Abstract
Purpose
To assess the validity of individual and combined prognostic effects of severe bronchopulmonary dysplasia (BPD), brain injury, retinopathy of prematurity (ROP), and parenteral nutrition associated cholestasis (PNAC).
Methods
We retrospectively analyzed the medical records of 80 extremely low birthweight (ELBW) infants admitted to the neonatal intensive care unit (NICU) of the Severance Children's Hospital, and who survived to a postmenstrual age of 36 weeks. We analyzed the relationship between 4 neonatal morbidities (severe BPD, severe brain injury, severe ROP, and severe PNAC) and poor outcome. Poor outcome indicated death after a postmenstrual age of 36 weeks or survival with neurosensory impairment (cerebral palsy, delayed development, hearing loss, or blindness) between 18 and 24 months of corrected age.
Results
Each neonatal morbidity correlated with poor outcome on univariate analysis. Multiple logistic regression analysis revealed that the odds ratios (OR) were 4.9 (95% confidence interval [CI], 1.0-22.6; P=0.044) for severe BPD, 13.2 (3.0-57.3; P<.001) for severe brain injury, 5.3 (1.6-18.1; P=0.007) for severe ROP, and 3.4 (0.5-22.7; P=0.215) for severe PNAC. Severe BPD, brain injury, and ROP were significantly correlated with poor outcome, but not severe PNAC. By increasing the morbidity count, the rate of poor outcome was significantly increased (OR 5.2; 95% CI, 2.2-11.9; P<.001). In infants free of the above-mentioned morbidities, the rate of poor outcome was 9%, while the corresponding rates in infants with 1, 2, and more than 3 neonatal morbidities were 46%, 69%, and 100%, respectively.
Conclusion
In ELBW infants 3 common neonatal mornidifies, severe BPD, brain injury and ROP, strongly predicts the risk of poor outcome.
Keywords: Extremely low birth weight infant, Morbidity, Outcome assessment, Mortality
Introduction
With advances in perinatal and neonatal intensive care, the survival rate of extremely low birthweight (ELBW) infants has increased remarkably. The estimated survival rate of ELBW infants was higher than 70% in Japan, America and other countries in early 20001-3), and 62.4% in Korea and 89% in Severance Hospital in 20071). The long term quality of life in ELBW infants therefore needs much consideration; survivors remain at high risk of late death and neurodevelopmental impairments, such as cerebral palsy (CP), cognitive delay, deafness and blindness4-6).
However, predicting the long term outcome of ELBW infants is still difficult7, 8). Current risk scoring systems such as the Score for Neonatal Acute Physiology (SNAP) and Clinical Risk Index for Babies (CRIP)9-12) are limited during the first few days and weeks after birth because their predictability is compounded by the ongoing influence of neonatal morbidities and adverse events due to prolonged hospitalization8,13). Bronchopulmonary dysplasia (BPD) is one of the major neonatal morbidities which have an impact on poor outcome, such as cerebral palsy, blindness and late death14,15). Severe intraventricular hemorrhage (IVH), extensive periventricular leukomalasia (PVL), and posthemorrhagic hydrocephalus requiring shunt insertion are also related to poor cognitive outcomes15,16). Retinopathy of prematurity (ROP) is associated with later visual impairment17). Additionally, recent reports have mentioned the association between undernutrition during brain growth and neurodevelopmental outcome18,19).
Since most ELBW infants are affected with several morbidities in the same period7, 8), it may not be possible to predict poor long term outcomes from individual neonatal morbidities. So Schmidt et al.7) made attempts to predict long term outcomes by the combination of 3 neonatal morbidities. They reported that 3 neonatal morbidities (severe BPD, severe brain injury and severe ROP) are individually or collectively correlated with poor outcomes such as late death or survival with CP, delayed development, hearing loss, and blindness at 18 months of corrected age. They showed that the higher the morbidity count, the higher the risk of poor outcome. Consequently they were able to establish a simple logistic model that predicts poor outcome using the morbidity count. Bassler et al.8) went on to report the mediated effects of neonatal infection on this logistic model.
