human IgE-allergen interaction and allergen-induced basophil degranulation. J. Immunol. 1998;160:6137–6144. [PubMed] [Google Scholar]
- 15.Bluestone JA, Clair EW, Turka LA. CTLA4Ig: Bridging the basic immunology with clinical application. Immunity. 2006;24:233–238. doi: 10.1016/j.immuni.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 16.Kurtz J, Ito H, Wekerle T, Shaffer J, Sykes M. Mechanisms involved in the establishment of tolerance through costimulatory blockade and BMT: Lack of requirement for CD40L-mediated signalling for tolerance or deletion or donor-reactive CD4+ cells. Am. J. Transplant. 2001;1:339–349. doi: 10.1034/j.1600-6143.2001.10409.x. [DOI] [PubMed] [Google Scholar]
- 17.Monk NJ, Hargreaves RE, Marsh JE, Farrar CA, Sacks SH, Millrain M, Simpson E, Dyson J, Jurcevic S. Fc-dependent depletion of activated T cells occurs through CD40L-specific Ab rather than costimulation blockade. Nat. Med. 2003;9:1275–1280. doi: 10.1038/nm931. [DOI] [PubMed] [Google Scholar]
- 18.Kremer JM, Westhovens R, Leon M, Di Giorgio E, Alten R, Steinfeld S, Russell A, Dougados M, Emery P, Nuamah IF, Williams GR, Becker JC, Hagerty DT, Moreland LW. Treatment of rheumatoid arthritis by selective inhibition of T-cell activation with fusion protein CTLA4Ig. N. Engl. J. Med. 2003;349:1907–1915. doi: 10.1056/NEJMoa035075. [DOI] [PubMed] [Google Scholar]
- 19.Vincenti F, Larsen C, Durrbach A, Wekerle T, Nashan B, Blancho G, Lang P, Gringo J, Halloran PF, Solez K, Hagerty D, Levy E, Zhou W, Natarajan K, Charpentier B, Belatacept Study Group Costimulation blockade with belatacept in renal transplantation. N. Engl. J. Med. 2005;353:770–781. doi: 10.1056/NEJMoa050085. [DOI] [PubMed] [Google Scholar]
- 20.Vrtala S, Susani M, Sperr WR, Valent P, Laffer S, Dolecek C, Kraft D, Valenta R. Immunologic characterization of purified recombinant timothy grass pollen (Phleum pratense) allergens (Phl p 1, Phl p 2, Phl p 5) J. Allergy Clin. Immunol. 1996;97:781–787. doi: 10.1016/s0091-6749(96)80156-4. [DOI] [PubMed] [Google Scholar]
- 21.Linsley PS, Wallace PM, Johnson J, Gibson MG, Greene JL, Ledbetter JA, Singh C, Tepper MA. Immunosuppression in vivo by a soluble form of the CTLA-4 T cell activation molecule. Science. 1992;257:792–795. doi: 10.1126/science.1496399. [DOI] [PubMed] [Google Scholar]
- 22.Blaha P, Bigenzahn S, Koporc Z, Schmid M, Langer F, Selzer E, Bergmeister H, Wrba F, Kurtz J, Kiss C, Roth E, Muehlbacher F, Sykes M, Wekerle T. The influence of immunosuppressive drugs on tolerance induction through bone marrow transplantation with costimulation blockade. Blood. 2003;101:2886–2893. doi: 10.1182/blood-2002-10-3014. [DOI] [PubMed] [Google Scholar]
- 23.Schaub M, Issazadeh S, Stadlbauer TH, Peach R, Sayegh MH, Khoury SJ. Costimulatory signal blockade in murine relapsing experimental autoimmune encephalomyelitis. J. Neuroimmunol. 1999;96:158–166. doi: 10.1016/s0165-5728(99)00022-3. [DOI] [PubMed] [Google Scholar]
- 24.Bigenzahn S, Blaha P, Koporc Z, Pree I, Selzer E, Bergmeister H, Wrba F, Heusser C, Wagner K, Muehlbacher F, Wekerle T. The role of non-deletional tolerance mechanisms in a murine model of mixed chimerism with costimulation blockade. Am. J. Transplant. 2005;5:1237–1247. doi: 10.1111/j.1600-6143.2005.00862.x. [DOI] [PubMed] [Google Scholar]
