Abstract
A new genus of specialized pro-resolving mediators (SPM) which include several families of distinct local mediators (lipoxins, resolvins, protectins, and maresins) are actively involved in the clearance and regulation of inflammatory exudates to permit restoration of tissue homeostasis. Classic lipid mediators that are temporally regulated are formed from arachidonic acid, and novel local mediators were uncovered that are biosynthesized from ω-3 poly-unsaturated fatty acids, such as eicosapentaenoic acid, docosapentaenoic acid and docosahexaenoic acid. The biosynthetic pathways for resolvins are constituted by fatty acid lipoxygenases and cyclooxygenase-2 via transcellular interactions established by innate immune effector cells which migrate from the vasculature to inflamed tissue sites. SPM provide local control over the execution of an inflammatory response towards resolution, and include recently recognized actions of SPM such as tissue protection and host defense. The structural families of the SPM do not resemble classic eicosanoids (PG or LT) and are novel structures that function uniquely via proresolving cellular and molecular targets. The extravasation of inflammatory cells expressing SPM biosynthetic routes are matched by the temporal provision of essential fatty acids from circulation needed as substrate for the formation of SPM. The present review provides an update and overview of the biosynthetic pathways and actions of SPM, and examines resolution as an integrated component of the inflammatory response and its return to homeostasis via biochemically active resolution mechanisms.
Keywords: Anti-inflammatory, apoptosis, aspirin, inflammation, leukocyte, lipid mediator, protectins, resolution, resolvins
The inflammatory response
The inflammatory response is a local reaction of the vasculature towards a disturbance of tissue homeostasis incurred by damage to tissue structure and infection [1,2,3]. Changes in local blood vessel perfusion and permeability permit the directional extravasation of circulating leukocytes that achieve tissue disinfection, and of a range of plasma proteins which play distinct roles in regulating the inflammatory process. Structural alterations of tissue structure that activate the inflammatory response can be brought about by a variety of energetic interactions between a tissue and an exogenous force that tears, shears, or wears on its integrity, e.g. radiation (sunlight), heat (a burn), a cut (a bite), enzymatic proteolysis (some allergens), and chemical modification. Superimposed infections by exogenous microorganisms are sensed by an array of innate immune receptors (toll-like receptors and C-type lectins), triggering the mounting of a granulocyte-dominated acute inflammatory response, as well as by adaptive immune responses that are appropriate for elimination of the inciting stimulus [4,5,6,7].
Exposure to noxious insults and infections is effectively minimized by learned behavior, through involuntary local and centrally mediated neural reflexes, and by conscious avoidance following their sensation. If tissue integrity is breached regardless, the inflammatory response is activated rapidly (within minutes). The principal objectives of this response are the delivery of blood-borne phagocytes (neutrophils and monocytes) to the affected tissue in order to increase the local tissue concentration of cells that can remove the inciting tissue-damaging stimulus, as well as the regulation of antigen-specific adaptive immune responses [8,9]. The directed migration of blood-borne leukocytes to the inflammatory locus, a transient increase in vascular permeability which facilitates plasma exudation, and phagocytosis of microbes and dying cells are central events during the pro-inflammatory phase of the inflammatory response (Fig. 1) [8,10,11]. Of importance, the acute inflammatory response is self-limiting and in a normal course of events should lead to complete tissue restoration and homeostasis. Specific pathogen-mediated modulation of the host immune response, unrelenting exposure to inflammatory stimuli, and molecular defects in the inflammatory response can redirect the physiological resolution of an inflammatory exudate towards the formation of a granuloma, fibrogenesis or scar formation, or towards chronic inflammation with associated tissue damage, structural remodelation and permanent loss of normal tissue function[12,13,14].
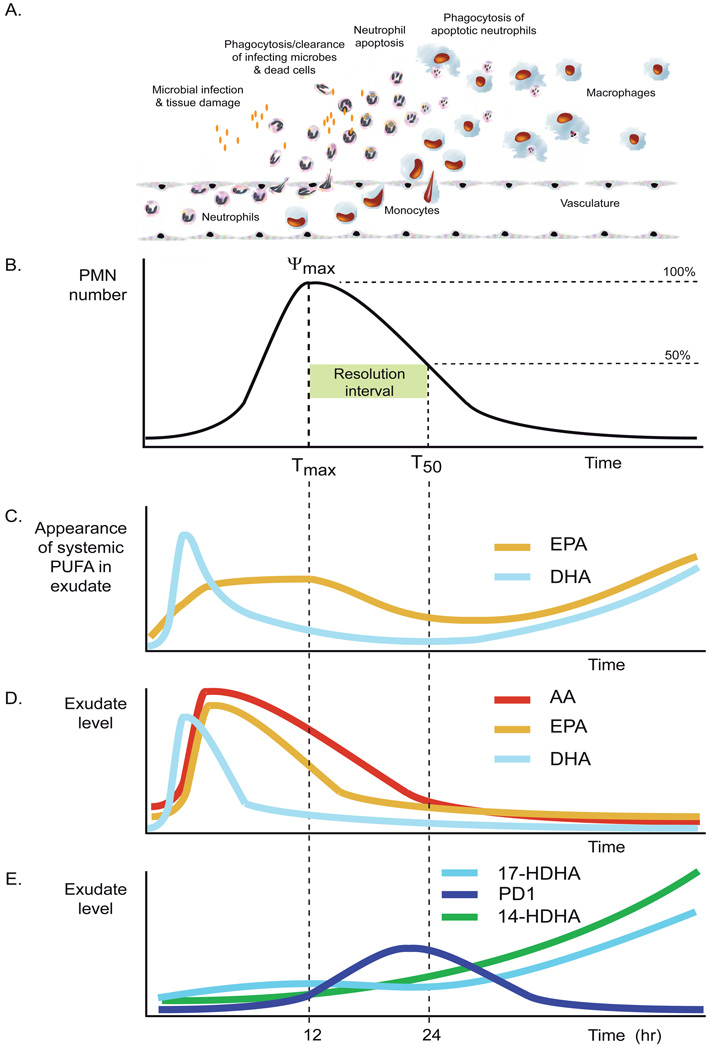
Figure 1.
Panel A. The inflammatory response comprises concerted actions by resident parenchymal cells, tissue-resident macrophages and the endothelium. The first steps comprise PMN adhesion to endothelium, diapedesis, chemotaxis towards microbes, and cell activation. The first cells recruited to a disturbed site are PMN, which remove infecting microbes and dead parenchymal cells by phagocytosis. After completion of their function or intrinsic timeline, PMNs undergo apoptosis and are phagocytosed in a non-phlogistic manner by infiltrating monocytes that differentiate into macrophages. Macrophages disappear by tissue egress or apoptosis.
Panel B. The inflammatory response can be defined in quantitatively measurable indices based on neutrophil kinetics in the inflammatory focus. Resolution is defined here as the disappearance of the neutrophilic infiltrate. Tmax is the time point at which neutrophil numbers are maximal (Ψmax), T50 is the time point when half of the neutrophils have disappeared. The time interval between Tmax and T50 is the resolution interval (Ri), and indicates the duration of the resolution process.
Panel C. Appearance of systemic ω-3 PUFA in the inflammatory exudate. Illustrated time courses are depicted graphically based on measurements of the levels of deuterium-labeled EPA and DHA administered systemically in mice 5 minutes prior to initiation of zymosan A-stimulated peritonitis [142].
Panel D. Levels of free arachidonic acid (AA) and ω-3 PUFA in inflammatory exudates during the inflammatory response. Illustrated time courses are depicted graphically based on measurements of cell-free exudate levels made during zymosan A-stimulated murine peritonitis [89].
Panel E. Formation of pro-resolution pathway biomarkers: 17S-hydroxydocosa-4Z,7Z,10Z,13Z,15E,19Z-hexaenoic acid (17-HDHA), protectin D1 and 14S-hydroxydocosa-4Z,7Z,10Z,12E,16Z,19Z-hexaenoic acid (14-HDHA) during the acute inflammatory response. In this model of inflammation the formation of protectin D1 was found to increase during the resolution interval. The time course is depicted graphically based on measurements of cell-free exudate levels made during zymosan A-stimulated murine peritonitis [89].
The inflammatory response resembles the execution of a molecular and cellular program which passes check-points that function in assessing the nature of the insult, monitoring the progress of leukocyte accumulation and microbial clearance, and in initiating resolution and tissue repair [4,15,16,17,18]. Specific eicosanoids, lipid mediators derived from arachidonic acid (AA), are well established to contribute to the initiation of inflammation and chronic inflammation [19]. These include leukotrienes generated by the fatty acid oxygenase 5-lipoxygenase (5-LO), such as leukotriene B4 (LTB4; 5S, 12R-dihydroxy-eicosa-6Z, 8E,10E,14Z-tetraenoic acid), a potent chemoattractant for neutrophils, and the cysteinyl leukotrienes, which are potent mediators of vascular permeability [10,20]. A number of prostaglandins (PG) also play important roles in the early phases of inflammation, such as PGE2, PGI2 and PGF2α, as they regulate changes in blood flow which promote leukocyte delivery and plasma exudation to inflamed tissue [21,22,23].
The “Front and Back” of Acute Inflammation
Conserved physiological mechanisms limit the extent and duration of the inflammatory response [4,24,25]. These mechanisms can counter-regulate the extent of inflammation (or anti-inflammation), and/or promote the active termination or resolution of inflammation [4,24]. The former encompasses those mechanisms activated to reduce the rate of granulocyte recruitment to the inflammatory focus and limit their state of activation. The latter comprises the active removal of the granulocytic infiltrate, permitting restoration of normal tissue structure and function. The counter-regulation of the inflammatory response is achieved by several physiological mechanisms acting at the systemic level; an increase in circulating levels of glucocorticoids, activation of the acute-phase response, and anti-inflammatory cholinergic efferent neural pathways provide systemically active and protective responses to stress and inflammation [26,27,28]. Of significance, counter-regulation and resolution of the inflammatory response is also operative at the local tissue level, providing control over tissue-specific regulatory actions required to limit inflammatory injury and restore tissue homeostasis [29]. Recent results establish key roles of specific lipid mediator autacoids derived from poly-unsaturated fatty acids (PUFA) in the endogenous counter-regulation of inflammation and activation of resolution, such as AA, eicosapentaenoic acid (EPA) and docosahexaenoic acid and (DHA) [30]. These lipid mediators, named lipoxins and resolvins, are formed via specific transcellular biosynthetic pathways established at strict temporal intervals during the inflammatory response. Without control of the inflammatory response, tissues would be overwhelmed by persistent inflammatory cell infiltrates, edema, and tissue damage incurred by activated inflammatory leukocytes [31,32,33]. Thus, active counter-regulation and resolution of inflammation are essential for the conservation of homeostasis and health [34].
Omega-3 fatty acids are dietary poly-unsaturated long-chain fatty acids essential for human health [35,36,37,38,39,40,41,42]. These can also be formed endogenously in men to a limited extent [43]. Phospholipids containing acylated EPA and DHA constitute a significant percentage of the fatty acid composition in specific locations of the body, e.g. the central nervous system, the retina, and sperm cells [44,45]. ω-3 PUFAs make an important contribution to structural and functional roles of specific subcellular membrane compartments [46,47]. It is now well documented that dietary ω-3 PUFAs impart protective actions in the cardiovascular and nervous systems, and can counteract a range of inflammatory diseases [30,35,36,37,38,39,40].
Anti-inflammatory and pro-resolving lipid mediators are derived from both ω-6 AA and ω-3 PUFA, which counteract the extent and regulate the pace of the inflammatory response at several critical cellular events. These include the down-regulation of cell adhesion molecules on both endothelial cells and leukocytes, reduced chemotaxis and transendothelial migration, reduced activation of neutrophils (measured by diminished degranulation and respiratory burst), inhibition of the formation and actions of pro-inflammatory mediators, the stimulation of non-phlogistic phagocytosis of apoptotic neutrophils and macrophages, as well as active egress of inflammatory leukocytes during resolution [48,49,50,51,52,53,54]. The transiently established transcellular biosynthetic pathways required for lipoxin and resolvin formation are assembled via heterotypic interactions of inflammatory leukocytes with endothelial cells, epithelial cells, macrophages and platelets that constitute an inflamed tissue. The formation of such lipid-derived cellular interaction products permits gauging the number and activation state of inflammatory leukocytes that participate in the inflammatory response. It is important to appreciate that lipoxins and resolvins act as endogenous receptor agonists at low concentrations (pM to low nM) and at specific G-protein coupled membrane-spanning receptors to actively down-regulate pro-inflammatory events, as well as stimulate the resolution of an inflammatory exudate [55]. In the following sections of this review, we summarize the biosynthesis of these endogenous lipid mediator autacoids, recently identified specific surface receptors, and their cellular actions.