This model suggested by Schmidt et al.7) is unique in that it applies a combination of neonatal morbidities to long term prognosis prediction. Such analysis has yet to be reported in Korea. In our study, severe parenteral nutrition associated cholestasis (PNAC) that reflects undernutrition was added to the three major neonatal morbidities. We undertook this study to examine the individual and combined prognostic impact of severe BPD, severe brain injury, severe ROP, and severe PNAC on the long term outcome of ELBW infants hospitalized at the Severance Neonatal Intensive Care Unit (NICU).
Materials and methods
1. Subjects
Two hundred and seventy-two ELBW infants weighing less than 1,000 g were hospitalized at the NCIU of Severance Children's Hospital, Yonsei University College of Medicine between January 1, 1997 and December 31, 2007. The medical records of 111 patients surviving longer than a postmenstrual age of 36 weeks were examined retrospectively. Of these, follow-up observation records were available for 80 patients. These 80 patients were enrolled in the study.
2. Methods
1) Examination of neonatal morbidities
The neonatal morbidities of ELBW infants were examined by dividing them to 4 categories: (1) BPD more than moderate severity, (2) severe brain injury such as PVL or IVH higher than grade 3, (3) severe ROP, and (4) severe PNAC.
BPD was defined as the need for supplemental oxygen at a postmenstrual age of 36 weeks. According to severity based classification20), severe BPD was defined as requiring either 28 days of supplemental oxygen therapy with more than 30% oxygen, or nasal continuous positive airway pressure (CPAP) or positive pressure ventilation at 36 weeks' postmenstrual age or discharge, whichever came first, in infants whose gestational age was younger than 32 weeks, or at 56 days' postnatal age or discharge, whichever came first, in infants whose gestational age was older than 32 weeks. Severe brain injury was defined as confirmed PVL by brain magnetic resonance imaging (cases only suspected by brain ultrasonography were excluded). Cases with IVH higher than grade 3 confirmed by brain ultrasonography or brain magnetic resonance imaging were included. ROP was diagnosed and classified according to the International Classification of Acute Retinopathy of Prematurity (ICROP)21). ROP cases showing the plus sign or those which underwent surgical treatment such as cryotherapy or laser photocoagulation were defined as severe ROP. Severe PNAC was defined as cholestasis development secondary to total parenteral nutrition, with a direct bilirubin value higher than 5.0 mg/dL and persistent for longer than one month, in association with increased liver enzyme values (>70 IU/L).
2) Examination of poor prognosis between 18 months and 24 months
The poor outcome of ELBW infants was divided broadly into two categories and examined. The two categories were (1) whether the infant died after a postmenstrual age of 36 weeks and (2) whether the findings of impairment such as CP, delayed development, hearing loss, blindness, etc. were found between the corrected ages of 18 months and 24 months. CP and delayed development were determined based on the evaluation of specialists of the rehabilitation department at our hospital. CP was defined as non-progressive motor disorder resulting in physical disability, chiefly in the area of body movement. In addition to such CP, the cases with developmental delay of more than 2 standard deviations (95%) on neurologic examination and which underwent rehabilitation as well as physical therapy were included in the 'delayed development' category. Hearing loss was defined as more than 55dB by brainstem evoked response audiometry (BERA)22). Blindness was defined as corrected vision of 20/200 or lower with almost absent peripheral vision based on the judgment of ophthalmologists of our hospital.
3) Statistical analysis
The association of the 4 neonatal severe morbidities with ELBW infants was analyzed by Chi-square test (or Fisher's exact test) and multiple logistic regression analysis was performed. For statistical analysis, the SAS v9.1 (SAS Institute Inc, Cary, NC) was used. A P value of less than 0.05 was considered to be statistically significant.
