Summary
Transmissible spongiform encephalopathies, or prion diseases, are caused by misfolding and aggregation of the prion protein PrP. These diseases can be hereditary in humans and four of the many disease-associated missense mutants of PrP are in the hydrophobic core: V180I, F198S, V203I and V210I. The T183A mutation is related to the hydrophobic core mutants as it is close to the hydrophobic core and known to cause instability. We have performed extensive molecular dynamics simulations of these five PrP mutants and compared their dynamics and conformations to wild-type PrP. The simulations highlight the changes that occur upon introduction of mutations and help to rationalize experimental findings. Changes can occur around the mutation site, but they can also be propagated over long distances. In particular, the F198S and T183A mutations lead to increased flexibility in parts of the structure that are normally stable, and the short β-sheet moves away from the rest of the protein. Mutations V180I, V210I and, to a lesser extent, V203I cause changes similar to those observed upon lowering the pH, which has been linked to misfolding. Early misfolding is observed in one V180I simulation. Overall, mutations in the hydrophobic core have a significant effect on the dynamics and stability of PrP, including the propensity to misfold, which helps to explain their role in the development of familial prion diseases.
Transmissible spongiform encephalopathies or prion diseases are neurodegenerative disorders that are fatal and occur in a range of mammals. These relatively rare disorders can arise spontaneously, through infection, or through inheritance of mutations in the gene encoding the prion protein (PrP). The majority of mutations related to prion disease identified in humans lead to single amino acid substitutions in the mature prion protein.1 These mutations can cause carriers to develop Creutzfeldt-Jacob disease (CJD), Gerstmann-Sträussler-Scheinker syndrome (GSS) or fatal familial insomnia (FFI), each of which is characterized by a different, but often overlapping, set of neurological symptoms.2 The disorders can usually further be identified by the accumulation of aggregates of PrP in neuronal tissue.3 Aggregation occurs due to conformational conversion of the normal cellular PrP (PrPC or PrPsen) to a pathogenic and largely proteinase K resistant form (PrPSc or PrPres), a process that involves an increase in β-structure.
PrP is primarily expressed in neuronal tissue. The mature protein is anchored to the outer cell membrane through a C-terminally linked glycosylphosphatidyl-inositol (GPI) anchor, and it can exist in un-, mono-, and diglycosylated forms. The exact function of PrPC is still elusive, although it is likely to be involved in neuronal signal transduction processes4,5 and metal metabolism.6–8 The protein is best known for its ability to misfold and form fibrillar aggregates. Such aggregates are linked to spongiform formation in the brain and severe neurotoxic effects in individuals that suffer from prion disease. Specific aggregates, prions, have the ability to infect other individuals.
Many questions remain about the process of PrP misfolding and aggregation, although simulation and modeling have provided important clues.9–14 How misfolding and aggregation are affected by disease-related mutations is largely unknown. Different mutations affect the stability of PrPC, increase its the propensity to aggregate and/or affect its cellular processing.1 It can be difficult to disentangle the multitude of potential effects in vivo or in cell models. Partly because of this, many studies focus on bacterially expressed recombinant PrP (recPrP), which is unglycosylated and lacks the GPI-anchor. The stability of this monomeric recombinant form must arise from interactions within the protein.
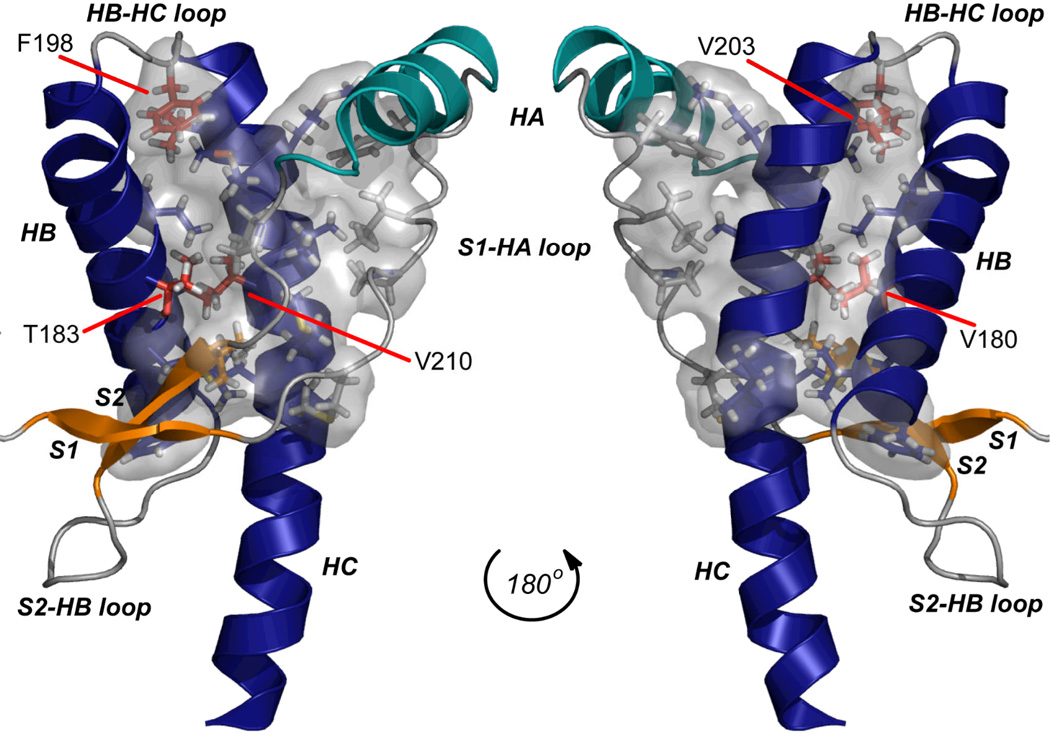
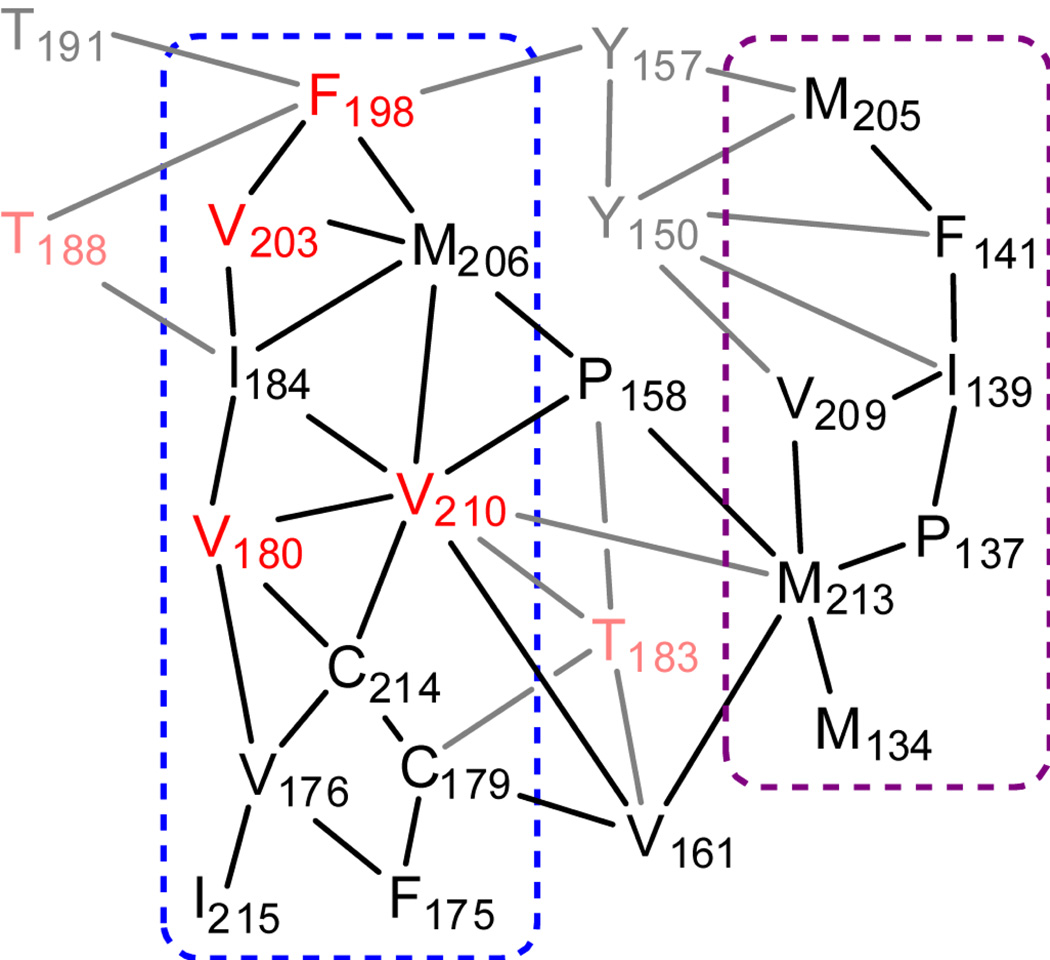
A number of disease-causing mutations significantly affect the thermodynamic stability15 and folding16 of recPrP. Several of these destabilizing mutations are located in the central hydrophobic core of PrP, a region that can be defined as interacting residues with purely hydrophobic side chains that form the core of the globular domain (Figure 1).17 It is likely that the hydrophobic core is important for stability of the globular domain of recPrP. The hydrophobic core provides tertiary interactions between helices HB and HC, between HC and the S1-HA loop, and between the short native β-sheet (through residues V161 and P158) and HB/HC (Figure 2). An extensive hydrophobic network exists between HB and HC, and it is in this region that all four disease-related mutations in the hydrophobic core reside: V180I (CJD),18 F198S (GSS),19 V203I (CJD)20 and V210I (CJD).21,22 The T183A mutation (CJD)23 affects a neighboring residue that provides further hydrophobic interactions in this region (Figure 2). Here, we study the influence of these five mutations on the structure, dynamics, and stability of recPrP through extensive molecular dynamics (MD) simulations.
Figure 1.
The globular domain of human PrP (PDB code 1QLX) with the hydrophobic core shown in space filling mode. Secondary structure elements are labeled (helices HA, HB, HC; β-strands S1, S2 and loops connecting them). The hydrophobic core was defined as the side chains of residues 134, 137, 139, 141, 158, 161, 175, 176, 179, 180, 184, 198, 203, 205, 206, 209, 210, and 213–215. It is shown as a translucent gray surface, with side chains displayed as sticks. Side chains of residues involved in disease-causing mutations studied here are colored red.
Figure 2.