Lipoxins and Aspirin
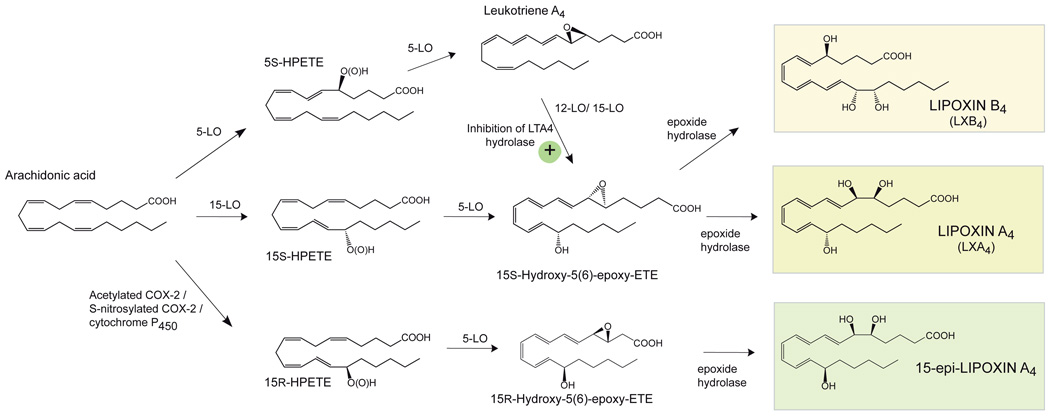
Lipoxin A4 (LXA4; (5S,6R,15S-trihydroxy-eicosa-7E,9E,11Z,13E-tetraenoic acid) is a central anti-inflammatory lipid mediator autacoid which plays an important function in determining the extent of granulocyte (neutrophil and eosinophil) accumulation and activation during inflammation. The formation of LXA4 is achieved by transcellular biosynthesis via two sequential oxygenation reactions of arachidonic acid (AA; Fig. 2) catalyzed by lipoxygenases present in interacting cell types, with one of the cell types often a neutrophil, eosinophil, or macrophage, and the other an endothelial, epithelial, or parenchymal cell or platelets [56,57]. LXA4 is a potent endogenous anti-inflammatory lipid mediator that activates the G-protein coupled receptor ALX/FPR2 (Kd ≈ 0.7 nM; Table 1) to reduce neutrophil chemotaxis, trans-endothelial migration and degranulation [58,59]. The transcellular formation of LXA4 likely constitutes a specific signal formed as a result of the physical proximity of inflammatory cells with cells in the inflammatory focus, and activates subsequent tissue responses which limit further inflammatory cell infiltration. LXA4 also exerts potent immuno-modulatory actions, promotes apoptosis of leukocytes in vivo by over-riding pro-survival signals, promotes migration of monocytes/macrophages to inflamed tissue, and stimulates the non-phlogistic phagocytosis of apoptotic leukocytes and lymphocytes by macrophages (efferocytosis) [50,60,61,62,63]. Taken together, LXA4 reduces inflammatory leukocyte accumulation and also promotes the active removal of inflammatory exudate cells and debris.
Figure 2.
Biosynthesis of lipoxins and aspirin-triggered lipoxins.
Table 1.
Summary of characterized G-protein–coupled receptors and selective binding sites for anti-inflammatory-pro-resolution lipid mediators.
| ANTI-INFLAMMATORY/ PRO-RESOLUTION LIPID MEDIATOR |
RECEPTOR |
Kd (nM) |
Ki (nM) |
|---|---|---|---|
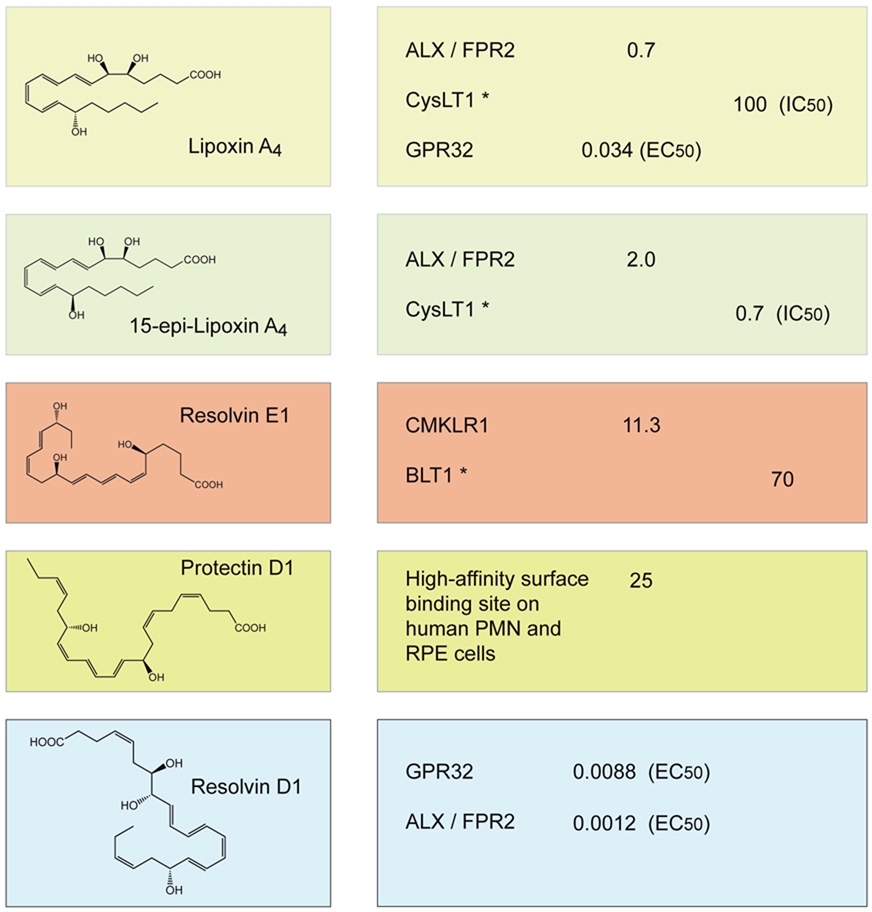 | |||
Known partial agonism or antagonism at indicated receptor. For those ligand-receptor interactions for which Kd or Ki values were not determined, their EC50 and IC50 values are indicated, which were obtained from a β-arrestin-based luminescence system and recombinant receptors, or competition binding studies at specific receptors and cellular binding sites [see [72,73,74] and text for further details]. (RPE; retinal pigment epithelium).
A unique feature of the non-steroidal anti-inflammatory drug aspirin (acetyl-salicylic acid) is its action on the second described isoform of cyclooxygenase (COX-2), a fatty acid oxygenase which catalyzes the stereospecific double oxygenation of AA to form prostaglandin H2, the central precursor for the formation of PGs. By acetylation of a conserved serine residue in the COX-2 active site, aspirin changes the enzymatic activity to allow the incorporation of one oxygen molecule to form 15R-hydroperoxy-eicosatetraenoic acid (15R-HpETE; 15R-hydroperoxy-5Z5Z,8Z,11Z,13E-tetraenoic acid) [64]. This product contains oxygen in the R configuration at carbon 15 and hence is epimeric and stereospecific compared to the oxygenation product formed during the second oxygenation step in PG biosynthesis. 15R-HpETE as well as 15R-HETE can be used as substrates by 5-LO to form 15-epi-LXA4 (5S,6R,15R-trihydroxy-eicosa-7E,9E,11Z,13E-tetraenoic acid) [65] (Fig. 2). This aspirin-triggered biosynthetic route can function efficiently between a COX-2 expressing cell type (e.g. endothelial cells) and a 5-LO-expressing cell type (e.g. neutrophils). The epimeric oxygenation product 15-epi-LXA4 has been termed an aspirin-triggered lipoxin (ATL). Recent results indicate that S-nitrosylation of COX-2 can also activate the epimeric oxygenation of AA to form 15R-HETE and initiate 15-epi-LXA4 biosynthesis [66]. Also, cytochrome P450 can contribute to 15-epi-LXA4 formation in vivo [67]. A recent study has shown that inhibition of the epoxide hydrolase LTA4-synthase promotes the formation of 15-epi-LXA4 most likely by increasing the steady state levels of LTA4 as substrate for 15-lipoxygenase (Fig. 2) [68]. Of particular interest are results from a human trial indicating that low dose aspirin triggers 15-epi-LXA4 production in human skin blisters that in turn stimulates local nitric oxide (NO) production and regulates PMN infiltration in vivo [69]. This confirms that 15-epi-lipoxin production reduces acute inflammation in vivo and this low dose aspirin’s protective actions in humans [70].
Like LXA4, 15-epi-LXA4 is an anti-inflammatory SPM that binds and activates ALX/FPR2 (Kd ≈ 2.0 nM; Table 1) [71]. It is noteworthy that the presence and chirality of the 15R-hydroxy group in 15-epi-LXA4 provides increased resistance to local tissue degradation catalyzed by 15-hydroxy prostaglandin dehydrogenases (15-PGDH) which contributes to augmenting the anti-inflammatory and pro-resolving potency of aspirin-triggered lipoxins in vivo [71]. Acting as an antagonist at the receptor CysLT1, ATL analogues have been instrumental in demonstrating that lipoxins can also counteract the pro-inflammatory actions of cysteinyl-leukotrienes (Table 1) [72,73]. Recently, LXA4 has been found to also bind to the human orphan receptor GPR32, a member of the chemoattractant receptor family (Table 1) [74].
Lipoxin B4 (LXB4; 5S,14R,15S-trihydroxy-eicosa-6E,8Z,10E,12E-tetraenoic acid) is a positional isomer of LXA4 which is also formed from endogenous sources of arachidonic acid through transcellular oxygenation pathways (Fig. 2) [75]. LXB4 and aspirin-triggered 15-epi-LXB4 stimulate monocyte chemotaxis and are non-phlogistic agonists for these cells [76,77]. LXB4 is rapidly inactivated via dehydrogenation [78]. LXB4 and aspirin-triggered LXB4 analogs have potent actions in vitro and in vivo [76,79]. LXB4 and its analogs demonstrate potent bioactions by both oral administration and topical application [79,80]. LXB4 does not interact with the sites or receptors that LXA4 or aspirin-triggered LXA4 act. The stereoselective actions of LXB4 indicate that it has its own receptor(s), which remains to be identified.
Specialized pro-resolving lipid mediators (SPM) derived from omega-3 polyunsaturated fatty acids
A number of enzymatically oxygenated lipid mediators derived from ω-3 poly-unsaturated fatty acids (PUFA), such as EPA and DHA, were recently identified to function as specialized pro-resolution mediators (SPM) that “turn off” the inflammatory response in an active fashion [30]. These mediators, named resolvins, protectins, and maresins, constitute novel families of autacoids with potent anti-inflammatory, tissue-protective, and resolution-stimulating functions. It is important to note that each SPM family has its own unique structural feature to evoke biological functions and that resolvins, protectins, and maresins do not resemble the structures of the classic eicosanoids, i.e., PG or LT. The formation of specific lipid mediator autacoids during resolution has been monitored by LC-MS-MS mass spectrometry-based lipidomic analysis of resolving, self-limited inflammatory exudates, via biosynthetic approaches that employed individual enzymes expressed during resolution, and matching of biological activity of novel compounds with that of synthetic standards with defined double bond geometry and hydroxyl group position and stereospecificity (reviewed in detail in [30] and references within).
It is only recently appreciated that specific autacoids activate the resolution of inflammation, since the resolution of acute inflammation was previously considered to be passive. It was recently discovered that omega-6 (ω-6) PUFA such as AA and docosapentaenoic acid (DPA ω-6; see below), and ω-3 PUFAs such as EPA, DHA and DPA (ω-3) are also substrates for temporally regulated cellular events and enzymatic oxygenation reactions that produce local acting lipid mediators with potent anti-inflammatory and pro-resolution actions. This was not clear earlier, because the cyclooxygenase enzymes known to efficiently oxygenate AA to form PGH2 had been found to oxygenate EPA or DHA at relatively low rates compared to AA. Furthermore, the cyclooxygenase products derived from EPA and DHA were found to activate known PG receptors with relatively weak affinities [81,82,83,84,85]. It appeared reasonable to doubt that EPA- and DHA-derived prostaglandins would constitute endogenously relevant mediators. Conceptual advances were made with the discovery that both cyclooxygenase and lipoxygenases can oxygenate ω-3 PUFAs to produce intermediate oxygenation products that can undergo transcellular biotransformation via a second fatty acid oxygenase [30]. These novel bioactive products are structurally unrelated to prostaglandins [30]. In the next sections, the biosynthesis and actions of the novel anti-inflammatory and pro-resolving lipid mediators will be reviewed in further detail.
