Abstract
All viruses require cellular ribosomes to translate their mRNAs. Viruses producing methyl-7 (m7) GTP-capped mRNAs, like Herpes Simplex Virus-1 (HSV-1), stimulate cap-dependent translation by activating mTORC1 to inhibit the translational repressor 4E-binding protein 1 (4E-BP1). Here, we establish that the HSV-1 kinase Us3 masquerades as Akt to activate mTORC1. Remarkably, Us3 displays no sequence homology with the cellular kinase Akt, yet directly phosphorylates tuberous sclerosis complex 2 (TSC2) on the same sites as Akt. TSC2 depletion rescued Us3-deficient virus replication, establishing that Us3 enhances replication by phosphorylating TSC2 to constitutively activate mTORC1, effectively bypassing S6K-mediated feedback inhibition. Moreover, Us3 stimulated Akt substrate phosphorylation in infected cells, including FOXO1 and GSK3. Thus, HSV-1 encodes an Akt surrogate with overlapping substrate specificity to activate mTORC1, stimulating translation and virus replication. This establishes Us3 as a unique viral kinase with promising drug development potential.
Keywords: Translational control, herpesvirus replication, viral kinase, mTOR activation, Akt signaling
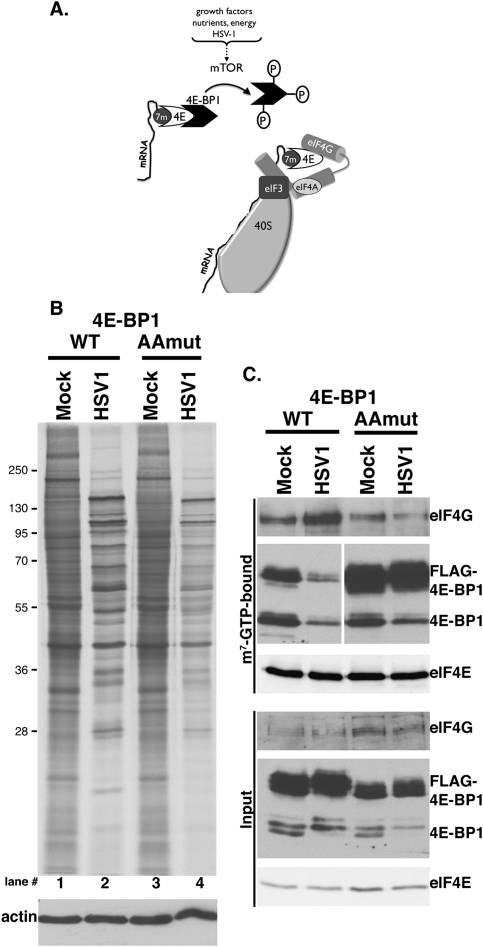
Recruiting 40S ribosome subunits to mRNA is a critical step controlling translation. For eukaryotic mRNAs, the vast majority of which have methyl-7 (m7) GTP caps at their 5′ termini, this involves assembling the multisubunit, cap-binding translation initiation factor eIF4F (Sonenberg and Hinnebusch 2009). Comprised of the cap recognition subunit eIF4E and an RNA helicase (eIF4A) bound to the eIF4G assembly platform, eIF4F associates with eIF3-bound 40S subunits and localizes them to the mRNA 5′ end. Interaction of eIF4E with eIF4G represents a pivotal step controlling eIF4F assembly, and is regulated in part by small 4E-binding proteins (4E-BPs) that sequester eIF4E, inhibit the eIF4E–eIF4G association, and thereby repress mRNA translation. Phosphorylation of 4E-BP by the cellular kinase mTORC1 releases eIF4E, a prerequisite for eIF4G binding and forming an active eIF4F complex. Integration of diverse signaling inputs by mTORC1 facilitates translation initiation factor complex remodeling and rapid response to nutrient availability, energy supplies, and a battery of environmental stresses, including viral infection. (Fig. 1A; Mohr et al. 2007; Buchkovich et al. 2008; Ma and Blenis 2009)
Figure 1.
Regulation of eIF4F assembly by site-specific 4E-BP1 phosphorylation in HSV-1-infected cells. (A) Inactivation of the translational repressor 4E-BP1 by mTOR. mTOR activation in response to different stimuli results in 4E-BP1 hyperphosphorylation and release from the cap-binding protein eIF4E. Subsequent eIF4E incorporation into a multisubunit initiation factor complex containing eIF4G and eIF4A allows recruitment of eIF3-bound 40S subunits to the mRNA 5′ end. (B) NHDFs stably expressing epitope-tagged wild-type (WT) or AA 4E-BP1 growth-arrested by serum deprivation were mock-infected or infected with HSV-1 (MOI = 5). Cultures were metabolically pulse-labeled for 1 h with 35S-amino acids at 14 hpi. Total protein was subsequently isolated and fractionated by SDS-PAGE, and the fixed, dried gel was exposed to X-ray film. Migration of molecular mass standards (in kilodaltons) appears to the left. (Bottom panel) Immunoblot loading control probed with anti-actin antibody. (C) Soluble extracts prepared from NHDFs described in B were incubated with m7GTP-Sepharose. After washing, input (bottom panel) and bound (top panel) fractions were separated by SDS-PAGE and analyzed by immunoblotting with the indicated antisera. Both anti-4E-BP1 panels in the m7GTP-bound group were from the same membrane.
Herpesvirus' lifelong latency within their cellular hosts is punctuated by productive viral replication episodes (Roizman et al. 2007). Successful completion of their replicative cycle during these outbreaks requires conscripting host ribosomes to translate viral mRNAs. This is achieved in part by viral functions that commandeer cellular translational control functions, one of which is ICP6. As a Herpes Simplex Virus-1 (HSV-1)-encoded eIF4G-associated protein that stimulates eIF4G binding to eIF4E, ICP6 mediates eIF4F assembly and eIF4E phosphorylation by the eIF4G-associated kinase Mnk. However, despite stimulating eIF4F assembly, ICP6 did not detectably contribute to the fate of the translational repressor 4E-BP1 in infected cells (Walsh and Mohr 2006).
Productive replication of representative α (HSV-1) and β (HCMV) herpesviruses, together with γ herpesvirus reactivation from latency, inactivate 4E-BP1 (Kudchodkar et al. 2004; Walsh and Mohr 2004; Walsh et al. 2005; Moorman et al. 2008; Arias et al. 2009). In HSV-1-infected primary human cells, this involves 4E-BP1 hyperphosphorylation, which requires viral gene expression, is sensitive to the mTORC1 inhibitor rapamycin, and is followed by proteasome-mediated degradation (Walsh and Mohr 2004). Here we show that phosphorylation of the 4E-BP1 translational repressor, an mTORC1 substrate, is required for eIF4F assembly and normal viral protein synthesis levels. Furthermore, we define the mechanism of mTORC1 activation and 4E-BP1 inactivation in HSV-1-infected cells by demonstrating that the virus-encoded Us3 Ser/Thr protein kinase is required. Surprisingly, Us3 catalytic function acts analogously to the cellular kinase Akt to promote mTORC1-mediated 4E-BP1 phosphorylation, enabling Akt-independent mTORC1 activation. By stimulating site-specific phosphorylation of the tuberous sclerosis complex (TSC) TSC2 subunit at S939 and T1462, Us3 ensures constitutive mTORC1 activation in infected cells by antagonizing TSC antiviral activity. Finally, siRNA-mediated TSC2 depletion selectively enhanced Us3-deficient virus replication, providing genetic evidence that TSC2 restricts mTOR activation, which is normally antagonized by Us3 in HSV-1-infected cells.
This establishes that the HSV-1 Us3 protein kinase overcomes host TSC antiviral activity and remodels host translational initiation factor complexes, defining a new strategy whereby protein kinases bearing little or no primary sequence homology with Akt regulate mTORC1. Remarkably, by encoding a constitutively active kinase to mimic Akt and activate mTORC1, HSV-1 bypasses feedback inhibitory circuits, antagonizes TSC2, and ensures that the 4E-BP1 translational repressor remains inactive throughout the productive growth cycle.
Results
Regulation of eIF4F assembly and protein synthesis in HSV-1-infected cells by mTORC1-mediated 4E-BP1 phosphorylation
Although 4E-BP1 phosphorylation in HSV-1-infected primary human cells was sensitive to the mTORC1 inhibitor rapamycin, significant reductions in viral replication or translation rates were not detected (Walsh and Mohr 2004). Recently developed mTOR active site inhibitors (PP242 and Torin) are superior to rapamycin in repressing translation by preventing 4E-BP1 phosphorylation (Feldman et al. 2009; Thoreen et al. 2009). Indeed, Torin reduced HSV-1 replication in immortalized mouse cells in a 4E-BP1-dependent manner (Moorman and Shenk 2010), and PP242 reduced HSV-1 replication in primary human fibroblasts (Supplemental Fig. S1). Thus, wild-type levels of HSV-1 replication and protein synthesis in normal, primary human cells required mTOR catalytic activity. However, the extent to which site-specific 4E-BP1 phosphorylation by mTOR restricted HSV-1 replication by regulating infected cell protein synthesis and, most significantly, the underlying mechanism responsible for activating mTOR in infected cells remained unexplored.