- 25.Flicker S, Vrtala S, Steinberger P, Vangelista L, Bufe A, Petersen A, Ghannadan M, Sperr WR, Valent P, Norderhaug L, Bohle B, Stockinger H, Suphioglu C, Ong EK, Kraft D, Valenta R. A Human Monoclonal IgE Antibody Defines a Highly Allergenic Fragment of the Major Timothy Grass Pollen Allergen, Phl p 5: Molecular, immunological, and structural characterization of the epitope-containing domain. J. Immunology. 2000;165:3849–3859. doi: 10.4049/jimmunol.165.7.3849. [DOI] [PubMed] [Google Scholar]
- 26.Vrtala S, Mayer P, Ferreira F, Susani M, Sehon AH, Kraft D, Valenta R. Induction of IgE antibodies in mice and rhesus monkeys with recombinant birch pollen allergens: different allergenicity of Bet v 1 and Bet v 2. J. Allergy Clin. Immunol. 1996;98:913–921. doi: 10.1016/s0091-6749(96)80007-8. [DOI] [PubMed] [Google Scholar]
- 27.Westritschnig K, Focke M, Verdino P, Goessler W, Keller W, Twardosz A, Mari A, Horak F, Wiedermann U, Hartl A, Thalhamer J, Sperr WR, Valent P, Valenta R. Generation of an allergy vaccine by disruption of the three-dimensional structure of the cross-reactive calcium-binding allergen, Phl p 7. J. Immunol. 2004;172:5684–5692. doi: 10.4049/jimmunol.172.9.5684. [DOI] [PubMed] [Google Scholar]
- 28.Valenta R. The future of allergen-specific immunotherapy. Nat. Rev. Immunol. 2002;2:446–453. doi: 10.1038/nri824. [DOI] [PubMed] [Google Scholar]
- 29.Abrams JR, Lebwohl MG, Guzzo CA, Jegasothy BV, Goldfarb MT, Goffe BS, Menter A, Lowe NJ, Krueger G, Brown MJ, Weiner RS, Birkhofer MJ, Warner GL, Berry KK, Linsley PS, Krueger JG, Ochs HD, Kelley SL, Kang S. CTLA4Ig-mediated blockade of T-cell costimulation in patients with psoriasis vulgaris. J. Clin. Invest. 1999;103:1243–1252. doi: 10.1172/JCI5857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Kooten C, Banchereau J. CD40-CD40 ligand. J. Leuoc. Biol. 2000;67:2–17. doi: 10.1002/jlb.67.1.2. [DOI] [PubMed] [Google Scholar]
- 31.Xu J, Foy TM, Laman JD, Elliott EA, Dunn JJ, Waldschmidt TJ, Elsemore J, Noelle RJ, Flavell RA. Mice deficient for the CD40 ligand. Immunity. 1994;1:423–431. doi: 10.1016/1074-7613(94)90073-6. [DOI] [PubMed] [Google Scholar]
- 32.Grewal IS, Xu J, Flavell RA. Impairment of antigen-specific T-cell priming in mice lacking CD40 ligand. Nature. 1995;378:617–620. doi: 10.1038/378617a0. [DOI] [PubMed] [Google Scholar]
- 33.Tang A, Judge TA, Nickoloff BJ, Turka LA. Suppression of murine allergic contact dermatitis by CTLA4Ig. Tolerance induction of Th2 responses requires additional blockade of CD40-ligand. J. Immunol. 1996;157:117–125. [PubMed] [Google Scholar]
- 34.Khoury SJ, Akalin E, Chandraker A, Turka LA, Linsley PS, Sayegh MH, Hancock WW. CD28-B7 costimulatory blockade by CTLA4Ig prevents actively induced experimental autoimmune encephalomyelitis and inhibits Th1 but spares Th2 cytokines in the central nervous system. J. Immunol. 1995;155:4521–4524. [PubMed] [Google Scholar]
- 35.Sayegh MH, Akalin E, Hancock WW, Russell ME, Carpenter CB, Linsley PS, Turka LA. CD28-B7 blockade after alloantigenic challange in vivo inhibits Th1 cytokines but spares Th2. J. Exp. Med. 1995;181:1869–1874. doi: 10.1084/jem.181.5.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vieira P, Rajewsky K. Persistence of memory B cells in mice deprived of T cell help. Int. Immunol. 1990;2:487–494. doi: 10.1093/intimm/2.6.487. [DOI] [PubMed] [Google Scholar]