E-series resolvins: resolvin E1 and resolvin E2
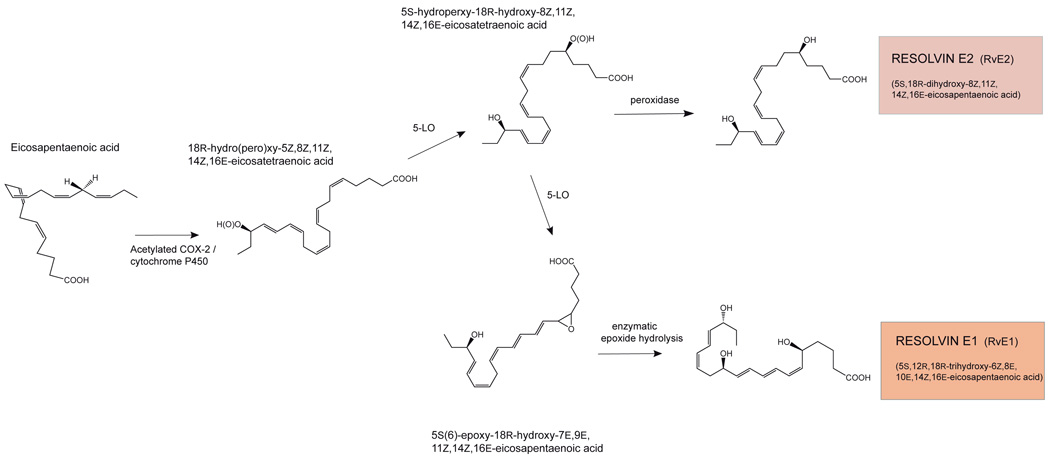
Specific bioactivity was uncovered in the resolving exudates that stopped PMN migration and was 100 times the potency as aspirin in vivo [86]. The active molecule was coined resolvin E1 based on its in vivo production in resolving murine exudates, potent stereoselective actions (demonstrated in vitro and in vivo) and its precursor substrate EPA. Hence, it was important to consider the biosynthetic routes involved in the production of this and related molecules in human cell types. After acetylation by aspirin, COX-2 acquires the capacity to oxygenate EPA to form 18R-hydro (pero) xy-5Z,8Z,11Z,14Z,16E-eicosapentaenoic acid (18R-H(p)EPE). Hypoxic endothelial cells convert EPA to 18R-H(p)EPE that is released and converted by activated human PMN to RvE1 in vitro. Neither unacetylated COX-2 nor COX-1 catalyzes this epimeric oxygenation reaction, and 18R-H(p)EPE formation should therefore be viewed as an aspirin-triggered epimeric oxygenation of EPA similar to 15R-HETE production from AA. The second oxygenation reaction established for 15-epi-LXA4 biosynthesis also proved to operate on 18R-HEPE with the formation of 5S,12R,18R-trihydroxy-6Z,8E,10E,14Z,16E-eicosapentaenoic acid via 5-LO, an epoxide intermediate, and subsequent enzymatic reactions (Fig. 3) [86,87]. This potent bioactive compound was named resolvin E1 (RvE1), as it is an EPA-derived (E-series) cellular interaction product which can be formed in resolving exudates in murine models of inflammation (zymosan-stimulated peritonitis and TNF-α-activated dorsal air pouch inflammation). The complete stereochemistry of RvE1 was determined by matching of chromatographic, spectroscopic, and mass physical properties, as well as biological activity of biogenically generated RvE1 with postulated geometric isomers made by total organic synthesis [87]. Synthetic RvE1 analogs that possessed double bond configurations, for example, 6E and 14E, or 6E and 8Z, could not be matched to the biogenic material and were thus excluded as being identical to the endogenously synthesized RvE1.
Figure 3.
Biosynthesis of E-series resolvins: RvE1 and RvE2.
A careful interpretation of the results of a large epidemiological study that reported the beneficial impact of enhanced ω-3 PUFA intake on mortality and recurring heart attacks in patients who experienced a prior myocardial infarction [88] provided important insight for the possible involvement of aspirin-acetylated COX-2 in the outcome of the study; in addition to EPA, many patients took aspirin as well [86]. Human plasma levels of RvE1 were measured up to 0.4 ng/ml after the dietary intake of EPA and DHA together with aspirin [87]. RvE1 potently reduces neutrophilic inflammation and activates the resolution of inflammation [89,90]. The general functions of RvE1 include reducing neutrophil-endothelial cell interactions and transmigration, stimulating non-phlogistic phagocytosis by macrophages, and reducing the release of pro-inflammatory cytokines [86,87,89,91,92,93,94,95,96]. RvE1 also potently inhibits inflammatory angiogenesis [97,98].
RvE1’s high potency in vivo, specific stereochemistry and double-bond geometry pointed to a selective recognition by an endogenous receptor. The binding of RvE1 with the G-protein coupled receptor CMKLR1 was identified in a screen for orphan-receptors (this receptor was formerly called ChemR23 and also binds the endogenous peptide chemerin) [87,99]. Tritium-labeled RvE1 was prepared by catalytic hydrogenation of a synthetic precursor and used to determine the binding constant of RvE1 with CMKLR1 (Kd ∼11 nM; Table 1). The functional importance of the RvE1-CMKLR1 interaction was demonstrated in murine dendritic cells (stimulating down-regulation of IL-12 formation and migration) [87] and in oral epithelial cells where activation of CMKLR1 by RvE1 stimulates transepithelial neutrophil migration [100]. A second receptor interaction was subsequently identified which mediates additional actions of RvE1, namely partial agonism of RvE1 at the LTB4 receptor BLT1 (Ki ∼ 70 nM; Table 1) [101]. Displacement of LTB4 constitutes a mechanism whereby RvE1 dampens the actions of this pro-inflammatory lipid mediator [86,101]. RvE1 also inhibits osteoclast differentiation via binding to BLT1 and can contribute to bone remodeling [102]. Taken together, RvE1 is the first identified ω-3 PUFA-derived lipid mediator with receptor-mediated anti-inflammatory and pro-resolving actions, and acts on two receptors, CMKLR1 and BLT1 [87] and potentially more stereoselective receptors in vivo [103] (Table 1).
In several in vivo models of inflammatory disease, RvE1 has now been demonstrated to exert potent anti-inflammatory and tissue-protective actions. For example, administration of RvE1 reduces ischemia/reperfusion (I/R)-induced kidney injury [104,105]. RvE1 can down-regulate obesity-induced inflammation in white adipose tissue of mice, which corrects a loss of insulin sensitivity and abrogates hepatic steatosis [106]. The potent counter-regulatory and pro-resolution actions of RvE1 in inflammation have been elegantly demonstrated in a rabbit model of periodontitis induced by application of the pathogenic bacterium Porphyromonas gingivalis; the topical application of RvE1 to inflamed gingival tissue stimulated restoration of lost bone and connective tissue and markedly reduced inflammation [107]. RvE1 promotes the resolution of allergic airway inflammation in mice by down-regulating IL-17, a cytokine which is important in sustaining allergen-induced airway inflammation [108]. RvE1 also down-regulates the formation of IL-6 and IL-23, two cytokines which are important in stimulating Th17-type responses. Through stimulating IFNγ formation, RvE1 also contributes to the resolution of airway inflammation by facilitating apoptosis [108]. Results of recent studies demonstrated a potent protective action in acute lung injury and pneumonia in mice [109]. Moreover, a recent clinical trial demonstrated that a synthetic RvE1 analogue reduced the signs and symptoms of ocular inflammation in patients with dry eye (Clinicaltrials.gov identifier:NCT00799552; http://www.scienceblog.com/cms/resolvyx-announces-positive-data-phase-2-trial-resolvin-rx-10045-dry-eye-syndrome-24358.html).
Additional mediators are produced by the E-series resolvin biosynthetic route. For example, the hydroperoxide intermediate 5S-hydroperoxy-18R-hydroxy-8Z,11Z,14Z,16E-eicosatetraenoic acid formed via the action of 5-lipoxygenase with 18R-HEPE, can also be directly reduced to a dihydroxy-containing product coined RvE2 (5S,18R-dihydroxy-8Z,11Z,14Z,16E-eicosapentaenoic acid) [110]. RvE2 constitutes a second member of the EPA-derived (E-series) resolvins, and likely regulates inflammatory targets which differ from those activated by RvE1 [110].
D-series resolvins
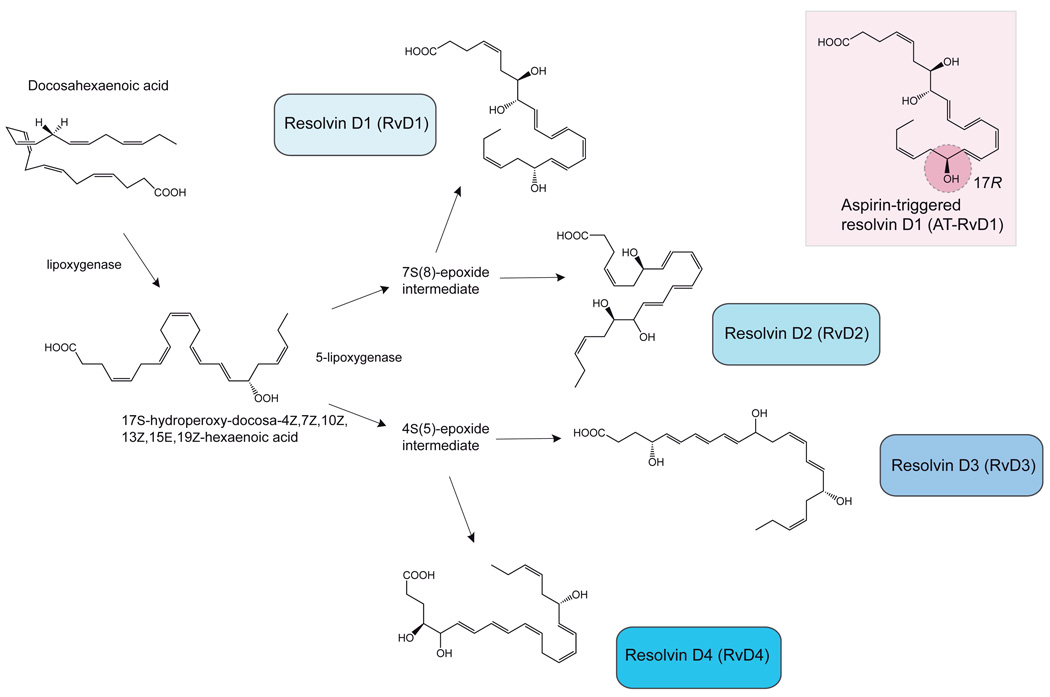
Given the ability of acetylated COX-2 to accept both AA and EPA as substrates for a single oxygenation reaction to form epimeric fatty acid hydroperoxides, DHA was subsequently tested and recognized as a substrate for acetylated COX-2 as well [92]. In this case oxygen is incorporated at carbon 17, forming a 17R-hydroperoxy group-containing DHA-derived intermediate that when reduced was shown to be 17R-hydroxydocosa-4Z,7Z,10Z,13Z,15E,19Z-hexaenoic acid (17R-HpDHA) (Fig. 4). The introduction of oxygen with the observed R stereospecificity is not expected to occur by a lipoxygenase-mediated oxygenation, as this typically occurs with S stereospecificity. 17R-HpDHA proved to be converted by 5-LO to a number of oxygenation products identified as trihydroxylated positional isomers, named aspirin-triggered resolvins (At-RvD1 is shown in Fig. 4) [92,111]. The DHA series resolvins are formed by hydrolysis of intermediate epoxides derived from one of two fatty acid peroxides formed by 5-LO, 4S-hydroperoxy-17R-hydroxy-docosa-5E,7Z,10Z,13Z,15E,19Z-hexaenoic acid and 7S-hydroxy-17R-hydroperoxy-docosa-4Z,8E,10Z,13Z,15E,19Z-hexaenoic acid.
Figure 4.
Biosynthesis of D-series resolvins. Inset, one example of an aspirin-triggered D-series resolvin, AT-RvD1 (7S,8,17R-trihydroxy-docosa-4Z,9E,11E,13Z,15E,19Z-hexaenoic acid), displaying predominately the R-stereospecificity at the carbon-17 alcohol group.
The LC-MS-MS based lipidomic analyses of DHA oxygenation products from incubations of human blood with DHA indicated that D-series resolvins are formed which possess a carbon 17 hydroxyl group with S stereospecificity. The apparent lack of requirement for acetylation by aspirin indicated that alternative endogenous biosynthetic routes were operative in the formation of DHA-derived resolvins (Fig. 4) [112]. It was found that 15-lipoxygenase can catalyze the oxygenation of DHA at carbon 17 with the more commonly observed S chirality and stereospecificity of lipoxygenases. Resolvin D1 (RvD1) and RvD2 are formed from the 7(8)S-epoxy-17S-hydroperoxy-docosa-4Z,9E,11E,13Z,15E,19Z-hexaenoic acid intermediate, whereas RvD3 and RvD4 are formed from the 4(5)S-epoxy-17S-hydroperoxy-docosa-6E,8E,10Z,13Z,15E,19Z-hexaenoic acid intermediate (Fig. 4) [111,112]. The absolute stereochemistry and double bond configuration of RvD1 has been determined by matching of the physical properties and biological activity of biogenically generated RvD1 with several isomers prepared by total organic synthesis [111]. The complete synthesis of RvD2 has also been achieved using this matching approach with total organic synthesis using starting materials with defined stereochemistry [113,114].
Results from recent studies with murine 12/15-lipoxygenase and retinal pigment epithelial cells lacking 15-lipoxygenase indicated that a rate-limiting factor for this biosynthetic pathway is the expression of 15-lipoxygenase activity to biosynthesize the 17S-hydroperoxydocosa-4Z,7Z,10Z,13Z,15E,19Z-hexaenoic acid intermediate [115,116]. Hence, D-series 17S-resolvins and aspirin-triggered resolvins can be formed endogenously but require the action of different oxygenases to initiate their biosynthesis.
The D-series resolvins and AT-resolvins display potent anti-inflammatory actions, such as reduced human neutrophil migration and inhibition of neutrophilic infiltration in murine models of inflammation [111,112]. RvD1 has been demonstrated in experimental periodontitis in the rabbit and ischemia/reperfusion (I/R)-induced kidney injury in mice [104,105,107]. RvD1 has recently been shown to potently reduce the inflammatory response and neutrophil oxidative burst that are generated in response to the 4-hydroxynonenal-glutathione adduct, a product that reflects the glutathione-dependent detoxification of a reactive lipid aldehyde generated during lipid peroxidation. This action by RvD1 may represent a mechanism to limit oxidative tissue injury imposed by neutrophil-mediated employment of oxygen and nitrogen radicals in the biocidal removal of infecting microbes [117].
Recent results indicate that RvD2 has extremely potent regulatory actions on neutrophil trafficking evident in the picogram range in vivo, stimulating resolution and enhancing innate host defense mechanisms [114]. The RvD2-stimulated inhibition of neutrophil-endothelial cells interactions and neutrophil trafficking were shown to be mediated at least in part by NO formation. Of importance, this study revealed a novel function of RvD2, namely the potent activation of microbial phagocytosis by monocytes/macrophages. In an experimental murine sepsis model initiated by a mid-grade surgical cecal ligation and puncture, RvD2 administration reduced the pro-inflammatory “cytokine storm” and also reduced the levels of IL-10, a cytokine that in human sepsis is associated with a detrimental outcome. The enhanced in vivo clearance of bacteria demonstrated that RvD2 promotes host defense and allowed the survival of animals which would have otherwise succumbed to the excessive inflammatory response during sepsis.