Results
1. General characteristic of the subject patients and their mother
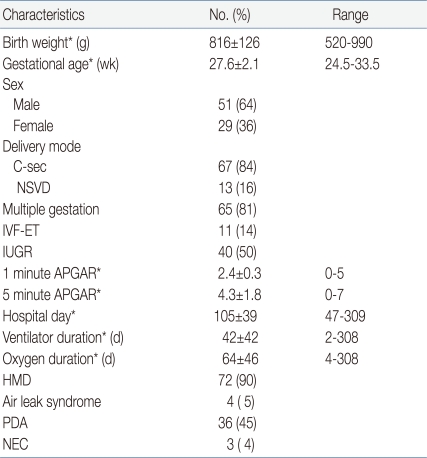
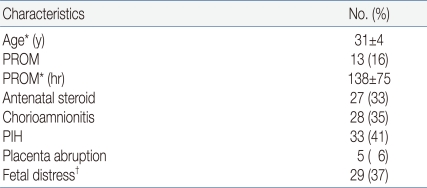
The average gestational age of the subject infants was 27.6±2.1 (24.5-33.5) weeks, and the mean birth weight was 816±126 g (520-990). 51 (64%) infants were male, 29 (36%) were female and 14% were born by the IVF-ET procedure (Table 1). The mean age of mothers was 31±4 years with premature rupture of membrane in 13 patients (16%); 27 mothers (33%) were administered steroids prior to birth, and 28 had chorioamnionitis confirmed pathologically or by bacteria culture (Table 2).
Table 1.
Baseline Characteristics of Infants
*Values are mean±SD
Abbreviations: SD, standard deviation; C-sec, cesarean delivery; NSVD, normal spontaneous vaginal delivery; IVF-ET, in vitro fertilization and embryo transfer; IUGR, intrauterine growth restriction; APGAR, Apgar score; HMD, hyaline membrane disease; PDA, patent ductus arteriosus; NEC, necrotizing enterocolitis
Table 2.
Baseline Characteristics of Mothers
*Values are mean±SD
†Increased or decreased fetal heart rate during a contraction or late deceleration on non-stress test (NST)
Abbreviations: SD, standard deviation; PROM, premature rupture of membranes; PIH, pregnancy induced hypertension
2. The relationship of neonatal morbidities and poor prognosis
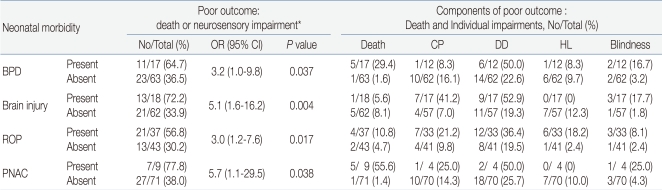
The association of the 4 neonatal morbidities (severe BPD, severe brain injury, severe ROP, and severe PNAC) with death occurred after the postmenstrual age of 36 weeks and CP, delayed development, blindness, and hearing loss developing between 18 months and 24 months were analyzed, and the odds ratio (OR) obtained. In univariate analysis, the OR of severe BPD was 3.2 (95% Confidence interval [CI], 1.0-9.8, P=0.037), the OR of severe brain injury was 5.1 (95% CI, 1.6-16.2, P=0.004), the OR of severe ROP was 3.0 (95% CI, 1.2-7.6, P=0.017), and the OR of severe PNAC was 5.7 (95% CI, 1.1-29.5, P=0.038), and these were significantly associated with poor long term outcomes.
The number of patients of each category of poor outcome and their percentage were distinguished and presented. Of the 17 ELBW infants who developed BPD, 5 infants died after a postmenstrual age of 36 weeks and thus the mortality rate was 29.4%. Of the 18 patients who developed brain injury, 1 patient died after a postmenstrual age of 36 weeks (5.6%). Of the 17 remaining patients, 7 patients had CP (41.2%). Of the 37 patients who developed ROP, 4 patients died (10.8%) and 3 showed blindness (8.1%) (Table 3).
