Abstract
Although previous studies have shown pointing errors and abnormal multijoint coordination in seated subjects with PD who cannot view their arm, the extent to which subjects with PD have problems using proprioception to coordinate equilibrium maintenance and goal-oriented task execution has not been adequately investigated. If a common motor program controls voluntary arm pointing movements and the accompanying postural adjustments, then impairments of proprioceptive integration in subjects with PD should have similar effects on pointing and body center of mass (CoM) control with eyes closed. Ten standing subjects with PD (OFF-medication) and 10 age-matched control subjects pointed to a target with their eyes closed and open. Although pointing accuracy was not significantly different between groups, body CoM displacements were reduced in subjects with PD, but not in controls, when eyes were closed. In addition, with eyes closed, PD subjects showed reduced temporal coupling between pointing and CoM velocity profiles and reduced spatial coupling between pointing and CoM endpoints. This poor coupling with eyes closed could be related to the PD subjects’ increased jerkiness of CoM displacements. The different effects of eye closure between control and PD subjects on the CoM displacements, but not pointing accuracy, are consistent with separate motor programs for the pointing and postural components of this task. Furthermore, the decoupling between the two movement components in subjects with PD when they could not use vision, suggests that the basal ganglia are involved in the integration of proprioceptive information for posture-movement coordination.
Keywords: Motor control, Pointing, Posture, Whole body, Basal ganglia, Complex movement
Patients with severe Parkinson’s disease (PD) have problems maintaining balance and coordinating complex goal-directed movements. Previous studies have shown that postural adjustments that accompany voluntary movements are bradykinetic (i.e. slow and weak) in subjects with PD (Horak et al. 1992; Gantchev et al. 1996; Frank et al. 2000; Rocchi et al. 2006). Subjects with PD also have smaller than normal limits of stability when asked to lean to their maximum limits (Mancini et al. 2008). Undershooting intended targets for arm pointing, locomotion, and stepping by subjects with PD with eyes closed has been hypothesized to be due to an underestimation of the motor command because of difficulty integrating proprioceptive information (Poizner et al. 1998; Adamovich et al. 2001b; Schubert et al. 2005; Almeida et al. 2005; Jacobs and Horak 2006). In contrast to smaller than normal intended movements, however, impaired upper-lower body coordination for axial movements during stance in subjects with PD often results in larger than normal center of mass (CoM) displacements, both in response to external perturbations and during voluntary axial movements (Alexandrov et al. 1998; Horak et al. 2005). Thus, although abnormal proprioceptive-motor integration in PD results in smaller than normal voluntary body CoM displacements during leaning and walking, it can also result in larger than normal CoM movements with reduced equilibrium control. It is unknown whether body CoM trajectories would be smaller or larger than normal when PD subjects are performing a voluntary, target-directed, arm pointing task requiring simultaneous control of axial postural coordination and postural equilibrium in stance (Stapley et al. 1999; Pozzo et al. 2002). Previous studies suggest that subjects with PD may have more difficulty coordinating multiple joints and multiple tasks than single joint single tasks (Poizner et al. 1998). Therefore, in the current study we asked standing subjects with PD and age-matched control subjects to point to a remembered visual target beyond their arm reach with their eyes open and eyes closed. Based on previous studies investigating movements to a target, we hypothesize that subjects with PD would undershoot their voluntary pointing endpoint and that the errors in endpoint will be greater in the subjects with PD when their eyes were closed, because they would need to depend on their impaired use of proprioceptive integration to estimate body motion (Jacobs and Horak 2006; Zia et al. 2000; Adamovich et al. 2001b). However, the analogies between the present experimental protocol and previous studies on axial synergies in subjects with PD would predict larger than normal body CoM displacements (Alexandrov et al. 1998).
Analysis of the conflict between hypometric voluntary (focal) arm movements and hypermetric postural (CoM) movements with loss of visual feedback in subjects with PD is especially interesting because this opposite prediction for the focal and postural components is not compatible with the hypothesis of a tightly coupled link between both the spatial (Stapley et al. 1999; Pozzo et al. 2002) and temporal (Frank et al. 2000; Patron et al. 2005) parameters of voluntary pointing and postural displacement in healthy subjects. If a single motor program controls the voluntary and postural components of a complex movement, it would not be possible for subjects with PD without visual feedback to decouple the close relationship between these two components (Massion 1992; Schepens and Drew 2003). However, a previous study suggests that subjects with PD show a less consistent coupling between the postural and voluntary components of a task (Frank et al. 2000). Specifically, unlike healthy subjects who consistently couple the offset of their preparatory postural, tibialis muscle activity with the onset of their focal, gastrocnemius muscle activity during a voluntary rise-to-toes task, subjects with PD showed late, inconsistent delays between the postural and the focal components (Frank et al. 2000). If PD decouples the normal tight link between the postural and voluntary components of a goal-directed pointing task, this is consistent with the hypothesis that the basal ganglia participate in this linkage and that posture and voluntary control are organized independently and then coupled downstream in subcortical areas (Massion 1992; Latash et al. 1995; Prentice and Drew 2001). If this uncoupling occurs only with loss of vision during the task, this is consistent with use of proprioceptive feedback in coupling postural with movement synergies (Adamovich et al. 2001a; Poizner et al. 2000).
1 Methods
1.1 Subjects
Ten subjects with idiopathic PD and 10 age-and sex-matched subjects gave informed consent to participate in the protocol approved by the institutional review board of Oregon Health & Science University. Subjects with neurological, muscular, or psychiatric disorders other than PD were excluded. All PD subjects showed bradykinesia and rigidity but little dyskinesia or tremor. Table 1 summarizes the characteristics of the PD subjects. To determine the effects of PD without the influence of dopaminergic medication, PD subjects were tested in the morning, at least 12 hours after their last consumption of anti-PD medication (OFF). There was no significant difference between the ages (PD: 61±7 vs CTR: 63±9 years) or weights (PD: 82±13 vs CTR: 85±11 kg) of subject groups.
Table 1.
Patient Characteristics. For each subject with PD the table reports the age, the years elapsed since the diagnosis of the PD, the score in the Hoehn & Yahr scale, the total and the axial score in the Unified Parkinson’s Disease Rating Scale and the Dyskinesia score in the off-medication state.
| Subject | Age | Since Diagnosis | H&Y | Motor UPDRS | Axial UPDRS | Dyskinesia |
|---|---|---|---|---|---|---|
| S1 | 55 | 5 | 2 | 19 | 10 | 0 |
| S2 | 65 | 7 | 2 | 25 | 10 | 0 |
| S3 | 52 | 8 | 2 | 28 | 14 | 4 |
| S4 | 65 | 8 | 2 | 34 | 18 | 0 |
| S5 | 69 | 9 | 2 | 27 | 12 | 0 |
| S6 | 71 | 10 | 2 | 58 | 24 | 0 |
| S7 | 50 | 10 | 3 | 27 | 13 | 0 |
| S8 | 62 | 15 | 4 | 59 | 28 | 4 |
| S9 | 60 | 16 | 2 | 39 | 23 | 0 |
| S10 | 58 | 18 | 4 | 60 | 34 | 0 |
1.2 Experimental protocol
The subjects performed whole-body pointing movements to a 2 cm diameter, reflective ball target suspended directly in front of them at a height from the floor and distance from their ankles of 50% of their body height (See Fig. 1). Subjects stood with their hands clasped together to point with both index fingertips at the target. Subjects repeated the task 5 times with their eyes closed, followed by 5 repetitions with their eyes open. For the eyes closed (EC) condition, subjects were asked to memorize the position of the target, close their eyes for approximately 3 seconds and then to reach to the target location, keeping the eyes closed. In order to avoid tactile feedback of task accomplishment, the target was moved aside once the subjects closed their eyes (the subjects were acquainted with it). The subjects held their final fingertip endpoint position for 3 seconds before returning to the starting position with eyes still closed. For the eyes open (EO) condition, the subjects touched the target with the tip of their fingers. Subjects were asked to move at their natural speed.
Fig. 1.