Recently the first experimental evidence for the existence of specific receptors for D-series resolvins has been obtained (Table 1). RvD1 label was synthesized and used to demonstrate RvD1 direct interactions with high affinity (Kd ≈ 0.2 nM) recognition sites on human neutrophils [74]. In a screening of G protein-coupled receptors two human receptors were identified to bind RvD1; GPR32 and ALX/FPR2 (Table 1) [74]. Although several peptide and synthetic ligands are known for ALX/FPR2 in addition to LXA4 and 15-epi-LXA4 [118,119], this study indicates for the first time the existence of two counter-regulatory lipid mediator ligands for the same receptor. It is not yet established if the ALX/FPR2 receptor binding by RvD1 and LXA4 is temporally or spatially dissociated. It has also been suggested that receptors for anti-inflammatory/pro-resolution lipid mediators may be co-organized in signaling clusters, possibly via receptor hetero-dimerization [74]. Specific receptor proteins which selectively recognize the other D-series resolvins continue to be of interest. Clearly, the identification of D-series resolvins has initiated a number of new avenues of research.
Protectins and Maresins
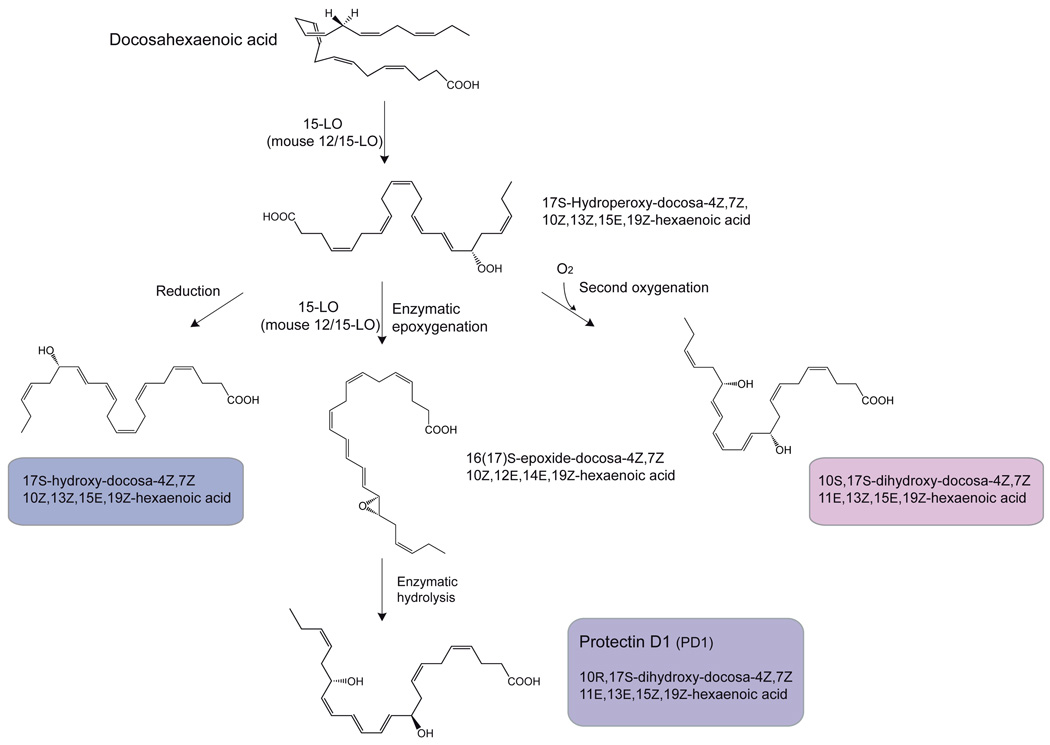
Although transcellular biosynthesis is recognized to be an important requirement for the formation of specific anti-inflammatory and pro-resolution lipid mediators, such as SPM, recent evidence is provided that a single cell type can also form oxygenated lipid mediators with potent counter-regulatory actions. The first pathway involves a 15-lipoxygenase-type I catalyzed formation of the same 17S-hydroperoxydocosa-4Z,7Z,10Z,13Z,15E,19Z-hexaenoic acid product required for D-series resolvin biosynthesis. Instead of undergoing a second oxygenation, the hydroperoxide intermediate product undergoes a second hydrogen abstraction by 15-lipoxygenase to form an intermediate epoxide (Fig. 5A) [112,120]. The epoxide is transformed to the dihydroxy-containing bioactive 10R,17S-dihydroxydocosa-4Z,7Z,11E,13E,15Z,19Z-hexaenoic acid, possibly through the formation of an enzyme-bound delocalized cation and attack by water [121,122,123]. This dihydroxy bioactive product was named protectin D1 because it affords potent tissue protective actions in brain, immune and retinal cells. In neural tissue PD1 is referred to as neuroprotectin D1 (NPD1) to reflect its site of biosynthesis and action (recently reviewed in Bazan et al [124]). In the innate immune system, PD1 exhibits potent anti-inflammatory activity, affords cell protective actions, and can activate the resolution of inflammation [89,90,112,122,125]. The 17S-hydroperoxydocosa-4Z,7Z,10Z,13Z,15E,19Z-hexaenoic acid intermediate can also be reduced to the corresponding alcohol (Fig. 5A), or undergo a second oxygenation by the same 15-lipoxygenase to form the dihydroxy acid 10S,17S-dihydroxy-docosa-4Z,7Z,11E,13Z,15E,19Z-hexaenoic acid (Fig. 5A). The epoxide intermediate can also be hydrolyzed to the vicinal diol 16,17S-dihydroxydocosa-4Z,7Z,10Z,12E,14E,19Z-hexaenoic acid. Both PD1 and the 16,17-dihydroxylated compound can be oxygenated at the ω22-carbon atom by cytochrome P450 enzymes to form the corresponding trihydroxylated products. Of note, a high-affinity binding site for NPD1/PD1 in human neutrophils (Kd ≈ 25 nM; Table 1) and in a retinal pigment epithelial cell line (31.3 pmol/mg cell protein) was recently demonstrated, which may constitute a novel receptor for NPD1/PD1 (Table 1) [126,127]. Other NPD1/PD1 isomers did not compete.
Figure 5.
A. Biosynthesis of protectins: protectin D1 (10R,17S-dihydroxy-docosa-4Z,7Z,11E,13E,15Z,19Z-hexaenoic acid), the mono-hydroxylated product 17S-hydroxy-docosa-4Z,7Z,10Z,13Z,15E,19Z-hexaenoic acid, and the double oxygenation product 10S,17S-dihydroxy-docosa-4Z,7Z,11E,13Z,15E,19Z-hexaenoic acid, an isomer of NPD1/PD1 (see text for details).
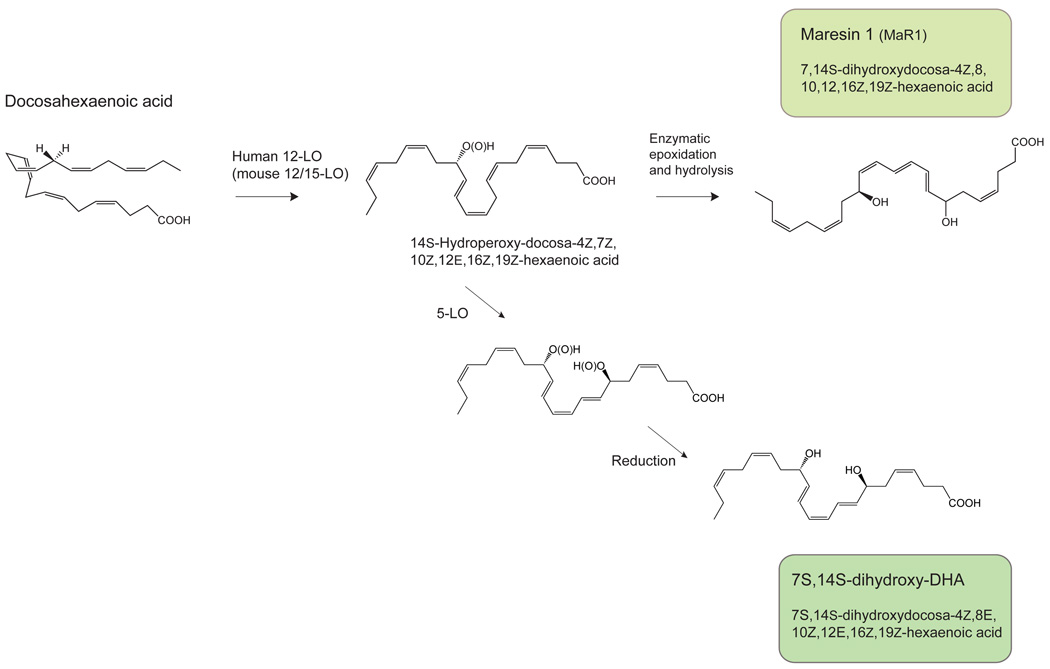
B. Formation of maresins: maresin 1 (7,14S-dihydroxydocosa-4Z,8,10,12,16Z,19Z-hexaenoic acid); the geometry of the double bonds depicted is tentative and in progress (see text for details). Also, an isomer 7S,14S-dihydroxydocosa-4Z,8E,10Z,12E,16Z,19Z-hexaenoic acid, a novel double dioxygenation product.
In addition, 17S-hydroperoxydocosa-4Z,7Z,10Z,13Z,15E,19Z-hexaenoic acid can be oxygenated a second time via 5-lipoxygenase to form two regio-isomeric dihydroxylated products formed without the formation of an enzyme-stabilized epoxide intermediate, namely 4S,17S-dihydroxy-docosa-5E,7Z,10Z,13Z,15E,19Z-hexaenoic acid, and resolvin D5 or 7S,17S-dihydroxy-docosa-4Z,8E,10Z,13Z,15E,19Z-hexaenoic acid (Fig. 5A). The latter also possess anti-inflammatory actions in vivo [112].
In the second single oxygenation route, the mediator coined maresin 1 (7S,14S-hydroxy-docosa-4Z,8E,10E,12Z,16Z,19Z-hexaenoic acid; MaR1) is formed via the action of human 12-lipoxygenase, an enzyme present in macrophages and platelets. DHA is first oxygenated to form a 14S-hydroperoxy-docosa-4Z,7Z,10Z,12E,16Z,19Z-hexaenoic acid intermediate that is further converted to an epoxide-containing intermediate, which is hydrolyzed to form the potent bioactive maresin 1 (Fig. 5B) [128]. The maresin biosynthetic pathway is activated in mouse and human macrophages during phagocytosis. MaR1 potently reduces neutrophil migration as well as stimulates phagocytosis by macrophages, demonstrating the hallmark actions of SPM. Additional products from 14S-hydroperoxy-docosahexaenoic acid were also identified, including the isomer of MaR1 double dioxygenation product 7S,14S-hydroxy-docosa-4Z,8E,10Z,12E,16Z,19Z-hexaenoic acid that proved to be less potent than either MaR1 or PD1 (Fig. 5B), as well as 4,14S-dihydroxy-docosa-5E,7Z,10Z,12Z,16Z,19Z-hexaenoic acid (Fig. 5B) and trihydroxy-containing products, and the direct reduction product of the C-14 hydroperoxide, forming the basis for an entire new family of lipid mediators termed maresins [128].
Docosapentaenoic acid-derived Resolvin–like products
Another ω-3 double bond-containing PUFA, ω-3 docosapentaenoic acid (DPA; 7Z,10Z,13Z,16Z,19Z-docosapentaenoic acid) has recently been found to be an efficient substrate for oxygenation by 5-, 12-, and 15-lipoxygenases. A number of mono- and dihydroxy fatty acids derived from DPA were identified [129,130]. Some of these hydroxy products display structural properties akin to pro-resolving protectin D1/NPD1 derived from DHA.
Also the ω-6 double bond isomer docosapentaenoic acid (ω-6 DPA; 4Z,7Z,10Z,13Z,16Z-docosapentaenoic acid) is a substrate for the formation of mono-and dihydroxylated products, such as ω-6 17S-hydroxy-pentaenoic acid and ω-6 10,17-dihydroxy-4Z,7Z,11E,13Z,15E-pentaenoic acid. These products, formed by the single and double oxygenation of ω-6 DPA by soybean 15-lipoxygenase, respectively, were shown to be potent anti-inflammatory lipid mediators [130]. Omega-6 17S-hydroxy-pentaenoic acid present in blood, cardiac tissue and trachea in rat, and can be formed in human blood from ω-6 DPA [129]. The further study of the formation and actions of these resolvin-like molecules in humans is of considerable interest since DPA is an intermediate in DHA biosynthesis.