To determine if preventing site-specific 4E-BP1 phosphorylation reduced protein synthesis in HSV-1-infected cells, normal human dermal fibroblasts (NHDFs) stably expressing epitope-tagged (Flag) wild-type 4E-BP1 or a double-mutant derivative (AA) with alanine substituted for each of the critical T37 and T46 phosphoacceptor residues were isolated. Unable to be inactivated by mTORC1, the AA mutant behaved as a constitutively activated translational repressor (Gingras et al. 1999). Growth-arrested NHDFs expressing wild-type or AA 4E-BP1 were either mock-infected or HSV-1-infected, and metabolically pulse-labeled proteins were analyzed by SDS-PAGE (Fig. 1B). Quantification of 35S amino acid incorporation in six independent experiments by TCA precipitation revealed a modest 10%–15% decrease in uninfected cells expressing AA versus wild-type 4E-BP1 (Fig. 1B, lanes 1,3). Under these conditions in growth-arrested primary fibroblasts, the introduced wild-type and AA 4E-BP1 were both active repressors that bound to eIF4E in mock-infected cells (Fig. 1C). Similar amounts of eIF4G were associated with eIF4E in mock-infected wild-type and AA 4E-BP1-expressing cells, correlating well with the similar rates of 35S amino acid incorporation observed (Fig. 1C). In contrast, translation rates were reduced by 30%–35% in HSV-1-infected AA compared with wild-type 4E-BP1-expressing cells (Fig. 1B, lanes 2,4). Under these conditions, wild-type was more abundant than AA 4E-BP1 (Fig. 1C, input panel), raising the possibility that further translational repression could be achieved if AA and wild-type 4E-BP1 were expressed equivalently. To determine how wild-type or AA 4E-BP1 expression influenced eIF4F assembly in HSV-1-infected cells, eIF4E-containing complexes bound to m7GTP-Sepharose were analyzed by immunoblotting. HSV-1 infection of NHDFs expressing Flag-wild-type 4E-BP1 substantially reduced endogenous and ectopically expressed 4E-BP1 binding to eIF4E, and concomitantly enhanced eIF4G-binding to eIF4E (Fig. 1C; Walsh and Mohr 2004; Dobrikova et al. 2009). However, AA 4E-BP1 remained efficiently bound to eIF4E in infected NHDFs even though endogenous 4E-BP1 was displaced, and prevented eIF4F assembly by inhibiting eIF4G binding to eIF4E (Fig. 1C). This provides the first evidence that site-specific phosphorylation and inactivation of the translational repressor 4E-BP1, an mTORC1 substrate, is important for eIF4F assembly and protein synthesis in HSV-1-infected cells.
Inactivation of 4E-BP1 in HSV-1-infected cells requires a virus-encoded Ser/Thr kinase
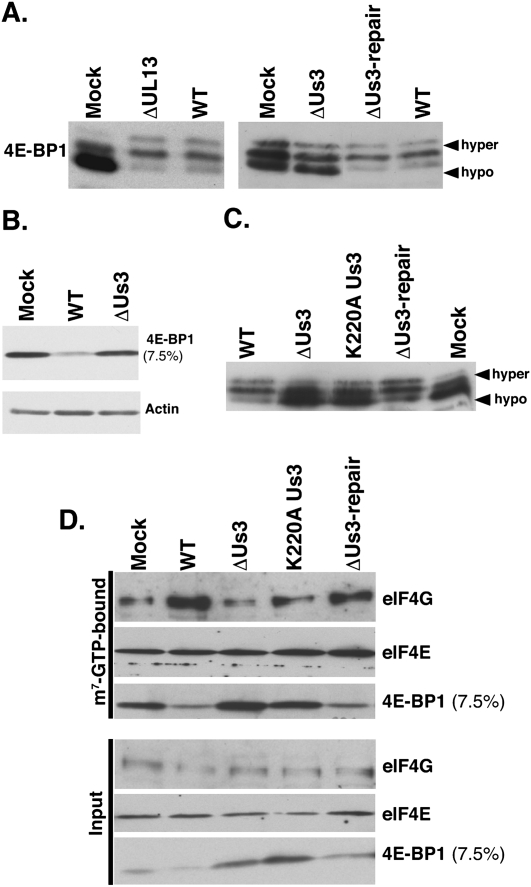
4E-BP1 phosphorylation in HSV-1-infected cells required viral gene expression and was sensitive to the mTOR active site inhibitor PP242 (Supplementary Fig. S1) as well as the mTORC1-selective inhibitor rapamycin (Walsh and Mohr 2004). mTORC1, in turn, is regulated by cellular kinases, notably PI3 kinase (PI3K) and Akt, which transduce receptor-mediated signals regulating protein synthesis (Ma and Blenis 2009). Given the role of protein phosphorylation in regulating mTORC1 and 4E-BP1, the possibility that one of two HSV-1-encoded Ser/Thr kinases intervened in this important cell signaling pathway was investigated. While some viral and cellular targets of the HSV-1 Us3 and UL13 protein kinases have been identified, the list of bona fide cellular substrates and their biological consequences is incomplete (for review, see Roizman et al. 2007). To determine if Us3 or UL13 were required to inactivate 4E-BP1 in infected cells, growth-arrested NHDFs were either mock-infected or infected with Us3-deficient (ΔUs3), UL13-deficient (ΔUL13), or wild-type HSV-1. At 18 h post-infection (hpi), total protein was isolated, fractionated by SDS-PAGE in a 17.5% gel to resolve hyper- and hypophosphorylated 4E-BP1 species, and analyzed by immunoblotting. While hypophosphorylated 4E-BP1 predominated in mock-infected cells, it was underrepresented in wild-type or ΔUL13-infected cells (Fig. 2A). In contrast, hypophosphorylated 4E-BP1 levels similar to mock-infected cells accumulated in ΔUs3-infected cells (Fig. 2A). To ensure this phenotype was due to the engineered Us3 deletion, NHDFs were infected with a recombinant in which the mutant Us3 allele was restored to wild type. Importantly, repairing the Us3 allele markedly decreased 4E-BP1 hypophosphorylation, correcting the mutant phenotype and proving it was due to the Us3 deficiency (Fig. 2A).
Figure 2.
Inactivation of 4E-BP1 in HSV-1-infected cells requires the virus-encoded Us3 Ser/Thr kinase. (A) Growth-arrested NHDFs were mock-infected (Mock) or infected (MOI = 5) with wild-type HSV-1 (WT), a UL13-deficient mutant (ΔUL13), a Us3-deficient virus (ΔUs3), or a virus where the Us3 deficiency was repaired (ΔUs3 REPAIR). At 18 hpi, total protein was isolated, fractionated by SDS-PAGE (17.5% gel), and analyzed by immunoblotting using anti-4E-BP1. Slow-migrating hyperphosphorylated (hyper) and fast-migrating hypophosphorylated (hypo) 4E-BP1 forms are noted on the right. (B) As in A, except total protein was fractionated using a 7.5% gel, which does not resolve phosphorylated 4E-BP1 isoforms. (C) As in A, except a virus expressing a catalytically inactive Us3 protein was included (Us3 K220A contains a single Lys-to-Ala substitution at residue 220). (D) Soluble extracts prepared from NHDFs described in A were incubated with m7GTP-Sepharose and analyzed as described in Figure 1C.
4E-BP1 hyperphosphorylation is followed by proteasome-mediated degradation in HSV-1-infected and uninfected cells (Walsh and Mohr 2004; Elia et al. 2008; Braunstein et al. 2009). Fractionating identical samples by SDS-PAGE in a 7.5% gel easily monitors steady-state 4E-BP1 abundance, as phosphorylated isoforms are not resolved and 4E-BP1 migrates as a single band. Indeed, overall 4E-BP1 abundance was reduced in wild-type virus-infected compared with mock-infected cells, whereas steady-state control antigen (actin) levels remained unchanged. However, overall 4E-BP1 levels in ΔUs3-infected cells remained similar to mock-infected cells (Fig. 2B). Thus, Us3-deficient viruses are unable to inactivate 4E-BP1 by promoting its phosphorylation and degradation in infected cells.
To determine if Us3 Ser/Thr kinase activity was required to inactivate 4E-BP1 in infected cells, NHDFs were either mock-infected or infected with ΔUs3, a recombinant virus expressing catalytically inactive Us3 (K220A), the Us3 repair virus, or wild-type HSV-1. Analysis of 4E-BP1 phosphorylation by SDS-PAGE followed by immunoblotting revealed that K220A and ΔUs3 were similarly defective in promoting 4E-BP1 phosphorylation (Fig. 2C). Finally, the ability of Us3 to interfere with eIF4E binding to the 4E-BP1 repressor in HSV-1-infected cells was evaluated by collecting eIF4E-bound proteins in cell-free extracts prepared from mock-infected or HSV-1-infected NHDFs on m7GTP-Sepharose. While the amount of 4E-BP1 associated with cap-bound eIF4E dropped significantly in lysates from wild-type or Us3 repair virus-infected cells, amounts of 4E-BP1 associated with cap-bound eIF4E in cells infected with either ΔUs3 or K220A remained similar to those in mock-infected cells (Fig. 2D). Furthermore, stimulation of eIF4G binding to eIF4E was detected only in cells infected with wild-type or Us3 repair viruses (Fig. 2D). Taken together, these results indicated that Us3 Ser/Thr kinase activity was required to stimulate 4E-BP1 phosphorylation in HSV-1-infected cells, thereby inactivating the translational repressor and disrupting the 4E-BP1-eIF4E complex to release the cap-binding, translation initiation factor eIF4E. Finally, Us3-mediated release of eIF4E from 4E-BP1 was required to recruit eIF4G, a critical eIF4F subunit required to recruit 40S ribosomes and initiate translation.