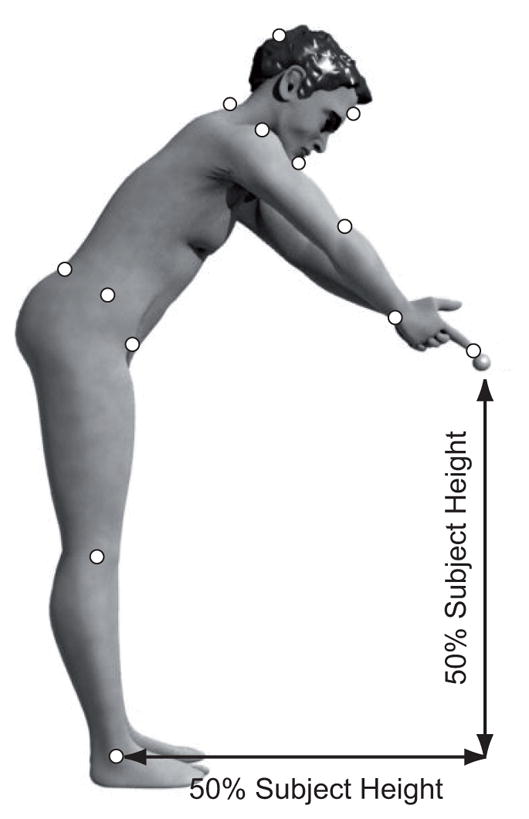
Whole body pointing protocol. Subjects reached with the apex of their joined index fingers to a target located at a distance and height proportional to their body height. Reflective markers (white circles) were placed at the following anatomical landmarks: nasion, occipital ridge, chin, 7th cervical segment of the spine, apex of index fingers, bilateral acromions, humeral lateral epicondyles, ulnar styloids, anterior superior iliac spines (ASIS), iliac crests, posterior superior iliac spines (PSIS), femoral lateral epicondyles, and lateral malleoli.
1.3 Data Collection
To quantify the subjects’ kinematic strategies for whole body pointing, 21 reflective markers were placed on the following anatomical landmarks: nasion, occipital ridge, chin, 7th cervical segment of the spine, apex of index fingers, bilateral acromions, humeral lateral epicondyles, ulnar styloids, anterior superior iliac spines (ASIS), iliac crests, posterior superior iliac spines (PSIS), femoral lateral epicondyles, and lateral malleoli. The three-dimensional position of the markers were recorded using a Motion Analysis system (Santa Rosa, CA, USA) with eight Falcon video cameras sampling at 60 Hz. Ground reaction forces and position of the center of foot pressure (CoP) were recorded with an AMTI force platform sampling at 480 Hz.
1.4 Data Analysis
The spatial coordinates of markers were projected onto the sagittal plane. For bilateral markers, their midpoints were projected onto this plane. In order to verify that whole-body pointing was actually constrained to the sagittal plane, a principal component analysis (PCA) was performed on the three-dimensional trajectories of all markers. PCA showed that the direction of minimal variability of the marker displacements was in the medio-lateral direction and the variability along this direction was negligible compared to the variability in the sagittal plane. Since the angle between the medio-lateral direction and the 3rd eigen-vector (i.e. the direction of minimal variability of the data) was less than 5 degrees, and the variability along this direction represented only 1.3% of the variability of the markers trajectories, we restricted the analysis to the sagittal plane.
A biomechanical model of the whole body was implemented and validated for each subject using the force platform data (Tagliabue et al. 2008). This 7-segment model was used to estimate the anterior-posterior position of the whole body CoM as well as the upper and lower body CoM (UCoM, LCoM, respectively). The upper-body consisted of head, trunk, arms, forearm and hands. The lower-body included pelvis, thighs, shanks and feet. To decouple the upper-and lower-body contributions, the position of the whole-body CoM and LCoM were calculated as the distance from the lateral malleolus, whereas the UCoM position was calculated as the distance from the lumbar spine midway between bilateral PSIS. All CoM displacements are reported as a percentage of foot length to compensate for between-subject differences in the length of base of support.
For each trial, movement onset was defined as the last motion capture frame when the velocity of all markers was less than 5% of their peak velocity. Task execution time was defined as the difference between the frame when the fingertip endpoint reached its final position and the movement onset.
The primary dependent measures were displacement of the fingertip endpoint (voluntary movement) and of the whole body CoM (postural adjustment) and the temporal and spatial relationship between them. The subjective pointing performances were evaluated with two types of endpoint errors: constant error and variable error (Adamovich et al. 2001b). Constant error (accuracy) and variable error (precision) are, respectively, the mean and the standard deviation of the length of the vector connecting the target with the final, pointing endpoint position. Pointing errors were calculated individually for each subject on the five trials performed in the EC condition. Endpoint error was not calculated for the EO condition because the fingertip of every subject accurately reached the target.
To determine whether control of the endpoint-or CoM-trajectory was impaired by lack of visual feedback, we calculated their mean jerk (i.e. the 3rd time derivative of position). Increased jerk (more frequent changes of acceleration) reflected increased corrections of the movement trajectory. Before the jerk was calculated, the fingertip and CoM trajectories were normalized in time and amplitude to compensate for the effects of different movement durations among trials and different displacement magnitudes between fingertip and CoM trajectories. In addition, the coefficient of correlation (R) between the velocity profiles of the pointing trajectories and CoM trajectories for each subject was taken as index of temporal coupling between the focal and postural components of the movement.
Spatial coupling between the focal and postural components was evaluated across subjects in each group. Spatial coupling was calculated as the R between the individual changes in horizontal pointing errors of the fingertip endpoint between the EO and EC conditions and the changes in horizontal body CoM displacement between the EO and EC conditions.
To address the hypothesis that pointing errors in the EC condition differed between groups, the nonparametric Mann-Whitney U test with group as a factor and the horizontal and vertical components of the pointing errors (in the sagittal plane) as dependent variables was used. For the other parameters describing the movement features, the Mann-Whitney U test was performed in both EO and EC conditions. The p values reported in the results section for the U test are exact probabilities for small samples.
To verify whether the lack of vision affects movement execution, the non-parametric Wilcoxon matched pairs test was performed for both CTR and PD subjects with vision condition (EO, EC) as factor and the parameter of interest as dependent variable. To determine possible differences in the effects of vision between the two groups, the difference between the median values of each parameter in the two visual conditions was computed for each subject and used as the dependent variable in a non-parametric Mann-Whitney U test, where the group was the independent factor.
To test whether the fatigue could affect the performances of the subject with PD the parameters a non-parametric Friedman test (χ2) and the Kendall’s Coefficient of Concordance (Kcc) were performed on the analyzed movement parameters with the trial number as independent variable, in both EC and EO conditions.
2 Results
2.1 Effects of PD and Vision on Pointing Errors
Although the bar graph in Fig. 2A shows that apparently the subjects with PD tend to take longer than CTR to complete the reach in both visual conditions, the differences between groups in pointing speed are not statistically significant. Fig. 2B shows that both groups of subjects had longer execution time in the EC, than in EO, condition (Z=2.80, p<0.01; Z=2.80, p<0.01 for CTR and PD respectively) and that the increases in the execution times without vision are not different for the two groups. The trial number does not affect the execution time of PD subjects either with EC (χ2(10,4)=1.52, p<0.82; Kcc=0.04) or with EO (χ2(10,4)=0.88, p<0.93; Kcc=0.02).
Fig. 2.
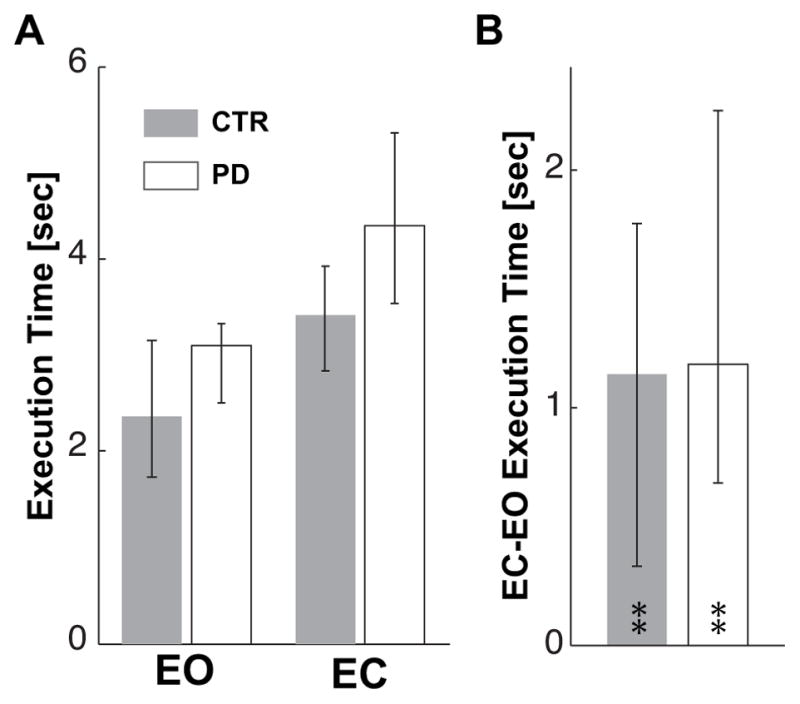
Execution time. A. Median values of pointing execution times for Eyes Open (EO) and Eyes Closed (EC) conditions and for CTR and PD subjects. B. Median value of the individual differences between EC and EO conditions for the CTR and PD groups. Zero on the ordinate axis corresponds to no effect of eyes closure.