Gauging resolution: Quantitative indices
Employing a widely used murine model of self-resolving acute inflammation activated by intraperitoneal administration of zymosan A, we earlier provided a definition of the inflammatory response in a set of quantifiable indices which can be relatively easily measured experimentally (Fig. 1) [89]. A close inspection of these indices for inflammation and resolution indicated that locally administered ATLa2, a stable 15-epi-LXA4 analogue, potently reduced inflammation, indicating a significant counter-regulatory role for LXA4 in this particular model of inflammation The EPA-derived lipid mediator RvE1 reduced inflammation and also activated resolution at an earlier time point. The DHA-derived lipid mediator PD1 activated an anti-inflammatory response and stimulated resolution and also accelerates resolution of the inflammatory exudate. These results revealed an important concept, namely that inflammation and resolution can be regulated separately, and, secondly, that counter-regulation of inflammation and the onset and pace of resolution appear to be independently regulated, at least to some extent [89]. A recent expansion for the use of resolution indices to gauge the inflammatory response has recently been provided by Gilroy and colleagues to allow the measurement of pro-resolving actions of treatments in a model that more closely resembles a chronic inflammatory setting [131]. Assessment of the sensitivity of an inflammatory response to pharmacological intervention and other treatments will be facilitated markedly when future studies will capture the modulation of the extent and pace of the response, including its resolution, in such quantifiable and comparative indices [90].
Specific molecular events during resolution of the inflammatory response
Recent studies indicate that distinct molecular processes are activated during the resolution phase of inflammation [89]. In addition to the cellular events which characterize resolution, such as leukocyte apoptosis, efferocytosis and egress, a parallel proteomic and mediator lipidomic study of the murine peritonitis model has indicated that distinct molecular events are also activated just prior to and during the resolution interval [89,90], which can be summarized as follows: i) Lipid mediators: the biosynthesis of PD1 is activated during the resolution interval (Fig. 1). PGD2 is maximal at the onset of resolution and remains high during resolution, possibly acting as a precursor for pro-resolving cyclopentenone prostaglandins [132]. ii) Proteins: A number of serum proteins extravasate and accumulate in the inflammatory exudate following the same time course as serum albumin. In contrast, the proteins haptoglobin and S100A9 displayed a delayed accumulation which reaches maximal level just prior to the onset of resolution, and thereafter remains high during resolution. iii) All major pro-inflammatory cytokines formed during the pro-inflammatory phase decreased to low (pre-inflammation) levels; only the level of transforming growth-factor-β increased during resolution. The time course of molecular changes determined in this model of inflammation has indicated that resolution of inflammation employs its own specific mechanisms to regulate cellular events required to return to homeostasis.
Further in-depth characterization of resolving exudates will reveal a more complete view of molecular mechanisms which operate during the resolution phase of the inflammatory response, the tissue-specificity in such regulation, and specific resolving mechanisms of inflammatory infiltrates mounted towards distinct inflammatory stimuli. Recent findings indicate that during a self-resolving inflammation of the tibio-tarsal joint in mice induced by the bacterium Borrelia burgdorferi marked temporal changes in levels of specific lipid mediators can be measured [133]. A comparison between a sensitive and resistant mouse strain revealed specific changes that may account for the differences in susceptibility; one important observation was the presence of PD1 and RvD1 in resistant mice [133]. These findings are of interest because this is a murine model of Lyme disease. Also, we can now appreciate the interdependence of pro-resolution lipid mediators and additional recognized endogenous counter-regulatory autacoids recently reported, such as NO and carbon monoxide [134,135].
In healthy conditions an inflammatory response will self limit or resolve completely, leading to full reconstitution of original tissue architecture and function. When inflammation is sustained due to persistent activation or defects in the resolution program described herein, a fibrogenic response may become prominent in the tissue. The fibrogenic response makes an important contribution to the overall remodeling of tissue structure and consequent loss of organ function. Sustained inflammation, cell proliferation and growth factor responses are intimately linked to promote the fibrogenic response. Recent results indicate that LXA4 also exerts important anti-fibrotic actions, thereby driving resolution towards tissue homeostasis to limit matrix deposition and remodeling of tissue [136,137]. The regulation of protein kinase activity downstream of receptor activation by several growth factors and pro-inflammatory autacoids such as leukotriene D4 has been shown to be an important mechanism whereby LXA4 regulates the inhibition of mitogenic responses [138,139,140]. Stimulation of FPR2/ALXR has recently been shown to antagonize the platelet-derived growth factor receptor β (PDGFRβ)-activated receptor tyrosine phosphorylation through the recruitment of the protein tyrosine-phosphatase SHP-2 [141]. The anti-fibrotic actions of LXA4 illustrate an additional manner in which an SPM directs the inflammatory response to proper resolution.
Substrate availability: Mobilization for Resolving Exudates
The rapid changes in local blood vessel perfusion and permeability which occur during the early phase of the inflammatory response not only permit directional extravasation of circulating leukocytes and plasma proteins, but also may play an important role in the provision of substrate for SPM biosynthesis. In zymosan-stimulated peritonitis exudate levels of free unacylated ω-3 PUFA AA, EPA, and DHA increase rapidly, reaching maximal levels around 2–4 hours (Fig. 1) [89]. Systemically administered isotopically labeled ω-3 PUFA rapidly appear in the developing infiltrate of inflammation and not at the onset of resolution [142]. A close inspection indicates that SPM precursors EPA and DHA appear in the inflammatory focus with similar kinetics as the extravasation of serum proteins and before the infiltration of neutrophils. These findings point to the existence of dedicated active transport processes or mass transport together with exudating serum proteins, to effectively deliver ω-3 PUFA to an inflamed tissue. The structural basis of the ω-3 PUFA delivery is of special interest. Several possible candidates can be considered for their involvement, such as blood lipoprotein particles, circulating or cell-released exosomes, albumin (known to bind PUFA and the most abundant), and specific PUFA-binding proteins such as S100A9, which we identified earlier in resolving inflammatory exudates [143,144,145].
The circulation likely constitutes an organ which is very sensitively responding to the state of homeostasis of the various perfused organs. Specific mechanisms must be operating which signal from a perturbed tissue towards the mobilization and delivery of ω-3 PUFA from the circulation. This does not exclude a role for reacylation of delivered PUFA (AA and ω-3 PUFA) into phospholipids, and posterior deacylation reaction to control SPM biosynthesis during specific time intervals of the inflammatory response. In addition, it is known that a first oxygenation step of AA by 15-lipoxygenase can be performed and the 15-hydro(pero)xy-eicosatetraenoic acid can be stored in phosphatidylinositol [146]. In this way, the direct substrate for LXA4 formation is effectively provided through signal-responsive deacylation reactions of a stored and singly oxygenated precursor, and presented for the required second oxygenation [146]. This can serve as a membrane priming event for SPM biosynthesis via deacylation of monohydroxy fatty acids from phospholipids. Furthermore, specific phospholipase enzymes are known to be activated at defined time intervals during the inflammatory response, some of which have specificity for long chain PUFAs and are activated during resolution, such as type VI iPLA2 which is important for LXA4 biosynthesis [147]. In this regard it is noteworthy that a second increase in the exudates levels of unesterified EPA and DHA commences at the end of the resolution interval, which may be the result of increased ω-3 PUFA deacylation (Fig. 1) [142].
Concluding remarks and further directions
The formation of endogenous autacoids derived from ω-3 PUFA may explain in part the well-known, essential roles of the ω-3 PUFA in human health and disease. More importantly the contribution of novel SPM to the benefits derived from dietary ω-3 FA are beginning to be appreciated with the identification of resolvins as potent autacoids that regulate the resolution phase of the acute inflammatory response [148]. The resolution phase of inflammation has its own regulation involving specific changes in cellular activity, and employment of specific lipid mediators and proteins. The novel endogenous structures of the resolvins, protectins and maresins as a genus function in this milieu, regulating both early (neutrophil traffic) and late responses (macrophage uptake and clearance of apoptotic granulocytes). Resolution is activated and accelerated by SPM, and both experimental animal systems and unbiased resolution indices are now in place to test the activity of pharmacological resolution-directed interventions and their impact in active resolution. Further studies which aim to understand lipid mediator biosynthesis, tissue- and stimulus-specific molecular signatures of resolution are likely to provide us with new ways to control inflammation and its unwanted side effect namely tissue damage and injury.
Acknowledgements
Work reviewed here in the CNS laboratory was sponsored in part by the National Institutes of Health USA grant nos. GM38765, DE 019938 and DK07448. G. Bannenberg was supported by a Postdoctoral Fellowship from the Arthritis Foundation, and is a Ramón y Cajal fellow at the Centro Nacional de Biotecnología/CSIC, Madrid, Spain. We thank Mary Small for excellent assistance in the preparation of this manuscript and our collaborators and colleagues for their efforts in the original reports reviewed herein.
Abbreviations
- AA
arachidonic acid
- ATL
aspirin-triggered lipoxin
- AT-RvD
aspirin-triggered D-series resolvin
- DHA
docosahexaenoic acid
- EPA
eicosapentaenoic acid
- FA
fatty acid
- I/R
ischemia/reperfusion
- LT
leukotriene
- LTB4
leukotriene B4 (5S,12R-dihydroxy-eicosa-6Z,8E,10E,14Z-tetraenoic acid)
- LXA4
lipoxin A4 (5S,6R,15S-trihydroxy-eicosa-7E, 9E,11Z,13E-tetraenoic acid)
- LXB4
lipoxin B4 (5S,14R,15S-trihydroxy-eicosa-6E,8Z,10E,12E-tetraenoic acid)
- MaR1
maresin 1
- NO
nitric oxide
- PD1/NPD1
protectin D1/neuroprotectin D1 (10R,17S-dihydroxy-docosa-4Z,7Z,11E,13E,15Z,19Z-hexaenoic acid)
- PG
prostaglandin
- PUFA
poly-unsaturated fatty acid
- RvD1
resolvin D1 (7S,8R,17R-trihydroxy-4Z,9E,11E,13Z,15E,19Z-docosahexaenoic acid)
- RvE1
resolvin E1 (5S,12R,18R-trihydroxy-eicosa-6Z,8E,10E,14Z,16E-pentaenoic acid)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement:
- The resolution of inflammation is actively regulated by specialized pro-resolving mediators (SPM).
- SPM are lipid mediators derived from arachidonic acid and omega-3 polyunsaturated fatty acids.
- SPM are formed enzymatically during resolution of inflammation.
- SPM include lipoxins, resolvins, protectins, and maresins.