Schematic depiction of hydrophobic interactions in human PrP. Names of residues that are part of the hydrophobic core (with purely hydrophobic side chains) are shown in black, or, if implicated in disease-causing mutations, in red; other residues involved in the hydrophobic core are shown in gray, or, if implicated in disease-causing mutations, in pink. The blue dashed line indicates interactions between residues in HB and HC and the purple dashed line indicates interactions between HC and the S1-HA loop.
Four of the mutations studied here are directly implicated in causing instability of recPrP. T183A and F198S significantly affect thermodynamic stability in mouse recPrP.15 V180I, V210 and F198S increase the population of a folding intermediate of recPrP with respect to the native form.16,24 Further, both T183A and F198S interfere with GPI-anchor attachment and cellular processing in cell models.25,26 T183A also disrupts the first of the two glycosylation sites, preventing the formation of diglycosylated PrP.27,28 The effect of V203I on PrP stability has not been studied experimentally, but the formation of cytosolic aggresomes upon proteasome inhibition (in contrast to WT PrP) may be indicative of destabilization.29
In this study, we present molecular dynamics (MD) simulations of five different mutant recPrPs that affect the hydrophobic core and are implicated in inherited prion disease. Three separate simulations of 50 ns were performed for each mutant in water at neutral pH, totaling 750 ns, or 0.75 µs of simulation time. We compare these simulations in detail with corresponding 50 ns runs of WT PrP, adding another 200 ns.14 All mutations affect protein conformation, dynamics, and/or stability, albeit to different degrees. Conformational effects distant from the mutation site occurred due to changes in interactions in the hydrophobic core. One of the simulations of V180I PrP reproduces early misfolding similar to WT PrP at decreased pH.11,14 Other simulations show related effects. Overall, the simulations are in agreement with experimental findings and provide detailed insight into the mechanism by which single residue mutations in the hydrophobic core can enhance instability and the subsequent misfolding propensity of PrP.
Results
Conformational changes and instability of the protein backbone
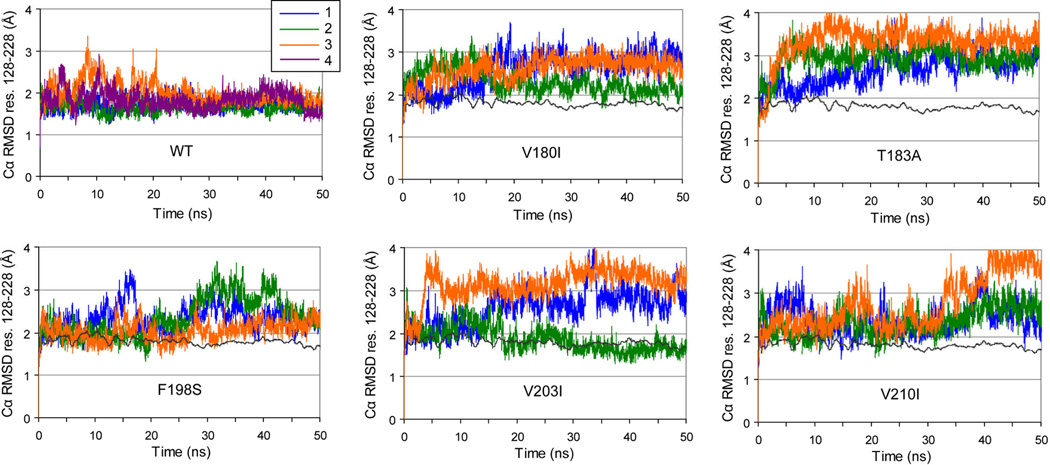
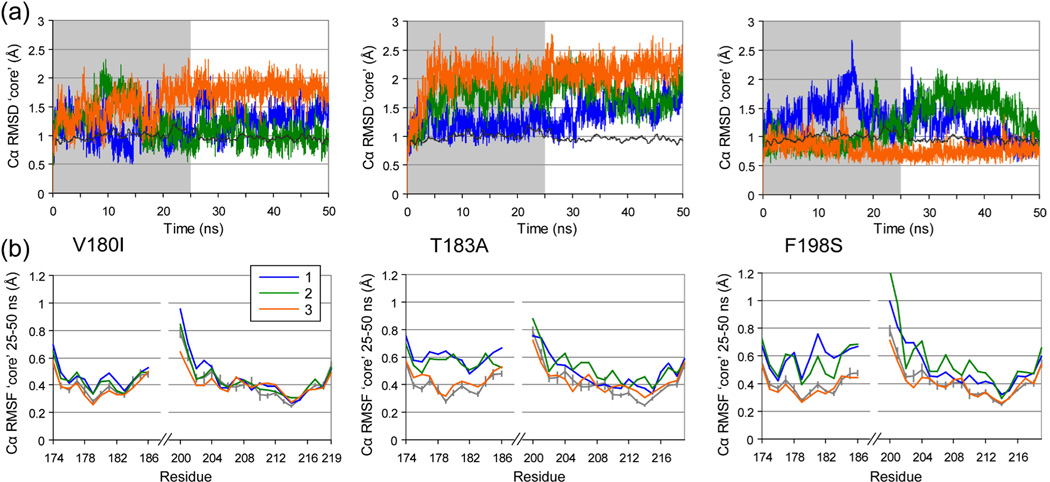
Cα RMSD calculations were performed for two regions: the globular domain (res. 128–228, Figure 3) and the ‘core’ domain (res. 174–186 and 200–219, Figure 4). The latter is a substructure consisting of parts of HB and HC that was shown to be stable in WT PrP by experiment30–33 and MD simulation.9,11,14 Average Cα RMSD values over the 25–50 ns time interval were compared (Table 1), to allow for sufficient equilibration of the structure after introducing the mutation. All mutant simulations showed increased globular Cα RMSD values compared with WT (apart from V203I run 2). Significant increases of the Cα RMSD of the core domain occurred for all T183A runs, 2 V180I runs and one F198S run (Table 1).
Figure 3.
Cα RMSD from the starting structure of the globular domain (res. 128–228) for all simulations. In mutant PrP plots, a windowed average (200 ps) of the WT PrP simulations is shown for reference as a dark gray line.
Figure 4.
Changes in the core domain. (a) Cα RMSD from the starting structure of the core domain (res. 174–186 & 200–219) for V180I, T183A and F198S PrP simulations. A windowed average (200 ps) of the WT PrP simulations is shown for reference (dark gray line). (b) Cα RMSF for the core domain (res. 174–186 & 200–219) over 25–50 ns of the trajectories (the non-shaded part in A) of V180I, T183A and F198S PrP. For reference, average Cα RMSF for the WT trajectories is shown in gray, with vertical bars indicating one standard deviation between the 4 runs.
Table 1.
Average properties of the trajectories from 25 to 50 ns.
| run | Cα RMSD globular (Å) a |
Cα RMSD ‘core’ (Å) b |
SASA globular (Å2) c |
SASA hydrophobic core (Å2) d |
# hphob contacts S1HA-HC e |
SASA 198 sc (Å2) f |
|
|---|---|---|---|---|---|---|---|
| WT | 1 | 1.72 | 0.98 | 6327 | 327 | 13.8 | 11.2 |
| 2 | 1.67 | 0.89 | 6242 | 349 | 23.5 | 8.7 | |
| 3 | 1.86 | 1.05 | 6213 | 375 | 27.4 | 14.2 | |
| 4 | 1.80 | 0.91 | 6203 | 326 | 28.8 | 6.4 | |
| WT average | 1.76 | 0.96 | 6246 | 344 | 23.4 | 10.1 | |
| V180I | 1 | 2.81 | 1.31 | 6486 | 598 | 6.3 | 82.9 |
| 2 | 2.17 | 1.01 | 6197 | 411 | 25.4 | 105.0 | |
| 3 | 2.77 | 1.82 | 6697 | 473 | 23.2 | 88.0 | |
| T183A | 1 | 2.86 | 1.46 | 6444 | 323 | 29.4 | 10.2 |
| 2 | 2.98 | 1.80 | 6346 | 428 | 17.9 | 88.4 | |
| 3 | 3.39 | 2.17 | 6324 | 501 | 21.7 | 80.4 | |
| F198S | 1 | 2.39 | 1.14 | 6459 | 356 | 22.4 | 7.5 |
| 2 | 2.71 | 1.49 | 6630 | 454 | 8.0 | 11.1 | |
| 3 | 2.12 | 0.74 | 6198 | 300 | 28.3 | 0.2 | |
| V203I | 1 | 2.84 | 0.93 | 6630 | 520 | 8.8 | 75.7 |
| 2 | 1.70 | 1.08 | 6459 | 379 | 17.3 | 16.4 | |
| 3 | 3.33 | 1.24 | 6383 | 366 | 33.1 | 78.4 | |
| V210I | 1 | 2.42 | 0.78 | 6856 | 594 | 4.8 | 81.3 |
| 2 | 2.50 | 1.02 | 6556 | 457 | 13.3 | 68.9 | |
| 3 | 3.13 | 1.01 | 6673 | 542 | 7.8 | 74.7 | |
Cα RMSD of the globular domain, res. 128–228.
Cα RMSD of the ‘core’ domain that is stable in WT recPrP, res. 174–186 and 200–219.
Solvent accessible surface area (SASA) of the whole globular domain, res 128–228.
SASA of the side chains of residues in the hydrophobic core (see Figure 1).
Number of atom-atom contacts between side chains of hydrophobic core res. 137, 139, 141 (in the S1-HA loop) and 205, 209, 213 (on HC).
SASA of res. 198 (in the HB-HC loop), indicating if it is still in the hydrophobic core (low values) or not (high values).
As well as changes in conformation, flexibility of the structure can be altered by mutations. Cα RMSF measurements give an indication of backbone mobility. For the 3 mutations that showed conformational changes in the ‘core’ domain (as indicated by Cα RMSD), only T183A and F198S showed an increase in Cα RMSF in 2 runs out of 3 (Figure 4). Notably, the Cα RMSF increased most significantly around the mutation site: res. 176–186 for T183A and 179–204 for F198S.