In A and B and following figures, upper and lower whiskers represent the 25th and 75th percentile of the data distribution respectively. The ** indicate significant (p<0.01) effects of eyes closure on the execution time for both groups.
Fig. 3 shows that the constant and variable components of the pointing errors in the EC condition are not statistically different between groups.
Fig. 3.
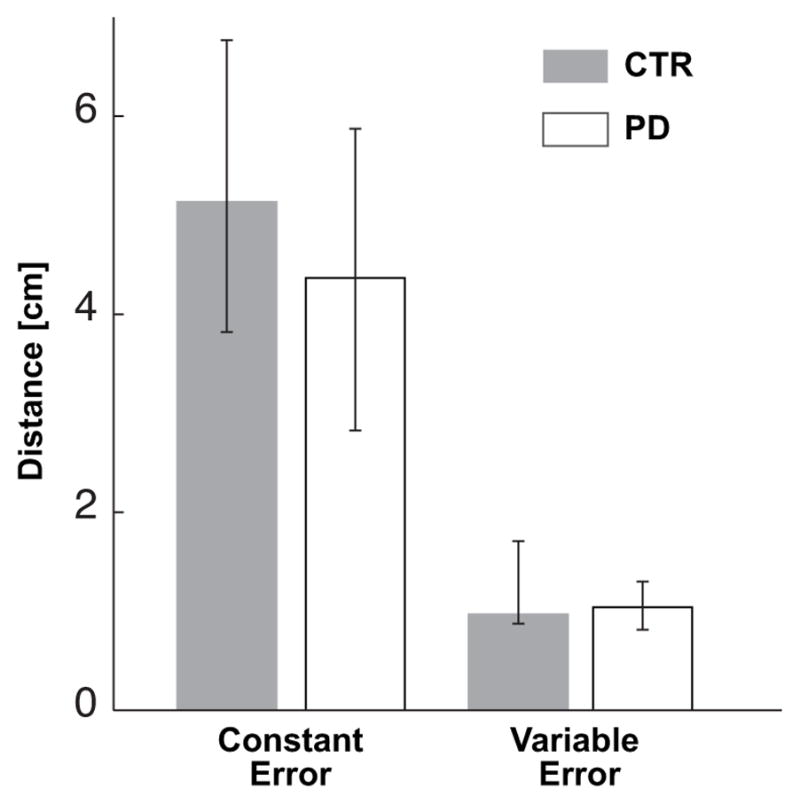
Median values of the constant and variable components of the pointing error for the CTR and PD groups in the EC condition.
These results are also supported by the error ellipse representation shown in Fig. 4, in which the CTR subjects appear to overshoot the target slightly more than the PD subjects with EC and the shape of the error ellipses appears a bit different between groups.
Fig. 4.
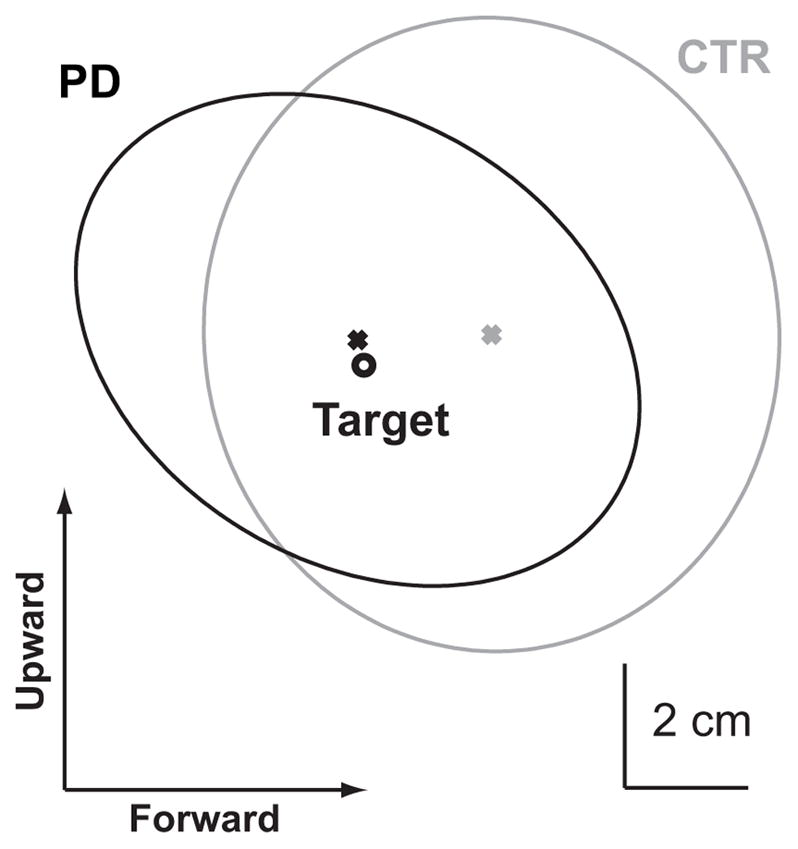
Distribution of pointing errors in the sagittal plane. The gray and black ellipses represent the 95% confidence interval of the pointing endpoint performed with eyes closed for the control (gray) and PD (black) groups. The black circle represents the target location. The ’x’ represents the center of the error ellipse. In this representation, subjects are located to the left of the target (see Fig. 1), therefore rightward and downward endpoints represent pointing overshoots, whereas upward and leftward endpoints represent undershoots.
Significant differences between the two groups were not found even when the vertical and anteroposterior components of the constant and variable pointing endpoint errors were considered separately.
2.2 Effects of PD and Vision on Body CoM control
Whole-body CoM
While pointing to the target with EO, the subjects with PD and the CTR subjects moved their body CoM forward the same distance. However, Figure 5A shows that in the EC condition, 7 out of 10 subjects with PD decreased their forward CoM displacements, whereas the CTR group slightly increased the forward CoM displacement. Fig. 5B compares the effect of EC on CoM displacement for the CTR and PD groups. EC did not significantly alter the CTR groups final CoM position. In contrast, EC significantly reduced the PD groups final CoM displacement (Z=2.60, p<0.01). The different effect of the lack of vision on the two groups is statistically significant (Z=2.04, p<0.05). However, the differences among subjects with PD shown in Fig. 5A does not depend on the severity of the disease. There is no significant correlation between the CoM displacement or change in displacement with eyes closed and either UPDRS or Hoehn & Yahr scores. The trial number does not affect the CoM displacement of PD subjects either with EC (χ2(10,4)=5.84, p<0.21; Kcc=0.15) or with EO (χ2(10,4)=3.2, p<0.52; Kcc=0.08).
Fig. 5.