Us3 acts analogously to Akt to stimulate mTORC1 signaling
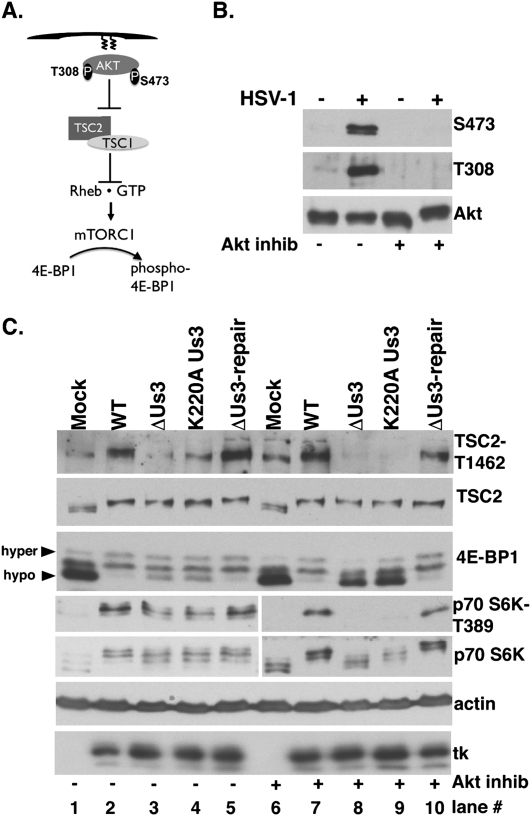
HSV-1 infection activated Akt in established, transformed cell lines (Benetti and Roizman 2006) and growth-arrested primary human cells (Fig. 3B). To determine if Akt is required to inactivate 4E-BP1 in HSV-1-infected cells, the extent of 4E-BP1 phosphorylation was evaluated after treatment with Akt inhibitor VIII, a highly selective allosteric Akt activation inhibitor (Fig. 3B; Calleja et al. 2009). Growth-arrested NHDFs were mock-infected or infected with wild-type or ΔUs3 virus and treated with Akt inhibitor, and, at 15 hpi, total protein was collected, fractionated by SDS-PAGE, and analyzed by immunoblotting. Significantly, phosphorylated 4E-BP1 and p70 S6K, another mTORC1 substrate, still accumulated in wild-type virus-infected cells treated with the Akt inhibitor (Fig. 3C, lanes 6 vs. 7), indicating that Akt activity is not required for HSV-1 to stimulate mTOR. p70 S6K and 4E-BP1 phosphorylation, however, was only partially impaired in ΔUs3 or K220A Us3-infected cells (Fig. 3C, lanes 3,4) compared with those infected with HSV-1-expressing wild-type Us3 (wild-type or ΔUs3 repair in Fig. 3C, lanes 2,5), suggesting that HSV-1 activates mTOR through both Akt-dependent and Akt-independent mechanisms. The Akt inhibitor did not detectably effect viral thymidine kinase (tk) accumulation, indicating that lack of detectable 4E-BP1 phosphorylation in ΔUs3 or K220A Us3 virus-infected cells did not result from nonspecific inhibition of viral gene expression (Fig. 3C). Thus, 4E-BP1 and p70 S6K phosphorylation in HSV-1-infected cells treated with an Akt inhibitor was Us3-dependent (Fig. 3C, lanes 8,9), suggesting that Us3 activates mTOR to phosphorylate p70 S6K and 4E-BP1 by phosphorylating a target downstream from and independent of Akt.
Figure 3.
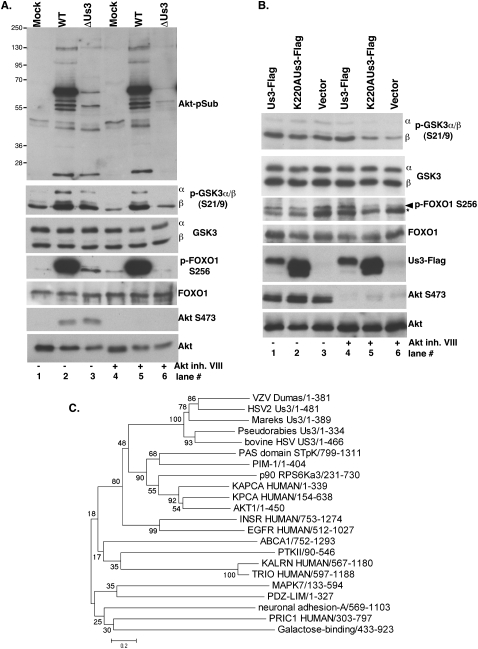
Inhibition of Akt does not suppress Us3-dependent 4E-BP1 and TSC2 phosphorylation in HSV-1-infected cells. (A) Activation of mTORC1 by Akt. Once plasma membrane localized via a lipid-binding plekstrin homology domain, Akt is activated by T308/S473 phosphorylation. Akt inhibits TSC rheb-GAP activity by phosphorylating TSC2 on T1462/S939, promoting rheb•GTP-mediated mTORC1 activation and subsequent 4E-BP1 hyperphosphorylation. (B) Growth-arrested NHDFs were mock-infected (−) or infected (MOI = 5) with wild-type (WT) HSV-1 (+). After 4 h, cells were treated with DMSO or Akt inhibitor VIII (5 μM). At 9 hpi, total protein was fractionated by SDS-PAGE and analyzed by immunoblotting with indicated antibodies (S473, T308-phospho specific Akt antibodies, and Akt total). (C) As in B, except ΔUs3, K220A Us3, and Us3 repair were included. Total protein was isolated at 15 hpi, fractionated by SDS-PAGE, and analyzed by immunoblotting with the indicated antisera: TSC2-T1462 (phospho-specific T1462 antibody), TSC2 (total TSC2), anti-4E-BP1, anti-HSV-1 tk antibody, S6K-T389 (phospho-specific T389 p70 S6K), and total p70 S6K. Different phospho-4E-BP1 forms were resolved (hyper vs. hypo) as described in Figure 2A.
Lying downstream from Akt, TSC is a Rheb•GTPase-activating protein (GAP) that negatively regulates mTORC1. TSC is a heterodimer composed of TSC1 and TSC2 subunits. TSC2 phosphorylation (T1462, S939) by Akt inhibits TSC GAP activity and allows Rheb•GTP accumulation and subsequent mTORC1 activation (Fig 3A; Ma and Blenis 2009). To determine if TSC2 phosphorylation on sites normally targeted by Akt occurred in HSV-1-infected cells, total protein isolated from NHDFs infected with wild-type or Us3 mutant viruses in the presence and absence of Akt inhibitor VIII was fractionated by SDS-PAGE, and TSC2 phosphorylation was analyzed by immunoblotting. In DMSO-treated cultures, reduced TSC2 p-T1462 levels accumulated in cells infected with Us3 mutant viruses (ΔUs3 or K220A), and this correlated with increased 4E-BP1 hypophosphorylation (Fig. 3C, lanes 3,4 vs. 2,5). However, TSC2 p-T1462 and hyperphosphorylated 4E-BP1 were detected only in Akt inhibitor-treated cultures infected with functional Us3-expressing viruses (wild-type or Us3 repair in Fig 3C, lanes 7,10 vs. lanes 8,9). Similar results were observed using antisera specific for TSC2 p-S939 (data not shown). This demonstrates that TSC2 Thr1462/Ser939 phosphorylation was Us3 Ser/Thr kinase-dependent in HSV-1-infected cells treated with an Akt inhibitor. Moreover, it suggested that TSC2 may be a substrate for the HSV-1-encoded kinase Us3, and this could be the mechanism underlying mTORC1 activation in infected cells.
Us3 phosphorylates TSC2 in vivo and in vitro
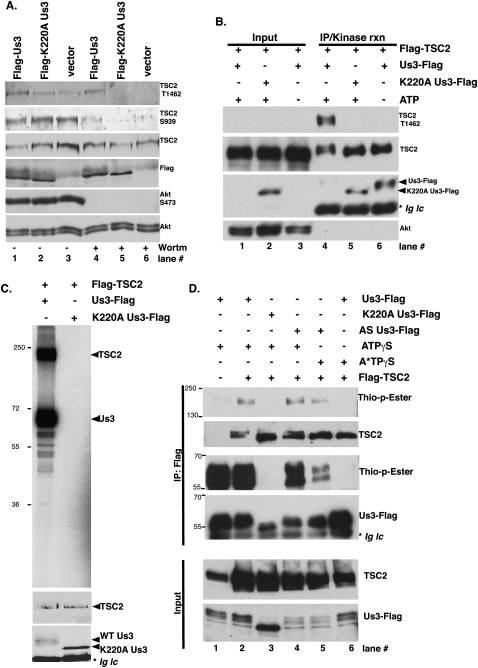
While phospho-TSC2 accumulation in HSV-1-infected cells was Us3-dependent, Us3 is itself regulated by another viral protein kinase (Kato et al. 2006). In addition, profound physiological changes in infected cells could conceivably contribute to TSC2 phosphorylation. To determine whether Us3 expression was sufficient to promote TSC2 phosphorylation in the absence of a concurrent HSV-1 infection, Us3 was transiently expressed in uninfected cells. Following transfection of U2OS cells with epitope-tagged vector expressing wild-type Us3, K220A catalytically inactive Us3, or empty vector, total protein was collected and fractionated by SDS-PAGE, and TSC2 phosphorylation along with Us3 expression were evaluated by immunoblotting (Fig. 4A). Despite being expressed to equivalent levels, wild-type Us3 was more effective than the K220A variant in promoting p-T1462 TSC2 accumulation in DMSO-treated cells (Fig. 4A, lanes 1–3). However, background p-S939 phosphorylation in this transformed, established osteosarcoma cell line was high. To eliminate any contribution of endogenous, constitutively activated Akt, the experiment was performed in parallel cultures treated with the PI3K inhibitor wortmannin. Under these conditions (1) Akt activation measured by S473 phosphorylation was not detected (Fig. 4A, lanes 4–6), and (2) p-TSC2 (T1462/S939) was detected only in cultures expressing wild-type Us3. Thus, Us3 expression was sufficient to promote p-TSC2 accumulation in uninfected cells; moreover, TSC2 phosphorylation in transiently transfected cells required Us3 Ser/Thr kinase catalytic activity.