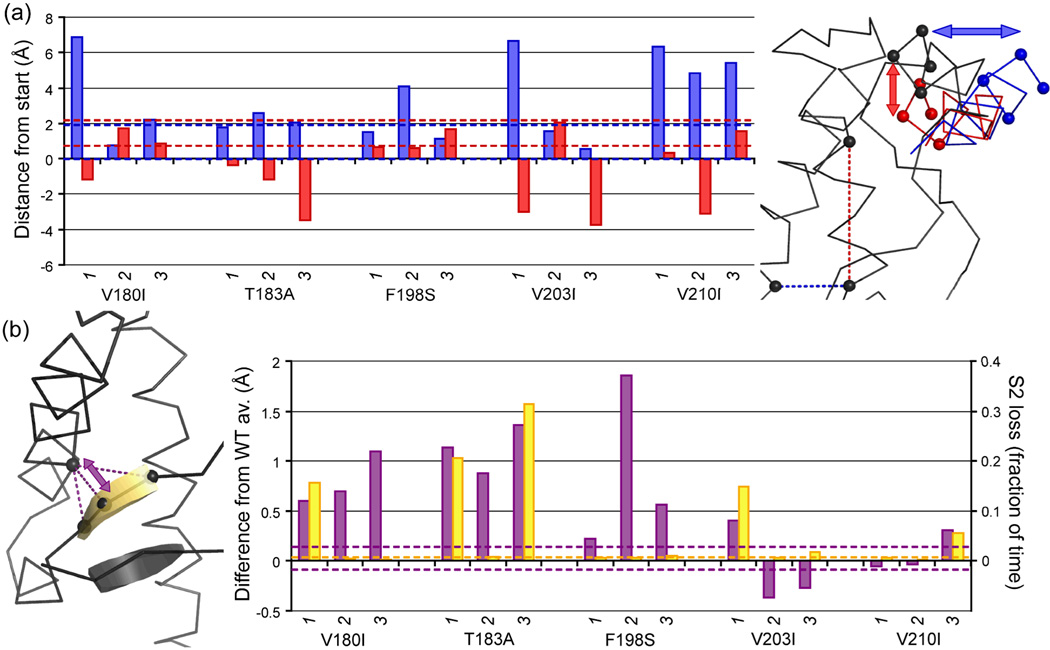
As found for WT PrP at low pH,14 the displacement of HA with respect to the rest of the structure was an important early conformational change. ‘Outward’ movement of the N-terminal region of HA (away from HB and HC, into solvent) was most significant (particularly in all V210I runs, V180I run 1 and V203I run 1), but ‘downward’ movement also occurred (Figure 5A). In WT PrP, HA ends in a 310 conformation. Outward movement of the N-terminal end of HA always caused the C-terminal residues to adopt an α-helical conformation.
Figure 5.
Displacement of HA and the native β-sheet. (a) Average outward (blue) and downward (red) displacement of the N-terminal part of HA (measured as displacement of Cα atoms of res. 144–147, shown as spheres) over 25–50 ns. Downward movement is relative to the axis through C214 and M206 (spheres connected with red dashed line), outward movement relative to axis through C214 and V176 (spheres connected with blue dashed line); structure alignment was performed on the ‘core’ region (res. 174–186 & 200–219). (b) Distance between S2 and Cys179 (purple) and the fraction of time S2 is lost (yellow; S2 is neither β-strand nor β-bridge according to DSSP) over 25–50 ns. Extreme values observed in the WT runs are shown as dashed lines in the histograms.
Further contributions to the increases in globular Cα RMSD were changes in 3 loops: the loop between S1 and HA (S1-HA loop), between S2 and HB (S2-HB loop) and between HB and HC (HB-HC loop). As the changes in these loops were related to instability in the hydrophobic core, a more detailed description follows below. No significant decrease in α-helical structure occurred: For the majority of the mutant trajectories, helical propensity was similar to WT simulations. In some cases, the population of i→i+4 hydrogen bonds decreased, but these changes didn’t lead to unfolding.
Changes in native β-sheet position and stability
Apart from HA moving away from HC, another change in tertiary structure was observed for several mutants: the sheet moved away from to the rest of the globular domain (Figure 5B). This change happened consistently for V180I, T183A and F198S, but not for V203I and V210I. For T183A, this behavior was expected, as the mutation abolishes the hydrogen bond between the T183 side chain and the Y162 backbone.1,14 This hydrogen bond was also lost in F198S (all three runs) and V180I run 3. Reduction of the hydrogen bond population was also found for other runs (including V203I and V210I), leading to a negative correlation between the population and shift in S2 for WT and mutant runs with a population >2% (R = −0.86). Instability of the β-sheet was also observed in some runs: two of the three hydrogen bonds between S1 and S2 were lost for part of the simulation in T183A runs 1 and 3, and to a lesser extent in one run each of V180I, V203I and V210I (Figure 5B).
Instability in the hydrophobic core affects loop conformations
Misfolding of PrP in response to decreased pH has been related to an increased exposure of hydrophobic residues.14,34 It is likely that such exposure is related to changes in the hydrophobic core. To measure the compactness of the hydrophobic core, its solvent-accessible surface area (SASA) was monitored throughout all simulations (Table 1). A significant increase (>20%) was detected in 10 of the 15 mutant runs. These runs include all V180I and V210I runs, two T183A runs and one run of F198S and V203I. The most extreme increases (>50%) were for V180I run 1, V210I runs 1 and 3 and V203I run 1. The major contributor to the increased exposure was a loss of hydrophobic contacts between the residues in the S1-HA loop and HC (Table 1; see also Figure 2). A significant reduction of contacts between the S1-HA loop and HC occurred and HA became displaced.
A further increase in hydrophobic surface exposure occurred in response to a change in the HB-HC loop. This change was marked by the expulsion of F198 from the hydrophobic core and increased conformational flexibility in the loop. In the starting structures and WT PrP, F198 interacts mostly with M206, through which it is connected to the tight cluster of hydrophobic contacts between HB and HC (Figures 1 and 2). This cluster includes all other disease-related mutation sites in the hydrophobic core and residues M206 and I184. The latter two residues displayed a marked change in position upon expulsion of F198. In all simulations in which F198 moved out of the hydrophobic core (all runs of V180I, V203I and V210I and runs 2 and 3 of T183A), the I184 side chain initially shifted outward, opening a gap in the hydrophobic core that was subsequently filled by M206. In order to do so, M206 shifted down and lost contact with F198, after which F198 swung out into solvent. The latter situation was maintained in all these runs (Figure 6, Table 1) apart from V203I run 2, where contact between M206 and F198 reformed after ~27 ns of simulation. The F198S mutation (which abolishes hydrophobic contacts with M206 and V203) caused a similar effect in the hydrophobic core: I184 can shift outwards and M206 downwards (Figure 6). Apart from run 3, which diverged little from the WT structure (Table 1, Figures 3 and 4), the HB-HC loop underwent a similar change in conformation as in the simulations where F198 moved out into solvent.
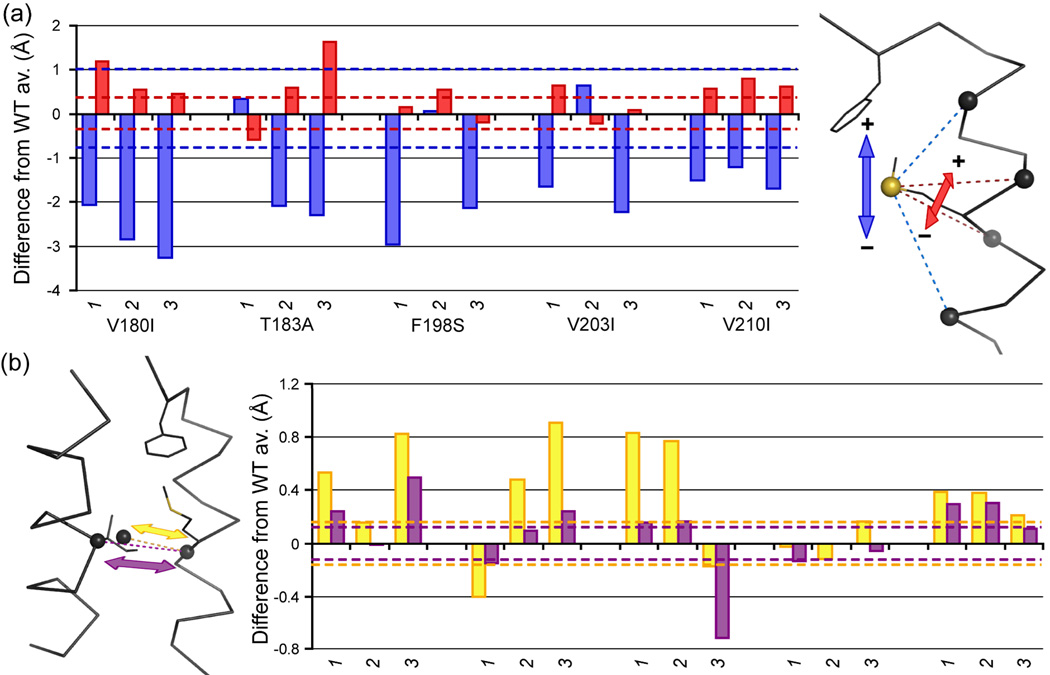
Figure 6.
Positioning of residues (a) M206 and (b) I184 compared to WT PrP simulations. (a) Vertical (blue) and horizontal (red) position of the M206 side chain Sδ. Vertical position is defined by the difference between d(SδM206—CαD202) and d(SδM206—CαV210), horizontal position as the difference between d(SδM206—CαM205) and d(SδM206—CαE207). (B) Shift of the side chain (center of mass, yellow) and Cα (purple) of I184 in relation to the center of mass of CαM206 and CαE207 (opposite from I184 on HC). Extreme values observed in the WT runs are shown as dashed lines in the histograms.
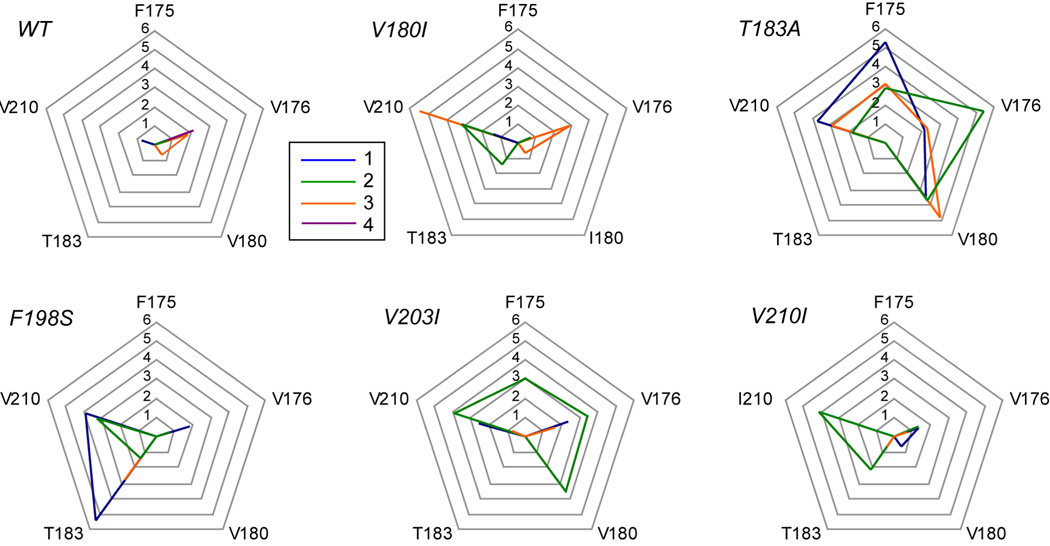
Apart from the changes in the hydrophobic core that caused changes in the S1-HA and HB-HC loops, subtle changes in the hydrophobic core were detected around the disulfide bond. Rotamer analysis of WT simulations indicated that the side chain positions around the disulfide bond (F175, V176, V180, T183 and V210) were stable; none or very few (max. 8, for V176) rotamer transitions occurred over 25–50 ns. This is in accord with hydrogen exchange studies that identified the residues around the disulfide bond as a ‘hyper-stable region’.31 In the mutant PrP simulations, rotamer changes increased significantly for many of these side chains; over 100 rotamer switches were not uncommon (Figure 7). This switching was particularly significant in the T183A simulations, but all mutants had at least one run with significantly more rotamer transitions than any of the WT runs.