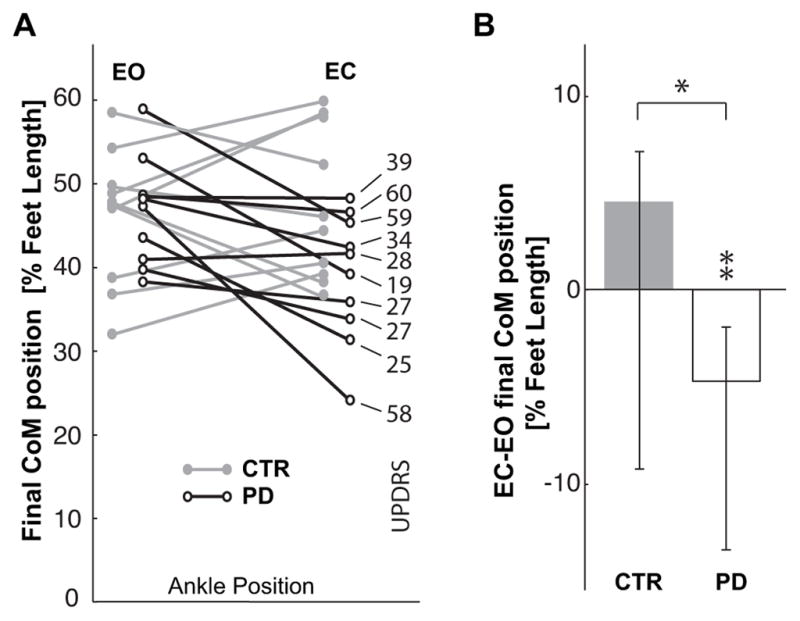
A. Comparison of final anterior-posterior position of the CoM in front of the ankle joints, CoMx, for the CTR and PD groups. Data are reported as percentage of foot length for the EC and EO conditions, so that the ordinate 0 value corresponds to the ankle position and 100 represents the limit of the base of support. On the right, the UPDRS score of each PD subject is related to their corresponding CoM displacement. B. Median values of the individual differences between the EC and EO condition for CTR and PD subjects. Zero on the ordinate axis corresponds to no effect of eyes closure. ** indicates a significant effect (p<0.01) of eyes closure for the subjects with PD. * indicates significant difference (p<0.05) in the effect of the eyes closure between the groups.
Panel A and B of figure 6 show that the jerkiness of the CoM trajectory increased when both CTR and PD subjects executed the pointing movement with EC (CTR: Z=2.08, p<0.05; PD: 2.70, p<0.01), but the increase observed for the subjects with PD was significantly greater than for CTRs (Z=2.72, p<0.01).
Fig. 6.
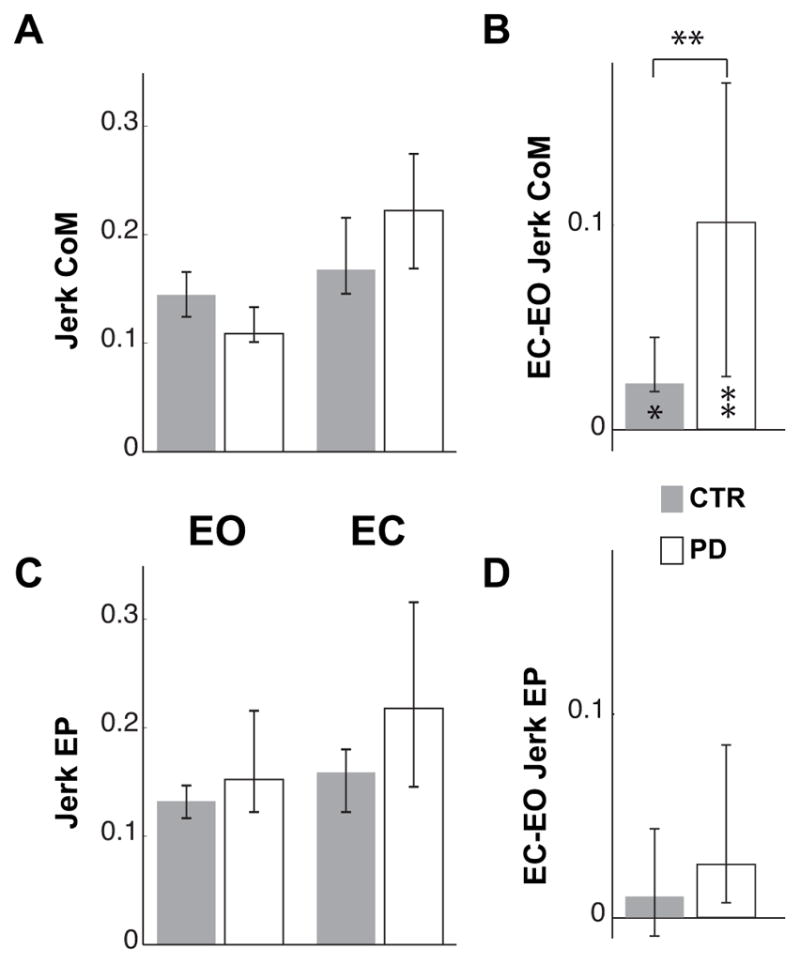
Normalized Jerk of CoM and finger trajectory. A and C represent the median Jerk of the CoM and finger endpoint (EP) in the EO and EC conditions for controls (CTR) and subjects with PD. Because of the time and amplitude normalization the values are dimensionless. B and D illustrate the median of the individual differences between EC and EO conditions of the CoM and EP Jerk. The zero value indicates no effect of eyes closure.
The upper and lower whiskers represent the 25th and 75th percentile of the data distribution respectively. In lower part of B, * and ** indicate significant (p<0.05 and p<0.01 respectively) effects of eyes closure on the Jerk of the CoM for both groups. In the upper part of B, ** indicates a significant difference (p<0.01) between the effects of the eyes closure on the CoM Jerk in CTR and PD subjects.
Panel C and D of Fig. 6 show that, unlike CoM jerk, the jerk of the pointing endpoint trajectory was not significantly affected by visual condition or by group and the effect of the lack of vision was similar for the two groups.
Upper-versus lower-body CoM
The similar forward displacements of the whole body CoM for both CTR and PD subjects in the EO condition are due to similar control of upper-and lower-body masses. Despite a stooped posture in the PD subjects, there was no significant difference in forward displacements of the upper body CoM or in backward displacements of the lower body CoM in the EO condition (Fig. 7A and C).
Fig. 7.
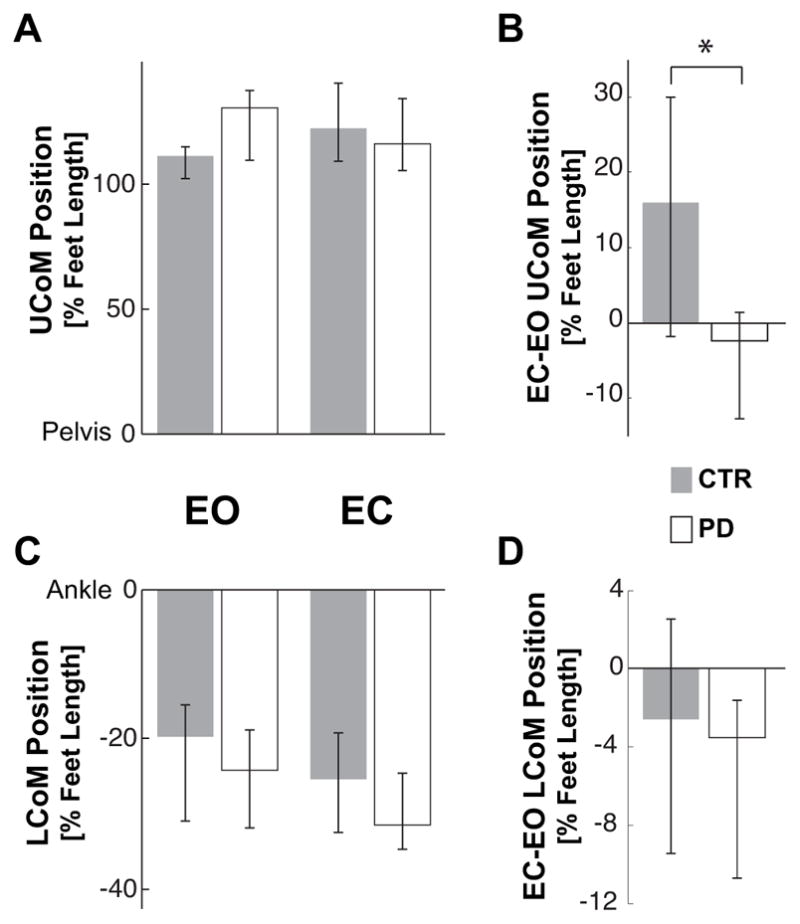
Final position of the Upper and Lower body CoM. A and C represent the median anterior-posterior position of the Upper body CoM (UCoM) and Lower body CoM (LCoM) in the EO and EC conditions for controls (CTR) and subjects with PD. The CoM position is reported as percentage of the foot length with zero corresponding to the pelvis position for the UCoM and ankle position for the LCoM. B and D illustrate the median of the individual differences between EC and EO conditions of the UCoM and LCoM. The zero value indicates no effect of eyes closure. * indicates a significant difference (p<0.05) between the effects of the eyes closure on the UCoM in CTR and PD subjects.