Table 3.
Univariate Relationships Between Individual Neonatal Morbidity and Outcome at 18-24 Months of Corrected Age
*The rate of poor outcome in the entire cohort was 34/80 (43%)
Abbreviations: OR, odds ratio; Cl, confidence interval; CP, cerebral palsy; DD, delayed development; HL, hearing loss; BPD, bronchopulmonary dysplasia; ROP, retinopathy of prematurity; PNAC, parenteral nutrition associated cholestasis
3. The significance of the combination of morbidities for long term prognosis
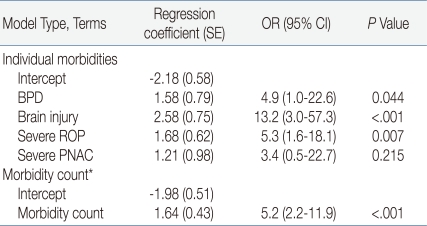
To adjust the effect of neonatal morbidities on each other, multiple logistic regression analysis was performed on the relationship of the 4 neonatal morbidities and poor long term prognosis. Severe BPD, severe brain injury, and severe ROP were significantly associated with poor long term outcomes, and the odds ratio was more increased [BPD, OR 4.9 (95% CI, 1.0-22.6), P=0.044; brain injury, OR 13.2 (95% CI, 3.0-57.3), P<.001; severe ROP, OR 5.3 (95% CI, 1.6-18.1), P=0.007]. After the removal of the effect of neonatal morbidities affecting each other, the correlation of the 3 neonatal morbidities to poor outcomes became higher than in univariate analysis. However, in multiple logistic regression analysis, PNAC was not significantly associated with poor long term outcomes [OR 3.4 (95% CI, 0.5-22.7), P=0.215]. As the morbidity count increased, the rate of poor long term outcomes also increased and they were significantly associated [OR 5.2 (95% CI, 2.2-11.9), P<0.001] (Table 4).
Table 4.
Logistic Regression Analysis of Long-term Outcome
*Comprises the number present of BPD, brain injury, severe ROP and severe PNAC.
Abbreviations: OR, odds ratio; Cl, confidence interval; BPD, bronchopulmonary dysplasia; ROP, retinopathy of prematurity; PNAC, parenteral nutrition associated cholestasis
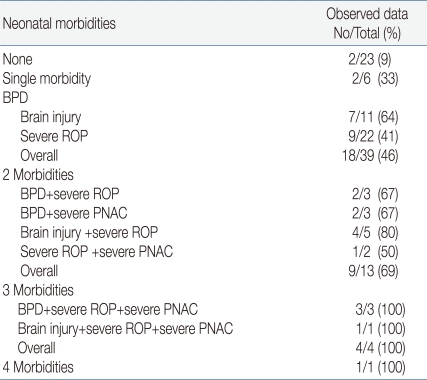
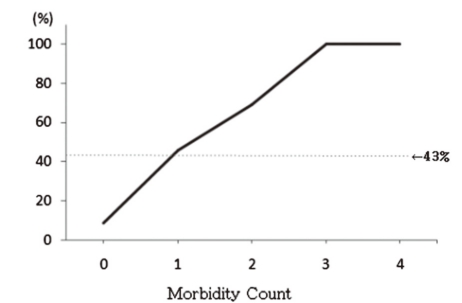
Examining the rate of poor outcomes according to the combination of neonatal morbidities, it was found that in cases who were not affected by the 4 neonatal morbidities, 9% showed poor outcomes. On the other hand, poor outcome was seen in 46% of cases affected with any one of these 4 morbidities, 69% of cases affected with 2 morbidities of any combination, and 100% in cases affected with 3 morbidities of any combination (Table 5, Fig. 1).
Table 5.