Figure 7.
Rotamer transitions in side chains around the disulfide bond connecting HB and HC. For five residues that form hydrophobic contacts with the disulfide bond (see Figure 2), the natural logarithm of the number of rotamer transitions between 25–50 ns of simulation is plotted, for each individual run. In order to make a fair comparison for res. 180 and 210 that are either an isoleucine or a valine, the number of major rotamer transitions is used (involving changes in the Cα-Cβ dihedral).
V180I: changes in the hydrophobic core and misfolding
The V to I mutation introduces an extra CH3 group in the structure. The effect of this change on the residue itself was small; its side chain maintained a similar position for the majority of the time; however, the mutation had a large effect on the position of I184. During the first 15 ns of all three V180I simulations, the position of the I184 side chain fluctuated significantly, moving out and back into the hydrophobic core. In runs 2 and 3, I184 settled after ~25 ns, but in different locations; in run 3, the side chain was more exposed to solvent than in WT PrP, whereas for run 2 the solvent exposure was similar (average side chain SASA 54 and 34 Å2, respectively, compared with an average of 30 Å2 for WT PrP). As mentioned above, displacement of I184 is related to F198 losing contact with the hydrophobic core (through a change in positioning of M206). In all three runs, F198 was fully solvated after 0.7 ns. In contrast to the WT runs, a capping box interaction between T199 and D202 never formed.14,35 At the opposite side of the hydrophobic core, another change occurred in all 3 simulations: S2 moved away from the core (Figure 5B). In run 3, the shift of S2 (around 22 ns) was most significant and it was preceded by a conformational change in the core domain (Figure 4): The N-terminal end of HC (res. 200–205) straightened out and the angle between the remaining stable parts of HC (res. 206–219) and HB (res. 174–186) was reduced by ~7°. This new conformation proved stable; the backbone RMSF for the ‘core’ domain was low from 25–50 ns (Figure 4). Overall, introduction of I180 caused changes at the edges of the hydrophobic core, about 15 Å (F198) and 9 Å (S2) away from the mutation site.
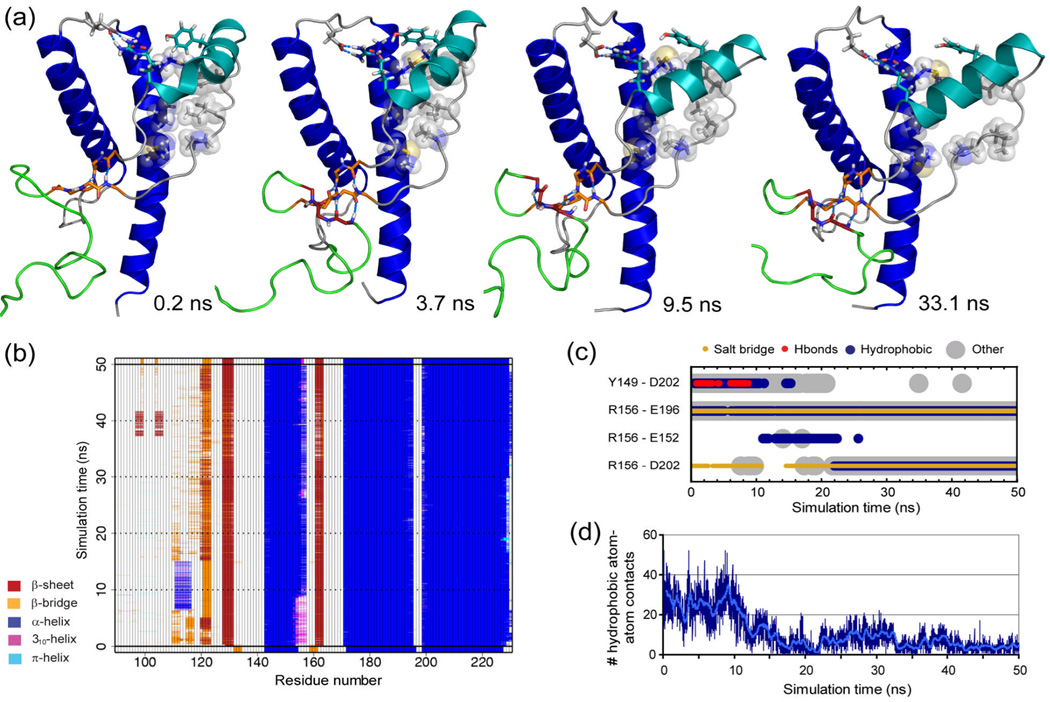
The most significant structural changes upon mutation of V180 to I were observed in run 1. This trajectory showed similar signs of early misfolding as observed for WT PrP at reduced pH:11,14 (1) an extra strand on the native sheet formed, (2) hydrophobic contacts between the S1-HA loop were lost, and (3) the N-terminal part of HA moved outward, exposing a large hydrophobic area to solvent (Figure 8). The extra strand appeared first, after only 0.3 ns of simulation. Hydrogen bonds were formed in this order: V121 O to L130 HN, G123 HN to Y128 O and V121 HN to L130 O. The first two hydrogen bonds were stable throughout the simulation, with the third occasionally breaking (e.g. between 4–16 ns, Figure 8B). Changes in the S1-HA loop started around 7 ns, with I139 moving away from V209. After 9 ns, the hydrophobic contacts between the S1-HA loop and HC decreased progressively, and at ~9.5 ns the N-terminal end of HA moved out into solvent. This change was accompanied by the permanent loss of the hydrogen bond between Y149 and D202, and res. 153–156 became fully α-helical. After 16 ns, the S1-HA loop had moved out to solvent. In contrast to the misfolding observed at reduced pH,14 the R156-E196 salt-bridge remained intact. It is possible, however, that this salt-bridge would break upon extension of the simulation.
Figure 8.
Early misfolding in V180I PrP. (a) Snapshots from run 1 at the indicated time points. Flexible N-terminus is shown from res. 105, in green; native β-sheet in orange, additional strand dark red, HA light blue and HB & HC blue. Relevant main chain and side chain atoms (in sticks) and hydrogen bond interactions (blue dashed lines) are shown. Side chains of res. P137, I139, F141 and M205, V209, M213, that make hydrophobic interactions in the starting structure, are also shown as transparent spheres. (b) Graphical representation of secondary structure analysis based on the DSSP algorithm13,72, with all residues participating in hydrogen bonding marked as part of the secondary structure element. (c) Selected residue-residue contacts between HA and the rest of the globular domain, over time. (d) Number of hydrophobic atom-atom contacts over time between res. 137,139,141 and 205,209,213.
T183A: increased flexibility and instability
Mutation of T183 to A affects the hydrophobic core and the hydrogen bond between T183 to the backbone of Y162, which is potentially important for the stability in the globular domain. In all three runs, hydrophobic contacts between res. 183 and P158 or V161 were reduced compared with WT PrP (74±27% and 89±5% of the time over 25–50 ns for P158 and V161, respectively). In combination with the abolished hydrogen bond, these contact changes had a significant effect on the position of the native sheet compared with the rest of the hydrophobic core (Figure 5). In run 1, contact between 183 and V210 was also reduced (69±2% reduction over 25–50 ns). In addition to these local effects, the flexibility and conformation of the globular domain were significantly affected in all three runs, including in the normally stable ‘core’ domain (Table1, Figure 4). The changes were different from most other mutant simulations; HA never moved out to solvent.
In run 3, a shift in conformation occurred in the core domain, similar to run 3 of V180I PrP. Furthermore, this simulation showed a significant reduction in helical stability for HB: over 25–50 ns, the population of i→i+4 backbone hydrogen bonds was reduced by 38% compared with WT. The change in helical stability was largest for the N-terminal part of HB (res. 172–182), because the first 2 turns bent out of the regular helix and into solvent. Run 3 and, to a lesser extent, run 2, showed an increase in exposure of hydrophobic surface area (Table 1). Apart from F198 moving out into solvent, this was largely due to exposure of V176, V180, I184 and I215.
F198S: increased flexibility and misfolding
Due to the positioning of F198 in 1QLX, the S198 side chain was directed down into the hydrophobic core. Its close proximity to T191 and D202 resulted in a hydrogen bonding network between these side chains in the 3rd run (permanently formed after 26.3 ns). The PrP conformation in this run stayed close to the WT PrP structure over the whole simulation, resulting in very similar characteristics (Table 1, Figure 3, Figure 4). An initial hydrogen bond between S198 and D202 was also formed in runs 1 and 2, but this interaction was lost after 2 and 13 ns, respectively. From this point onwards, the flexibility in the HB-HC loop was high, presumably due to the lack of interactions anchoring the HB-HC loop to the hydrophobic core. The increased flexibility extended to the upper parts of HB and HC, which showed large positional fluctuations compared with WT PrP (Figure 4). In the C-terminal part of HB (res. 182–194), the population of α-helical hydrogen bonding (i→i+4) decreased compared with WT (on average 12 and 10% lower than WT over 25–50 ns for run 1 and 2, respectively).