Figure 4.
Us3 Ser/Thr kinase directly phosphorylates TSC2. (A) U20S cells were transfected with plasmids expressing Flag-Us3, Flag-K220AUs3, or an empty vector control (vector). After 24 h, cells were treated with DMSO or 200 nM wortmannin for 30 min to eliminate PI3K-mediated, Akt-dependent TSC2 phosphorylation. Total protein was isolated, fractionated by SDS-PAGE, and analyzed by immunoblotting with the indicated antibodies. (B) Us3 stimulates TSC2 phosphorylation in vitro. HEK 293 cells were transiently transfected with plasmids expressing Flag-TSC2 or individual Us3 expression plasmids described in A. After 24 h, soluble cell-free fractions from Flag-TSC2 and Flag-Us3 transfected cells were mixed and immunoprecipitated using anti-Flag antibody. After a high-salt wash to reduce nonspecific binding, immune complexes were re-equilibrated in kinase buffer (±ATP) and incubated for 30 min at 30°C. Samples were subsequently fractionated by SDS-PAGE and analyzed by immunoblotting using the indicated antibodies. Wild-type Flag-Us3 autophosphorylates in the presence of ATP and thus migrates slower (Sagou et al. 2009). The band marked with an asterisk represents immunoglobulin light chain (Ig lc). (C, top panel) As in B, except kinase reactions contained [γ32P]-ATP and the fixed dried gel exposed to X-ray film. (Middle panel) TSC2 immunoblot. (Bottom panel) Anti-Flag immunoblot. The band marked with an asterisk represents immunoglobulin light chain. (D) Soluble cell-free fractions from cells transfected and immunoprecipitated as in B were incubated with the indicated ATP derivatives for 60 min at 30°C. Following thiophosphate alkylation using p-nitrobenzylmesylate (PNBM), samples were fractionated by SDS-PAGE and analyzed by immunoblotting using the indicated antibodies. The band marked with an asterisk in the anti-Flag blot represents immunoglobulin light chain (Ig lc).
To refine requirements for TSC2 phosphorylation, Us3-dependent TSC2 phosphorylation was reconstituted in vitro using immunopurified components. Transiently expressed epitope-tagged Us3 and TSC2 were immunopurified using protein G-Sepharose bound to anti-Flag antibody. TSC2-expressing cultures were wortmannin-treated to enrich for unphosphorylated TSC2. Furthermore, to reduce nonspecific protein binding, immune complexes were washed with high-salt buffer prior to equilibrating the beads in kinase reaction buffer. A similar procedure established that TSC2 was a bona fide Akt substrate (Manning et al. 2002). After incubating at 30°C, reactions were fractionated by SDS-PAGE and TSC2 phosphorylation was evaluated by immunoblotting using phospho-specific antibodies (Fig. 4B). Phospho-TSC2 accumulation was both Us3- and ATP-dependent, and was not detected in reactions containing catalytically inactive K220A Us3 (Fig. 4B, lanes 4–6). While Akt was present in the initial cell-free lysate, it was not detected in the immunopurified kinase reactions, demonstrating the effectiveness of the purification procedure in depleting the endogenous cellular TSC2 kinase (Fig. 4B, cf. lanes 1–3 and 4–6). To further control for specificity, samples programmed with [γ32P]-ATP were fractionated by SDS-PAGE to visualize all radiolabeled products. Remarkably, only two major 32P-incorporated products were observed on overexposed films in reactions that contained wild-type but not K220A Us3. The largest product corresponds in size to TSC2, while the smaller one corresponds to the Us3 autophosphorylation product (Fig. 4C; Sagou et al. 2009). Thus, Us3 stimulated TSC2 phosphorylation in vitro and the reaction required catalytically active Us3 and ATP.
TSC2 is a substrate phosphorylated directly by Us3
While Us3 stimulated TSC2 phosphorylation in vivo and in vitro, other cellular Us3-responsive or copurifying kinase contaminants might still be responsible. To evaluate if a direct kinase–substrate interaction accounted for Us3-mediated TSC2 phosphorylation, Us3 was engineered to accept a bio-orthogonal ATP analog not used by the remainder of the kinome (Allen et al. 2007). This was achieved by introducing a Leu → Gly substitution at residue 259, and allows the Us3 active site to accept an unusual bulky ATP analog. While wild-type Us3 can use ATPγS to thiophosphorylate substrates, only analog-sensitive Us3 (AS-Us3) can use N6-alkylated ATPγS (A*TPγS) to thiophosphorylate its substrates. Thiophosphorylated proteins are subsequently reacted with a thiol-specific alkylating agent that generates a bio-orthogonal thiophosphate ester. The resulting labeled proteins are readily detected by immunoblotting using a thiophosphate ester-specific antibody (Allen et al. 2007). Following immunopurification from transfected 293 cells, wild-type and AS-Us3 used ATPγS to thiophosphorylate themselves and TSC2 (Fig. 4D, lanes 1,2,4). Both Us3 and TSC2 thiophosphorylation were not detected in reactions containing K220A catalytically inactive Us3 (Fig. 4D, lane 3). Significantly, whereas AS-Us3 accepted ATPγS and A*TPγS to thiophosphorylate both Us3 and TSC2, wild-type Us3 used ATPγS but not A*TPγS (Fig. 5, lanes 5 vs. 6). This demonstrated that A*TPγS is bio-orthogonal, as it was detectably used by only AS-Us3 and not wild-type Us3. Moreover, it established that Us3 directly phosphorylates TSC2, since AS-Us3 is the only kinase in the reaction capable of accepting and using the A*TPγS analog to thiophosphorylate TSC2.
Figure 5.
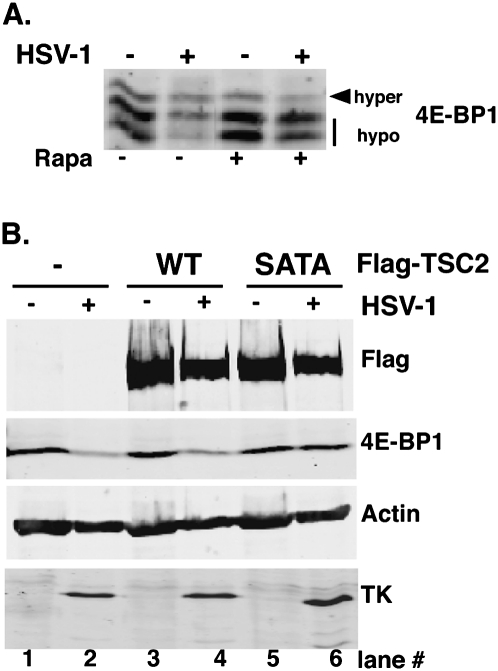
Inactivation of the 4E-BP1 translational repressor in HSV-1-infected cells requires site-specific TSC2 phosphorylation. (A) 293 cells were mock-infected (−) or infected (+) with HSV1 (MOI = 10) and, after 3 h, were treated with DMSO or rapamycin. Total protein was collected at 12 hpi, fractionated by SDS-PAGE (17.5% gel), and immunoblotted with anti-4E-BP1 antibody. (B) 293 cells were transiently transfected with either empty vector (−), pcDNA3-Flag-TSC2 (wild type [WT]), or pcDNA3-Flag-TSC2SATA (SATA). After 6 h, cells were serum-starved for 18 h and subsequently mock-infected or infected (MOI = 10) with HSV-1. At 12 hpi, total protein was fractionated by SDS-PAGE (7.5% gel) and analyzed by immunoblotting with the indicated antibodies.
