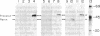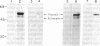Abstract
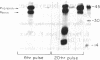
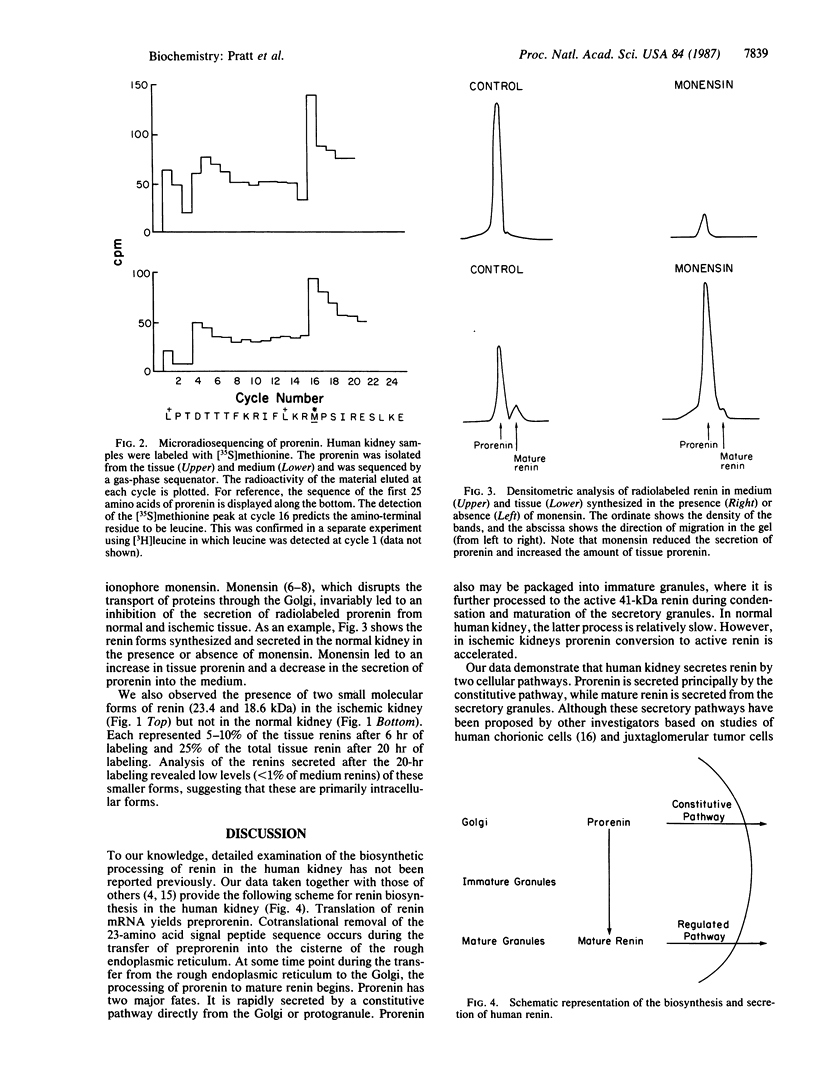
The pathway of renin biosynthesis and secretion in normal and ischemic human kidneys has been investigated by pulse-labeling experiments. The results indicate that in normal human kidney, preprorenin is rapidly processed to 47-kDa prorenin. Microradiosequencing showed that this molecule was generated by cleavage between Gly-23 and Leu-24, yielding a 43-amino acid proregion. Analysis of prorenin secreted by the kidney tissue yielded an identical sequence, indicating that prorenin is secreted without any further proteolysis. An examination of the kinetics of processing and secretion suggested that a majority of the newly synthesized prorenin is quickly secreted, while only a small fraction is processed intracellularly to the mature renin. The differences in secretion kinetics between prorenin and mature renin and the selective inhibition of prorenin secretion by monensin suggest that they are secreted independently via two pathways: a constitutive pathway probably from the Golgi or protogranules that rapidly release prorenin and a regulated pathway that secretes mature renin from the mature granules. A comparison of the kinetics of processing between normal and ischemic tissues suggests that renal ischemia leads to an overall increase in the rate of processing of prorenin to mature renin. In addition, prolonged biosynthetic labeling of renin in the ischemic kidney yielded two smaller molecular weight immunoreactive forms suggestive of renin fragments that may be degradative products. These fragments were not detected in normal kidney tissue labeled for similar lengths of time.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acker G. M., Galen F. X., Devaux C., Foote S., Papernik E., Pesty A., Menard J., Corvol P. Human chorionic cells in primary culture: a model for renin biosynthesis. J Clin Endocrinol Metab. 1982 Nov;55(5):902–909. doi: 10.1210/jcem-55-5-902. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P., Wilchek M., Anfinsen C. B. Selective enzyme purification by affinity chromatography. Proc Natl Acad Sci U S A. 1968 Oct;61(2):636–643. doi: 10.1073/pnas.61.2.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day R. P., Hui K. Y., Gure M., Carlson W. D., Dzau V. J., Haber E. A monoclonal antibody specific for the amino terminal sequence of human prorenin identifies a common epitope on renal and amniotic fluid inactive renins. J Hypertens. 1986 Jun;4(3):375–381. doi: 10.1097/00004872-198606000-00020. [DOI] [PubMed] [Google Scholar]
- Do Y. S., Shinagawa T., Tam H., Inagami T., Hsueh W. A. Characterization of pure human renal renin. Evidence for a subunit structure. J Biol Chem. 1987 Jan 25;262(3):1037–1043. [PubMed] [Google Scholar]