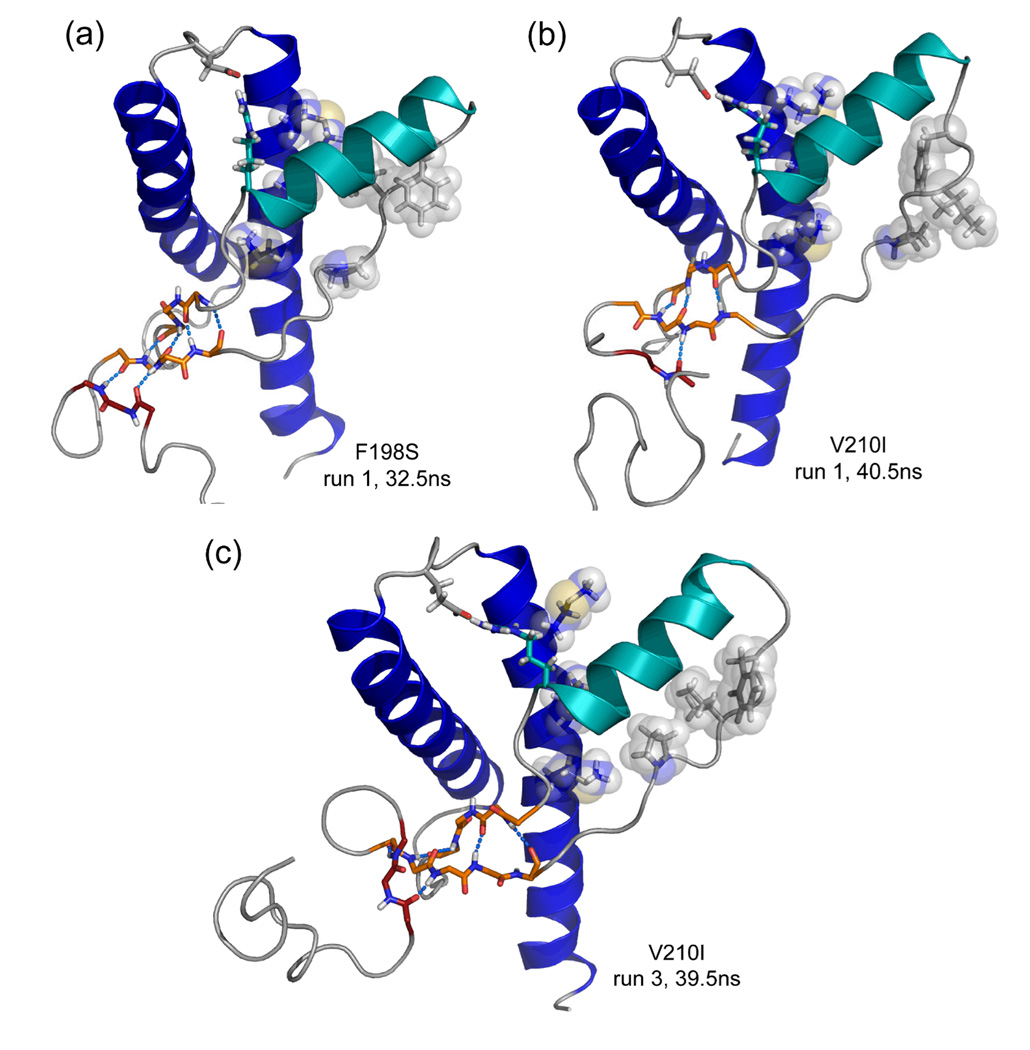
In run 2, D202 formed a stable capping box interaction with T199 after 14.2 ns, and S198 moved permanently out to solvent at 20 ns, triggering and increase the Cα RMSDs of the globular and core domains (Figures 3 and 4). The main change in the backbone of the core domain was a slight twist of the N-terminal end of HC (res. 200–210). In the globular domain, two significant changes occurred. First, the native sheet moved away from HB (~20.7 ns), resulting in the largest shift observed in all simulations performed (Figure 5B). This shows that the F198S mutation not only increases flexibility around the mutation site, but it can also cause effects ~20 Å away. Second, the S1-HA loop lost the majority of its hydrophobic contacts with HC (~30 ns), allowing the N-terminal part of HA to move outward and back again during the remainder of the simulation. In addition, a β-bridge formed in res. 120–122, as an additional strand to the native sheet. Combined with outward movement of HA and exposure of hydrophobic surface on the S1-HA loop and HC, a conformation similar to the early misfolding of V180I PrP formed between 32–37 ns (Figure 9A).
Figure 9.
Signs of early misfolding. Coloring as in Figure 8. Relevant main chain and side chain atoms (in sticks) and hydrogen bond interactions (blue dashed lines) are shown. Side chains of res. P137, I139, F141 and M205, V209, M213 are also shown as transparent spheres. (a) Run 1 of F198S PrP, 32.5 ns. (b) Run 1 of V210I PrP, 40.5 ns. (c) Run 3 of V210I PrP, 39.5 ns.
V203I: a variety of subtle effects
The starting structure of V203I was very similar to WT, as the extra CH3 group in V203I was directed into solvent. The three separate runs showed a variety of effects. The main commonality between the runs was that F198 moved out into solvent within the first 0.7 ns. In run 2, however, F198 returned to the hydrophobic core after 27 ns and the interaction between F198 and M206 was restored (Figure 7). Soon thereafter (~28.8 ns), the capping box at the N-terminal end of HC was formed. From that point onwards, the overall conformation of the globular domain was similar to WT PrP for this run (Figure 3). In the other runs, the N-terminal end of HA was significantly displaced: outward and downward in run 1 and downward only in run 3. As in other simulations that showed outward movement of HA, hydrophobic contacts between the S1-HA loop and HC in run 1 decreased significantly. Run 3 showed some signs of backbone instability (particularly at the N-terminal end of HB, approx. 20 Å from the mutation site) and increased flexibility in the hydrophobic core around the disulfide bond (Figure 7).
V210I: steric crowding may cause misfolding
Introduction of the Ile side chain caused steric crowding in a crucial part of the hydrophobic core – the interface between two hydrophobic networks (Figure 2). In all simulations, I210 formed direct hydrophobic contacts with V209. This interaction affected both the I184 and the V209 side chains, which were pushed out of their preferred positions. The former had a similar effect as observed for V180I: M206 moved ino the gap left by I184, thereby losing contact with F198. The latter had a significant effect on the hydrophobic interactions between HC and the S1-HA loop; the number of interactions declined rapidly in all three simulations. Although res. 210 is also directly in contact with V161, the effect on the positioning of S2 with respect to the rest of the molecule was negligible. Despite the changes in the S1-HA loop, HA and the HB-HC loop, the core domain was stable and similar to WT PrP (Table 1).
In runs 1 and 3, most hydrophobic contacts between S1-HA and HC were lost (Table 1), causing a similar exposure of hydrophobic residues to that of V180I run 1. In addition, these V210I simulations showed the beginnings of an extra strand forming on the native sheet (Figure 9). Although in run 2 hydrophobic contacts were lost only between I139 and HC, a stable β-bridge between res. 117–119 and S1 appeared after 0.5 ns and remained present throughout. Overall, the introduction of the V210I mutation caused similar conformational changes as described for V180I (Figure 8) and in WT at low pH.11,14
Discussion
Starting structures and conformations sampled
When simulating mutant proteins based on a structure of the WT protein, one can never be certain that the relevant conformational ensemble for the mutant protein is sampled in the MD simulations. However, the same can be true of WT simulations, due to experimental uncertainty (e.g. lack of NOE restraints to determine side chain positions) or differential structural context (e.g. dimerization and other inter-molecule contacts in protein crystals). For WT PrP we have shown, by extensive comparison with experiment, that our simulations sample relevant conformational ensembles14 Interestingly, the NMR solution structure we used as the starting point for our simulations33 does not contain the capping box interaction between T199 and D202 on top of HC, whereas this interaction is always formed in simulation. In crystal structures of human PrP this interaction also exists,36,37 confirming that the simulations converged to an experimentally observed local conformation.
We introduce mutations by selecting the rotamer that best fits the WT starting structure based on interactions with its surroundings. This starting structure may not represent the highest populated conformer of the mutant protein, but during simulation the protein structure may adopt the correct conformation. We have therefore focused our analysis on the second half of our MD trajectories, 25–50 ns. Our simulations, performed at room temperature and neutral pH, do not show large changes in secondary structure, such as the unfolding of helices. Such changes are not expected: far- and near UV measurements of mouse mutant recPrPs V180I, T183A, F198S and V210I are essentially identical to wild-type.15 For V180I, T183A, V203I and V210I PrP, no high-resolution structural information from experiment is available. Recently (after we performed our simulations), two crystal structures for F198S (either with M or V at position 129) were reported.37 These structures are dimers, however, and the dimer interface includes the mutation site. To allow for dimerization, HB and the HB-HC loop need to shift.37 The structure is therefore not representative of monomeric F198S PrP in aqueous solution. Our simulations indicate that the flexibility of the HB-HC loop, as well as the adjoining parts of HB and HC, are significantly increased compared with WT, which will allow for the conformational shift observed in the dimeric crystal structures and are in line with the flexibility inferred from the X-ray diffraction data (no distinct density for res. 193–197 and high B-factors for residues around this region). The crystal structures and our simulations further indicate that side chain rearrangements occur due to the absence of the F198 side chain, such as a downwards shift of M206. In the crystal structures, S198 is directed either towards the hydrophobic core or away from it, and we observe both positions in our simulations. These observations confirm that the conformations sampled in simulation are consistent with experiment.
Protein instability and aggregation
Urea-induced unfolding of mouse recPrP (res. 121–231) indicate that the T183A and F198S mutations significantly decrease thermodynamic stability, whereas a small effect and no effect were observed for V180I and V210I, respectively.15 Our simulations agree with these results, and show that the stability of the core domain of PrP (the stable parts of HB and HC) is a key determinant of thermodynamic stability. In addition, the displacement and instability of the native sheet correlates with instability of the core domain and is primarily seen in T183A and F198S, and to a lesser extent in V180I. β-sheet displacement is an early event upon addition of urea,38 and simulations indicate that the sheet could be a “weak spot”.39 The sheet displacement and core instability in T183A and F198S observed in our simulations therefore explain the instability relative to WT upon addition of denaturant. The observed instability of T183A and F198S PrP is likely also present in vivo, because interference with normal cellular processing, such as GPI-anchor attachment, occurs in cell models.25,40
Instability of the PrP globular domain can lead to aggregation. Upon production of mutant PrP in E. coli, Liemann and Glockshuber found that all mutations that significantly decreased thermodynamic stability, including T183A and F198S, caused the prion protein to be present in inclusion bodies.15 For F198S recPrP, PrPSc-like oligomers formed without denaturant and faster than WT recPrP treated with denaturant.41 The oligomers show a loss of α-helical structure and a gain of β-sheet. The increased flexibility in the HB-HC loop and the adjoining parts of the HB and HC helices in our simulations indicate a likely starting point for this conversion. Our explicit all-atom simulations further show how the instability of T183A PrP is related to the loss of the hydrogen bond to the native sheet, as well as instability of the hydrophobic core.