Inactivation of 4E-BP1 requires TSC2 phosphorylation in HSV-1-infected cells
Stimulation of TSC2 T1462/S939 phosphorylation by Us3 suggested that TSC inactivation was required to activate mTORC1 and in turn stimulate 4E-BP1 phosphorylation in HSV-1-infected cells. To test this, epitope-tagged wild-type TSC2 or a double-mutant derivative with alanine substituted at each of the critical S939 and T1462 phospho-acceptor residues (SATA) were transiently expressed in 293 cells. While both ectopically expressed wild-type and SATA TSC2 subunits bind TSC1, SATA-TSC2 was unable to be inactivated by Akt, and behaves as a constitutively activated Rheb-GAP that prevents mTORC1 activation (Manning et al. 2002). HSV-1 infection of 293 cells resulted in rapamycin-sensitive 4E-BP1 hyperphosphorylation followed by proteasome-mediated degradation, as reported in NHDFs (Fig. 5A; Walsh and Mohr 2004). Cells transiently transfected with vector alone or TSC2 expression plasmids (wild type or SATA) were infected with wild-type HSV-1, and, at 12 hpi, total protein was isolated, fractionated by SDS-PAGE, and analyzed by immunoblotting. As expected, HSV-1 infection reduced 4E-BP1 steady-state levels in cells transfected with either vector alone or wild-type TSC2 expression plasmid, while control antigen abundance (actin) remained relatively constant (Fig. 5B, cf. lanes 1 and 2, and lanes 3 and 4). However, 4E-BP1 levels were not detectably reduced in HSV-1-infected cells expressing TSC2 SATA (Fig. 5B, lanes 5 vs. 6). Both wild-type and SATA proteins accumulated to similar levels, and comparable viral tk levels confirmed the cultures were infected equivalently (Fig. 5B, lanes 2,4,6). This established that TSC2 S939/T1462 phosphorylation was required for 4E-BP1 inactivation and degradation in HSV-1-infected cells, and that viral mTORC1 activation proceeded via TSC2 phosphorylation.
TSC2 restricts replication of a Us3-deficient virus
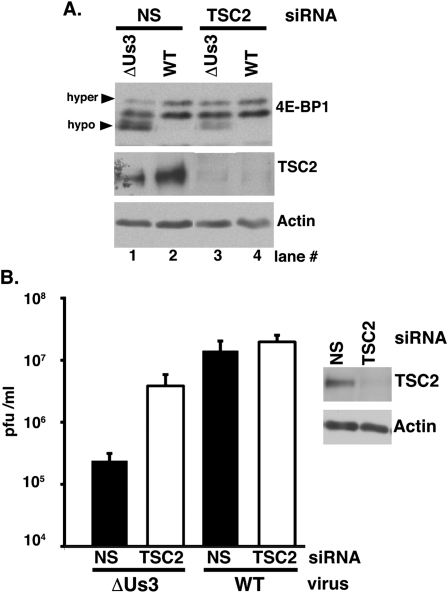
Collectively, the data suggested that (1) TSC2 is an integral node through which HSV-1 manipulates eIF4F assembly in infected cells; (2) Us3-deficient virus growth was restricted by host TSC2, which limited 4E-BP1 inactivation and replication in the absence of Us3; and (3) Us3 antagonized the antiviral actions of TSC2. To test this hypothesis, 4E-BP1 phosphorylation and viral replication were evaluated in TSC2-depleted NHDFs. Following treatment with either nonsilencing siRNA or a TSC2 siRNA pool, NHDFs were infected with ΔUs3 or wild-type virus. At 15 hpi, total protein was isolated, fractionated by SDS-PAGE, and analyzed by immunoblotting to verify TSC2 depletion and evaluate 4E-BP1 phosphorylation. 4E-BP1 was largely hypophosphorylated in ΔUs3-infected cells treated with nonsilencing siRNA, as evidenced by the relative abundance of fast-migrating, hypophosphorylated forms compared with the slower-migrating hyperphosphorylated forms (Fig. 6A, lane 1). In contrast, relatively greater amounts of slower-migrating, hyperphosphorylated 4E-BP1 accumulated in ΔUs3-infected cultures, where TSC2 protein levels were effectively depleted by TSC2 siRNA treatment (Fig. 6A, cf. lanes 3 and 1). Furthermore, hyperphosphorylated 4E-BP1 levels achieved by silencing TSC2 in ΔUs3-infected cells were similar to those in wild-type virus-infected cells treated with nonsilencing siRNA (Fig. 6A, lanes 2 vs. 3). This established that endogenous host TSC2 restricted 4E-BP1 inactivation by Us3-deficient HSV-1, and provided genetic evidence that the Us3–TSC2 interaction is required to activate mTORC1.
Figure 6.
TSC2 restricts 4E-BP1 repressor inactivation and viral replication in cells infected with a Us3-deficient virus. (A) NHDFs treated with nonsilencing (NS) or TSC2-specific siRNAs were either mock-infected or infected (MOI = 5) with Us3-deficient (ΔUs3) or wild-type (WT) HSV-1. After 15 h, total protein was harvested, fractionated by SDS-PAGE, and analyzed by immunoblotting with the indicated antisera. Phospho-4E-BP1 forms were resolved (hyper vs. hypo) as described in Figure 2A. (B) NHDFs treated with siRNAs as in A were infected (MOI = 1 × 10−4) with the indicated viruses. After 4 d, the virus produced was quantified by plaque assay in Vero cells. (Right panel) Immunoblot illustrating efficiency and persistence of TSC2 siRNA depletion after 4 d.
To determine the impact of TSC2 depletion on replication and spread of Us3-deficient virus, NHDFs treated with either nonsilencing or a TSC2 siRNA pool were infected with either ΔUs3 or wild-type HSV-1. After 4 d, the virus produced was quantified by plaque assay in Vero cells. Both wild-type HSV-1 and encephalomyocarditis (EMCV), an unrelated +-strand RNA virus, replicated equivalently in the presence of either nonsilencing or TSC2 siRNA, demonstrating that TSC2 depletion did not generally increase viral replication in mammalian cells (Fig. 6B; Supplemental Fig. S2). In contrast, TSC2 siRNA selectively enhanced ΔUs3 replication by ∼20-fold (Fig. 6B). While substantial, TSC2 depletion did not fully restore ΔUs3 replication to wild-type levels. This was not surprising, since Us3 is multifunctional and, as a Ser/Thr kinase, has numerous viral and cellular substrates (Mou et al. 2007). Without Us3, critical virus functions, including those needed for egress, remain impaired and are unaffected by TSC2 depletion. Nevertheless, these results established that endogenous cellular TSC2 restricted Us3-deficient virus replication in NHDFs. Furthermore, they suggest that Us3 stimulates HSV-1 replication in part by antagonizing TSC2 function, which in turn stimulates mTORC1, promotes 4E-BP1 translational repressor inactivation, and facilitates eIF4F assembly.
Stimulation of Us3-dependent, Akt-substrate phosphorylation in HSV-1-infected cells
Having established Us3 phosphorylated TSC2 on identical residues targeted by the cellular kinase Akt, the possibility that other Akt substrates were phosphorylated in a Us3-dependent manner in infected cells was investigated. An Akt-phosphosubstrate (Akt-pSub) antibody used to identify TSC2 as an Akt substrate and that recognizes the RxRxxpS/T Akt substrate motif in a growth factor- and PI3K-dependent manner was employed (Manning et al. 2002). Akt-pSub accumulation was stimulated in HSV-1-infected NHDFs in the presence and absence of Akt inhibitor VIII (Fig. 7A). Significantly, while similar profiles of Akt-pSub immunoreactive proteins were detected in ΔUs3-infected cells, their accumulation was Akt inhibitor VIII-sensitive, consistent with their being Akt substrates. This suggested that Akt-pSub accumulation in HSV-1-infected cells was Us3-dependent. Indeed, phosphorylation of Akt substrates GSK3 and FOXO1 on Akt target sites was stimulated in HSV-infected cells and fully dependent on Us3 in the presence of the Akt inhibitor (Fig. 7A). However, unlike Akt, which requires PI3K-dependent activation, GSK3 and 4E-BP1 phosphorylation in HSV-1-infected cells was insensitive to the PI3K inhibitor wortmannin (Supplemental Fig. S3). Finally, wild-type but not K220A Us3 effectively stimulated FOXO1 and GSK3 phosphorylation in uninfected, Akt inhibitor-treated cells (Fig. 7B, lanes 4–6), demonstrating that Us3 was necessary and sufficient to promote phosphorylation of these well-characterized Akt substrates in uninfected cells. Despite stimulating Akt substrate phosphorylation, α herpesvirus Us3-like Ser/Thr kinases occupy a separate branch distinct and equidistant from other cellular kinases. In particular, the Akt/ PKA subfamily is not more closely related to Us3 than any other subfamily (Fig. 7C). Overall 28% identity between Akt1 and Us3, along with a 3e-15 blast E-value, suggests that, while both are functional Ser/Thr protein kinases, they are at best very distantly related at the phylogenetic level.
Figure 7.