References
- 1.Cotran RS, Kumar V, T C. Robbins Pathologic Basis of Disease. 6th ed. Philadelphia: W.B. Saunders Co; 1999. [Google Scholar]
- 2.Gallin JL, Snyderman R. Inflammation, Basic principles and clinical correlates. 3rd ed. Philadelphia: Lippincott Williams & Wilkins; 1999. [Google Scholar]
- 3.Plytycz B, Seljelid R. From inflammation to sickness: historical perspective. Arch. Immunol. Ther. Exp. (Warsz) 2003;51:105–109. [PubMed] [Google Scholar]
- 4.Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nat. Immunol. 2005;6:1191–1197. doi: 10.1038/ni1276. [DOI] [PubMed] [Google Scholar]
- 5.Fabry Z, Schreiber HA, Harris MG, Sandor M. Sensing the microenvironment of the central nervous system: immune cells in the central nervous system and their pharmacological manipulation. Curr. Opin. Pharmacol. 2008;8:496–507. doi: 10.1016/j.coph.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gronert K. Lipid autacoids in inflammation and injury responses: a matter of privilege. Mol. Interv. 2008;8:28–35. doi: 10.1124/mi.8.1.7. [DOI] [PubMed] [Google Scholar]
- 7.Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428–435. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- 8.Metchnikoff E. Lectures on Comparative Pathology of Inflammation. New York: 1893. [Google Scholar]
- 9.Iwasaki A, Medzhitov R. Regulation of adaptive immunity by the innate immune system. Science. 2010;327:291–295. doi: 10.1126/science.1183021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kolaczkowska E. Shedding light on vascular permeability during peritonitis: role of mast cell histamine versus macrophage cysteinyl leukotrienes. Inflamm Res. 2002;51:519–521. doi: 10.1007/pl00012422. [DOI] [PubMed] [Google Scholar]
- 11.Newman SL, Henson JE, Henson PM. Phagocytosis of senescent neutrophils by human monocyte-derived macrophages and rabbit inflammatory macrophages. J. Exp. Med. 1982;156:430–442. doi: 10.1084/jem.156.2.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tobin DM, Vary JCJ, Ray JP, Walsh GS, Dunstan SJ, Bang ND, Hagge DA, Khadge S, King MC, Hawn TR, Moens CB, Ramakrishnan L. The lta4h locus modulates susceptibility to mycobacterial infection in zebrafish and humans. Cell. 2010;140:717–730. doi: 10.1016/j.cell.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diegelmann RF, Evans MC. Wound healing: an overview of acute, fibrotic and delayed healing. Front Biosci. 2004;1:283–289. doi: 10.2741/1184. [DOI] [PubMed] [Google Scholar]
- 14.MacKay AR, Sedgwick AD, Dunn CJ, Fleming WE, Willoughby DA. The transition from acute to chronic inflammation. Br. J. Dermatol. 1985;113:34–48. doi: 10.1111/j.1365-2133.1985.tb15624.x. [DOI] [PubMed] [Google Scholar]
- 15.Nathan C, Ding A. Nonresolving inflammation. Cell. 2010;140:871–882. doi: 10.1016/j.cell.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 16.Serhan CN, Brain SD, Buckley CD, Gilroy DW, Haslett C, O’Neill LA, Perretti M, Rossi AG, Wallace JL. Resolution of inflammation: state of the art, definitions and terms. FASEB J. 2007;21:325–332. doi: 10.1096/fj.06-7227rev. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hao S, Baltimore D. The stability of mRNA influences the temporal order of the induction of genes encoding inflammatory molecules. Nat Immunol. 2009;10:281–288. doi: 10.1038/ni.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.White AJ, Gompertz S, Bayley DL, Hill SL, O’Brien C, Unsal I, Stockley RA. Resolution of bronchial inflammation is related to bacterial eradication following treatment of exacerbations of chronic bronchitis. Thorax. 2003;58:680–685. doi: 10.1136/thorax.58.8.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Samuelsson B. Arachidonic acid metabolism: role in inflammation. Z. Rheumatol. 1991;50:3–6. [PubMed] [Google Scholar]
- 20.Lee TH, Woszczek G, Farooque SP. Leukotriene E4: perspective on the forgotten mediator. J. Allergy Clin. Immunol. 2009;124:417–421. doi: 10.1016/j.jaci.2009.04.020. [DOI] [PubMed] [Google Scholar]
- 21.Hedqvist P, Raud J, Dahlén SE. Dual action of prostaglandin E2 in allergic inflammation. Adv. Prostaglandin Thromboxane Leukot. Res. 1989;19:539–542. [PubMed] [Google Scholar]
- 22.Harada Y, Tanaka K, Uchida Y, Ueno A, Oh-Ishi S, Yamashita K, Ishibashi M, Miyazaki H, Katori M. Changes in the levels of prostaglandins and thromboxane and their roles in the accumulation of exudate in rat carrageenin-induced pleurisy--a profile analysis using gas chromatography-mass spectrometry. Prostaglandins. 1982;23:881–895. doi: 10.1016/0090-6980(82)90131-9. [DOI] [PubMed] [Google Scholar]
- 23.Williams TJ. Prostaglandin E2, prostaglandin I2 and the vascular changes of inflammation. Br. J. Pharmacol. 1979;65:517–524. doi: 10.1111/j.1476-5381.1979.tb07860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Serhan CN. Lipoxins and aspirin-triggered 15-epi-lipoxins are the first lipid mediators of endogenous anti-inflammation and resolution. Prostaglandins Leukot. Essent. Fatty Acids. 2005;73:141–162. doi: 10.1016/j.plefa.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 25.Haslett C. Resolution of acute inflammation and the role of apoptosis in the tissue fate of granulocytes. Clin. Sci. (Lond) 1992;83:639–648. doi: 10.1042/cs0830639. [DOI] [PubMed] [Google Scholar]
- 26.Berczi I, Quintanar-Stephano A, Kovacs K. Neuroimmune regulation in immunocompetence, acute illness, and healing. Ann N Y Acad Sci. 2009;1153:220–239. doi: 10.1111/j.1749-6632.2008.03975.x. [DOI] [PubMed] [Google Scholar]
- 27.Sternberg EM. Neuroendocrine regulation of autoimmune/inflammatory disease. J Endocrinol. 2001;169:429–435. doi: 10.1677/joe.0.1690429. [DOI] [PubMed] [Google Scholar]
- 28.Tracey KJ. Physiology and immunology of the cholinergic antiinflammatory pathway. J. Clin. Invest. 2007;117:289–296. doi: 10.1172/JCI30555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Willoughby DA, Moore AR, Colville-Nash PR, Gilroy D. Resolution of inflammation. Int. J. Immunopharmacol. 2000;22:1131–1135. doi: 10.1016/s0192-0561(00)00064-3. [DOI] [PubMed] [Google Scholar]
- 30.Serhan CN. Resolution phases of inflammation: Novel endogenous anti- inflammatory and proresolving lipid mediators and pathways. Annu. Rev. Immunol. 2006;25:101–137. doi: 10.1146/annurev.immunol.25.022106.141647. [DOI] [PubMed] [Google Scholar]
- 31.Varani J, Ward PA. Mechanisms of endothelial cell injury in acute inflammation. Shock. 1994;2:311–319. doi: 10.1097/00024382-199411000-00001. [DOI] [PubMed] [Google Scholar]
- 32.Dallegri F, Ottonello L. Tissue injury in neutrophilic inflammation. Inflamm. Res. 1997:382–391. doi: 10.1007/s000110050208. [DOI] [PubMed] [Google Scholar]
- 33.Weiss SJ. Tissue destruction by neutrophils. N. Engl. J. Med. 1989;320:365–376. doi: 10.1056/NEJM198902093200606. [DOI] [PubMed] [Google Scholar]
- 34.Han J, Ulevitch RJ. Limiting inflammatory responses during activation of innate immunity. Nat. Immunol. 2005;6:1198–1205. doi: 10.1038/ni1274. [DOI] [PubMed] [Google Scholar]
- 35.Lands WE. Biochemistry and physiology of n-3 fatty acids. FASEB J. 1992;6:2530–2536. doi: 10.1096/fasebj.6.8.1592205. [DOI] [PubMed] [Google Scholar]
- 36.Bazan NG. Omega-3 fatty acids, pro-inflammatory signaling and neuroprotection. Curr. Opin. Clin. Nutr. Metab. Care. 2007;10:136–141. doi: 10.1097/MCO.0b013e32802b7030. [DOI] [PubMed] [Google Scholar]
- 37.Rose DP, Connolly JM. Omega-3 fatty acids as cancer chemopreventive agents. Pharmacol Ther. 1999;83:217–244. doi: 10.1016/s0163-7258(99)00026-1. [DOI] [PubMed] [Google Scholar]
- 38.Burr GO, Burr MM. A new deficiency disease produced by the rigid exclusion of fat from the diet. J. Biol. Chem. 1929;82:345–367. doi: 10.1111/j.1753-4887.1973.tb06008.x. [DOI] [PubMed] [Google Scholar]
- 39.Calder PC. Polyunsaturated fatty acids and inflammatory processes: New twists in an old tale. Biochimie. 2009;91:791–795. doi: 10.1016/j.biochi.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 40.von Schacky C. Omega-3 fatty acids and cardiovascular disease. Curr. Opin. Clin. Nutr. Metab. Care. 2007;10:129–135. doi: 10.1097/MCO.0b013e3280127af0. [DOI] [PubMed] [Google Scholar]
- 41.Kuratko CN, Salem NJ. Biomarkers of DHA status. Prostaglandins Leukot. Essent. Fatty Acids. 2009;81:111–118. doi: 10.1016/j.plefa.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 42.Simopoulos AP, Leaf A, Salem NJ. Workshop on the essentiality of and recommended dietary intakes for omega-6 and omega-3 fatty acids. J. Am. Coll. Nutr. 1999;18:487–489. doi: 10.1080/07315724.1999.10718888. [DOI] [PubMed] [Google Scholar]
- 43.Burdge GC, Calder PC. Conversion of alpha-linolenic acid to longer-chain polyunsaturated fatty acids in human adults. Reprod. Nutr. Dev. 2005;45:581–597. doi: 10.1051/rnd:2005047. [DOI] [PubMed] [Google Scholar]
- 44.Aksoy Y, Aksoy H, Altinkaynak K, Aydin HR, Ozkan A. Sperm fatty acid composition in subfertile men. Prostaglandins Leukot. Essent. Fatty Acids. 2006;75:75–79. doi: 10.1016/j.plefa.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 45.Bazan NG. Cellular and molecular events mediated by docosahexaenoic acid-derived neuroprotectin D1 signaling in photoreceptor cell survival and brain protection. Prostaglandins Leukot. Essent. Fatty Acids. 2009;81:205–211. doi: 10.1016/j.plefa.2009.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bouvier J, Zemski Berry KA, Hullin-Matsuda F, Makino A, Michaud S, Geloën A, Murphy RC, Kobayashi T, Lagarde M, Delton-Vandenbroucke I. Selective decrease of bis(monoacylglycero)phosphate content in macrophages by high supplementation with docosahexaenoic acid. J. Lipid Res. 2009;50:243–255. doi: 10.1194/jlr.M800300-JLR200. [DOI] [PubMed] [Google Scholar]
- 47.Kagan VE, Tyurina YY, Bayir H, Chu CT, Kapralov AA, Vlasova II, Belikova NA, Tyurin VA, Amoscato A, Epperly M, Greenberger J, Dekosky S, Shvedova AA, Jiang J. The "pro-apoptotic genies" get out of mitochondria: oxidative lipidomics and redox activity of cytochrome c/cardiolipin complexes. Chem. Biol. Interact. 2006;163:15–28. doi: 10.1016/j.cbi.2006.04.019. [DOI] [PubMed] [Google Scholar]
- 48.Lee TH, Horton CE, Kyan-Aung U, Haskard D, Crea AE, Spur BW. Lipoxin A4 and lipoxin B4 inhibit chemotactic responses of human neutrophils stimulated by leukotriene B4 and N-formyl-L-methionyl-L-leucyl-L-phenylalanine. Clin. Sci. (Lond) 1989;77:195–203. doi: 10.1042/cs0770195. [DOI] [PubMed] [Google Scholar]
- 49.Uller L, Persson CG, Erjefält JS. Resolution of airway disease: removal of inflammatory cells through apoptosis, egression or both? Trends Pharmacol Sci. 2006;27:461–466. doi: 10.1016/j.tips.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 50.Godson C, Mitchell S, Harvey K, Petasis NA, Hogg N, Brady HR. Lipoxins rapidly stimulate nonphlogistic phagocytosis of apoptotic neutrophils by monocyte-derived macrophages. J. Immunol. 2000;164:1663–1667. doi: 10.4049/jimmunol.164.4.1663. [DOI] [PubMed] [Google Scholar]
- 51.Kolaczkowska E, Koziol A, Plytycz B, Arnold B. Inflammatory macrophages, and not only neutrophils, die by apoptosis during acute peritonitis. Immunobiology. 2010;215:492–504. doi: 10.1016/j.imbio.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 52.Papayianni A, Serhan CN, Brady HR. Lipoxin A4 and B4 inhibit leukotriene-stimulated interactions of human neutrophils and endothelial cells. J. Immunol. 1996;156:2264–2272. [PubMed] [Google Scholar]
- 53.Gewirtz AT, McCormick B, Neish AS, Petasis NA, Gronert K, Serhan CN, Madara JL. Pathogen-induced chemokine secretion from model intestinal epithelium is inhibited by lipoxin A4 analogs. J. Clin. Invest. 1998;101:1860–1869. doi: 10.1172/JCI1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Colgan SP, Serhan CN, Parkos CA, Delp-Archer C, Madara JL. Lipoxin A4 modulates transmigration of human neutrophils across intestinal epithelial monolayers. J. Clin. Invest. 1993;92:75–82. doi: 10.1172/JCI116601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Serhan CN, Yacoubian S, Yang R. Anti-inflammatory and proresolving lipid mediators. Annu. Rev. Pathol. 2008;3:279–312. doi: 10.1146/annurev.pathmechdis.3.121806.151409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Serhan CN, Hamberg M, Samuelsson B. Lipoxins: novel series of biologically active compounds formed from arachidonic acid in human leukocytes. Proc. Natl. Acad. Sci. U.S.A. 1984;81:5335–5339. doi: 10.1073/pnas.81.17.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gronert K, Clish CB, Romano M, Serhan CN. Transcellular regulation of eicosanoid biosynthesis. Methods Mol. Biol. 1999;120:119–144. doi: 10.1385/1-59259-263-5:119. [DOI] [PubMed] [Google Scholar]