Observed and Predicted Poor Outcome at 18-24 Months of Corrected Age in the Case of Combined Neonatal Morbidities
Abbreviations: BPD, bronchopulmonary dysplasia; ROP, retinopathy of prematurity; PNAC, parenteral nutrition associated cholestasis
Fig. 1.
Probability of poor outcome in the study infants with 0, 1, 2, 3 and all 4 neonatal morbidities. Observed rates of poor outcome between 18-24 months of corrected age. Solid line indicates predictions based on the fitted morbidity count model; dotted line (←), the overall probability of a poor outcome between 18-24 month of corrected age (43%).
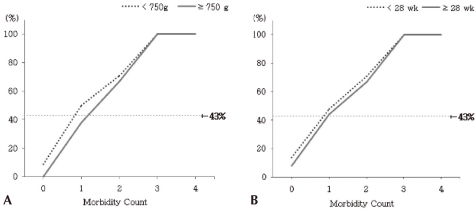
Additionally, the morbidity count predicted the poor outcomes equally well in each of the 2 strata for birth weight (<750 g) and gestational age (<28 weeks) (Fig. 2).
Fig. 2.
Probability of poor outcome by morbidity count, stratified by birth weight (A) and gestational age (B). Observed rates of poor outcome. Dotted lines (←) indicate the overall probability of a poor 18-24 month outcome (43%).
Discussion
The increased survival rate of ELBW infants highlights the necessity of the consideration of quality of life in ELBW infants1-6). We examined the prognostic impact of severe BPD, severe brain injury, severe ROP, and severe PNAC on poor outcomes such as late death or neurodevelopmental impairment in ELBW infants between 18 and 24 months of corrected age.
Until now, birth weight and gestational age have traditionally been used as strong indicators for the risk of neonatal death13). Scoring systems use physiologic abnormalities (hypotension-hypertension, acidosis, hypoxia, hypercapnia, anemia, neutropenia), as in the Score for Neonatal Acute Physiology (SNAP)9), or clinical parameters (gestational age, birth weight, anomalies, acidosis, FiO2), as in the Clinical Risk Index for Babies (CRIB)10). CRIB includes six parameters collected in the first 12 hour after birth, and SNAP has 26 variables collected in the first 24 hours. While these risk scoring systems may provide prognostic information for mortality, objective risk scores provide similar predictability when compared with the clinical judgment of experienced neonatologists based on birth weight, illness severity, low Apgar score, and therapeutic requirements9-13). Furthermore, such scores may not be useful for predicting long term outcome in survivors because their predictability is confused by the ongoing influence of neonatal morbidities during prolonged hospitalization8, 13, 15).
BPD has been associated with low psychomotor development index scores and neurologic abnormalities including CP, poor mental development, blindness and late death. Frequent episodes of hypoxia secondary to airway constriction, poor caloric intake due to prolonged ventilation, frequent respiratory diseases, increase of the use of corticosteroid after birth, accompanying diseases such as ischemic encephalopathy, PVL, and IVH in BPD survivors could be causative factors in developmental delay14, 15). Severe brain injury is associated with poorer intellectual outcome. Severe IVH (grade III or IV), progressive hydrocephalus requiring ventricular peritoneal shunting, intraparenchymal hemorrhage, and extensive PVL are each independently associated with a poor prognosis (CP and abnormal psychomotor, or cognitive outcome) while the majority of infants with grade I or II IVH have normal neurodevelopmental outcome15, 16). ROP is also associated with later visual impairment and functional disability17).
However, the clinical usefulness of each of these morbidities in predicting outcome is limited8). Up to 30% of ELBW infants with normal cranial ultrasound have CP or low cognitive performance at 18 months of corrected age16). In our study, similarly, approximately 35.3% of patients affected by BPD showed good prognosis and 36.5% of patients unaffected by BPD showed poor outcomes such as death or neurological developmental impairment. This is because most ELBW infants have not only one morbidity but several neonatal morbidities in the same period8).