Although both groups similarly increased the amplitude of the backward lower-body CoM displacement with EC (see Fig. 7D), only the CTR subjects compensated for this backward motion by increasing the forward displacement of their upper-body CoM (Fig. 7B). The difference of the effect of vision on the upper-body CoM replacement for the two groups is statistically significant (Z=2.27, p=0.02).
2.3 Coordination of Pointing with Posture
No differences were observed between CTR and PD groups’ temporal correlation between pointing- and CoM-trajectory velocities in the EO or EC conditions (Fig. 8A). However, the effect of visual deprivation on the temporal coupling between the voluntary and postural components of the task was significantly different between groups. That is, while CTR subjects tended to increase the correlation between the pointing and CoM-trajectory velocity in the EC condition, the subjects with PD showed a decrease of this coordination (Fig. 8B).
Fig. 8.
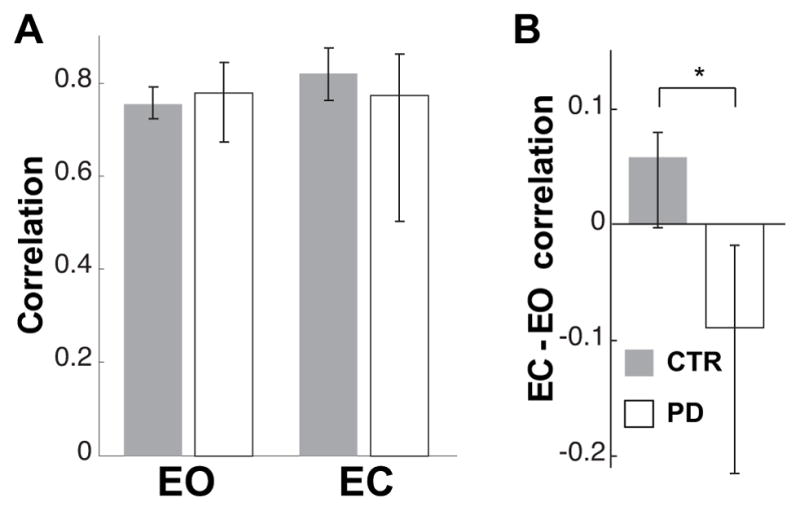
Temporal coupling between postural and voluntary components of the task. A. Median values of correlation between CoM and fingertip velocity profile in EO and EC conditions for controls and subjects with PD. Values equal to 1 indicate a perfect correlation. B. Median values of the individual differences in correlation between the EC and EO conditions for CTR and subjects with PD. Zero on the ordinate represents no effect of eye closure. * indicates significant difference (p<0.05) in the effect of eyes closure between the groups.
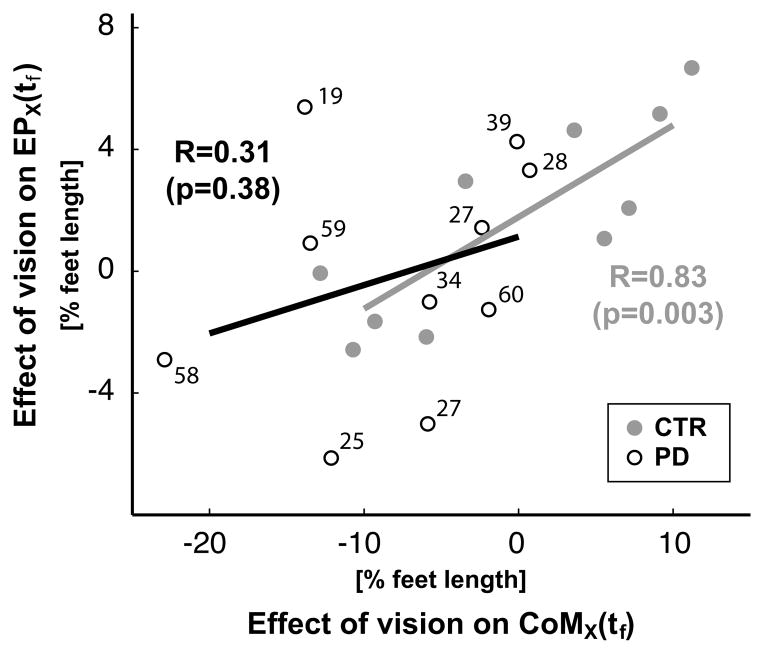
Spatial coupling between the voluntary and postural components of the task were affected by eye closure in the PD, but not the control subjects. The relative effect of eye closure on fingertip and CoM displacement were significantly correlated across the CTR, but not the PD, subjects. As shown in Figure 9, the more each control subject increased their pointing endpoint displacement with EC, the more they also increased their CoM displacement (R=0.83, p=0.003). In contrast, the correlation between the effects of vision on pointing endpoint and CoM displacements was not significant for the subjects with PD (R=0.31, p=0.38). For example, of the 3 PD subjects who moved their CoM backward 10% more with EC than EO, one subject increased endpoint displacement, one decreased endpoint displacement and one did not change endpoint displacement. Besides, Figure 9 shows that the decline of the coupling between the effect of the eye closure on the voluntary and postural components of the movement can not be ascribed to the PD severity.
Fig. 9.
The effect of eyes closure on spatial coupling between the final horizontal CoM position, CoMx, and on the pointing error, EPx. Both variables are represented as percentage of subject foot length. Each point represents a subject. The UPDRS score is reported for each subject with PD. The coefficient of correlation, R, between the vision effect on CoMx and EPx, as well as the p-values for testing the hypothesis of no correlation are reported for the PD and CTR groups in black and in gray respectively.
3 Discussion
This study demonstrates the importance of an intact basal ganglia and proprioceptive feedback for the precision and coordination of the postural (body CoM) with the voluntary (fingertip pointing) components of a whole-body pointing task while standing. The key effects of PD were: (1) no deterioration in precision of pointing to targets with eyes closed and with eyes open; (2) undershooting of whole body CoM displacement with eyes closed; (3) impaired temporal coordination between the fingertip endpoint and body CoM velocity profiles; (4) increased jerkiness of CoM displacement with eyes closed; and (5) poor spatial coupling between effects of eyes closure on body CoM and pointing error across subjects with PD. The significant effects of removing visual feedback on postural control and postural-voluntary movement coordination, but not on pointing accuracy in subjects with PD suggest that the basal ganglia are more important for proprioceptive mapping of whole-body coordination than for visual-proprioceptive mapping for pointing to a remembered endpoint.
3.1 Pointing movement
Previous studies of pointing by sitting PD subjects with similar disease severity have reported significantly more slowing and significantly larger than normal constant and variable pointing errors (Flowers 1975; Poizner et al. 1998; Adamovich et al. 2001b; Schubert et al. 2002; Maschke et al. 2003; Keijsers et al. 2005). Decrements in performance of PD subjects with eyes closed have been ascribed to impairment of proprioceptive integration for precise, voluntary movements (Adamovich et al. 2001b; Maschke et al. 2003). Analogous observations of impaired performance with eyes closed, compared to eyes open, in subject with PD have also been reported recently for compensatory stepping and locomotion (Almeida et al. 2005; Jacobs and Horak 2006). In the present study, however, even in the eyes closed condition, when PD subjects could not compensate with vision for unreliable proprioception, the subjects with PD were able to point to the target with similar slowing, accuracy and precision as then control subjects. How is this possible?