Previously, we identified likely early events of prion protein misfolding as triggered by a decrease in pH:11,14,42 outwards movement of HA accompanied by a significant increase in exposed hydrophobic surface and the addition of an extra strand onto the native β-sheet. Here we show that V180I, F198S and V210I can also cause these effects at neutral pH. The key conformational change in HA and hydrophobic exposure is particularly prevalent in V210I and is also observed in one V203I PrP simulation. These events may be important for aggregation; a model for early aggregates (or protofibrils) based on such structures was proposed and found to be in agreement with the available experimental data.10,12,13 Structures from the V180I simulation in which these early misfolding events occur also fit this model. Interestingly, the F198S, V180I and V210I mutations increase the population of a folding intermediate that may be relevant for misfolding, and a decrease in pH had the same effect.16,24 Little experimental information is available for V203I, but aggreasome formation of V203I PrP upon prolonged inhibition of the proteasome points to a subtle increase in aggregation propensity compared with WT.29 Our simulations indicate that misfolding of V203I PrP is less likely than misfolding of, for example, V210I PrP, but a pathway similar to pH-induced misfolding may be followed. In contrast, we show that T183A causes instability and conformational changes, but the effects are not similar to those observed for WT PrP at low pH. In this light, it is important to note that the urea-induced (un)folding pathway15 and aggregate formation43 of T183A PrP appear to be different from WT PrP.
Relation to familial prion diseases
We have described how our simulations provide insight into effects at the molecular level that can be used to understand observations of in vitro. Ultimately, the question is how prion disease emerges in individuals that carry these predisposing mutations. Based on our simulations and previous experimental findings, we can hypothesize that the mutations in the hydrophobic core can cause disease in two main ways. First, mutations can destabilize PrPC. In addition to promoting conformational rearrangements, destabilization can affect cellular processing, such as GPI-anchor attachment, as well as aggregation. This may be the case for T183A and F198S PrP. Second, mutations do not affect thermodynamic stability or cellular processing of PrP, but they promote its misfolding while attached to a membrane, either on the cell surface or in endosomal compartments. The prime example for this mechanism would be V210I. The finding that brain extracts from patients with V210I CJD contain PrPSc aggregates with both WT and V210I PrP, indicates that misfolding and aggregation are likely to be similar.44 Interestingly, disease progression and symptoms can also be distinguished between these two different groups. The T183A and F198S mutations are linked to slow disease progression compared with sporadic CJD (average durations are 49 and 75 months for T183A and F198S, respectively, and 3–6 months for sporadic CJD) and an unusually high occurrence of extrapyramidal symptoms.2 In contrast, disease duration and symptoms in patients carrying the V210I mutation are very similar to typical cases of sporadic CJD.22,45–47 The rarely found V203I mutation caused relatively subtle effects on recPrP dynamics and conformation in this study, mostly similar to those found with V210I. The mutation likely has low penetrance,20 but it may promote prion disease in a similar fashion, causing typical CJD symptoms.20,48,49
There appears to be a difference between causing instability of the globular fold (T183A, F198S) and promoting typical disease-related misfolding (V210I, V203I). In this light, it is interesting to note that an inverse correlation between conversion efficiency in a seeded assay and thermal denaturation was recently found for engineered mutations in mouse PrP.50 It is not clear which category V180I PrP belongs to. Thermodynamic stability of recPrP is somewhat affected15 but cell-surface GPI-anchored PrP is generated efficiently,51 suggesting that misfolding happens whilst attached to a membrane. Our simulations indicate that misfolding may happen in a similar fashion as WT PrP, but conformational changes unlike those observed in WT PrP were found as well. In contrast to sporadic or V210I CJD, deposits of V180I PrPSc are never diglycosylated52 and V180I CJD cannot be transmitted to mice expressing WT PrP.53 Clinical and pathological symptoms as well as the average duration of disease (>16 months) also differ from sporadic CJD.54,55 It appears that although WT-like misfolding may occur, the aggregates differ in conformation, leading to a symptomatically abnormal, non-transmissible, form of CJD.
Conclusions
Using extensive MD simulations, we have investigated the influence of 5 disease-related mutations (V180I, T183A, F198S, V203I and V210I) on stability, flexibility and conformation of the prion protein. These mutations all affect the hydrophobic core of PrP, increase structural flexibility and, in some cases, the propensity to misfold. The effects on the conformation of the protein can occur far from the mutation site (up to ~20 Å) and are mediated through changes in interactions in the hydrophobic core. Such long-range effects are not uncommon for single residue mutations56–58 and emphasize the importance of MD simulations to assess the effect of disease-related mutations on protein structure, dynamics and stability.59 The T183A and F198S mutations cause an overall decrease in the stability of the protein fold, in agreement with experiment. This decrease is accompanied by a shift of the native sheet away from the rest of the globular domain. V210I and V203I do not significantly affect the overall stability the protein; instead, specific conformational changes are observed that are similar to the effect of lowering pH, and possibly related to typical PrP misfolding. V180I falls between these two extremes and in one of the simulations, early misfolding occurred. Overall, we show that realistic atomistic simulations can provide insight into the effects of disease-related mutations on prion protein dynamics and stability, which in turn adds to our understanding of how these mutations may cause disease.
Methods
Starting structures
The starting structure for all simulations was based on the wild-type human prion protein representative NMR-structure obtained at pH 4.5 (PDB code 1QLX33). Residues (res.) 90–127, which form the disordered N-terminus important for conversion of PrPC to PrPSc,34,60 and res. 229–230 were added to the structure, as described previously.14 The WT PrP structure (res. 90–230) was then subjected to a procedure to introduce mutations, making use of a rotamer library built from the protein native state simulations in the Dynameomics database.59 First, the side chain of a target residue is replaced by all defined rotamers. Then, 100 steps of steepest descents minimization of all side chains in the resulting set of structures was performed (using in lucem molecular mechanics, ilmm61). The structure with the most favorable interaction energy between the side chain of the mutated residue and its surroundings was selected and confirmed by visual inspection.
All simulations were performed at near-physiological or ‘neutral’ pH, as described previously.14 In reported disease cases of V180I, T183A, V203I and V210I, the allele carrying the mutation coded for M129 of the M/V polymorphic site.23,49,54 In contrast, F198S has been reported to occur with V129.62 This polymorphic site is implicated in disease susceptibility63 and phenotypical differences.64–67 However, studies with F198S recPrP are usually performed with M129 in the protein construct.15,16,24,41,68 We therefore used M129 in all our starting structures, as found in the NMR structure 1QLX.33
Molecular dynamics simulations
The MD simulations were performed using ilmm,61 employing the Levitt et al. force field69 and the flexible F3C70 water model, as described previously.14 Three 50 ns MD simulations were performed for all five mutants, using different initial velocities for each. The simulations were compared with 4 published simulations of WT PrP simulated at the same pH range for 50 ns each:14 In the previous study 5 simulations of WT PrP were performed, but one simulation was identified as a significant outlier (run 1). This simulation was therefore not considered in the current study.
Analysis of MD trajectories
A range of analysis methods were applied, most of which have been described before.14 In short, root-mean square deviations (RMSD) of Cα atoms were calculated for the globular domain (res. 128–228) and a core domain consisting of parts of helices HB and HC connected by the disulfide bond (res. 174–186 and 200–219). All backbone and side chain dihedral angles were measured and used to assign side chain rotamers (Scouras and Daggett, unpublished work). Solvent accessible surface areas (SASAs) were calculated using the method of Lee and Richards71 and secondary structure assignments were performed using the DSSP (Define Secondary Structure of Proteins) algorithm72 with additional definitions.13 Non-hydrogen atoms were considered to be in contact when ≤ 5.4 Å apart (for pairs of carbon atoms) or ≤ 4.6 Å apart (for all other pairs). Two interactions were defined specifically: salt bridge – Glu/Asp oxygen(s) in contact with Arg/Lys nitrogens and hydrophobic – contacts between CHn / S groups. Hydrogen bonds were defined by a H-acceptor distance ≤ 2.6 Å and a donor-H-acceptor angle > 135°.