Schmidt et al.7) therefore made attempts to predict long term outcomes by the combination of 3 neonatal morbidities (severe BPD, severe brain injury and severe ROP). They showed that 3 neonatal morbidities are individually or collectively correlated with poor outcomes such as late death or survival with CP, delayed development, hearing loss, and blindness at 18 months of corrected age. More specifically, in infants who were free of severe BPD, brain injury and ROP, the rate of poor long term outcome was 18% and corresponding rates with any 1, any 2, and all neonatal morbidities were 42%, 62% and 88%, respectively. This shows that the higher the morbidity count, the higher the risk of poor outcome. A simple count of 3 neonatal morbidities strongly predicts the risk of later death or neurosensory impairment. Therefore they established a simple logistic model that predicts poor outcome by using morbidity count. After this, Bassler et al.8) reported the mediated effects of neonatal infection on this logistic model. Neonatal infection is also significantly associated with poor outcomes such as late death or survival with neurosensory impairment. However, infection was a weaker predictor of poor outcome than BPD, brain injury, and severe ROP. When any type of infection was added to the 3 morbidity-count model suggested by Schmidt et al., the rates of poor prognosis were further increased. More specifically, in the sepsis group, the rate of poor long term outcome in infants who were free of severe BPD, brain injury, and ROP was 18% and corresponding rates with any 1, any 2 and all neonatal morbidities were 50%, 68% and 100%, respectively8).
Related studies have yet to be reported in Korea. Our study was conducted to see if the morbidity count model of Schmidt et al.7) could be applied to ELBW infants hospitalized at the Severance NICU. Additionally, we added severe PNAC to this model based on recent reports that undernutrition during brain growth is associated with neurodevelopmental deficits as we thought that severe PNAC may reflect undernutrition18, 19). Latal-Hajnal et al.18) have demonstrated that postnatal growth pattern, not being small for gestational age (SGA) at birth, is significantly associated with poor neurodevelopmental outcome at 24 months of corrected age. SGA infants who underwent insufficient catch-up growth and appropriate for gestational age (AGA) infants who underwent catch-down growth had significantly poorer mental and motor function than SGA infants who underwent substantial catch-up growth and AGA infants whose weight remained appropriate for age from birth to 24 months. The most recent reviews note that glutathione, ascorbic acid (vitamin C), riboflavin (vitamin B2) and tocopherol (vitamin E) deficiency during brain growth may induce brain injury secondary to oxidative stress and free radicals. These nutrients are major components of the body's antioxidant system19).
Our study confirms previous reports that severe BPD, severe brain injury, and severe ROP increase the risk of poor outcome in ELBW infants. Severe PNAC was significantly associated with poor outcome in univariate analysis, but was not significantly associated with poor outcome in multiple logistic regression analysis performed to eliminate the effect of the 3 other neonatal morbidities. In our study, in infants who were free of severe BPD, brain injury, ROP and PNAC, the rate of poor outcomes was 9%. Corresponding rates with any 1, any 2 and more than 3 neonatal morbidities were 46%, 69% and 100%, respectively. It also was found that higher morbidity counts were associated with greater risk of poor outcome. Compared with infants with no morbidity, infants with 1 morbidity have approximately double the risk of poor outcome, while 2 morbidities approximately triple the risk. This implies that 4 morbidities would have a similar effect on long term outcome.
In infants who were not affected by any one of these 4 morbidities, the rate of poor prognosis was 9% in our study (18% was shown in the study of Schmidt et al.). According to our definition, this portion may include infants affected by less than severe BPD, brain injury, ROP, or PNAC or neonatal infection and sepsis which are closely associated with poor outcome.
The significance of this study is the predictability of poor outcome for ELBW infants from measurement of simple morbidity count. Moreover, even the smallest and most immature infants have a favorable long term outcome if they survive the NICU stay without serious adverse events and morbidities such as BPD or ROP.