- 58.Fiore S, Maddox JF, Perez HD, Serhan CN. Identification of a human cDNA encoding a functional high affinity lipoxin A4 receptor. J. Exp. Med. 1994;180:253–260. doi: 10.1084/jem.180.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chiang N, Arita M, Serhan CN. Anti-inflammatory circuitry: lipoxin, aspirin-triggered lipoxins and their receptor ALX. Prostaglandins Leukot. Essent. Fatty Acids. 2005;73:163–177. doi: 10.1016/j.plefa.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 60.Filep JG, Kebir DE. Neutrophil apoptosis: a target for enhancing the resolution of inflammation. J. Cell. Biochem. 2009;108:1039–1046. doi: 10.1002/jcb.22351. [DOI] [PubMed] [Google Scholar]
- 61.Parkinson JF. Lipoxin and synthetic lipoxin analogs: an overview of anti-inflammatory functions and new concepts in immunomodulation. Inflamm. Allergy Drug Targets. 2006;5:91–106. doi: 10.2174/187152806776383125. [DOI] [PubMed] [Google Scholar]
- 62.Maddox JF, Serhan CN. Lipoxin A4 and B4 are potent stimuli for human monocyte migration and adhesion: selective inactivation by dehydrogenation and reduction. J. Exp.Med. 1996;183:137–146. doi: 10.1084/jem.183.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Reville K, Crean JK, Vivers S, Dransfield I, Godson C. Lipoxin A4 redistributes myosin IIA and Cdc42 in macrophages: implications for phagocytosis of apoptotic leukocytes. J. Immunol. 2006;176:1878–1888. doi: 10.4049/jimmunol.176.3.1878. [DOI] [PubMed] [Google Scholar]
- 64.Lecomte M, Laneuville O, Ji C, DeWitt DL, Smith WL. Acetylation of human prostaglandin endoperoxide synthase-2 (cyclooxygenase-2) by aspirin. J. Biol. Chem. 1994;269:13207–13215. [PubMed] [Google Scholar]
- 65.Clària J, Serhan CN. Aspirin triggers previously undescribed bioactive eicosanoids by human endothelial cell-leukocyte interactions. Proc. Natl. Acad. Sci. U.S.A. 1995;92:9475–9479. doi: 10.1073/pnas.92.21.9475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Birnbaum Y, Ye Y, Lin Y, Freeberg SY, Huang MH, Perez-Polo JR, Uretsky BF. Aspirin augments 15-epi-lipoxin A4 production by lipopolysaccharide, but blocks the pioglitazone and atorvastatin induction of 15-epi-lipoxin A4 in the rat heart. Prostaglandins Other Lipid Mediat. 2007;83:89–98. doi: 10.1016/j.prostaglandins.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 67.Titos E, Chiang N, Serhan CN, Romano M, Gaya J, Pueyo G, Clària J. Hepatocytes are a rich source of novel aspirin-triggered 15-epi-lipoxin A4. Am. J. Physiol. 1999;277:C870–C877. doi: 10.1152/ajpcell.1999.277.5.C870. [DOI] [PubMed] [Google Scholar]
- 68.Rao NL, Riley JP, Banie H, Xue X, Sun B, Crawford S, Lundeen KA, Yu F, Karlsson L, Fourie AM, Dunford PJ. Leukotriene A4 hydrolase inhibition attenuates allergic airway inflammation and hyperresponsiveness. Am. J. Respir. Crit. Care Med. 2010;181:899–907. doi: 10.1164/rccm.200807-1158OC. [DOI] [PubMed] [Google Scholar]
- 69.Morris T, Stables M, Hobbs A, de Souza P, Colville-Nash P, Warner T, Newson J, Bellingan G, Gilroy DW. Effects of low-dose aspirin on acute inflammatory responses in humans. J. Immunol. 2009;183:2089–2096. doi: 10.4049/jimmunol.0900477. [DOI] [PubMed] [Google Scholar]
- 70.Chiang N, Bermudez EA, Ridker PM, Hurwitz S, Serhan CN. Aspirin triggers antiinflammatory 15-epi-lipoxin A4 and inhibits thromboxane in a randomized human trial. Proc. Natl. Acad. Sci. U.S.A. 2004;101:15178–15183. doi: 10.1073/pnas.0405445101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Takano T, Fiore S, Maddox JF, Brady HR, Petasis NA, Serhan CN. Aspirin-triggered 15-epi-lipoxin A4 (LXA4) and LXA4 stable analogues are potent inhibitors of acute inflammation: evidence for anti-inflammatory receptors. J. Exp. Med. 1997;185:1693–1704. doi: 10.1084/jem.185.9.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gronert K, Martinsson-Niskanen T, Ravasi S, Chiang N, Serhan CN. Selectivity of recombinant human leukotriene D4, leukotriene B4, and lipoxin A4 receptors with aspirin-triggered 15-epi-LXA4 and regulation of vascular and inflammatory responses. Am. J. Pathol. 2001;158:3–9. doi: 10.1016/S0002-9440(10)63937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Badr KF, DeBoer DK, Schwartzberg M, Serhan CN. Lipoxin A4 antagonizes cellular and in vivo actions of leukotriene D4 in rat glomerular mesangial cells: evidence for competition at a common receptor. Proc. Natl. Acad. Sci. USA. 1989;86:3438–3442. doi: 10.1073/pnas.86.9.3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Krishnamoorthy S, Recchiuti A, Chiang N, Yacoubian S, Lee CH, Yang R, Petasis NA, Serhan CN. Resolvin D1 binds human phagocytes with evidence for proresolving receptors. Proc. Natl. Acad. Sci. U S A. 2010;107:1660–1665. doi: 10.1073/pnas.0907342107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fiore S, Serhan CN. Formation of lipoxins and leukotrienes during receptor-mediated interactions of human platelets and recombinant human granulocyte/macrophage colony-stimulating factor-primed neutrophils. J. Exp. Med. 1990;172:1451–1457. doi: 10.1084/jem.172.5.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Maddox JF, Hachicha M, Takano T, Petasis NA, Fokin VV, Serhan CN. Lipoxin A4 stable analogs are potent mimetics that stimulate human monocytes and THP-1 cells via a G-protein-linked lipoxin A4 receptor. J. Biol. Chem. 1997;272:6972–6978. doi: 10.1074/jbc.272.11.6972. [DOI] [PubMed] [Google Scholar]
- 77.Romano M, Maddox JF, Serhan CN. Activation of human monocytes and the acute monocytic leukemia cell line (THP-1) by lipoxins involves unique signaling pathways for lipoxin A4 versus lipoxin B4: evidence for differential Ca2+ mobilization. J. Immunol. 1996;157:2149–2154. [PubMed] [Google Scholar]
- 78.Maddox JF, Colgan SP, Clish CB, Petasis NA, Fokin VV, Serhan CN. Lipoxin B4 regulates human monocyte/neutrophil adherence and motility: design of stable lipoxin B4 analogs with increased biologic activity. FASEB J. 1998;12:487–494. doi: 10.1096/fasebj.12.6.487. [DOI] [PubMed] [Google Scholar]
- 79.Takano T, Clish CB, Gronert K, Petasis N, Serhan CN. Neutrophil-mediated changes in vascular permeability are inhibited by topical application of aspirin-triggered 15-epi-lipoxin A4 and novel lipoxin B4 stable analogues. J. Clin. Invest. 1998;101:819–826. doi: 10.1172/JCI1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bannenberg G, Moussignac RL, Gronert K, Devchand PR, Schmidt BA, Guilford WJ, Bauman JG, Subramanyam B, Perez HD, Parkinson JF, Serhan CN. Lipoxins and novel 15-epi-lipoxin analogs display potent anti-inflammatory actions after oral administration. Br. J. Pharmacol. 2004;143:43–52. doi: 10.1038/sj.bjp.0705912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Larsen LN, Dahl E, Bremer J. Peroxidative oxidation of leuco-dichlorofluorescein by prostaglandin H synthase in prostaglandin biosynthesis from polyunsaturated fatty acids. Biochim. Biophys. Acta. 1996;1299:47–53. doi: 10.1016/0005-2760(95)00188-3. [DOI] [PubMed] [Google Scholar]
- 82.Hornstra G, Christ-Hazelhof E, Haddeman E, ten Hoor F, Nugteren DH. Fish oil feeding lowers thromboxane- and prostacyclin production by rat platelets and aorta and does not result in the formation of prostaglandin I3. Prostaglandins. 1981;21:727–738. [Google Scholar]
- 83.Hudert CA, Weylandt KH, Lu Y, Wang J, Hong S, Dignass A, Serhan CN, Kang JX. Transgenic mice rich in endogenous omega-3 fatty acids are protected from colitis. Proc. Natl. Acad. Sci. USA. 2006;103:11276–11281. doi: 10.1073/pnas.0601280103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Terano T, Salmon JA, Moncada S. Biosynthesis and biological activity of leukotriene B5. Prostaglandins. 1984;27:217–232. doi: 10.1016/0090-6980(84)90075-3. [DOI] [PubMed] [Google Scholar]
- 85.Whitaker MO, Wyche A, Fitzpatrick F, Sprecher H, Needleman P. Triene prostaglandins: prostaglandin D3 and icosapentaenoic acid as potential antithrombotic substances. Proc. Natl. Acad. Sci. USA. 1979;76:5919–5923. doi: 10.1073/pnas.76.11.5919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Serhan CN, Clish CB, Brannon J, Colgan SP, Chiang N, Gronert K. Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processing. J. Exp. Med. 2000;192:1197–1204. doi: 10.1084/jem.192.8.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Arita M, Bianchini F, Aliberti J, Sher A, Chiang N, Hong S, Yang R, Petasis NA, Serhan CN. Stereochemical assignment, antiinflammatory properties, and receptor for the omega-3 lipid mediator resolvin E1. J. Exp. Med. 2005;201:713–722. doi: 10.1084/jem.20042031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.GISSI-Prevenzione_Investigators. Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto miocardico. Lancet. 1999;354:447–455. [PubMed] [Google Scholar]
- 89.Bannenberg* GL, Chiang* N, Ariel A, Arita M, Tjonahen E, Gotlinger KH, Hong S, Serhan CN. Molecular circuits of resolution: formation and actions of resolvins and protectins. J. Immunol. 2005;174:4345–4355. doi: 10.4049/jimmunol.174.7.4345. (* shared first authors) [DOI] [PubMed] [Google Scholar]
- 90.Schwab JM, Chiang N, Arita M, Serhan CN. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature. 2007;447:869–874. doi: 10.1038/nature05877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Haas-Stapleton EJ, Lu Y, Hong S, Arita M, Favoreto S, Nigam S, Serhan CN, Agabian N. Candida albicans modulates host defense by biosynthesizing the proresolving mediator resolvin E1. PLoS ONE. 2007;2:e1316. doi: 10.1371/journal.pone.0001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Serhan CN, Hong S, Gronert K, Colgan SP, Devchand PR, Mirick G, Moussignac R-L. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J. Exp. Med. 2002;196:1025–1037. doi: 10.1084/jem.20020760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hong S, Porter TF, Lu Y, Oh SF, Pillai PS, Serhan CN. Resolvin E1 metabolome in local inactivation during inflammation-resolution. J. Immunol. 2008;180:3512–3519. doi: 10.4049/jimmunol.180.5.3512. [DOI] [PubMed] [Google Scholar]
- 94.Dona M, Fredman G, Schwab JM, Chiang N, Arita M, Goodarzi A, Cheng G, von Andrian UH, Serhan CN. Resolvin E1, an EPA-derived mediator in whole blood, selectively counterregulates leukocytes and platelets. Blood. 2008;112:848–855. doi: 10.1182/blood-2007-11-122598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ariel A, Fredman G, Sun YP, Kantarci A, Van Dyke TE, Luster AD, Serhan CN. Apoptotic neutrophils and T cells sequester chemokines during immune response resolution through modulation of CCR5 expression. Nat. Immunol. 2006;7:1209–1216. doi: 10.1038/ni1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Arita M, Oh SF, Chonan T, Hong S, Elangovan S, Sun YP, Uddin J, Petasis NA, Serhan CN. Metabolic inactivation of resolvin E1 and stabilization of its anti-inflammatory actions. J. Biol. Chem. 2006;281:22847–22854. doi: 10.1074/jbc.M603766200. [DOI] [PubMed] [Google Scholar]
- 97.Jin Y, Arita M, Zhang Q, Saban DR, Chauhan SK, Chiang N, Serhan CN, Dana R. Anti-angiogenesis effect of the novel anti-inflammatory and proresolving lipid mediators. Invest. Ophthalmol. Vis. Sci. 2009;50:4743–4752. doi: 10.1167/iovs.08-2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Connor KM, SanGiovanni JP, Löfqvist C, Aderman CM, Chen J, Higuchi A, Hong S, Pravda EA, Majchrzak S, Carper D, Hellström A, Kang JX, Chew EY, Salem NJ, Serhan CN, Smith LE. Increased dietary intake of omega-3-polyunsaturated fatty acids reduces pathological retinal angiogenesis. Nat. Med. 2007;13:868–873. doi: 10.1038/nm1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wittamer V, Franssen JD, Vulcano M, Mirjolet JF, Le Poul E, Migeotte I, Brezillon S, Tyldesley R, Blanpain C, Detheux M, Mantovani A, Sozzani S, Vassart G, Parmentier M, Communi D. Specific recruitment of antigen-presenting cells by chemerin, a novel processed ligand from human inflammatory fluids. J. Exp. Med. 2003;198:977–985. doi: 10.1084/jem.20030382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Campbell EL, Louis NA, Tomassetti SE, Canny GO, Arita M, Serhan CN, Colgan SP. Resolvin E1 promotes mucosal surface clearance of neutrophils: a new paradigm for inflammatory resolution. FASEB J. 2007;21:3162–3170. doi: 10.1096/fj.07-8473com. [DOI] [PubMed] [Google Scholar]
- 101.Arita M, Ohira T, Sun Y-P, Elangovan S, Chiang N, Serhan CN. Resolvin E1 selectively interacts with leukotriene B4 receptor BLT1 and ChemR23 to regulate inflammation. J. Immunol. 2007;178:3912–3917. doi: 10.4049/jimmunol.178.6.3912. [DOI] [PubMed] [Google Scholar]
- 102.Herrera BS, Ohira T, Gao L, Omori K, Yang R, Zhu M, Muscara MN, Serhan CN, Van Dyke TE, Gyurko R. An endogenous regulator of inflammation, resolvin E1, modulates osteoclast differentiation and bone resorption. Br. J. Pharmacol. 2008;155:1214–1223. doi: 10.1038/bjp.2008.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ohira T, Arita M, Omori K, Recchiuti A, Van Dyke TE, Serhan CN. Resolvin E1 receptor activation signals phosphorylation and phagocytosis. J. Biol. Chem. 2010;285:3451–3461. doi: 10.1074/jbc.M109.044131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Duffield JS, Hong S, Vaidya VS, Lu Y, Fredman G, Serhan CN, Bonventre JV. Resolvin D series and protectin D1 mitigate acute kidney injury. J. Immunol. 2006;177:5902–5911. doi: 10.4049/jimmunol.177.9.5902. [DOI] [PubMed] [Google Scholar]