The most likely explanation for excellent pointing performance by moderately severe PD subjects with eyes closed in the Off state is that our subjects with PD formed accurate internal representation of target location from visual-spatial maps memorized prior to the movement. Consistent with our study, a previous study in sitting subjects who could view both their initial hand position and the position of the target, showed no larger pointing errors in subjects with PD than in controls (Poizner et al. 1998). Furthermore, the large head displacements associated with pointing allowed subjects to use vestibular information, instead of proprioception, to track the visual representation of the target. This hypothesis is consistent with recent studies suggesting that subjects with PD have an increased gain of vestibulospinal and visual control of posture, secondary to impaired proprioceptive control (Pastor et al. 1993; Smiley-Oyen et al. 2002; Maurer et al. 2004). Better than normal use of vestibular inputs for postural control to compensate for impaired use of proprioception also explains why subjects with PD show increased sway during quiet stance on a firm surface, but better than normal sway on an unstable surface (Chastan et al. 2008). In fact, our results are consistent with another study in which trunk and head movements were involved when subjects with PD pointed from a seated position (Poizner et al. 2000). Even while sitting, the vestibular information produced by the head movements associated with pointing could be used to update the visual representation of the target. Alternatively, the subjects with PD may have used preprogrammed trunk-arm movement synergies to compensate for the body displacements (Poizner et al. 2000; Adamovich et al. 2001a).
The lack of differences in the pointing endpoint accuracy between groups suggests that this task did not represent a particular challenge for subjects with moderately severe PD. In fact, the need to control stance equilibrium simultaneously while pointing did not seem to impair the voluntary pointing movement, as would be expected if the task was associated with fear of falling (Adkin et al. 2003) or required divided attention between the postural and voluntary components (Woollacott and Shumway-Cook 2002).
3.2 Equilibrium control
Despite the relative lack of effects of PD on the voluntary, pointing component of the movement, the postural strategy was affected by PD, but only when visual feedback was not allowed. With eyes closed, PD but not CTR subjects showed increased jerk in their CoM trajectory, consistent with increased active corrections to their movement. In addition, whereas control subjects tended to slightly increase the forward displacement of their body CoM when they closed their eyes, subjects with PD showed a reduction (undershooting) of their forward CoM displacement with eyes closed. This difference in whole body CoM displacement between groups was due to the fact that the CTR, but not the PD subjects, changed kinematic strategies when closing their eyes. With eyes closed, the controls increased the backward displacement of their lower-body CoM while concomitantly increasing the forward displacement of their upper-body CoM. In contrast, the subjects with PD increased the backward displacement of their lower-body CoM, but did not compensate by increasing the forward displacement of their upper-body CoM. Similar inflexibility of postural strategies with changes in support, instruction, and environmental conditions in subjects with PD has been demonstrated previously for responses to external perturbations (Horak et al. 1992; Chong et al. 1999–2000).
With eyes closed, the subjects with PD may have exaggerated their backward postural adjustments to increase their equilibrium safety margin from the front of foot support (Mancini et al. 2008). Without visual feedback, PD subjects’ proprioceptive deficits become more relevant and, thus, a more conservative postural strategy may be desirable (Jacobs and Horak 2006). Alternatively, the undershooting of forward CoM displacement in the EC condition may be because PD subjects were not able to accurately use proprioception to map how far their body CoM was moving forward. In fact, undershooting of intended limb and body motion associated with the bradykinesia of PD subjects has been proposed to be due to overestimation of movements due to impaired proprioception (Adamovich et al. 2001b; Almeida et al. 2005). Subjects with PD also undershoot body CoM movements while walking to remembered targets in the dark but improve their locomotion endpoint positions to remembered targets when they can view their bodies (Almeida et al. 2005). Similarly, the PD subjects undershoot their compensatory, postural stepping in response to external perturbations and increased their stepping accuracy to a visual target if they could view their legs (Jacobs and Horak 2006).
Surprisingly, the postural deficits of subjects with PD in this pointing task were not related to disease progression, although postural problems increase with severity of disease (Bloem et al. 2004). The inability to integrate proprioception for control posture could be a very early symptom of Parkinson’s disease, consistent with increased postural sway in newly-diagnosed, untreated subjects with PD (Chastan et al. 2008).
3.3 Movement-posture coordination
A coupling of the voluntary, with the postural, components of the whole body pointing movement could be seen in the control subjects by the strong temporal relationship between the velocity profiles of the pointing endpoint and body CoM displacement and by the strong correlation between the effects of vision on both the endpoint pointing and CoM displacements. Specifically, loss of visual feedback resulted in the same amount of over-shooting of the pointing endpoint and the forward CoM displacement in the control, but not the PD subjects. Similarly, control, but not PD subjects, kept the same temporal synergy between the finger and CoM displacement with eyes closed. These results are consistent with the hypothesis that the voluntary (focal pointing) and postural (body CoM) components of the whole-body pointing task are normally tightly coupled (Stapley et al. 1999; Kerlirzin et al. 1999; Pozzo et al. 2002), but they also suggest that this coupling can be disrupted by PD when subjects need to rely on proprioception (Massion 1992; Frank et al. 2000).
The lack of relation of the UPDRS with the decrease in focal-postural coupling suggests that these coordination problems are not simply rooted in rigidity, bradykinesia or tremor, since all of these symptoms were related to the UPDRS. Since PD subjects with mild or more severe symptoms could show just as disordered posture-voluntary coordination, this may suggest a problem in pontomedulary postural integration sites (Massion 1992; Schepens and Drew 2004, Braak and Tredici 2008) or in the mesocortical pathway (Owen 2004) that are affected early in the disease.
Although the substantial heterogeneity of disease severity in the patients involved in this study could be considered as a potential issue for the interpretation of the results, it allowed to show that fundamental aspects of the motor behavior, such as the coordination between movement and posture, can be affected independently from the classic symptoms used to classify the stage of Parkinson’s disease.
Disruption of focal-postural coupling in the subjects with PD occurred only when they closed their eyes, suggesting that this coupling depends, at least partly, upon proprioceptive feedback, which is impaired by PD. When visual information is not available, healthy subjects could still use proprioceptive control for this coupling, whereas subjects with PD must depend upon memorized visual information and vestibular inputs to compensate for their difficulty integrating proprioceptive information. Many studies have demonstrated impaired joint, force, and cutaneous kinesthesia as well as impaired motor coordination with eye closure in patients with PD, consistent with poor use of propoprioception (Zia et al. 2000; Poizner et al. 2000; Adamovich et al. 2001a; Maschke et al. 2006; Konczak et al. 2007). It is possible that the disruption in coupling between the CoM and finger pointing components of the task is due to the role of the basal ganglia in using proprioceptive feedback to formulate internal representation of body maps to help control multisegmental movements. Studies on human and animal subjects with proprioceptive loss and lack of visual feedback are consistent with an important role of proprioceptive pathways for multisegmental coordination when the body cannot be seen (Fung and Macpherson 1999; Horak 2001; Cordo and Gurfinkel 2004; Vaugoyeau et al. 2007). The poor temporal coordination between the voluntary and postural components of the movement in our standing subjects with PD was not related to any degradation of synchrony between pointing and postural movement onsets however, as suggested for pointing from a seated position involving the trunk (Poizner et al. 2000). In the present study, the impaired voluntary-postural coupling in PD appeared due to increasing jerk of the CoM trajectory without similar effects of loss of vision on pointing endpoint trajectory. The difference between the present and previous findings reflects a fundamental difference in the motor control involved in executing a pointing movement while seated or standing. While standing, the CNS must coordinate movement execution with balance maintenance, so we can investigate the effects of PD and vision on the coordination between movement and posture.
Although proprioceptive feedback aids voluntary-postural coupling, central nervous system coupling of voluntary-postural components is also involved, consistent with the hypothesis of Massion (1992). In fact, recently, separate populations of brainstem neurons have been identified that are active in relation to either the postural or the voluntary component of a paw lift in cats (Schepens and Drew 2004). Since the brainstem reticular formation in which these neurons can be found receive descending projections from the basal ganglia, it is not surprising that PD may disrupt this coupling between the postural and voluntary components of the task.