- 37.Hebeis BJ, Klenovsek K, Rohwer P, Ritter U, Schneider A, Mach M, Winkler TH. Activation of virus-specific memory B cells in the absence of T cell help. J. Exp. Med. 2004;199:593–602. doi: 10.1084/jem.20030091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bernasconi NL, Traggiai E, Lanzavecchia A. Maintenance of serological memory by polyclonal activation of human memory B cells. Science. 2002;298:2199–2202. doi: 10.1126/science.1076071. [DOI] [PubMed] [Google Scholar]
- 39.Ochsenbein AF, Pinschewer DD, Sierro S, Horvath E, Hengartner H, Zinkernagel RM. Protective long-term antibody memory by antigen-driven and T help-dependent differentiation of long-lived memory B cells to short-lived plasma cells independent of secondary lymphoid organs. Proc. Natl. Acad. Sci. USA. 2000;97:13263–13268. doi: 10.1073/pnas.230417497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bell EB, Hayes S, McDonagh M, Bunce C, Yang C, Sparshott SM. Both CD45R(low) and CD45R(high) ‘revertant’ CD4 memory T cells provide help for memory B cells. Eur. J. immunol. 2001;31:1685–1695. doi: 10.1002/1521-4141(200106)31:6<1685::aid-immu1685>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 41.Larche M, Wraith DC. Peptide-based therapeutic vaccines for allergic and autoimmune disease. Nat. Med. 2005;11:S69–76. doi: 10.1038/nm1226. [DOI] [PubMed] [Google Scholar]
- 42.Simons FE, Imada M, Li Y, Watson WT, HayGlass KT. Fel d 1 peptides: effect on skin tests and cytokine synthesis in cat-allergic human subjects. Int. Immunol. 1996;8:1937–1945. doi: 10.1093/intimm/8.12.1937. [DOI] [PubMed] [Google Scholar]
- 43.Larche M, Till SJ, Haselden BM, North J, Barkans J, Corrigan CJ, Kay AB, Robinson DS. Costimulation through CD86 is involved in airway antigen-presenting cell and T cell responses to allergen in atopic asthmatics. J. Immunol. 1998;161:6375–6382. [PubMed] [Google Scholar]
- 44.Krinzman SJ, De Sanctis GT, Cernadas M, Mark D, Wang Y, Listman J, Kobzik L, Donovan C, Nassr K, Katona I, Christiani DC, Perkins DL. Inhibition of T cell costimulation abrogates airway hyperresponsiveness in a murine model. J. Clin. Invest. 1996;98:2693–2699. doi: 10.1172/JCI119093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Harris N, Peach R, Naemura J, Linsley PS, Le Gros G, Ronchese F. CD80 costimulation is essential for the induction of airway eosinophilia. J. Exp. Med. 1997;185:177–182. doi: 10.1084/jem.185.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tsuyuki S, Tsuyuki J, Einsle K, Kopf M, Coyle AJ. Costimulation through B7-2 (CD86) is required for the induction of lung mucosal T helper cell 2 (TH2) immune response and altered airway responsiveness. J. Exp. Med. 1997;185:1671–1679. doi: 10.1084/jem.185.9.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Keane-Myers A, Gause WC, Linsley PS, Chen S, Wills-Karp M. B7-CD28/CTLA-4 costimulatory pathways are required for the development of T helper cell 2-mediated allergic airway responses to inhaled antigens. J. Immunol. 1997;158:2042–2049. [PubMed] [Google Scholar]
- 48.Kroczek R, Hamelmann E. T-cell costimulatory molecules: optimal targets for the treatment of allergic airway disease with monoclonal antibodies. J. Allergy Clin. Immunol. 2005;116:906–909. doi: 10.1016/j.jaci.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 49.Kurtz J, Wekerle T, Sykes M. Tolerance in mixed chimerism – a role for regulatory cells? Trends Immunol. 2004;25:518–523. doi: 10.1016/j.it.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 50.Coffman RL. Origins of the T(H)1-T(H)2 model: a personal perspective. Nat. Immunol. 2006;7:539–541. doi: 10.1038/ni0606-539. [DOI] [PubMed] [Google Scholar]