In this study, we were not able to establish a logistic morbidity model due to the small cohort size. However, in terms of baseline characteristics, the incidences were comparable to other studies23, 24).
In the future, the morbidity count model needs to be proven to be practical by a prospective randomized large scale cohort study. It may then become a useful tool for medical teams planning long term treatment and parent counseling for ELBW infants.
References
- 1.Kim KS, Bae CW. Trends in survival rate for very low birth weight infants and extremely low birth weight infants in Korea (1967-2007) Korean J Pediatr. 2008;51:237–242. [Google Scholar]
- 2.Kusuda S, Fugimura M, Sakuma I, Aotani H, Kabe K, Itani Y, et al. Morbidity and mortality of infants with very low birth weight in Japan: center variation. Pediatrics. 2006;118:e1130–e1138. doi: 10.1542/peds.2005-2724. [DOI] [PubMed] [Google Scholar]
- 3.Fanaroff AA, Stoll BJ, Wright LL, Carlo WA, Ehrenkranz RA, Stark AR, et al. Trends in neonatal morbidity and mortality for very low birth weight infants. Am J Obstet Gynecol. 2007;196:147.e1-8. doi: 10.1016/j.ajog.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 4.Wilson-Costello D, Friedman H, Minich N, Siner B, Taylor G, Schluchter M, et al. Improved neurodevelopmental outcomes for extremely low birth weight infants in 2000-2002. Pediatrics. 2007;119:37–45. doi: 10.1542/peds.2006-1416. [DOI] [PubMed] [Google Scholar]
- 5.Valcamonico A, Accorsi P, Sanzeni C, Martelli P, La-Boria P, Cavazza A, et al. Mid- and long term outcome of extremely low birth weight (ELBW) infants: an analysis of prognostic factors. J Matern Fetal Neonatal Med. 2007;20:465–471. doi: 10.1080/14767050701398413. [DOI] [PubMed] [Google Scholar]
- 6.Wood NS, Marlow N, Costeloe K, Gibson AT, Wilkinson AR. Neurologic and developmental disability after extremely preterm birth. N Engl J Med. 2000;343:378–384. doi: 10.1056/NEJM200008103430601. [DOI] [PubMed] [Google Scholar]
- 7.Schmidt B, Asztalos EV, Roberts RS, Robertson CM, Sauve RS, Whitfield MF. Impact of bronchopulmonary dysplasia, brain injury, and severe retinopathy on the outcome of extremely low-birth-weight infants at 18 months. JAMA. 2003;289:1124–1129. doi: 10.1001/jama.289.9.1124. [DOI] [PubMed] [Google Scholar]
- 8.Bassler D, Stoll BJ, Schmidt B, Asztalos EV, Roberts RS, Robertson CMT, et al. Using a count of neonatal morbidities to predict poor outcome in extremely low birth weight infants: added role of neonatal infection. Pediatrics. 2009;123:313–318. doi: 10.1542/peds.2008-0377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maiya PP, Nagashree S, Shaik MS. Role of score for neonatal acute physiology (SNAP) in predicting neonatal mortality. Indian J Pediatr. 2001;68:829–834. doi: 10.1007/BF02762105. [DOI] [PubMed] [Google Scholar]
- 10.Kaaresen PI, Døhlen G, Fundingsrud HP, Dahl LB. The use of CRIB (clinical risk index for babies) score in auditing the performance of one neonatal intensive care unit. Acta Paediatr. 1998;87:195–200. doi: 10.1080/08035259850157660. [DOI] [PubMed] [Google Scholar]