Stimulation of Akt substrate phosphorylation by Us3. (A) NHDFs were mock-infected (Mock) or infected (MOI = 5) with Us3-deficient (ΔUs3) or wild-type HSV-1 (WT) in the presence (+) or absence (−) of Akt inhibitor VIII. At 16 hpi, total protein was isolated, fractionated by SDS-PAGE, and analyzed by immunoblotting with the indicated antisera. (B) U2OS cells transfected with the indicated plasmids (described in Fig. 4A) were treated with DMSO (−) or Akt inhibitor VIII (+). Proteins were analyzed as described in Figure 4A. In the p-FOXO immunoblot, the asterisk represents a nonspecific band insensitive to Akt inhibitor routinely observed in U2OS cells. (C) α Herpesvirus Us3 protein sequences (HSV-1, VZV, HSV-2, bovine herpesvirus, Mareks, and Pseudorabies) were aligned using ClustalW2 (Larkin et al. 2007), with a broad selection of kinases representing the range of subfamilies in the InterPro Protein Kinase superfamily (CL0016), including AKT1 (AAL55732), KAPCA (P17612), KPCA (P17252), p90_RPS6Ka3 (NP_004577), PIM-1 (P11309), ABCA1 (NP_005493), PAS_domain_STpK (NP_055963), PTKII (NP_751911), MAPK7 (O43318), INSR (P06213), EGFR (P00533), KALRN (O60229), TRIO (O75962), neuronal adhesion A (NP_001032209), Galactose-binding (O14786), PDZ-LIM (O00151), and PRIC1 (Q96MT3). The evolutionary history was inferred using the Minimum Evolution (ME) method (Rzhetsky and Nei 1992). The optimal tree with the sum of the branch length = 16.70884448 is shown. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (500 replicates) are shown next to the branches (Felsenstein 1985). The tree is drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree. The evolutionary distances were computed using the Poisson correction method and are in the units of the number of amino acid substitutions per site. The ME tree was searched using the Close Neighbor Interchange (CNI) algorithm (Nei and Kumar 2000) at a search level of 1. The neighbor-joining algorithm (Saitou and Nei 1987) was used to generate the initial tree. All positions containing gaps and missing data were eliminated from the data set (complete deletion option). There were a total of 137 positions in the final data set. Phylogenetic analyses were conducted in MEGA4 (Tamura et al. 2007).
Discussion
By preventing eIF4G from associating with the cap-binding protein eIF4E and restricting assembly of a multisubunit initiation factor complex, the translation repressor 4E-BP1 regulates 40S ribosome subunit recruitment to mRNA. Moreover, having 4E-BP repressor activity responsive to mTORC1 allows translational output to respond to changes in intracellular and environmental status. For example, viruses whose mRNAs contain a 5′ m7GTP cap must ensure that translational repression by 4E-BP1 does not limit viral protein production (Mohr et al. 2007). Here, we establish that the HSV-1 Us3 Ser/Thr kinase is required to inactivate 4E-BP1 in infected cells. Acting analogously to the cellular kinase Akt, Us3 stimulated accumulation of phosphorylated Akt substrates, including FOXO1, GSK3, and TSC2. Direct, site-specific TSC2 phosphorylation by Us3 turned on mTORC1, inactivating the translational repressor 4E-BP1 in infected cells. Finally, TSC2 depletion increased Us3-deficient virus replication in normal human fibroblasts, illustrating that TSC restricts replication of a virus unable to activate mTORC1 and that Us3 is required to antagonize TSC antiviral activity. This demonstrates that the HSV-1 Us3 Ser/Thr protein kinase can remodel host translational initiation factor complexes, and establishes a new strategy whereby protein kinases bearing little or no primary sequence homology with Akt are used to regulate mTORC1.
By encoding a Ser/Thr protein kinase like Us3, HSV-1 capitalizes on protein phosphorylation as a potent cell signaling mechanism. Moreover, by imbuing this kinase with a broad yet specific substrate specificity, this single gene product is able to subvert signaling within multiple, fundamental pathways to favor viral productive replication without occupying substantial space in the viral genome (Munger and Roizman 2001; Ogg et al. 2004; Poon et al. 2006; Leach et al. 2007; Mou et al. 2007). While not essential for replication, Us3-deficient viruses exhibit cell type-specific replication defects in culture and are severely impaired in mouse pathogenesis models (Meignier et al. 1988; Nishiyama et al. 1992; Ryckman and Roller 2004). In addition, Us3 bears little resemblance to any specific cellular Ser/Thr kinase obstructing target substrate prediction. Despite phosphorylating some peptide substrates shared with PKA and PKC in vitro, Us3 exhibited distinct synthetic peptide substrate specificity (Leader et al. 1991). One study suggested Us3 specificity overlapped with PKA, whereas another found this too limiting (Benetti and Roizman 2004; Mou et al. 2007). Nevertheless, recognition motifs are unlikely to provide the specificity required in vivo, since (1) many kinases share in vitro recognition motifs (i.e., Arg residues N-terminal to phospho-acceptor site for p90 RSK, PKA, and Akt), (2) recognition motifs may not be physiologically phosphorylated, (3) peptides may not mimic intact protein phosphorylation kinetics, and (4) not all kinases have a clear consensus motif in peptide substrates (Biondi and Nebreda 2003). Instead, biological specificity is often achieved by interactions outside the active site that dock the kinase to physiological substrates (Pawson and Nash 2000; Remenyi et al. 2006). To further complicate matters, Us3 also targets viral substrates and is itself phosphorylated by a second HSV-1 protein kinase, UL13 (Kato et al. 2006). While UL13 might alter Us3 specificity, Us3 alone is sufficient to promote TSC2, GSK3, and FOXO1 phosphorylation. Us3 not only facilitates nuclear lamina disassembly and egress of newly assembled progeny virions, it has anti-apoptotic activity and stimulated phosphorylation of the Akt substrate BAD in vitro (Munger and Roizman 2001; Kato et al. 2005). Our findings that Us3 stimulates site-specific, phosphorylated Akt substrate accumulation—including FOXO1, GSK3, and TSC2–now raise the possibility that Us3 may in fact be a mimic for this critical cellular regulator. Importantly, although Us3 targets Akt substrates and constitutively activates Akt signaling, it is unrelated to Akt at the primary sequence level and therefore is unresponsive to endogenous regulatory pathways that limit Akt activity.
By restricting mTORC1 activation, TSC controls an Akt-regulated antiviral checkpoint limiting access to the host cap-dependent translation machinery. Indeed, co-opting control of Akt signaling by inactivating TSC2 figures prominently in the biology of different representative α, β, and γ herpesvirus subfamily members. However, distinct mechanisms of targeting the TSC2 subunit are used by each subfamily. The β herpesvirus HCMV encodes a TSC2-binding protein (UL38) required for mTORC1 activation (Moorman et al. 2008), whereas the γ herpesvirus KSHV stimulates TSC2 phosphorylation via a virus-encoded GPCR (Sodhi et al. 2006). We now define Us3 as the first viral Ser/Thr kinase capable of directly phosphorylating TSC2 on sites normally targeted by Akt. Notably, only α herpesvirus subfamily members (HSV-1, PRV, and VZV) encode a Us3-related Ser/Thr protein kinase. Conceivably, the diverse mechanisms by which TSC is inactivated by different herpesviruses could reflect the specialized relationship each virus has with the differentiated host cells it colonizes. For example, the Ser/Thr kinase is encoded uniquely by α herpesviruses (which colonize neurons), while an inhibitory TSC2-binding protein is specified by HCMV (which colonizes monocyte/macrophage precursors), and a GPCR is encoded by KSHV (which targets B cells and endothelial cells).
Regardless of their TSC inactivation mechanism, the fact that representative herpesvirus family members all target TSC signaling to manipulate mTORC1 is striking, highlighting the significance of the TSC regulatory intersection in regulating mTORC1 and implying that it is an intrinsic host defense component whose actions must be antagonized by a viral function. Other unrelated DNA viruses that produce capped, polyadenylated mRNAs also target TSC2, as the HPV E6 protein promotes TSC2 degradation (Lu et al. 2004). Furthermore, physiological dampening of cap-dependent translation to stresses—including hypoxia and energy depletion via AMPK and REDD1, respectively—all proceed through TSC (for review, see Ma and Blenis 2009). Importantly, having a virus-encoded function activate mTORC1 through TSC overrides p70 S6K-mediated cellular feedback controls in place to limit both receptor-mediated activation of the PI3K/Akt/mTORC1 signaling axis (Harrington et al. 2005) and mTORC2-mediated Akt activation (Dibble et al. 2009). Thus, activating mTORC1 via a PI3K-coupled cellular receptor would ultimately only be transient. Indeed, transient Akt activation early in the replication cycle is observed in primary human fibroblasts infected with wild-type HSV-1 or HCMV (Benetti and Roizman 2006; C McKinney, C Perez, and I Mohr, in prep. ; this study), although the precise mechanism responsible remains unknown. Intervening downstream from PI3K-linked receptors ensures constitutive mTORC1 activation throughout the viral replication cycle, while targeting TSC2 by a viral function allows Akt itself to potentially remain inactive. Such a design allows selective regulation of some, but not all, Akt targets by herpesvirus functions, and raises the possibility that not all Akt targets will be Us3 substrates. Nevertheless, inhibition of TSC function by Us3-stimulated TSC2 phosphorylation constitutively activates mTORC1, which in turn inactivates the 4E-BP1 translational repressor. This guarantees that the cap-binding protein eIF4E remains available for assembly into a multisubunit initiation factor complex critical for recruiting host 40S ribosomes, and defines a new strategy whereby herpesvirus-encoded functions intervene in critical cell signaling pathways that regulate mRNA translation. Finally, a new opportunity for preventing virus-mediated mTORC1 activation has been identified. Us3 represents a unique, potentially druggable viral kinase that lacks primary sequence homology with the cellular kinase Akt. While catalytic site mTOR inhibitors effectively antagonize viral replication in vitro, their anti-proliferative activity makes it likely that they will have substantial immunosuppressive side effects in vivo (Seghal 2003). Targeting the viral mTORC1 trigger bypasses this dilemma by selectively preventing mTORC1 activation in infected cells.