- 105.Hassan IR, Gronert K. Acute changes in dietary omega-3 and omega-6 polyunsaturated fatty acids have a pronounced impact on survival following ischemic renal injury and formation of renoprotective docosahexaenoic acid-derived protectin D1. J. Immunol. 2009;182:3223–3232. doi: 10.4049/jimmunol.0802064. [DOI] [PubMed] [Google Scholar]
- 106.González-Périz A, Horrillo R, Ferré N, Gronert K, Dong B, Morán-Salvador E, Titos E, Martínez-Clemente M, López-Parra M, Arroyo V, Clària J. Obesity-induced insulin resistance and hepatic steatosis are alleviated by omega-3 fatty acids: a role for resolvins and protectins. FASEB J. 2009;23:1946–1957. doi: 10.1096/fj.08-125674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hasturk H, Kantarci A, Goguet-Surmenian E, Blackwood A, Andry C, Serhan CN, Van Dyke TE. Resolvin E1 regulates inflammation at the cellular and tissue level and restores tissue homeostasis in vivo. J. Immunol. 2007;179:7021–7029. doi: 10.4049/jimmunol.179.10.7021. [DOI] [PubMed] [Google Scholar]
- 108.Haworth O, Cernadas M, Yang R, Serhan CN, Levy BD. Resolvin E1 regulates interleukin 23, interferon-γ and lipoxin A4 to promote the resolution of allergic airway inflammation. Nat. Immunol. 2008;9:873–879. doi: 10.1038/ni.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Seki H, Fukunaga K, Arita M, Arai H, Nakanishi H, Taguchi R, Miyasho T, Takamiya R, Asano K, Ishizaka A, Takeda J, Levy BD. The anti-inflammatory and proresolving mediator resolvin E1 protects mice from bacterial pneumonia and acute lung injury. J. Immunol. 2010;184:836–843. doi: 10.4049/jimmunol.0901809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tjonahen E, Oh SF, Siegelman J, Elangovan S, Percarpio KB, Hong S, Arita M, Serhan CN. Resolvin E2: identification and anti-inflammatory actions: pivotal role of human 5-lipoxygenase in resolvin E series biosynthesis. Chem Biol. 2006;13:1193–1202. doi: 10.1016/j.chembiol.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 111.Sun YP, Oh SF, Uddin J, Yang R, Gotlinger K, Campbell E, Colgan SP, Petasis NA, Serhan CN. Resolvin D1 and its aspirin-triggered 17R epimer. Stereochemical assignments, anti-inflammatory properties, and enzymatic inactivation. J. Biol. Chem. 2007;282:9323–9334. doi: 10.1074/jbc.M609212200. [DOI] [PubMed] [Google Scholar]
- 112.Hong S, Gronert L, Devchand PR, Moussignac R-L, Serhan CN. Serhan, Novel docosatrienes and 17S-resolvins generated from docosahexaenoic acid in murine brain, human blood, and glial cells. J. Biol. Chem. 2003;278:14677–14687. doi: 10.1074/jbc.M300218200. [DOI] [PubMed] [Google Scholar]
- 113.Rodriguez AR, Spur BW. First total synthesis of 7(S),16(R),17(S)-resolvin D2, a potent anti-inflammatory lipid mediator. Tetrahedron Lett. 2004;45:8717–8720. [Google Scholar]
- 114.Spite M, Norling LV, Summers L, Yang R, Cooper D, Petasis NA, Flower RJ, Perretti M, Serhan CN. Resolvin D2 is a potent regulator of leukocytes and controls microbial sepsis. Nature. 2009;461:1287–1291. doi: 10.1038/nature08541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Merched AJ, Ko K, Gotlinger KH, Serhan CN, Chan L. Atherosclerosis: evidence for impairment of resolution of vascular inflammation governed by specific lipid mediators. FASEB J. 2008;22:3595–3606. doi: 10.1096/fj.08-112201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Calandria JM, Marcheselli VL, Mukherjee PK, Uddin J, Winkler JW, Petasis NA, Bazan NG. Selective survival rescue in 15-lipoxygenase-1 deficient retinal pigment epithelial cells by the novel docosahexaenoic acid-derived mediator, neuroprotectin D1. J. Biol. Chem. 2009;284:17877–17882. doi: 10.1074/jbc.M109.003988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Spite M, Summers L, Porter TF, Srivastava S, Bhatnagar A, Serhan CN. Resolvin D1 controls inflammation initiated by glutathione-lipid conjugates formed during oxidative stress. Br. J. Pharmacol. 2009;158:1062–1073. doi: 10.1111/j.1476-5381.2009.00234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chiang N, Serhan CN, Dahlén SE, Drazen JM, Hay DW, Rovati GE, Shimizu T, Yokomizo T, Brink C. The lipoxin receptor ALX: potent ligand-specific and stereoselective actions in vivo. Pharmacol. Rev. 2006;58:463–487. doi: 10.1124/pr.58.3.4. [DOI] [PubMed] [Google Scholar]
- 119.Bannenberg GL. Anti-inflammatory actions of lipoxins. Exp. Opin. Ther. Patents. 2007;17:591–605. [Google Scholar]
- 120.Hong S, Lu Y, Yang R, Gotlinger KH, Petasis NA, Serhan CN. Resolvin D1, protectin D1, and related docosahexaenoic acid-derived products: Analysis via electrospray/low energy tandem mass spectrometry based on spectra and fragmentation mechanisms. J. Am. Soc. Mass Spectrom. 2007;18:128–144. doi: 10.1016/j.jasms.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Creo EJ, Mehrotra MM. A stereoselective an practical synthesis of 5,6(S,S)-epoxy-15(S)-hydroxy-7(E),9(E),11(Z),13(E)-eicosatetraenoic acid (4), a possible precursor of the lipoxins. Tetrah. Lett. 1986;27:5173–5176. [Google Scholar]
- 122.Serhan CN, Gotlinger K, Hong S, Lu Y, Siegelman J, Baer T, Yang R, Colgan SP, Petasis NA. Anti-inflammatory actions of neuroprotectin D1/protectin D1 and its natural stereoisomers: assignments of dihydroxy-containing docosatrienes. J. Immunol. 2006;176:1848–1859. doi: 10.4049/jimmunol.176.3.1848. [DOI] [PubMed] [Google Scholar]
- 123.Serhan CN. Lipoxin biosynthesis and its impact in inflammatory and vascular events. Biochim. Biophys. Acta. 1994;1212:1–25. doi: 10.1016/0005-2760(94)90185-6. [DOI] [PubMed] [Google Scholar]
- 124.Bazan NG, Calandria JM, Serhan CN. Rescue and repair during photoreceptor cell renewal mediated by docosahexaenoic acid-derived neuroprotectin D1. J Lipid Res. 2010;51:2018–2031. doi: 10.1194/jlr.R001131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Antony R, Lukiw WJ, Bazan NG. Neuroprotectin D1 induces dephosphorylation of BCL-XL in a PP2A-dependent manner during oxidative stress and promotes retinal pigment epithelial cell survival. J. Biol. Chem. 2010;285:18301–18308. doi: 10.1074/jbc.M109.095232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Calandria JM, Marcheselli VL, Mukherjee PK, Uddin J, Winkler JW, Petasis NA, Bazan NG. Selective survival rescue in 15-lipoxygenase-1 deficient retinal pigment epithelial cells by the novel docosahexaenoic acid-derived mediator, neuroprotectin D1. J. Biol. Chem. 2009;284:17877–17882. doi: 10.1074/jbc.M109.003988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Marcheselli VL, Mukherjee PK, Arita M, Hong S, Antony R, Sheets K, Winkler JW, Petasis NA, Serhan CN, Bazan NG. Neuroprotectin D1/protectin D1 stereoselective and specific binding with human retinal pigment epithelial cells and neutrophils. Prostaglandins Leukot. Essent. Fatty Acids. 2010;82:27–34. doi: 10.1016/j.plefa.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Serhan CN, Yang R, Martinod K, Kasuga K, Pillai PS, Porter TF, Oh SF, Spite M. Maresins: novel macrophage mediators with potent antiinflammatory and proresolving actions. J. Exp. Med. 2009;206:15–23. doi: 10.1084/jem.20081880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Dangi B, Obeng M, Naurothm M, CG J, Bailey-Hall E, Hallenbeck T, Arterburn LM. Metabolism and biological production of resolvins derived from docosapentaenoic acid (DPAn-6) Biochem. Pharmacol. 2009;79:251–260. doi: 10.1016/j.bcp.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 130.Dangi B, Obeng M, Nauroth JM, Teymourlouei M, Needham M, Raman K, Arterburn LM. Biogenic synthesis, and chemical characterization of anti-inflammatory resolvins derived from docosapentaenoic acid (DPAn-6) J. Biol. Chem. 2009;284:14744–14759. doi: 10.1074/jbc.M809014200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Navarro-Xavier RA, Newson J, Flor Silveira VL, Farrow SN, Gilroy DW, Byström J. A new strategy for the identification of novel molecules with targeted pro-resolution of inflammatory properties. J. Immunol. 2010;184:1516–1525. doi: 10.4049/jimmunol.0902866. [DOI] [PubMed] [Google Scholar]
- 132.Colville-Nash PR, Gilroy DW. COX-2 and the cyclopentenone prostaglandins - a new chapter in the book of inflammation? Prostaglandins Other Lipid Mediat. 2000;62:33–43. doi: 10.1016/s0090-6980(00)00074-5. [DOI] [PubMed] [Google Scholar]
- 133.Blaho VA, Buczynski MW, Brown CR, Dennis EA. Lipidomic analysis of dynamic eicosanoid responses during the induction and resolution of Lyme arthritis. J. Biol. Chem. 2009;284:21599–21612. doi: 10.1074/jbc.M109.003822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Biteman B, Hassan IR, Walker E, Leedom AJ, Dunn M, Seta F, Laniado-Schwartzman M, Gronert K. Interdependence of lipoxin A4 and heme-oxygenase in counter-regulating inflammation during corneal wound healing. FASEB J. 2007;21:2257–2266. doi: 10.1096/fj.06-7918com. [DOI] [PubMed] [Google Scholar]
- 135.Paul-Clark MJ, Van Cao T, Moradi-Bidhendi N, Cooper D, Gilroy DW. 15-epi-lipoxin A4-mediated induction of nitric oxide explains how aspirin inhibits acute inflammation. J. Exp. Med. 2004;200:69–78. doi: 10.1084/jem.20040566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Rodgers K, McMahon B, Mitchell D, Sadlier D, Godson C. Lipoxin A4 modifies platelet-derived growth factor-induced pro-fibrotic gene expression in human renal mesangial cells. Am. J. Pathol. 2005;167:683–694. doi: 10.1016/S0002-9440(10)62043-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Martins V, Valença SS, Farias-Filho FA, Molinaro R, Simões RL, Ferreira TP, e Silva PM, Hogaboam CM, Kunkel SL, Fierro IM, Canetti C, Benjamim CF. ATLa, an aspirin-triggered lipoxin A4 synthetic analog, prevents the inflammatory and fibrotic effects of bleomycin-induced pulmonary fibrosis. J. Immunol. 2009;182:5374–5381. doi: 10.4049/jimmunol.0802259. [DOI] [PubMed] [Google Scholar]
- 138.McMahon B, Mitchell D, Shattock R, Martin F, Brady HR, Godson C. Lipoxin, leukotriene, and PDGF receptors cross-talk to regulate mesangial cell proliferation. FASEB J. 2002;16:1817–1819. doi: 10.1096/fj.02-0416fje. [DOI] [PubMed] [Google Scholar]
- 139.Mitchell D, Rodgers K, Hanly J, McMahon B, Brady HR, Martin F, Godson C. Lipoxins inhibit Akt/PKB activation and cell cycle progression in human mesangial cells. Am. J. Pathol. 2004;164:937–946. doi: 10.1016/S0002-9440(10)63181-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ryan A, Godson C. Lipoxins: regulators of resolution. Curr. Opin. Pharmacol. 2010;10:166–172. doi: 10.1016/j.coph.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 141.Mitchell D, O’Meara SJ, Gaffney A, Crean JK, Kinsella BT, Godson C. The Lipoxin A4 receptor is coupled to SHP-2 activation: implications for regulation of receptor tyrosine kinases. J. Biol. Chem. 2007;282:15606–15618. doi: 10.1074/jbc.M611004200. [DOI] [PubMed] [Google Scholar]
- 142.Kasuga K, Yang R, Porter TF, Agrawal N, Petasis NA, Irimia D, Toner M, Serhan CN. Rapid appearance of resolvin precursors in inflammatory exudates: novel mechanisms in resolution. J. Immunol. 2008;181:8677–8687. doi: 10.4049/jimmunol.181.12.8677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Siegenthaler G, Roulin K, Chatellard-Gruaz D, Hotz R, Saurat J, Hellman U, Hagens G. A heterocomplex formed by the calcium-binding proteins MRP8 (S100A8) and MRP14 (S100A9) binds unsaturated fatty acids with high affinity. J. Biol. Chem. 1997;272:9371–9377. doi: 10.1074/jbc.272.14.9371. [DOI] [PubMed] [Google Scholar]
- 144.Harrington MG, Fonteh AN, Oborina E, Liao P, Cowan RP, McComb G, Chavez JN, Rush J, Biringer RG, F A. Hühmer, The morphology and biochemistry of nanostructures provide evidence for synthesis and signaling functions in human cerebrospinal fluid. Cerebrospinal Fluid Res. 2009;6:10. doi: 10.1186/1743-8454-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Huang BX, Dass C, Kim HY. Probing conformational changes of human serum albumin due to unsaturated fatty acid binding by chemical cross-linking and mass spectrometry. Biochem. J. 2005;387:695–702. doi: 10.1042/BJ20041624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Brezinski ME, Serhan CN. Selective incorporation of (15S)-hydroxyeicosatetraenoic acid in phosphatidylinositol of human neutrophils: agonist-induced deacylation and transformation of stored hydroxyeicosanoids. Proc. Natl. Acad. Sci. U.S.A. 1990;87:6248–6252. doi: 10.1073/pnas.87.16.6248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Gilroy DW, Newson J, Sawmynaden P, Willoughby DA, Croxtall JD. A novel role for phospholipase A2 isoforms in the checkpoint control of acute inflammation. Faseb J. 2004;18:489–498. doi: 10.1096/fj.03-0837com. [DOI] [PubMed] [Google Scholar]
- 148.Bannenberg GL. Resolvins: Current understanding and future potential in the control of inflammation. Curr. Opin. Drug Discov. Devel. 2009;12:644–658. [PubMed] [Google Scholar]