4 Conclusions
The effect of vision deprivation on the postural, but not the voluntary, component of the movement and disruption of the posture-movement coupling by a basal ganglia disease, such as PD, could be explained by the existence of separate pathways for programming the voluntary and postural components of whole-body goal-oriented tasks, (Massion 1992; Slijper et al. 2002, Massion et al. 2004, Drew et al. 2004, Robert et al. 2007), and by a role of basal ganglia in coordinating program selection and coupling (Massion 1992; Grasso et al. 1999; Poizner et al. 2000). The fact that the decoupling was observed in subjects with PD only when they could not use visual feedback suggests that coordination between the voluntary and postural motor commands involves not only the basal ganglia, but also proprioceptive pathways, critical for postural control (Pigeon and Feldman 1998; Poizner et al. 2000; Maurer et al. 2006). In contrast, when subjects have prior visual spatial information about hand and target location, the voluntary component of whole-body pointing movement did not seem to require proprioceptive integration, but could be carried out via remembered visual-motor transformations, assisted by vestibular feedback.
Acknowledgments
We thank Jesse Jacobs, Dr. Patricia Carlson-Kuhta, Dr. Charles Russell and Triana Nagel Nelson for assistance with data collection and subject recruitment.
Supported by a grant from the National Institute on Aging of NIH (AG06457), by the Italian Space Agency DCMC contract and the Italian Institute of Technology.
List of Abbreviations
- CoM
center of mass
- CoP
center of pressure
- CTR
control
- EO
eyes open
- EC
eyes closed
- LCoM
lower center of mass
- PCA
principal component analysis
- PD
Parkinson’s disease
- R
coefficient of correlation
- UCoM
upper center of mass
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Michele Tagliabue, Email: michele.tagliabue@parisdescartes.fr, LNRS (UMR CNRS 7060), University Paris Descartes, 45 rue Des Saints Pères, 75270 Paris Cedex 06, France, Phone: +33 (0)1 4286 3315, Fax: +33 (0)1 4286 3399.
Giancarlo Ferrigno, Politecnico di Milano, Bioengineering Department, Neuroengineering and Medical Robotics Laboratory, Milano, Italy.
Fay Horak, Department of Neurology, Oregon Health & Science University, Beaverton, OR, USA.
References
- Adamovich S, Archambault P, Ghafouri M, Levin M, Poizner H, Feldman A. Hand trajectory invariance in reaching movements involving the trunk. Exp Brain Res. 2001a;138(3):288–303. doi: 10.1007/s002210100694. [DOI] [PubMed] [Google Scholar]
- Adamovich SV, Berkinblit MB, Hening W, Sage J, Poizner H. The interaction of visual and proprioceptive inputs in pointing to actual and remembered targets in parkinson’s disease. Neuroscience. 2001b;104(4):1027–1041. doi: 10.1016/s0306-4522(01)00099-9. [DOI] [PubMed] [Google Scholar]
- Adkin AL, Frank JS, Jog MS. Fear of falling and postural control in parkinson’s disease. Mov Disord. 2003;18(5):496–502. doi: 10.1002/mds.10396. [DOI] [PubMed] [Google Scholar]
- Alexandrov A, Aurenty R, Massion J, Mesure S, Viallet F. Axial synergies in parkinson patients during voluntary trunk bending. Gait and Posture. 1998;8:124–135. doi: 10.1016/s0966-6362(98)00026-5. [DOI] [PubMed] [Google Scholar]
- Almeida Q, Frank J, Roy E, Jenkins M, Spaulding S, Patla A, Jog M. An evaluation of sensorimotor integration during locomotion toward a target in parkinson’s disease. Neuroscience. 2005;134:283–293. doi: 10.1016/j.neuroscience.2005.02.050. [DOI] [PubMed] [Google Scholar]
- Bloem BR, Hausdorff JM, Visser JE, Giladi N. Falls and freezing of gait in parkinson’s disease: a review of two interconnected, episodic phenomena. Mov Disord. 2004;19(8):871–884. doi: 10.1002/mds.20115. [DOI] [PubMed] [Google Scholar]
- Braak H, Tredici KD. Invited article: Nervous system pathology in sporadic Parkinson disease. Neurology 2008 [Google Scholar]
- Chastan N, Debono B, Maltte D, Weber J. Discordance between measured postural instability and absence of clinical symptoms in parkinson’s disease patients in the early stages of the disease. Mov Disord. 2008;23(3):366–372. doi: 10.1002/mds.21840. [DOI] [PubMed] [Google Scholar]
- Chong RK, Horak FB, Woollacott MH. Parkinson’s disease impairs the ability to change set quickly. J Neurol Sci. 2000;175(1):57–70. doi: 10.1016/s0022-510x(00)00277-x. [DOI] [PubMed] [Google Scholar]
- Chong RK, Jones CL, Horak FB. Postural set for balance control is normal in alzheimer’s but not in parkinson’s disease. J Gerontol A Biol Sci Med Sci. 1999;54(3):M129–M135. doi: 10.1093/gerona/54.3.m129. [DOI] [PubMed] [Google Scholar]
- Cordo PJ, Gurfinkel VS. Motor coordination can be fully understood only by studying complex movements. Prog Brain Res. 2004;143:29–38. doi: 10.1016/S0079-6123(03)43003-3. [DOI] [PubMed] [Google Scholar]
- Drew T, Prentice S, Schepens B. Cortical and brainstem control of locomotion. Prog Brain Res. 2004;143:251–261. doi: 10.1016/S0079-6123(03)43025-2. [DOI] [PubMed] [Google Scholar]
- Flowers K. Ballistic and corrective movements on an aiming task. intention tremor and parkinsonian movement disorders compared. Neurology. 1975;25(5):413–421. doi: 10.1212/wnl.25.5.413. [DOI] [PubMed] [Google Scholar]
- Frank JS, Horak FB, Nutt J. Centrally initiated postural adjustments in parkinsonian patients on and off levodopa. J Neurophysiol. 2000;84(5):2440–2448. doi: 10.1152/jn.2000.84.5.2440. [DOI] [PubMed] [Google Scholar]
- Fung J, Macpherson JM. Attributes of quiet stance in the chronic spinal cat. J Neurophysiol. 1999;82(6):3056–3065. doi: 10.1152/jn.1999.82.6.3056. [DOI] [PubMed] [Google Scholar]
- Gantchev N, Viallet F, Aurenty R, Massion J. Impairment of posturo-kinetic co-ordination during initiation of forward oriented stepping movements in parkinsonian patients. Electroencephalogr Clin Neurophysiol. 1996;101(2):110–120. doi: 10.1016/0924-980x(95)00253-h. [DOI] [PubMed] [Google Scholar]
- Grasso R, Peppe A, Stratta F, Angelini D, Zago M, Stanzione P, Lacquaniti F. Basal ganglia and gait control: apomorphine administration and internal pallidum stimulation in parkinson’s disease. Exp Brain Res. 1999;126(2):139–148. doi: 10.1007/s002210050724. [DOI] [PubMed] [Google Scholar]
- Horak FB. Postural ataxia related to somatosensory loss. Adv Neurol. 2001;87:173–182. [PubMed] [Google Scholar]
- Horak FB, Dimitrova D, Nutt JG. Direction-specific postural instability in subjects with parkinson’s disease. Exp Neurol. 2005;193(2):504–521. doi: 10.1016/j.expneurol.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Horak FB, Nutt JG, Nashner LM. Postural inflexibility in parkinsonian subjects. J Neurol Sci. 1992;111(1):46–58. doi: 10.1016/0022-510x(92)90111-w. [DOI] [PubMed] [Google Scholar]
- Jacobs JV, Horak FB. Abnormal proprioceptive-motor integration contributes to hypo-metric postural responses of subjects with parkinson’s disease. Neuroscience. 2006;141(2):999–1009. doi: 10.1016/j.neuroscience.2006.04.014. [DOI] [PubMed] [Google Scholar]
- Keijsers NLW, Admiraal MA, Cools AR, Bloem BR, Gielen CCAM. Differential progression of proprioceptive and visual information processing deficits in parkinson’s disease. Eur J Neurosci. 2005;21(1):239–248. doi: 10.1111/j.1460-9568.2004.03840.x. [DOI] [PubMed] [Google Scholar]