Acknowledgements
We are grateful for support provided by the National Institutes of Health (GM 81407) and for computer resources provided by the NIH through the Multi-Tiered Proteomic Compute Cluster (NCRR 1S10RR023044-01). Molecular images were created using PyMOL.73
Abbreviations used
- PrP
prion protein
- recPrP
recombinant prion protein
- CJD
Creutzfeldt-Jakob disease
- GSS
Gerstmann-Sträussler-Scheinker
- FFI
fatal familial insomnia
- GPI
glycosylphosphatidyl-inositol
- MD
molecular dynamics simulation
- WT
wild-type
- ns
nanoseconds
- ps
picoseconds
- res
residues
- PDB
Protein Data Bank
- Cα RMSD
root-mean-square deviation of Cα atom coordinates from the starting structure
- Cα RMSF
root-mean-square deviation of Cα atom coordinates about the mean structure
- SASA
solvent accessible surface area
References
- 1.Van der Kamp MW, Daggett V. The consequences of pathogenic mutations to the human prion protein. Protein Eng. Des. Sel. 2009;22:461–468. doi: 10.1093/protein/gzp039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kovacs GG, Trabattoni G, Hainfellner JA, Ironside JW, Knight RSG, Budka H. Mutations of the prion protein gene: Phenotypic spectrum. J Neurol. 2002;249:1567–1582. doi: 10.1007/s00415-002-0896-9. [DOI] [PubMed] [Google Scholar]
- 3.Kovacs GG, Budka H. Molecular pathology of human prion diseases. Int. J. Mol. Sci. 2009;10:976–999. doi: 10.3390/ijms10030976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Linden R, Martins VR, Prado MAM, Cammarota M, Izquierdo I, Brentani RR. Physiology of the prion protein. Physiol. Rev. 2008;88:673–728. doi: 10.1152/physrev.00007.2007. [DOI] [PubMed] [Google Scholar]
- 5.Le Pichon CE, Firestein S. Expression and localization of the prion protein PrPc in the olfactory system of the mouse. J. Comp. Neurol. 2008;508:487–499. doi: 10.1002/cne.21698. [DOI] [PubMed] [Google Scholar]
- 6.Millhauser GL. Copper and the prion protein: Methods, structures, function, and disease. Annu. Rev. Phys. Chem. 2007;58:299–320. doi: 10.1146/annurev.physchem.58.032806.104657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Viles JH, Klewpatinond M, Nadal RC. Copper and the structural biology of the prion protein. Biochem. Soc. Trans. 2008;36:1288–1292. doi: 10.1042/BST0361288. [DOI] [PubMed] [Google Scholar]
- 8.Singh A, Mohan ML, Isaac AO, Luo X, Petrak J, Vyoral D, Singh N. Prion protein modulates cellular iron uptake: a novel function with implications for prion disease pathogenesis. PLoS ONE. 2009;4:e4468. doi: 10.1371/journal.pone.0004468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alonso DOV, DeArmond SJ, Cohen FE, Daggett V. Mapping the early steps in the pH-induced conformational conversion of the prion protein. Proc. Natl. Acad. Sci. USA. 2001;98:2985–2989. doi: 10.1073/pnas.061555898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeMarco ML, Daggett V. From conversion to aggregation: Protofibril formation of the prion protein. Proc. Natl. Acad. Sci. USA. 2004;101:2293–2298. doi: 10.1073/pnas.0307178101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeMarco ML, Daggett V. Molecular mechanism for low pH triggered misfolding of the human prion protein. Biochemistry. 2007;46:3045–3054. doi: 10.1021/bi0619066. [DOI] [PubMed] [Google Scholar]
- 12.DeMarco ML, Silveira J, Caughey B, Daggett V. Structural properties of prion protein protofibrils and fibrils: An experimental assessment of atomic models. Biochemistry. 2006;45:15573–15582. doi: 10.1021/bi0612723. [DOI] [PubMed] [Google Scholar]
- 13.Scouras AD, Daggett V. Species variation in PrPSc protofibril models. J. Mater. Sci. 2008;43:3625–3637. [Google Scholar]
- 14.Van der Kamp MW, Daggett V. The influence of pH on the human prion protein: Insights into the early steps of misfolding. Biophys. J. 2010;99 doi: 10.1016/j.bpj.2010.07.063. in press (scheduled for October issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liemann S, Glockshuber R. Influence of amino acid substitutions related to inherited human prion diseases on the thermodynamic stability of the cellular prion protein. Biochemistry. 1999;38:3258–3267. doi: 10.1021/bi982714g. [DOI] [PubMed] [Google Scholar]
- 16.Apetri AC, Surewicz K, Surewicz WK. The effect of disease-associated mutations on the folding pathway of human prion protein. J. Biol. Chem. 2004;279:18008–18014. doi: 10.1074/jbc.M313581200. [DOI] [PubMed] [Google Scholar]
- 17.Riek R, Wider G, Billeter M, Hornemann S, Glockshuber R, Wüthrich K. Prion protein NMR structure and familial human spongiform encephalopathies. Proc. Natl. Acad. Sci. USA. 1998;95:11667–11672. doi: 10.1073/pnas.95.20.11667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kitamoto T, Ohta M, Dohura K, Hitoshi S, Terao Y, Tateishi J. Novel missense variants of prion protein in Creutzfeldt-Jakob disease or Gerstmann-Straüssler syndrome. Biochem. Biophys. Res. Commun. 1993;191:709–714. doi: 10.1006/bbrc.1993.1275. [DOI] [PubMed] [Google Scholar]
- 19.Hsiao K, Dlouhy SR, Farlow MR, Cass C, Dacosta M, Conneally PM, Hodes ME, Ghetti B, Prusiner SB. Mutant prion proteins in Gerstmann-Straüssler-Scheinker disease with neurofibrillary tangles. Nat. Genet. 1992;1:68–71. doi: 10.1038/ng0492-68. [DOI] [PubMed] [Google Scholar]
- 20.Peoc'h K, Manivet P, Beaudry P, Attane F, Besson G, Hannequin D, Delasnerie-Lauprêtre N, Laplanche J-L. Identification of three novel mutations (E196K, V203I, E211Q) in the prion protein gene PRNP in inherited prion diseases with Creutzfeldt-Jakob disease phenotype. Hum. Mutat. 2000;15:482. doi: 10.1002/(SICI)1098-1004(200005)15:5<482::AID-HUMU16>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 21.Pocchiari M, Salvatore M, Cutruzzola F, Genuardi M, Allocatelli CT, Masullo C, Macchi G, Alema G, Galgani S, Xi YG, Petraroli R, Silvestrini MC, Brunori M. A new point mutation of the prion protein gene in Creutzfeldt-Jakob-disease. Ann. Neurol. 1993;34:802–807. doi: 10.1002/ana.410340608. [DOI] [PubMed] [Google Scholar]
- 22.Ripoll L, Laplanche JL, Salzmann M, Jouvet A, Planques B, Dussaucy M, Chatelain J, Beaudry P, Launay JM. A new point mutation in the prion protein gene at codon 210 in Creutzfeldt-Jakob disease. Neurology. 1993;43:1934–1938. doi: 10.1212/wnl.43.10.1934. [DOI] [PubMed] [Google Scholar]
- 23.Nitrini R, Rosemberg S, PassosBueno MR, daSilva LST, Iughetti P, Papadopoulos M, Carrilho PM, Caramelli P, Albrecht S, Zatz M, LeBlanc A. Familial spongiform encephalopathy associated with a novel prion protein gene mutation. Ann. Neurol. 1997;42:138–146. doi: 10.1002/ana.410420203. [DOI] [PubMed] [Google Scholar]
- 24.Apetri AC, Maki K, Roder H, Surewicz WK. Early intermediate in human prion protein folding as evidenced by ultrarapid mixing experiments. J. Am. Chem. Soc. 2006;128:11673–11678. doi: 10.1021/ja063880b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kiachopoulos S, Bracher A, Winklhofer KF, Tatzelt J. Pathogenic mutations located in the hydrophobic core of the prion protein interfere with folding and attachment of the glycosylphosphatidylinositol anchor. J. Biol. Chem. 2005;280:9320–9329. doi: 10.1074/jbc.M412525200. [DOI] [PubMed] [Google Scholar]
- 26.Zaidi SIA, Richardson SL, Capellari S, Song L, Smith MA, Ghetti B, Sy MS, Gambetti P, Petersen RB. Characterization of the F198S prion protein mutation: Enhanced glycosylation and defective refolding. J. Alzheimer's Dis. 2005;7:159–171. doi: 10.3233/jad-2005-7209. [DOI] [PubMed] [Google Scholar]
- 27.Grasbon-Frödl E, Lorenz H, Mann U, Nitsch RM, Windl O, Kretzschmar HA. Loss of glycosylation associated with the T183A mutation in human prion disease. Acta Neuropathol. (Berl) 2004;108:476–484. doi: 10.1007/s00401-004-0913-4. [DOI] [PubMed] [Google Scholar]
- 28.Rogers M, Taraboulos A, Scott M, Groth D, Prusiner SB. Intracellular accumulation of the cellular prion protein after mutagenesis of its Asn-linked glycosylation sites. Glycobiology. 1990;1:101–109. doi: 10.1093/glycob/1.1.101. [DOI] [PubMed] [Google Scholar]
- 29.Mishra RS, Bose S, Gu Y, Li R, Singh N. Aggresome formation by mutant prion proteins: The unfolding role of proteasomes in familial prion disorders. J. Alzheimer's Dis. 2003;5:15–23. doi: 10.3233/jad-2003-5103. [DOI] [PubMed] [Google Scholar]
- 30.Calzolai L, Zahn R. Influence of pH on NMR structure and stability of the human prion protein globular domain. J. Biol. Chem. 2003;278:35592–35596. doi: 10.1074/jbc.M303005200. [DOI] [PubMed] [Google Scholar]
- 31.Hosszu LLP, Baxter NJ, Jackson GS, Power A, Clarke AR, Waltho JP, Craven CJ, Collinge J. Structural mobility of the human prion protein probed by backbone hydrogen exchange. Nat. Struct. Biol. 1999;6:740–743. doi: 10.1038/11507. [DOI] [PubMed] [Google Scholar]
- 32.Viles JH, Donne D, Kroon G, Prusiner SB, Cohen FE, Dyson HJ, Wright PE. Local structural plasticity of the prion protein. Analysis of NMR relaxation dynamics. Biochemistry. 2001;40:2743–2753. doi: 10.1021/bi002898a. [DOI] [PubMed] [Google Scholar]
- 33.Zahn R, Liu AZ, Luhrs T, Riek R, von Schroetter C, Garcia FL, Billeter M, Calzolai L, Wider G, Wüthrich K. NMR solution structure of the human prion protein. Proc. Natl. Acad. Sci. USA. 2000;97:145–150. doi: 10.1073/pnas.97.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Swietnicki W, Petersen R, Gambetti P, Surewicz WK. pH-dependent stability and conformation of the recombinant human prion protein PrP(90–231) J. Biol. Chem. 1997;272:27517–27520. doi: 10.1074/jbc.272.44.27517. [DOI] [PubMed] [Google Scholar]
- 35.Harper ET, Rose GD. Helix stop signals in proteins and peptides: The capping box. Biochemistry. 1993;32:7605–7609. doi: 10.1021/bi00081a001. [DOI] [PubMed] [Google Scholar]