- Fritz L. C., Arfsten A. E., Dzau V. J., Atlas S. A., Baxter J. D., Fiddes J. C., Shine J., Cofer C. L., Kushner P., Ponte P. A. Characterization of human prorenin expressed in mammalian cells from cloned cDNA. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4114–4118. doi: 10.1073/pnas.83.12.4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galen F. X., Devaux C., Guyenne T., Menard J., Corvol P. Multiple forms of human renin. Purification and characterization. J Biol Chem. 1979 Jun 10;254(11):4848–4855. [PubMed] [Google Scholar]
- Galen F. X., Devaux C., Houot A. M., Menard J., Corvol P., Corvol M. T., Gubler M. C., Mounier F., Camilleri J. P. Renin biosynthesis by human tumoral juxtaglomerular cells. Evidences for a renin precursor. J Clin Invest. 1984 Apr;73(4):1144–1155. doi: 10.1172/JCI111300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose S., Kim S., Miyazaki H., Park Y. S., Murakami K. In vitro biosynthesis of human renin and identification of plasma inactive renin as an activation intermediate. J Biol Chem. 1985 Dec 25;260(30):16400–16405. [PubMed] [Google Scholar]
- Hobart P. M., Fogliano M., O'Connor B. A., Schaefer I. M., Chirgwin J. M. Human renin gene: structure and sequence analysis. Proc Natl Acad Sci U S A. 1984 Aug;81(16):5026–5030. doi: 10.1073/pnas.81.16.5026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunkapiller M. W., Lujan E., Ostrander F., Hood L. E. Isolation of microgram quantities of proteins from polyacrylamide gels for amino acid sequence analysis. Methods Enzymol. 1983;91:227–236. doi: 10.1016/s0076-6879(83)91019-4. [DOI] [PubMed] [Google Scholar]
- Imai T., Miyazaki H., Hirose S., Hori H., Hayashi T., Kageyama R., Ohkubo H., Nakanishi S., Murakami K. Cloning and sequence analysis of cDNA for human renin precursor. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7405–7409. doi: 10.1073/pnas.80.24.7405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Madoff D. H., Lenard J. A membrane glycoprotein that accumulates intracellularly: cellular processing of the large glycoprotein of LaCrosse virus. Cell. 1982 Apr;28(4):821–829. doi: 10.1016/0092-8674(82)90061-7. [DOI] [PubMed] [Google Scholar]
- Slater E. E., Strout H. V., Jr Pure human renin. Identification and characterization and of two major molecular weight forms. J Biol Chem. 1981 Aug 10;256(15):8164–8171. [PubMed] [Google Scholar]
- Strous G. J., Lodish H. F. Intracellular transport of secretory and membrane proteins in hepatoma cells infected by vesicular stomatitis virus. Cell. 1980 Dec;22(3):709–717. doi: 10.1016/0092-8674(80)90547-4. [DOI] [PubMed] [Google Scholar]
- Tartakoff A., Vassalli P., Détraz M. Comparative studies of intracellular transport of secretory proteins. J Cell Biol. 1978 Dec;79(3):694–707. doi: 10.1083/jcb.79.3.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taugner R., Kim S. J., Murakami K., Waldherr R. The fate of prorenin during granulopoiesis in epithelioid cells. Immunocytochemical experiments with antisera against renin and different portions of the renin prosegment. Histochemistry. 1987;86(3):249–253. doi: 10.1007/BF00490255. [DOI] [PubMed] [Google Scholar]