- Kerlirzin Y, Pozzo T, Dietrich G, Vieilledent S. Effects of kinematics constraints on hand trajectory during whole-body lifting tasks. Neurosci Lett. 1999;277(1):41–44. doi: 10.1016/s0304-3940(99)00843-5. [DOI] [PubMed] [Google Scholar]
- Konczak J, Krawczewski K, Tuite P, Maschke M. The perception of passive motion in parkinson’s disease. J Neurol. 2007;254(5):655–663. doi: 10.1007/s00415-006-0426-2. [DOI] [PubMed] [Google Scholar]
- Latash ML, Aruin AS, Neyman I, Nicholas JJ, Shapiro MB. Feedforward postural adjustments in a simple two-joint synergy in patients with parkinson’s disease. Electroencephalogr Clin Neurophysiol. 1995;97(2):77–89. doi: 10.1016/0924-980x(94)00272-9. [DOI] [PubMed] [Google Scholar]
- Mancini M, Rocchi L, Horak FB, Chiari L. Effects of parkinson’s disease and levodopa on functional limits of stability. Clin Biomech. 2008;23(4):450–458. doi: 10.1016/j.clinbiomech.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maschke M, Gomez CM, Tuite PJ, Konczak J. Dysfunction of the basal ganglia, but not the cerebellum, impairs kinaesthesia. Brain. 2003;126(Pt 10):2312–2322. doi: 10.1093/brain/awg230. [DOI] [PubMed] [Google Scholar]
- Maschke M, Tuite PJ, Krawczewski K, Pickett K, Konczak J. Perception of heaviness in parkinson’s disease. Mov Disord. 2006;21(7):1013–1018. doi: 10.1002/mds.20876. [DOI] [PubMed] [Google Scholar]
- Massion J. Movement, posture and equilibrium: interaction and coordination. Prog Neurobiol. 1992;38(1):35–56. doi: 10.1016/0301-0082(92)90034-c. [DOI] [PubMed] [Google Scholar]
- Massion J, Alexandrov A, Peterka RJ. Why and how are posture and movement coordinated? Prog Brain Res. 2004;143:13–27. doi: 10.1016/S0079-6123(03)43002-1. [DOI] [PubMed] [Google Scholar]
- Maurer C, Mergner T, Peterka RJ. Abnormal resonance behavior of the postural control loop in parkinson’s disease. Exp Brain Res. 2004;157(3):369–376. doi: 10.1007/s00221-004-1852-y. [DOI] [PubMed] [Google Scholar]
- Maurer C, Mergner T, Peterka RJ. Multisensory control of human upright stance. Exp Brain Res. 2006;171(2):231–250. doi: 10.1007/s00221-005-0256-y. [DOI] [PubMed] [Google Scholar]
- Owen AM. Cognitive dysfunction in parkinson’s disease: the role of frontostrialtal circuitry. Neuroscientist. 2004;10(6):525–537. doi: 10.1177/1073858404266776. [DOI] [PubMed] [Google Scholar]
- Pastor MA, Day BL, Marsden CD. Vestibular induced postural responses in parkinson’s disease. Brain. 1993;116 ( Pt 5):1177–1190. doi: 10.1093/brain/116.5.1177. [DOI] [PubMed] [Google Scholar]
- Patron J, Stapley P, Pozzo T. Human whole-body reaching in normal gravity and micro-gravity reveals a strong temporal coordination between postural and focal task components. Exp Brain Res. 2005;165(1):84–96. doi: 10.1007/s00221-005-2283-0. [DOI] [PubMed] [Google Scholar]
- Pigeon P, Feldman AG. Compensatory arm-trunk coordination in pointing movements is preserved in the absence of visual feedback. Brain Res. 1998;802(1–2):274–280. doi: 10.1016/s0006-8993(98)00616-7. [DOI] [PubMed] [Google Scholar]
- Poizner H, Feldman AG, Levin MF, Berkinblit MB, Hening WA, Patel A, Adamovich SV. The timing of arm-trunk coordination is deficient and vision-dependent in parkinson’s patients during reaching movements. Exp Brain Res. 2000;133(3):279–292. doi: 10.1007/s002210000379. [DOI] [PubMed] [Google Scholar]
- Poizner H, Fookson OI, Berkinblit MB, Hening W, Feldman G, Adamovich S. Pointing to remembered targets in 3-d space in parkinson’s disease. Motor Control. 1998;2(3):251–277. doi: 10.1123/mcj.2.3.251. [DOI] [PubMed] [Google Scholar]
- Pozzo T, Stapley PJ, Papaxanthis C. Coordination between equilibrium and hand trajectories during whole body pointing movements. Exp Brain Res. 2002;144(3):343–350. doi: 10.1007/s00221-002-1052-6. [DOI] [PubMed] [Google Scholar]
- Prentice SD, Drew T. Contributions of the reticulospinal system to the postural adjustments occurring during voluntary gait modifications. J Neurophysiol. 2001;85(2):679–698. doi: 10.1152/jn.2001.85.2.679. [DOI] [PubMed] [Google Scholar]
- Robert G, Blouin J, Ruget H, Mouchnino L. Coordiantion between postural and movement controls: effect of changes in body mass distribution on postural and focal component characteristics. Exp Brain Res. 2007;181(1):159–171. doi: 10.1007/s00221-007-0916-1. [DOI] [PubMed] [Google Scholar]
- Rocchi L, Chiari L, Mancini M, Carlson-Kuhta P, Gross A, Horak FB. Step initiation in parkinson’s disease: influence of initial stance conditions. Neurosci Lett. 2006;406(1–2):128–132. doi: 10.1016/j.neulet.2006.07.027. [DOI] [PubMed] [Google Scholar]
- Schepens B, Drew T. Strategies for the integration of posture and movement during reaching in the cat. J Neurophysiol. 2003;90(5):3066–3086. doi: 10.1152/jn.00339.2003. [DOI] [PubMed] [Google Scholar]
- Schepens B, Drew T. Independent and convergent signals from the pontomedullary reticular formation contribute to the control of posture and movement during reaching in the cat. J Neurophysiol. 2004;92(4):2217–2238. doi: 10.1152/jn.01189.2003. [DOI] [PubMed] [Google Scholar]
- Schubert M, Prokop T, Brocke F, Berger W. Visual kinesthesia and locomotion in parkinson’s disease. Mov Disord. 2005;20(2):141–150. doi: 10.1002/mds.20281. [DOI] [PubMed] [Google Scholar]
- Schubert T, Volkmann J, Mller U, Sturm V, Voges J, Freund H-J, Cramon DYV. Effects of pallidal deep brain stimulation and levodopa treatment on reaction-time performance in parkinson’s disease. Exp Brain Res. 2002;144(1):8–16. doi: 10.1007/s00221-002-1020-1. [DOI] [PubMed] [Google Scholar]
- Slijper H, Latash ML, Mordkoff JT. Anticipatory postural adjustements under simple and choice reaction time conditions. Brain Res. 2002;924(2):184–197. doi: 10.1016/s0006-8993(01)03233-4. [DOI] [PubMed] [Google Scholar]
- Smiley-Oyen AL, Cheng H-YK, Latt LD, Redfern MS. Adaptation of vibration-induced postural sway in individuals with parkinson’s disease. Gait Posture. 2002;16(2):188–197. doi: 10.1016/s0966-6362(02)00005-x. [DOI] [PubMed] [Google Scholar]
- Stapley PJ, Pozzo T, Cheron G, Grishin A. Does the coordination between posture and movement during human whole-body reaching ensure center of mass stabilization? Exp Brain Res. 1999;129(1):134–146. doi: 10.1007/s002210050944. [DOI] [PubMed] [Google Scholar]
- Tagliabue M, Pedrocchi A, Pozzo T, Ferrigno G. A mathematical tool to generate complex whole body motor tasks and test hypotheses on underlying motor planning. Med Biol Eng Comput. 2008;46(1):11–22. doi: 10.1007/s11517-007-0252-4. [DOI] [PubMed] [Google Scholar]
- Vaugoyeau M, Viel S, Assaiante C, Amblard B, Azulay JP. Impaired vertical postural control and proprioceptive integration deficits in parkinson’s disease. Neuroscience. 2007;146(2):852–863. doi: 10.1016/j.neuroscience.2007.01.052. [DOI] [PubMed] [Google Scholar]
- Woollacott M, Shumway-Cook A. Attention and the control of posture and gait: a review of an emerging area of research. Gait Posture. 2002;16(1):1–14. doi: 10.1016/s0966-6362(01)00156-4. [DOI] [PubMed] [Google Scholar]
- Zia S, Cody F, O’Boyle D. Joint position sense is impaired by parkinson’s disease. Ann Neurol. 2000;47(2):218–228. [PubMed] [Google Scholar]