- 36.Antonyuk SV, Trevitt CR, Strange RW, Jackson GS, Sangar D, Batchelor M, Cooper S, Fraser C, Jones S, Georgiou T, Khalili-Shirazi A, Clarke AR, Hasnain SS, Collinge J. Crystal structure of human prion protein bound to a therapeutic antibody. Proc. Natl. Acad. Sci. USA. 2009;106:2554–2558. doi: 10.1073/pnas.0809170106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee S, Antony L, Hartmann R, Knaus KJ, Surewicz K, Surewicz WK, Yee VC. Conformational diversity in prion protein variants influences intermolecular beta-sheet formation. EMBO J. 2010;29:251–262. doi: 10.1038/emboj.2009.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Julien O, Chatterjee S, Thiessen A, Graether SP, Sykes BD. Differential stability of the bovine prion protein upon urea unfolding. Protein Sci. 2009;18:2172–2182. doi: 10.1002/pro.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blinov N, Berjanskii M, Wishart DS, Stepanova M. Structural domains and main-chain flexibility in prion proteins. Biochemistry. 2009;48:1488–1497. doi: 10.1021/bi802043h. [DOI] [PubMed] [Google Scholar]
- 40.Lehmann S, Harris DA. Blockade of glycosylation promotes acquisition of scrapie-like properties by the prion protein in cultured cells. J. Biol. Chem. 1997;272:21479–21487. doi: 10.1074/jbc.272.34.21479. [DOI] [PubMed] [Google Scholar]
- 41.Vanik DL, Surewicz WK. Disease-associated F198S mutation increases the propensity of the recombinant prion protein for conformational conversion to scrapie-like form. J. Biol. Chem. 2002;277:49065–49070. doi: 10.1074/jbc.M207511200. [DOI] [PubMed] [Google Scholar]
- 42.Alonso DOV, An C, Daggett V. Simulations of biomolecules: Characterization of the early steps in the pH-induced conformational conversion of the hamster, bovine and human forms of the prion protein. Philos. Trans. R. Soc. Lond. A. Math. Phys. Eng. Sci. 2002;360:1165–1178. doi: 10.1098/rsta.2002.0986. [DOI] [PubMed] [Google Scholar]
- 43.Yuan J, Dong Z, Guo JP, McGeehan J, Xiao X, Wang J, Cali I, McGeer PL, Cashman NR, Bessen R, Surewicz WK, Kneale G, Petersen RB, Gambetti P, Zou WQ. Accessibility of a critical prion protein region involved in strain recognition and its implications for the early detection of prions. Cell. Mol. Life Sci. 2008;65:631–643. doi: 10.1007/s00018-007-7478-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Silvestrini MC, Cardone F, Maras B, Pucci P, Barra D, Brunori M, Pocchiari M. Identification of the prion protein allotypes which accumulate in the brain of sporadic and familial Creutzfeldt-Jakob disease patients. Nat. Med. 1997;3:521–525. doi: 10.1038/nm0597-521. [DOI] [PubMed] [Google Scholar]
- 45.Furukawa H, Kitamoto T, Hashiguchi H, Tateishi J. A Japanese case of Creutzfeldt-Jakob disease with a point mutation in the prion protein gene at codon 210. J. Neurol. Sci. 1996;141:120–122. doi: 10.1016/0022-510x(96)00157-8. [DOI] [PubMed] [Google Scholar]
- 46.Huang N, Marie SKN, Kok F, Nitrini R. Familial Creutzfeldt-Jakob disease associated with a point mutation at codon 210 of the prion protein gene. Arq. Neuropsiquiatr. 2001;59:932–935. doi: 10.1590/s0004-282x2001000600017. [DOI] [PubMed] [Google Scholar]
- 47.Mouillet-Richard S, Teil C, Lenne M, Hugon S, Taleb O, Laplanche JL. Mutation at codon 210 (V210I) of the prion protein gene in a North African patient with Creutzfeldt-Jakob disease. J. Neurol. Sci. 1999;168:141–144. doi: 10.1016/s0022-510x(99)00179-3. [DOI] [PubMed] [Google Scholar]
- 48.Byung-Hoon J, Yong-Chul J, Yun-Jung L, Han-Jeong C, Seok-Ju P, Dan-Il C, Juhan K, Seung Hyun K, Hee-Tae K, Eun-Kyoung C, Kyung-Chan C, Richard IC, Yong-Sun K. Creutzfeldt-Jakob disease with the V203I mutation and M129V polymorphism of the prion protein gene PRNP and a 17 kDa prion protein fragment. Neuropathol. Appl. Neurobiol. 2010 doi: 10.1111/j.1365-2990.2010.01094.x. doi: 10.1111/j.1365–2990.2010.01094.x. [DOI] [PubMed] [Google Scholar]
- 49.Ladogana A, Puopolo M, Poleggi A, Almonti S, Mellina V, Equestre M, Pocchiari M. High incidence of genetic human transmissible spongiform encephalopathies in Italy. Neurology. 2005;64:1592–1597. doi: 10.1212/01.WNL.0000160118.26865.11. [DOI] [PubMed] [Google Scholar]
- 50.Kirby L, Agarwal S, Graham JF, Goldmann W, Gill AC. Inverse correlation of thermal lability and conversion efficiency for five prion protein polymorphic variants. Biochemistry. 2010;49:1448–1459. doi: 10.1021/bi901855z. [DOI] [PubMed] [Google Scholar]
- 51.Jodoin J, Laroche-Pierre S, Goodyer CG, LeBlanc AC. Defective retrotranslocation causes loss of anti-bax function in human familial prion protein mutants. J. Neurosci. 2007;27:5081–5091. doi: 10.1523/JNEUROSCI.0957-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chasseigneaux S, Haik S, Laffont-Proust I, De Marco O, Lenne M, Brandel JP, Hauw JJ, Laplanche JL, Peoc'h K. V180I mutation of the prion protein gene associated with atypical PrPSc glycosylation. Neurosci. Lett. 2006;408:165–169. doi: 10.1016/j.neulet.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 53.Tateishi J, Kitamoto T, Hoque MZ, Furukawa H. Experimental transmission of Creutzfeldt-Jakob disease and related diseases to rodents. Neurology. 1996;46:532–537. doi: 10.1212/wnl.46.2.532. [DOI] [PubMed] [Google Scholar]
- 54.Jin K, Shiga Y, Shibuya S, Chida K, Sato Y, Konno H, Doh-ura K, Kitamoto T, Itoyama Y. Clinical features of Creutzfeldt-Jakob disease with V180I mutation. Neurology. 2004;62:502–505. doi: 10.1212/01.wnl.0000106954.54011.80. [DOI] [PubMed] [Google Scholar]
- 55.Mutsukura K, Satoh K, Shirabe S, Tomita I, Fukutome T, Morikawa M, Iseki M, Sasaki K, Shiaga Y, Kitamoto T, Eguchi K. Familial Creutzfeldt-Jakob disease with a V180I mutation: Comparative analysis with pathological findings and diffusion-weighted images. Dement. Geriatr. Cogn. Disord. 2009;28:550–557. doi: 10.1159/000254842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Anderson PC, Daggett V. The R46Q, R131Q and R154H polymorphs of human DNA glycosylase/beta-Lyase hOgg1 severely distort the active site and DNA recognition site but do not cause unfolding. J. Am. Chem. Soc. 2009;131:9506–9515. doi: 10.1021/ja809726e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rutherford K, Daggett V. A hotspot of inactivation: The A22S and V108M polymorphisms individually destabilize the active site structure of catechol O-methyltransferase. Biochemistry. 2009;48:6450–6460. doi: 10.1021/bi900174v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schmidlin T, Kennedy BK, Daggett V. Structural changes to monomeric CuZn superoxide dismutase caused by the familial Amyotrophic Lateral Sclerosis-associated mutation A4V. Biophys. J. 2009;97:1709–1718. doi: 10.1016/j.bpj.2009.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Van der Kamp MW, Schaeffer RD, Jonsson AL, Scouras AD, Simms AM, Toofanny RD, Benson NC, Anderson PC, Merkley ED, Rysavy S, Bromley D, Beck DAC, Daggett V. Dynameomics: A comprehensive database of protein dynamics. Structure. 2010;18:423–435. doi: 10.1016/j.str.2010.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lührs TT, Zahn R, Wüthrich K. Amyloid formation by recombinant full-length prion proteins in phospholipid bicelle solutions. J. Mol. Biol. 2006;357:833–841. doi: 10.1016/j.jmb.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 61.Beck DAC, Alonso DOV, Daggett V. in lucem molecular mechanics. Seattle, WA: University of Washington; 2000–2010. [Google Scholar]
- 62.Ghetti B, Miravalle L, Yamaguchi K, Epperson F, Murrell JR, Perkins T, Hui S, Farlow MR, Piccardo P, Dlouhy SR. Role of the polymorphism at codon 129 of the Prion protein gene in the phenotypic expression of Gerstmann-Straussler-Scheinker disease associated with the F198s mutation. J. Neuropathol. Exp. Neurol. 2004;63:72. [Google Scholar]
- 63.Alperovitch A, Zerr I, Pocchiari M, Mitrova E, Cuesta JD, Hegyi I, Collins S, Kretzschmar H, van Duijn C, Will RG. Codon 129 prion protein genotype and sporadic Creutzfeldt-Jakob disease. Lancet. 1999;353:1673–1674. doi: 10.1016/s0140-6736(99)01342-2. [DOI] [PubMed] [Google Scholar]
- 64.Gambetti P, Parchi P, Petersen RB, Chen SG, Lugaresi E. Fatal familial insomnia and familial Creutzfeldt-Jakob disease: clinical, pathological and molecular-features. Brain Pathol. 1995;5:43–51. doi: 10.1111/j.1750-3639.1995.tb00576.x. [DOI] [PubMed] [Google Scholar]
- 65.Goldfarb LG, Petersen RB, Tabaton M, Brown P, Leblanc AC, Montagna P, Cortelli P, Julien J, Vital C, Pendelbury WW, Haltia M, Wills PR, Hauw JJ, Mckeever PE, Monari L, Schrank B, Swergold GD, Autiliogambetti L, Gajdusek DC, Lugaresi E, Gambetti P. Fatal Familial Insomnia and familial Creutzfeldt-Jakob disease: Disease phenotype determined by a DNA polymorphism. Science. 1992;258:806–808. doi: 10.1126/science.1439789. [DOI] [PubMed] [Google Scholar]
- 66.Hauw JJ, Sazdovitch V, Laplanche JL, Peoc'h K, Kopp N, Kemeny J, Privat N, Delasnerie-Laupretre N, Brandel JP, Deslys JP, Dormont D, Alperovitch A. Neuropathologic variants of sporadic Creutzfeldt-Jakob disease and codon 129 of PrP gene. Neurology. 2000;54:1641–1646. doi: 10.1212/wnl.54.8.1641. [DOI] [PubMed] [Google Scholar]
- 67.Puoti G, Rossi G, Giaccone G, Awan T, Lievens PMJ, Defanti CA, Tagliavini F, Bugiani O. Polymorphism at codon 129 of PRNP, affects the phenotypic expression of Creutzfeldt-Jakob disease linked to E200K mutation. Ann. Neurol. 2000;48:269–270. [PubMed] [Google Scholar]
- 68.Cereghetti GM, Schweiger A, Glockshuber R, Van Doorslaer S. Stability and Cu(II) binding of prion protein variants related to inherited human prion diseases. Biophys. J. 2003;84:1985–1997. doi: 10.1016/S0006-3495(03)75007-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Levitt M, Hirshberg M, Sharon R, Daggett V. Potential-energy function and parameters for simulations of the molecular-dynamics of proteins and nucleic-acids in solution. Comput. Phys. Commun. 1995;91:215–231. [Google Scholar]
- 70.Levitt M, Hirshberg M, Sharon R, Laidig KE, Daggett V. Calibration and testing of a water model for simulation of the molecular dynamics of proteins and nucleic acids in solution. J. Phys. Chem. B. 1997;101:5051–5061. [Google Scholar]
- 71.Lee B, Richards FM. Interpretation of protein structures: Estimation of static accessibility. J. Mol. Biol. 1971;55:379–400. doi: 10.1016/0022-2836(71)90324-x. [DOI] [PubMed] [Google Scholar]
- 72.Kabsch W, Sander C. Dictionary of protein secondary structure: Pattern-recognition of hydrogen-bonded and geometrical features. Biopolymers. 1983;22:2577–2637. doi: 10.1002/bip.360221211. [DOI] [PubMed] [Google Scholar]
- 73.DeLano WL. The PyMOL Molecular Graphics System. Palo Alto, CA, USA: DeLano Scientific; 2002. [Google Scholar]