Materials and methods
Cell culture, viruses, and chemicals
All cells were propagated in 5% CO2 with Dulbecco's modified Eagle's medium (DMEM) supplemented with 2 mM glutamine, 50 U of penicillin per milliliter, and 50 μg of Streptomycin per milliliter, plus serum as specified: primary NHDFs (Clonetics) were maintained in 5% fetal bovine serum (FBS) and serum-deprived in 0.2% FBS as described (Walsh and Mohr 2004); U20S cells (American Type Culture Collection [ATCC]) were propagated in 10% FBS; 293 cells were propagated in 5% Fetalplex (Gemini) and serum-deprived for 24 h in 0.2% Fetalplex; Vero (ATCC) cells were propagated in 5% calf serum. F-strain wild-type and ΔUs3 (VRR1202), K220A Us3 mutant (VRR1204), and ΔUs3 repair (VRR1202repair) HSV1 were described (Ryckman and Roller 2004). UL13-deficient virus (d 13 lac z) was a gift from S. Rice. Antibodies Akt (#9272), Akt-pSer473 (#9271), Akt-pThr 308 (#9275), TSC2 (#3611), TSC2-pSer939 (#3615), TSC2-pThr1462 (#3611), AktpSub (#9611), p70 S6K T389 and total (#9430), FOXO1 (#2488), FOXO1-pSer256 (#9461), and GSK3α/β-pSer9/21 (#9331) were from Cell Signaling; anti-GSK3α/β (#05-903) was from Millipore; anti-thiophosphate ester was from Epitomics (2686-1); anti-HSV-1 tk was a gift from B. Roizman; and anti-FlagM2 Monoclonal and ATPγS (AI388) were from Sigma. All other antibodies were described (Walsh and Mohr 2004). Rapamycin, wortmannin, puromycin, and Akt inhibitor VIII were from Calbiochem; PP242 was described (Feldman et al. 2009); PNBM was from Epitomics (3700-1); and N6-Phenylethyl-ATPγS was from BioLog (P026).
Western blotting and m7GTP-Sepharose chromatography
These procedures were performed as described (Walsh and Mohr 2004; Walsh et al. 2005).
siRNA and plasmids
siGENOME SMARTpool targeting human TSC2 and siGENOME Non-Targeting siRNA #2 were from ThermoScientific. pcDNA3-FlagTSC2 (14129), pcDNA3-FlagTSC2SATA (14131), and pRK7-FlagTSC2 (8996) were from AddGene. pcDNA3-Us3-Flag was described previously (Ogg et al. 2004). pcDNA3-Us3-Flag K220A and L256G were constructed by standard techniques and verified by sequencing (R Roller and U Chuluunbaatar, unpubl.). Flag-HA-4E-BP1 wild-type and AA mutant cloned into the retroviral vector pBABE-puro were from R. Schneider.
Cell transfection and infection
NHDFs were seeded at 3 × 105cells per well in six-well dishes. The next day, cells were washed with PBS, serum-deprived for 72 h as described (Walsh and Mohr 2004), and then infected with HSV-1 (multiplicity of infection [MOI] = 5). HEK293 cells were seeded (4 × 105 cells per well in a 12-well dish), and the following day were transfected using Lipofectamine 2000 (Invitrogen) at 1:2.5 (w/v) ratio for 6 h, serum-deprived for 18 h, and infected with HSV-1 (MOI = 10). U20S cells were seeded (1.5 × 105 cells per well in a 12-well dish), and the next day were transfected as described above. After 6 h, fresh media was added. At 24 h post-transfection, cells were treated with 200 nM wortmannin, Akt inhibitor VIII, or DMSO for 30 min before collection. For multicycle growth experiments, cultures were infected with 100 plaque-forming units (pfu) per well. After 4 d, cell-free lysates were prepared by freeze–thawing and the virus produced was quantified by plaque assay in Vero cells.
siRNA depletion
NHDFs were seeded at 2.7 × 104 cells per well in 12-well dishes, and the next day were transfected with siRNA using DharmaFECTDuo (ThermoScientific) according to the manufacturer. After 24 h, the transfection procedure was repeated. Forty-eight hours later, cultures were serum-deprived as described (Walsh and Mohr 2004) and then infected with HSV-1 or EMCV.
Immunoprecipitation and in vitro kinase assay
HEK293 cells (60-mm dishes) were transfected with pRK7-FlagTSC2, pcDNA3-Us3Flag, or pcDNA3-K220AUs3Flag. DNA quantity was adjusted to achieve comparable Us3 and K220A expression. Twenty-four hours post-transfection, FlagTSC2-expressing cells were treated with wortmannin (200 nM for 30 min). Cells were lysed in 50 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1% NP-40, 0.5% deoxycholate, and 0.1% SDS plus Complete Mini protease inhibitor (Roche), and extracts were clarified by centrifugation (12,000g, for 10 min at 4°C). Soluble fractions from Flag TSC2 transfected cells were mixed 1:1 with an equivalent fraction from Us3Flag or K220AUs3Flag transfected cells. Protein G-Sepharose conjugated to anti-Flag antibody (10 μL of settled bed volume) was added and the mixture was rocked (2 h at 4°C). Immune complexes were collected by brief centrifugation, resuspended in buffer (10 mM Tris-HCl at pH 8.0, 1 M NaCl, 0.2% NP-40), rocked gently (15 min at 4°C), and then washed three times with 0.5 mL of Us3 kinase buffer (50 mM Tris-HCl at pH 9.0, 20 mM MgCl2, 0.1% NP-40) (Kato et al. 2005). Kinase reactions were performed in 30 μL of Us3 kinase buffer supplemented with 10 μM ATP, 0.5 mM DTT, and 10 μCi [γ-32P]ATP for 30 min at 30°C and stopped by adding equal volume of 2× sample buffer (125 mM Tris-HCl at pH 6.8, 4% SDS, 20% glycerol, 10% β-mercaptoethanol). Reaction mixtures were fractionated by SDS-PAGE and processed for immunoblotting or autoradiography. Two-hundred-fifty micromolar ATPγS or N6-Phenylethyl ATPγS was included in place of ATP where indicated. ATP analog-containing reactions were incubated for 1 h at 30°C, terminated by adding EDTA to 40 mM, and alkylated with 2.5 mM p-nitrobenzyl mesylate (PNBM) for 2 h at room temperature. After SDS-PAGE and immunoblotting, alkylated proteins were detected using anti-thiophosphate ester antibody.
Retroviral expression
HEK293 Gag-Pol packaging cells (80% confluent on 10-cm dishes) were transfected with 8 μg each of pCMV-VSV-G and pBABE-puro vectors using Lipofectamine 2000 (Invitrogen). Retrovirus-containing supernatants were harvested after 48 h, filtered (0.45 μm), and frozen at −80°C until use. Early passage NHDFs were infected with retrovirus-containing media plus polybrene (10 μg/mL) for 6 h. Puromycin (2 μg/mL) was added 48 hpi and the population was maintained in the presence of drug. Puromycin was removed from cultures before infecting with HSV-1.
Acknowledgments
We are grateful to B. Roizman, S. Rice, and R. Schneider for generously providing viruses and constructs. In addition, we thank C. Perez, C. McKinney, M. Kobayashi, M. Garabedian, and A. Wilson for many helpful discussions. This work was supported by grants from the NIH to I.M. (GM056927 and AI073898) and R.R. (AI41478). U.C. was supported in part by an NIH training grant (T32 AI07180). I.M. is a scholar of the Irma T. Hirshl Trust.
Footnotes
Article is online at http://www.genesdev.org/cgi/doi/10.1101/gad.1978310.
Supplemental material is available at http://www.genesdev.org.