- 11.Parry G, Tucker J, Tarnow-Mordi W UK Neonatal Staffing Study Collaborative Group. CRIB II: an update of the clinical risk index for babies score. Lancet. 2003;361:1789–1791. doi: 10.1016/S0140-6736(03)13397-1. [DOI] [PubMed] [Google Scholar]
- 12.Slater A, Shann F, Pearson G PIM Study Group. PIM2: a revised version of the paediatric index of mortality. Intensive Care Med. 2003;29:278–285. doi: 10.1007/s00134-002-1601-2. [DOI] [PubMed] [Google Scholar]
- 13.Stoll BJ, Adams-Chapman I. The high risk infants. In: Kliegman RM, Behrman RE, Jenson HB, Stanton BF, editors. Nelson textbook of pediatrics. 18th ed. Philadelphia: Saunders Co; 2007. pp. 698–711. [Google Scholar]
- 14.Bregman J, Farrell EE. Neurodevelopmental outcome in infants with bronchopulmonary dysplasia. Clin Perinatol. 1992;19:673–694. [PubMed] [Google Scholar]
- 15.Vohr BR, Wright LL, Dusick AM, Mele L, Verter J, Steichen JJ, et al. Neurodevelopmental and functional outcomes of extremely low birth weight infants in the national institute of child health and human development neonatal research network, 1993-1994. Pediatrics. 2000;105:1216–1226. doi: 10.1542/peds.105.6.1216. [DOI] [PubMed] [Google Scholar]
- 16.Adams-Chapman I, Stoll BJ. Nervous system disorders. In: Kliegman RM, Behrman RE, Jenson HB, Stanton BF, editors. Nelson textbook of pediatrics. 18th ed. Philadelphia: Saunders Co; 2007. pp. 713–722. [Google Scholar]
- 17.Olitsky SE, Hug D, Smith LP. Disorders of the retina and vitreous. In: Kliegman RM, Behrman RE, Jenson HB, Stanton BF, editors. Nelson textbook of pediatrics. 18th ed. Philadelphia: Saunders Co; 2007. pp. 2598–2605. [Google Scholar]
- 18.Yeung MY. Postnatal growth, neurodevelopment and altered adiposity after preterm birth-from a clinical nutrition perspective. Acta Paediatr. 2006;95:909–917. doi: 10.1080/08035250600724507. [DOI] [PubMed] [Google Scholar]
- 19.Yeung MY. Influence of early postnatal nutritional management on oxidative stress and antioxidant defence in extreme prematurity. Acta Paediatr. 2006;95:153–163. doi: 10.1080/08035250500301133. [DOI] [PubMed] [Google Scholar]
- 20.Ehrenkranz RA, Walsh MC, Vohr BR, Jobe AH, Wright LL, Fanaroff AA, et al. Validation of the national institutes of health consensus definition of bronchopulmonary dysplasia. Pediatrics. 2005;116:1353–1360. doi: 10.1542/peds.2005-0249. [DOI] [PubMed] [Google Scholar]
- 21.The Committee for the Classification of Retinopathy of Prematurity. An international classification of retinopathy of prematurity. Arch Ophthalmol. 1984;102:1130–1134. doi: 10.1001/archopht.1984.01040030908011. [DOI] [PubMed] [Google Scholar]
- 22.Kryter KD. Hearing loss from gun and railroad noise-relations with ISO standard 1999. J Acoust Soc Am. 1991;90:3180–3195. doi: 10.1121/1.401427. [DOI] [PubMed] [Google Scholar]
- 23.Hack M, Fanaroff AA. Outcomes of children of extremely low birthweight and gestational age in the 1990s. Semin Neonatol. 2000;5:89–106. doi: 10.1053/siny.1999.0001. [DOI] [PubMed] [Google Scholar]
- 24.Tommiska V, Heinonen K, Lehtonen L, Renlund M, Saarela T, Tammela O, et al. No improvement in outcome of nationwide extremely low birth weight infant population between 1996-1997 and 1999-2000. Pediatrics. 2007;119:29–36. doi: 10.1542/peds.2006-1472. [DOI] [PubMed] [Google Scholar]