References
- Allen JJ, Li M, Brinkworth CS, Paulson JL, Wang D, Hübner A, Chou WH, Davis RJ, Burlingame AL, Messing RO, et al. 2007. A semisynthetic epitope for kinase substrates. Nat Methods 4: 511–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias C, Walsh D, Harbell J, Wilson AC, Mohr I 2009. Activation of host translational control pathways by a viral developmental switch. PLoS Pathog 5: e1000334 doi: 10.1371/journal.ppat.1000334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benetti L, Roizman B 2004. Herpes simplex virus protein kinase US3 activates and functionally overlaps protein kinase A to block apoptosis. Proc Natl Acad Sci 101: 9411–9416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benetti L, Roizman B 2006. Protein kinase B/Akt is present in activated form throughout the entire replicative cycle of ΔU(S)3 mutant virus but only at early times after infection with wild-type herpes simplex virus 1. J Virol 80: 3341–3348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biondi RM, Nebreda AR 2003. Signaling specificity of ser/thr protein kinases through docking-site mediated interactions. Biochem J 372: 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braunstein S, Badura ML, Xi Q, Formenti SC, Schneider RJ 2009. Regulation of protein synthesis by ionizing radiation. Mol Cell Biol 29: 5645–5656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchkovich NJ, Yu Y, Zampieri CA, Alwine JC 2008. The TORrid affairs of viruses: Effects of mammalian DNA viruses on the PI3K–Akt–mTOR signaling pathway. Nat Rev Microbiol 6: 266–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calleja V, Laguerre M, Parker PJ, Larijani B 2009. Role of a novel PH-kinase domain interface in PKB/Akt regulation: Structural mechanism for allosteric inhibition. PLoS Biol 7: e17 doi: 10.1371/journal.pbio.1000017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dibble CC, Asara JM, Manning BD 2009. Characterization of Rictor phosphorylation sites reveals direct regulation of mTOR complex 2 by S6K1. Mol Cell Biol 29: 5657–5670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrikova E, Shveygert M, Walters R, Gromeier M 2009. Herpes simplex virus proteins ICP27 and UL47 associate with polyadenylate-binding protein and control its sub-cellular distribution. J Virol 84: 270–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elia A, Constantinou C, Clemens MJ 2008. Effects of protein phosphorylation on ubiquitination and stability of the translational inhibitor protein 4E-BP1. Oncogene 27: 811–822 [DOI] [PubMed] [Google Scholar]
- Feldman ME, Apsel B, Uotila A, Loewith R, Knight ZA, Ruggero D, Shokat KM 2009. Active-site inhibitors of mTOR target rapamycin-resistant outputs of mTORC1 and mTORC2. PLoS Biol 7: e38 doi: 10.1371/journal.pbio.1000038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsenstein J 1985. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 39: 783–791 [DOI] [PubMed] [Google Scholar]
- Gingras AC, Gygi SP, Raught B, Polakiewicz RD, Abraham RT, Hoekstra MF, Aebersold R, Sonenberg N 1999. Regulation of 4E-BP1 phosphorylation: A novel two-step mechanism. GenesDev 13: 1422–1437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington LS, Findlay GM, Lamb RF 2005. Restraining PI3K: mTOR signaling goes back to the membrane. Trends Biochem Sci 30: 35–42 [DOI] [PubMed] [Google Scholar]
- Kato A, Yamamoto M, Ohno T, Kodaira H, Nishiyama Y, Kawaguchi Y 2005. Identification of proteins phosphorylated directly by the Us3 protein kinase encoded by herpes simplex virus 1. J Virol 79: 9325–9331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato A, Yamamoto M, Ohno T, Tanaka M, Sata T, Nishiyama Y, Kawaguchi Y 2006. Herpes simplex virus 1-encoded protein kinase UL13 phosphorylates viral Us3 protein kinase and regulates nuclear localization of viral envelopment factors UL34 and UL31. J Virol 80: 1476–1486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudchodkar SB, Yu Y, Maguire TG, Alwine JC 2004. Human cytomegalovirus infection induces rapamycin-insensitive phosphorylation of downstream effectors of mTOR kinase. J Virol 78: 11030–11039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, et al. 2007. ClustalW and ClustalX version 2. Bioinformatics 23: 2947–2948 [DOI] [PubMed] [Google Scholar]
- Leach N, Bjerke SL, Christensen DK, Bouchard JM, Mou F, Park R, Baines J, Haraguchi T, Roller RJ 2007. Emerin is hyperphosphorylated and redistributed in herpes simplex virus type 1-infected cells in a manner dependent on both UL34 and US3. J Virol 81: 10792–10803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leader DP, Deana AD, Marchiori F, Purves FC, Pinna LA 1991. Further definition of the substrate specificity of the α-herpesvirus protein kinase and comparison with protein kinases A and C. Biochim Biophys Acta 1091: 426–431 [DOI] [PubMed] [Google Scholar]
- Lu Z, Hu X, Li Y, Zheng L, Zhou Y, Jiang H, Ning T, Basang Z, Zhang C, Ke Y 2004. Human papillomavirus 16 E6 oncoprotein interferences with insulin signaling pathway by binding to tuberin. J Biol Chem 279: 35664–35670 [DOI] [PubMed] [Google Scholar]
- Ma XM, Blenis J 2009. Molecular mechanisms of mTOR-mediated translational control. Nat Rev Mol Cell Biol 10: 307–318 [DOI] [PubMed] [Google Scholar]
- Manning BD, Tee AR, Logsdon MN, Blenis J, Cantley LC 2002. Identification of the tuberous sclerosis complex-2 tumor suppressor gene product tuberin as a target of the phosphoinositide 3-kinase/akt pathway. Mol Cell 10: 151–162 [DOI] [PubMed] [Google Scholar]
- Meignier B, Longnecker R, Mavromara-Nazos P, Sears AE, Roizman B 1988. Virulence of and establishment of latency by genetically engineered deletion mutants of herpes simplex virus 1. Virology 162: 251–254 [DOI] [PubMed] [Google Scholar]
- Mohr IJ, Pe'ery T, Mathews MB 2007. Protein synthesis and translational control during viral infection. In Translational control in biology and medicine (ed. Mathews MB, Sonenberg N, Hershey JWB), pp. 545–595 Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- Moorman NJ, Shenk T 2010. Rapamycin-resistant mTORC1 kinase activity is required for herpesvirus replication. J Virol 84: 5260–5269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorman NJ, Cristea IM, Terhune SS, Rout MP, Chait BT, Shenk T 2008. Human cytomegalovirus protein UL38 inhibits host cell stress responses by antagonizing the tuberous sclerosis protein complex. Cell Host Microbe 3: 253–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mou F, Forest T, Baines JD 2007. US3 of herpes simplex virus type 1 encodes a promiscuous protein kinase that phosphorylates and alters localization of lamin A/C in infected cells. J Virol 81: 6459–6470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munger J, Roizman B 2001. The US3 protein kinase of herpes simplex virus 1 mediates the posttranslational modification of BAD and prevents BAD-induced programmed cell death in the absence of other viral proteins. Proc Natl Acad Sci 98: 10410–10415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nei M, Kumar S 2000. Molecular evolution and phylogenetics. Oxford University Press, New York [Google Scholar]
- Nishiyama Y, Yamada Y, Kurachi R, Daikoku T 1992. Construction of a US3 lacZ insertion mutant of herpes simplex virus type 2 and characterization of its phenotype in vitro and in vivo. Virology 190: 256–268 [DOI] [PubMed] [Google Scholar]
- Ogg PD, McDonell PJ, Ryckman BJ, Knudson CM, Roller RJ 2004. The HSV-1 Us3 protein kinase is sufficient to block apoptosis induced by over-expression of a variety of Bcl-2 family members. Virology 319: 212–224 [DOI] [PubMed] [Google Scholar]
- Pawson T, Nash P 2000. Protein–protein interactions define specificity in signal transduction. GenesDev 14: 1027–1047 [PubMed] [Google Scholar]
- Poon AP, Gu H, Roizman B 2006. ICP0 and the US3 protein kinase of herpes simplex virus 1 independently block histone deacetylation to enable gene expression. Proc Natl Acad Sci 103: 9993–9998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remenyi A, Good MC, Lim WA 2006. Docking interactions in protein kinase and phosphatase networks. Curr Opin Struct Biol 16: 676–685 [DOI] [PubMed] [Google Scholar]
- Roizman B, Knipe DM, Whitley R 2007. Herpes simplex viruses. In Fields' virology, 5th ed (ed. Knipe DM, Howley PM), pp. 2501–2602 Lippincott, Williams & Wilkins, Philadelphia, PA [Google Scholar]
- Ryckman BJ, Roller RJ 2004. Herpes simplex virus type 1 primary envelopment: UL34 protein modification and the US3–UL34 catalytic relationship. J Virol 78: 399–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rzhetsky A, Nei M 1992. A simple method for estimating and testing minimum evolution trees. Mol Biol Evol 9: 945–967 [Google Scholar]
- Sagou K, Imai T, Sagara H, Uema M, Kawaguchi Y 2009. Regulation of the catalytic activity of herpes simplex virus 1 protein kinase Us3 by autophosphorylation and its role in pathogenesis. J Virol 83: 5773–5783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitou N, Nei M 1987. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol Biol Evol 4: 406–425 [DOI] [PubMed] [Google Scholar]
- Seghal SN 2003. Sirolimus: Its discovery, biological properties and mechanism of action. Transplant Proc 35: S7–S14 doi: 10.1016/S0041-1345(03)00211-2 [DOI] [PubMed] [Google Scholar]
- Sodhi A, Chaisuparat R, Hu J, Ramsdell AK, Manning BD, Sausville EA, Sawai ET, Molinolo A, Gutkind JS, Montaner S 2006. The TSC2/mTOR pathway drives endothelial cell transformation induced by the Kaposi's sarcoma-associated herpesvirus G protein-coupled receptor. Cancer Cell 10: 133–143 [DOI] [PubMed] [Google Scholar]
- Sonenberg N, Hinnebusch AG 2009. Regulation of translation initiation in eukaryotes: Mechanisms and biological targets. Cell 136: 731–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K, Dudley J, Nei M, Kumar S 2007. MEGA4: Molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol 24: 1596–1599 [DOI] [PubMed] [Google Scholar]
- Thoreen CC, Kang SA, Chang JW, Liu Q, Zhang J, Gao Y, Reichling LJ, Sim T, Sabatini DM, Gray NS 2009. An ATP-competitive mammalian target of rapamycin inhibitor reveals rapamycin-resistant functions of mTORC1. J Biol Chem 284: 8023–8032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh D, Mohr I 2004. Phosphorylation of eIF4E by Mnk-1 enhances HSV-1 translation and replication in quiescent cells. Genes Dev 18: 660–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh D, Mohr I 2006. Assembly of an active translation initiation factor complex by a viral protein. Genes Dev 20: 461–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh D, Perez C, Notary J, Mohr I 2005. Regulation of the translation initiation factor eIF4F by multiple mechanisms in human cytomegalovirus-infected cells. J Virol 79: 8057–8064 [DOI] [PMC free article] [PubMed] [Google Scholar]